Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Crystallographic Properties
2.2. Textural Properties and Morphology
2.3. Optical Properties
2.4. Sample Composition
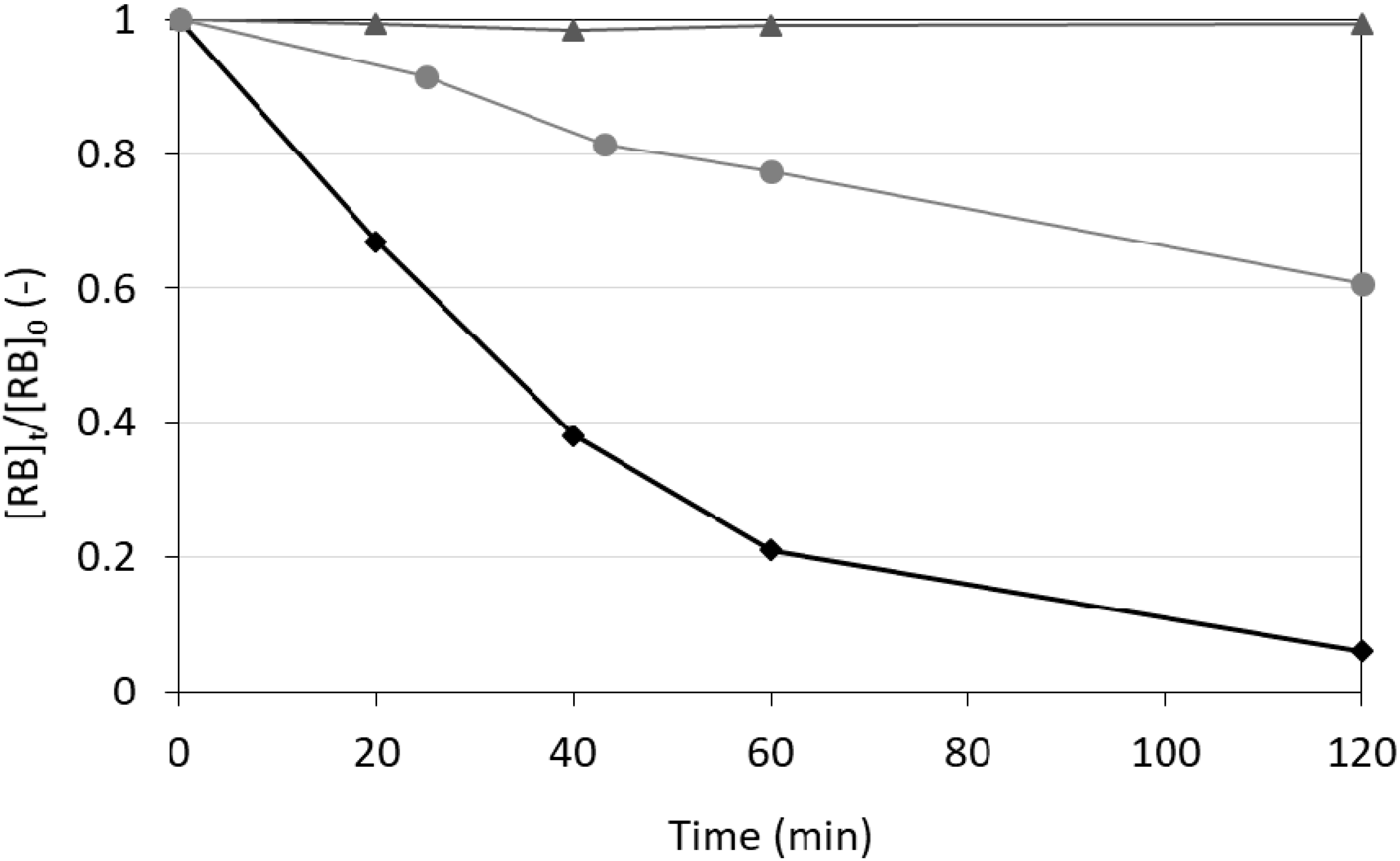
2.5. Photocatalytic Activity of the Powders
2.6. Film Crystallinity and Thickness
2.7. Photocatalytic Activity of the Films
2.8. Large-Scale Synthesis of TiO2/Fe0.5
3. Materials and Methods
3.1. Pure TiO2 Synthesis
3.2. Doped and Co-Doped TiO2 Synthesis
3.3. Film Deposition
3.4. Powder Characterization
3.5. Film Characterization
3.6. Photocatalytic Activity of the Powders under Visible Light
3.7. Photocatalytic Activity of the Films
3.8. Large-Scale Synthesis of TiO2/Fe0.5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, M.A.; Ghouri, A.M. Environmental Pollution: Its Effects on Life and Its Remedies. Res. World J. Arts Sci. Commer. 2011, 2, 276–285. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B. Application of Rhodococcus in Bioremediation of Contaminated Environments. In Biology of Rhodococcus; Springer: Berlin/Heidelberg, Germany, 2010; pp. 231–262. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; Bkc: Tokyo, Japan, 1999; ISBN 493905103X 9784939051036. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Subagio, D.P.; Srinivasan, M.; Lim, M.; Lim, T.-T. Photocatalytic degradation of bisphenol-A by nitrogen-doped TiO2 hollow sphere in a vis-LED photoreactor. Appl. Catal. B Environ. 2010, 95, 414–422. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Ofomaja, A.E. Study on light emission diode/carbon modified TiO2 system for tetracycline hydrochloride degradation. J. Photochem. Photobiol. A Chem. 2018, 360, 242–248. [Google Scholar] [CrossRef]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.T.; Allsopp, D.W.E.; Bowen, C.R.; van Grieken, R.; Marugán, J. Design and validation of a LED-based high intensity photocatalytic reactor for quantifying activity measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Hossaini, H.; Moussavi, G.; Farrokhi, M. Oxidation of diazinon in cns-ZnO/LED photocatalytic process: Catalyst preparation, photocatalytic examination, and toxicity bioassay of oxidation by-products. Sep. Purif. Technol. 2017, 174, 320–330. [Google Scholar] [CrossRef]
- Chevremont, A.-C.; Farnet, A.-M.; Coulomb, B.; Boudenne, J.-L. Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Sci. Total Environ. 2012, 426, 304–310. [Google Scholar] [CrossRef]
- Ghosh, J.P.; Langford, C.H.; Achari, G. Characterization of an LED Based Photoreactor to Degrade 4-Chlorophenol in an Aqueous Medium Using Coumarin (C-343) Sensitized TiO2. J. Phys. Chem. A 2008, 112, 10310–10314. [Google Scholar] [CrossRef]
- Takano, T.; Mino, T.; Sakai, J.; Noguchi, N.; Tsubaki, K.; Hirayama, H. Deep-ultraviolet light-emitting diodes with external quantum efficiency higher than 20% at 275 nm achieved by improving light-extraction efficiency. Appl. Phys. Express 2017, 10, 031002. [Google Scholar] [CrossRef]
- Epifani, M.; Giannini, C.; Tapfer, L.; Vasanelli, L. Sol–Gel Synthesis and Characterization of Ag and Au Nanoparticles in SiO2, TiO2, and ZrO2 Thin Films. J. Am. Ceram. Soc. 2000, 83, 2385–2393. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navío, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B Environ. 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Ofiarska, A.; Pieczyńska, A.; Fiszka Borzyszkowska, A.; Stepnowski, P.; Siedlecka, E.M. Pt–TiO2-assisted photocatalytic degradation of the cytostatic drugs ifosfamide and cyclophosphamide under artificial sunlight. Chem. Eng. J. 2016, 285, 417–427. [Google Scholar] [CrossRef]
- Semlali, S.; Pigot, T.; Flahaut, D.; Allouche, J.; Lacombe, S.; Nicole, L. Mesoporous Pt-TiO2 thin films: Photocatalytic efficiency under UV and visible light. Appl. Catal. B Environ. 2014, 150–151, 656–662. [Google Scholar] [CrossRef]
- Di Paola, A.; Marcì, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, S.; Ohtani, B. Preparation of Polycrystalline TiO2 Photocatalysts Impregnated with Various Transition Metal Ions: Characterization and Photocatalytic Activity for the Degradation of 4-Nitrophenol. J. Phys. Chem. B 2002, 106, 637–645. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Malengreaux, C.M.; Pirard, S.L.; Léonard, G.; Mahy, J.G.; Herlitschke, M.; Klobes, B.; Hermann, R.; Heinrichs, B.; Bartlett, J.R. Study of the photocatalytic activity of Fe3+, Cr3+, La3+ and Eu3+ single-doped and co-doped TiO2 catalysts produced by aqueous sol-gel processing. J. Alloys Compd. 2017, 691, 726–738. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Léonard, G.L.-M.; Malengreaux, C.M.; Mélotte, Q.; Lambert, S.D.; Bruneel, E.; Van Driessche, I.; Heinrichs, B. Doped sol–gel films vs. powders TiO2: On the positive effect induced by the presence of a substrate. J. Environ. Chem. Eng. 2016, 4, 449–459. [Google Scholar] [CrossRef]
- Léonard, G.L.-M.; Pàez, C.A.; Ramírez, A.E.; Mahy, J.G.; Heinrichs, B. Interactions between Zn2+ or ZnO with TiO2 to produce an efficient photocatalytic, superhydrophilic and aesthetic glass. J. Photochem. Photobiol. A Chem. 2018, 350, 32–43. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Tilkin, R.G.; Wolfs, C.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Douven, S. Ambient temperature ZrO2-doped TiO2 crystalline photocatalysts: Highly efficient powders and films for water depollution. Mater. Today Energy 2019, 13, 312–322. [Google Scholar] [CrossRef]
- Papadimitriou, V.C.; Stefanopoulos, V.G.; Romanias, M.N.; Papagiannakopoulos, P.; Sambani, K.; Tudose, V.; Kiriakidis, G. Determination of photo-catalytic activity of un-doped and Mn-doped TiO2 anatase powders on acetaldehyde under UV and visible light. Thin Solid Films 2011, 520, 1195–1201. [Google Scholar] [CrossRef]
- Malengreaux, C.M.; Douven, S.; Poelman, D.; Heinrichs, B.; Bartlett, J.R. An ambient temperature aqueous sol-gel processing of efficient nanocrystalline doped TiO2-based photocatalysts for the degradation of organic pollutants. J. Sol-Gel Sci. Technol. 2014, 71, 557–570. [Google Scholar] [CrossRef]
- Granados, G.; Martínez, F.; Páez-Mozo, E.A. Photocatalytic degradation of phenol on TiO2 and TiO2/Pt sensitized with metallophthalocyanines. Catal. Today 2005, 107–108, 589–594. [Google Scholar] [CrossRef]
- Mahy, J.G.; Paez, C.A.; Carcel, C.; Bied, C.; Tatton, A.S.; Damblon, C.; Heinrichs, B.; Man, M.W.C.; Lambert, S.D. Porphyrin-based hybrid silica-titania as a visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 373, 66–76. [Google Scholar] [CrossRef]
- Tasseroul, L.; Pirard, S.L.; Lambert, S.D.; Páez, C.A.; Poelman, D.; Pirard, J.-P.; Heinrichs, B. Kinetic study of p-nitrophenol photodegradation with modified TiO2 xerogels. Chem. Eng. J. 2012, 191, 441–450. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhang, J.; Zhang, Q.; Liu, Z. An N-doped anatase/rutile TiO2 hybrid from low-temperature direct nitridization: Enhanced photoactivity under UV-/visible-light. RSC Adv. 2014, 4, 420–427. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly efficient low-temperature N-doped TiO2 catalysts for visible light photocatalytic applications. Materials (Basel) 2018, 11, 584. [Google Scholar] [CrossRef]
- Iwase, M.; Yamada, K.; Kurisaki, T.; Prieto-Mahaney, O.O.; Ohtani, B.; Wakita, H. Visible-light photocatalysis with phosphorus-doped titanium(IV) oxide particles prepared using a phosphide compound. Appl. Catal. B Environ. 2013, 132–133, 39–44. [Google Scholar] [CrossRef]
- Bodson, C.J.; Heinrichs, B.; Tasseroul, L.; Bied, C.; Mahy, J.G.; Man, M.W.C.; Lambert, S.D. Efficient P- and Ag-doped titania for the photocatalytic degradation of waste water organic pollutants. J. Alloys Compd. 2016, 682, 144–153. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, H.; Chen, Q.; Li, J.; Wang, P. Construction of N, S codoped TiO2 NCs decorated TiO2 nano-tube array photoelectrode and its enhanced visible light photocatalytic mechanism. Electrochim. Acta 2013, 103, 134–142. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Lecloux, A. Exploitation des isothermes d’adsorption et de désorption d’azote pour l’étude de la texture des solides poreux. Mém. Soc. R. Des Sci. Liège 6ème série 1971, 1, 169–209. [Google Scholar]
- Mahy, J.G.; Léonard, G.L.-M.; Pirard, S.; Wicky, D.; Daniel, A.; Archambeau, C.; Liquet, D.; Heinrichs, B. Aqueous sol–gel synthesis and film deposition methods for the large-scale manufacture of coated steel with self-cleaning properties. J. Sol-Gel Sci. Technol. 2017, 81, 27–35. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Shi, Y.; Liu, Y. Preparation of N, Fe co-doped TiO2 with visible light response. Powder Technol. 2011, 207, 165–169. [Google Scholar] [CrossRef]
- Kim, T.-H.; Rodríguez-González, V.; Gyawali, G.; Cho, S.-H.; Sekino, T.; Lee, S.-W. Synthesis of solar light responsive Fe, N co-doped TiO2 photocatalyst by sonochemical method. Catal. Today 2013, 212, 75–80. [Google Scholar] [CrossRef]
- Su, Y.; Xiao, Y.; Li, Y.; Du, Y.; Zhang, Y. Preparation, photocatalytic performance and electronic structures of visible-light-driven Fe–N-codoped TiO2 nanoparticles. Mater. Chem. Phys. 2011, 126, 761–768. [Google Scholar] [CrossRef]
- Aba-Guevara, C.G.; Medina-Ramírez, I.E.; Hernández-Ramírez, A.; Jáuregui-Rincón, J.; Lozano-Álvarez, J.A.; Rodríguez-López, J.L. Comparison of two synthesis methods on the preparation of Fe, N-Co-doped TiO2 materials for degradation of pharmaceutical compounds under visible light. Ceram. Int. 2017, 43, 5068–5079. [Google Scholar] [CrossRef]
- Azouani, R.; Tieng, S.; Chhor, K.; Bocquet, J.-F.; Eloy, P.; Gaigneaux, E.M.; Klementiev, K.; Kanaev, A.V. TiO2 doping by hydroxyurea at the nucleation stage: Towards a new photocatalyst in the visible spectral range. Phys. Chem. Chem. Phys. 2010, 12, 11325–11334. [Google Scholar] [CrossRef]
- Bittencourt, C.; Rutar, M.; Umek, P.; Mrzel, A.; Vozel, K.; Arčon, D.; Henzler, K.; Krüger, P.; Guttmann, P. Molecular nitrogen in N-doped TiO2 nanoribbons. RSC Adv. 2015, 5, 23350–23356. [Google Scholar] [CrossRef]
- Livraghi, S.; Chierotti, M.R.; Giamello, E.; Magnacca, G.; Paganini, M.C.; Cappelletti, G.; Bianchi, C.L. Nitrogen-Doped Titanium Dioxide Active in Photocatalytic Reactions with Visible Light: A Multi-Technique Characterization of Differently Prepared Materials. J. Phys. Chem. C 2008, 112, 17244–17252. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Boningari, T.; Damma, D.; Inturi, S.N.R. Single-step rapid aerosol synthesis of N-doped TiO2 for enhanced visible light photocatalytic activity. Catal. Commun. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Gil, A.; García, A.M.; Fernández, M.; Vicente, M.A.; González-Rodríguez, B.; Rives, V.; Korili, S.A. Effect of dopants on the structure of titanium oxide used as a photocatalyst for the removal of emergent contaminants. J. Ind. Eng. Chem. 2017, 53, 183–191. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Léonard, G.L.-M.; Zubiaur, A.; Olu, P.-Y.; Mahmoud, A.; Boschini, F.; Heinrichs, B. Towards a large scale aqueous sol-gel synthesis of doped TiO2: Study of various metallic dopings for the photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. A Chem. 2016, 329, 189–202. [Google Scholar] [CrossRef]
- El Koura, Z.; Cazzanelli, M.; Bazzanella, N.; Patel, N.; Fernandes, R.; Arnaoutakis, G.E.; Gakamsky, A.; Dick, A.; Quaranta, A.; Miotello, A. Synthesis and Characterization of Cu and N Codoped RF-Sputtered TiO2 Films: Photoluminescence Dynamics of Charge Carriers Relevant for Water Splitting. J. Phys. Chem. C 2016, 120, 12042–12050. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Hildebrand, J.P.; Taffa, D.H.; Wark, M.; Kamonsuangkasem, K.; Chirawatkul, P.; Wantala, K. Visible light-induced degradation of antibiotic ciprofloxacin over Fe–N–TiO2 mesoporous photocatalyst with anatase/rutile/brookite nanocrystal mixture. J. Photochem. Photobiol. A Chem. 2020, 391, 112371. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Mertes, A.; Vreuls, C.; Drot, S.; Smeets, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Lambert, S.D. Advanced photocatalytic oxidation processes for micropollutant elimination from municipal and industrial water. J. Environ. Manag. 2019, 250, 109561. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Wu, Q.; Yu, H.; Zhao, Y.; Qu, J.; Huo, M.; Yuan, X. Synthesis of Cu2O nanocrystals/TiO2 photonic crystal composite for efficient p-nitrophenol removal. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 291–300. [Google Scholar] [CrossRef]
- Kothavale, V.P.; Patil, T.S.; Patil, P.B.; Bhosale, C.H. Photoelectrocatalytic degradation of Rhodamine B using N doped TiO2 thin Films. Mater. Today Proc. 2020, 23, 382–388. [Google Scholar] [CrossRef]
- Gastello, E.; Estrada, D.; Estrada, W.; Luyo, C.; Espinoza, J.; Ponce, S.; de Oca, J.M.; Rodriguez, J.M. TiO2 films on CoFe2O4 nanoparticles for the Photocatalytic oxidation of Rhodamine B: Influence of the alcoholic solutions. Proc. SPIE 2019, 11371, 113710C. [Google Scholar] [CrossRef]
- Hao, S.; Lin, T.; Ning, S.; Qi, Y.; Deng, Z.; Wang, Y. Research on cracking of SiO2 nanofilms prepared by the sol-gel method. Mater. Sci. Semicond. Process. 2019, 91, 181–187. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, D.M.; Piccirillo, C.; Rozman, N.; Pullar, R.C.; Seabra, M.P.; Škapin, A.S.; Castro, P.M.L.; Labrincha, J.A. Effects of Cu, Zn and Cu-Zn addition on the microstructure and antibacterial and photocatalytic functional properties of Cu-Zn modified TiO2 nano-heterostructures. J. Photochem. Photobiol. A Chem. 2016, 330, 44–54. [Google Scholar] [CrossRef]
- Lopes, D.; Daniel-da-Silva, A.L.; Sarabando, A.R.; Arias-Serrano, B.I.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E.; Trindade, T.; Frade, J.R.; Kovalevsky, A.V. Design of Multifunctional Titania-Based Photocatalysts by Controlled Redox Reactions. Materials 2020, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farban striche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Bryson, C.E. Surface potential control in XPS. Surf. Sci. 1987, 189–190, 50–58. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]








| Sample | Fe Content mol/mol% | dXRD nm ± 1 | SBET m² g−1 ± 5 | VDR cm3 g−1 ± 0.01 | Vp cm3 g−1 ± 0.01 | dBET nm ± 1 | Eg,direct eV ± 0.01 | Eg,indirect eV ± 0.01 |
|---|---|---|---|---|---|---|---|---|
| P25 | – 1 | 182–83 | 47 | 0.03 | – 1 | 31 | 3.45 | 3.05 |
| Pure TiO2 | – 1 | 5 | 180 | 0.09 | 0.10 | 9 | 3.25 | 2.90 |
| TiO2/Fe0.25 | 0.32 | 4 | 195 | 0.10 | 0.10 | 8 | 3.25 | 2.80 |
| TiO2/Fe0.5 | 0.56 | 5 | 180 | 0.10 | 0.10 | 9 | 3.20 | 2.75 |
| TiO2/N10 | – 1 | 5 | 185 | 0.10 | 0.10 | 8 | 3.15 | 2.90 |
| TiO2/N30 | – 1 | 4 | 185 | 0.09 | 0.09 | 8 | 3.15 | 2.90 |
| TiO2/N43 | – 1 | 5 | 220 | 0.11 | 0.11 | 7 | 3.20 | 2.90 |
| TiO2/N75 | – 1 | 4 | 210 | 0.11 | 0.11 | 7 | 3.20 | 2.90 |
| TiO2/Fe0.25/N10 | 0.28 | 4 | 155 | 0.08 | 0.08 | 10 | 3.10 | 2.80 |
| TiO2/Fe0.25/N30 | 0.27 | 4 | 200 | 0.10 | 0.10 | 8 | 3.10 | 2.75 |
| TiO2/Fe0.25/N43 | 0.27 | 4 | 240 | 0.12 | 0.12 | 6 | 3.10 | 2.80 |
| TiO2/Fe0.25/N75 | 0.31 | 4 | 235 | 0.12 | 0.12 | 7 | 3.15 | 2.70 |
| TiO2/Fe0.5/N10 | 0.54 | 5 | 185 | 0.10 | 0.10 | 8 | 3.05 | 2.70 |
| TiO2/Fe0.5/N30 | 0.54 | 4 | 210 | 0.11 | 0.11 | 7 | 3.1 | 2.65 |
| TiO2/Fe0.5/N43 | 0.55 | 4 | 230 | 0.12 | 0.12 | 7 | 3.05 | 2.65 |
| TiO2/Fe0.5/N75 | 0.53 | 4 | 220 | 0.11 | 0.11 | 7 | 3.05 | 2.65 |
| Sample | Anatase Content % | Brookite Content % | Rutile Content % |
|---|---|---|---|
| P25 | 80 | – | 20 |
| Pure TiO2 | 89 | 11 | – |
| TiO2/Fe0.25 | 90 | 10 | – |
| TiO2/Fe0.5 | 90 | 10 | – |
| TiO2/N10 | 91 | 9 | – |
| TiO2/N30 | 89 | 11 | – |
| TiO2/N43 | 91 | 9 | – |
| TiO2/N75 | 90 | 10 | – |
| TiO2/Fe0.25/N10 | 90 | 10 | – |
| TiO2/Fe0.25/N30 | 88 | 12 | – |
| TiO2/Fe0.25/N43 | 91 | 10 | – |
| TiO2/Fe0.25/N75 | 89 | 11 | – |
| TiO2/Fe0.5/N10 | 88 | 12 | – |
| TiO2/Fe0.5/N30 | 89 | 11 | – |
| TiO2/Fe0.5/N43 | 90 | 10 | – |
| TiO2/Fe0.5/N75 | 92 | 8 | – |
| Sample | PNP Degradation after 72 h (%) ± 3 |
|---|---|
| Brushed steel | 26 |
| Brushed steel + SiO2 sublayer | 19 |
| Brushed steel + TiO2 sublayer | 32 |
| Bare steel | 18 |
| Bare steel + SiO2 sublayer | 15 |
| Bare steel + TiO2 sublayer | 12 |
| Glass | 26 |
| Glass + TiO2 sublayer | 68 |
| TiO2/Fe0.5–PNP | TiO2/Fe0.5/N43–RB | |
| k (h−1) | 6.52 × 10−3 | 1.43 |
| k’ (h−1 m3solution/m2cata) | 9.73 × 10−5 | 2.13 × 10−2 |
| R² (correlation coefficient) | 0.9996 | 0.9948 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douven, S.; Mahy, J.G.; Wolfs, C.; Reyserhove, C.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Lambert, S.D. Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films. Catalysts 2020, 10, 547. https://doi.org/10.3390/catal10050547
Douven S, Mahy JG, Wolfs C, Reyserhove C, Poelman D, Devred F, Gaigneaux EM, Lambert SD. Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films. Catalysts. 2020; 10(5):547. https://doi.org/10.3390/catal10050547
Chicago/Turabian StyleDouven, Sigrid, Julien G. Mahy, Cédric Wolfs, Charles Reyserhove, Dirk Poelman, François Devred, Eric M. Gaigneaux, and Stéphanie D. Lambert. 2020. "Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films" Catalysts 10, no. 5: 547. https://doi.org/10.3390/catal10050547
APA StyleDouven, S., Mahy, J. G., Wolfs, C., Reyserhove, C., Poelman, D., Devred, F., Gaigneaux, E. M., & Lambert, S. D. (2020). Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films. Catalysts, 10(5), 547. https://doi.org/10.3390/catal10050547








