Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds
Abstract
:1. Introduction
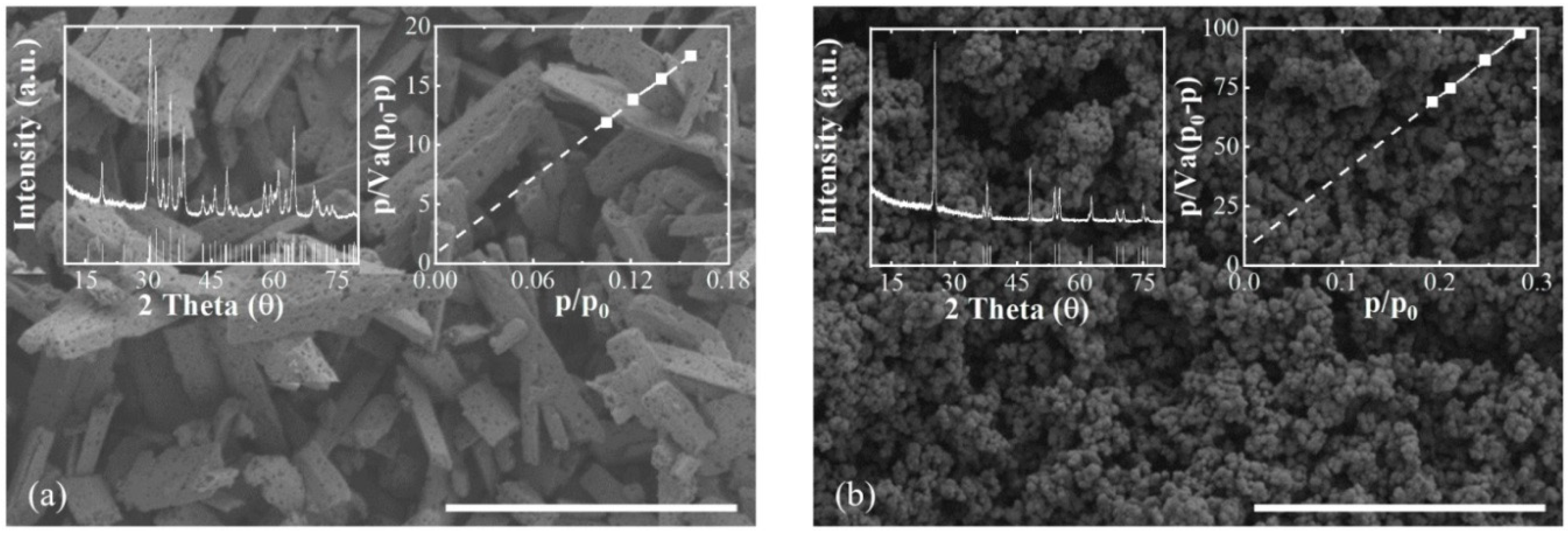
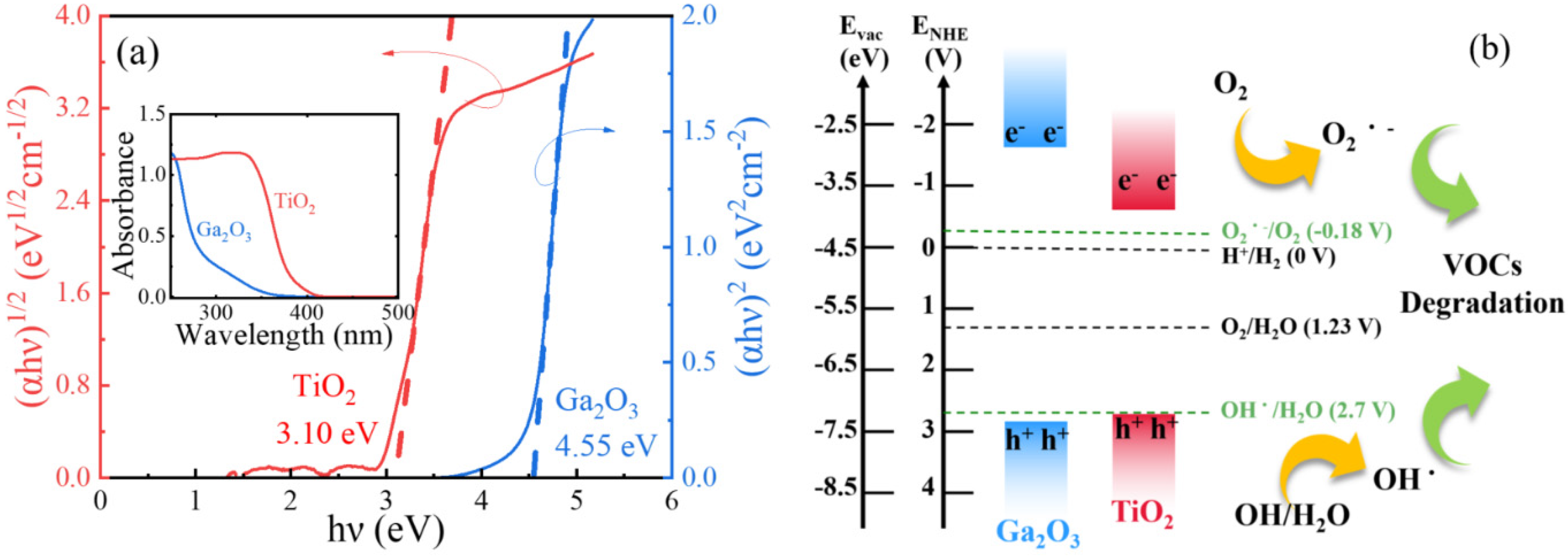
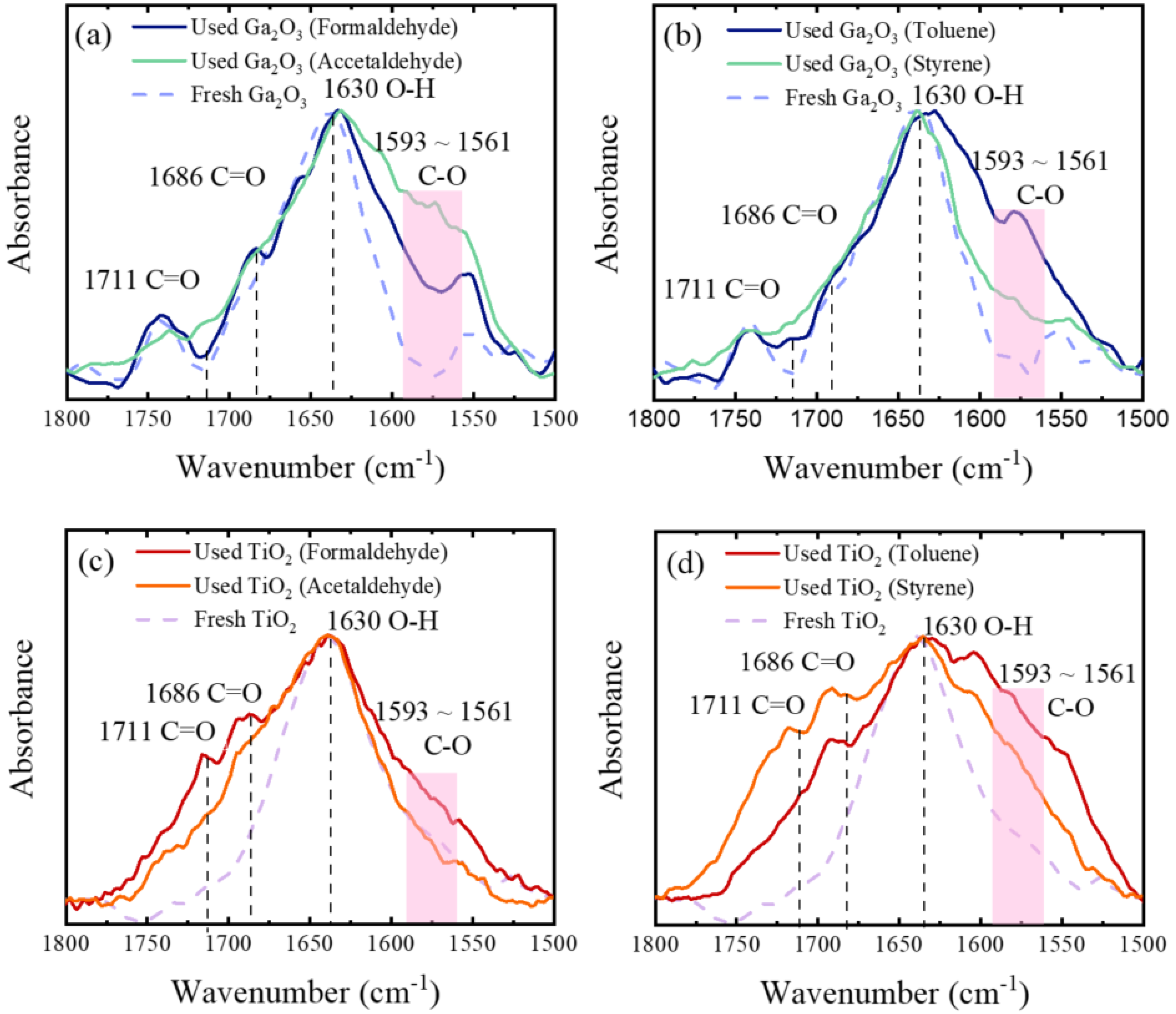
2. Results and Discussion
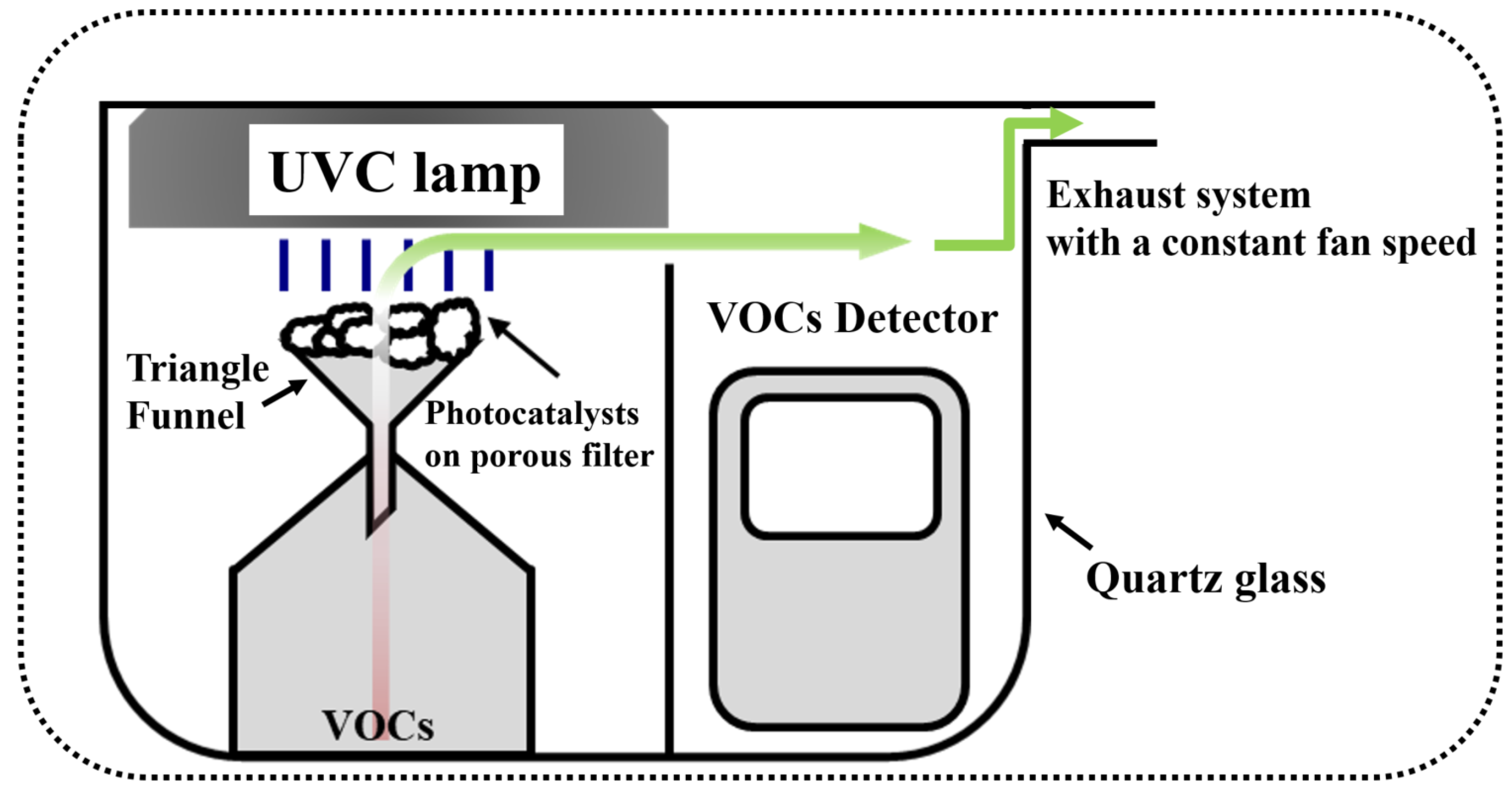
3. Materials and Methods
3.1. Characterization of Ga2O3 and TiO2 Photocatalysts
3.2. Photocatalytic Degradation
3.3. Kinetic Models Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cincinelli, A.; Martellini, T. Indoor air quality and health. Int. J. Environ. Res. Public Health 2017, 14, 1286–1290. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [Green Version]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, B.J.; Hwang, W.S. Enhanced Photocatalytic Activity of Electrospun β-Ga2O3 Nanofibers via In-Situ Si Doping. Using Tetraethyl Orthosilicate. Catalysts 2019, 9, 1005. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.J.; Yoo, T.H.; Kim, S.; Choi, W.; Song, Y.S.; Kwon, D.K.; Hwang, W.S. Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide Under Ultraviolet-C Irradiation. Catalysts 2019, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Photolysis-decomposition of water at the surface of an irradiated semiconductor. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Zhang, Z. TiO2-based catalysts for photocatalytic reduction of aqueous oxyanions: State-of-the-art and future prospects. Environ. Int. 2020, 136, 105453. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Wu, L.; Ding, Z.; Fu, X. Efficient decomposition of benzene over a β-Ga2O3 photocatalyst under ambient conditions. Environ. Sci. Technol. 2006, 40, 5799–5803. [Google Scholar] [CrossRef]
- Martra, G.; Coluccia, S.; Marchese, L.; Augugliaro, V.; Loddo, V.; Palmisano, L.; Schiavello, M. The role of H2O in the photocatalytic oxidation of toluene in vapour phase on anatase TiO2 catalyst: A FTIR study. Catal. Today 1999, 53, 695–702. [Google Scholar] [CrossRef]
- Mendez-Roman, R.; Cardona-Martínez, N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Patra, A.K.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Enhanced photocatalytic performance of novel self-assembled floral β-Ga2O3 nanorods. Curr. Appl. Phys. 2013, 13, 652–658. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, J.; Ding, Z.; Wu, L. Synthesis, characterization and photocatalytic activity of β-Ga2O3 nanostructures. Powder Technol. 2010, 203, 440–446. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, L.; Wang, X.; Ding, Z.; Li, Z.; Fu, X. Photocatalytic performance of α-, β-, and γ-Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–18. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics, 100th ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781138367296. [Google Scholar]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase–a review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Ryou, H.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Shin, M.; Cho, J.; Cho, B.J.; Hwang, W.S. Hydrothermal Synthesis and Photocatalytic Property of Sn-doped β-Ga2O3 Nanostructure. ECS J. Solid State Sci. Technol. 2020, 9, 045009. [Google Scholar] [CrossRef]
- Cho, J.; Sheng, A.; Suwandaratne, N.; Wangoh, L.; Andrews, J.L.; Zhang, P.; Banerjee, S. The middle road less taken: Electronic-structure-inspired design of hybrid photocatalytic platforms for solar fuel generation. Acc. Chem. Res. 2018, 52, 645–655. [Google Scholar] [CrossRef]
- Andrews, J.L.; Cho, J.; Wangoh, L.; Suwandaratne, N.; Sheng, A.; Chauhan, S.; Thakur, P.K. Hole extraction by design in photocatalytic architectures interfacing CdSe quantum dots with topochemically stabilized tin vanadium oxide. J. Am. Chem. Soc. 2018, 140, 17163–17174. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol–gel route. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Service, R.J.; Hillier, W.; Debus, R.J. Evidence from FTIR difference spectroscopy of an extensive network of hydrogen bonds near the oxygen-evolving Mn4Ca cluster of photosystem II involving D1-Glu65, D2-Glu312, and D1-Glu329. Biochemistry 2010, 49, 6655–6669. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849373664. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of specific surface by the BET method. Mat. Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Ghodsi, V.; Jin, S.; Byers, J.C.; Pan, Y.; Radovanovic, P.V. Anomalous photocatalytic activity of nanocrystalline γ-phase Ga2O3 enabled by long-lived defect trap states. J. Phys. Chem. C 2017, 121, 9433–9441. [Google Scholar] [CrossRef]
- Rochetto, U.L.; Tomaz, E. Degradation of volatile organic compounds in the gas phase by heterogeneous photocatalysis with titanium dioxide/ultraviolet light. J. Air. Waste. Manag. Assoc. 2015, 65, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]


| Formaldehyde | Acetaldehyde | Toluene | Styrene | ||
|---|---|---|---|---|---|
| k | Ga2O3 | 0.182 | 0.048 | 0.220 | 0.097 |
| TiO2 | 0.058 | 0.024 | 0.031 | 0.012 | |
| No photocatalyst (only UVC) | 0.016 | 0.009 | 0.025 | 0.006 | |
| R2 | Ga2O3 | 0.998 | 0.998 | 0.985 | 0.997 |
| TiO2 | 0.986 | 0.994 | 0.998 | 0.927 | |
| No photocatalyst (only UVC) | 0.999 | 0.997 | 0.999 | 0.939 |
| Compound | Formula | Molecular Weight (g/mol) | Dissociation Energy (kJ/mol) | Diffusion Coefficient in Air (cm2/s) |
|---|---|---|---|---|
| Formaldehyde | CH2O | 30.03 | 1,552 | 0.18 |
| Acetaldehyde | C2H4O | 44.05 | 2,722 | 0.12 |
| Toluene | C7H8 | 92.14 | 6,513 | 0.09 |
| Styrene | C8H8 | 104.15 | 7,124 | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, J.; Cho, B.J.; Hwang, W.S. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts 2020, 10, 545. https://doi.org/10.3390/catal10050545
Yoo TH, Ryou H, Lee IG, Cho J, Cho BJ, Hwang WS. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts. 2020; 10(5):545. https://doi.org/10.3390/catal10050545
Chicago/Turabian StyleYoo, Tae Hee, Heejoong Ryou, In Gyu Lee, Junsang Cho, Byung Jin Cho, and Wan Sik Hwang. 2020. "Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds" Catalysts 10, no. 5: 545. https://doi.org/10.3390/catal10050545
APA StyleYoo, T. H., Ryou, H., Lee, I. G., Cho, J., Cho, B. J., & Hwang, W. S. (2020). Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts, 10(5), 545. https://doi.org/10.3390/catal10050545






