New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma–Wood Fiber Combination
Abstract
1. Introduction
2. Results
2.1. Effect of Wood Fiber in NOx Removal
2.2. Mechanism of NOx Removal in Presence of Wood Fiber
2.3. Effect of Wood Fiber Loaded Carbon Soot on NOx Removal
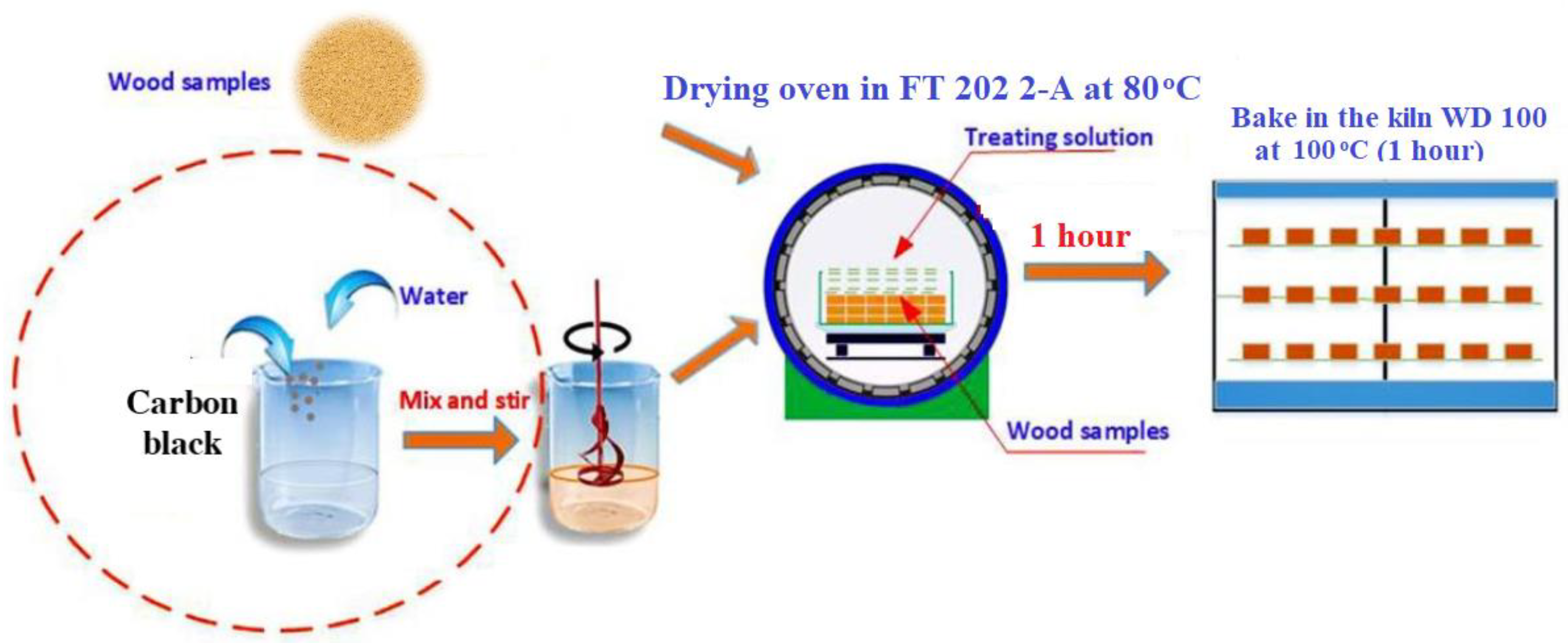
3. Materials and Methods
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiaqiang, E.; Pham, M.; Zhao, D.; Deng, Y.; Le, D.; Zuo, W.; Zhu, H.; Liu, T.; Peng, Q.; Zhang, Z. Effect of different technologies on combustion and emissions of the diesel engine fueled with biodiesel: A review. Renew. Sustain. Energy Rev. 2017, 80, 620–647. [Google Scholar]
- Prasad, R.; Bella, V.R. A review on diesel soot emission, its effect and control. Bull. Chem. React. Eng. Catal. 2010, 5, 69. [Google Scholar] [CrossRef]
- Wade, J.; Farrauto, R. Controlling emissions of pollutants in urban areas. In Metropolitan Sustainability; Elsevier: Amsterdam, The Netherlands, 2012; pp. 260–291. [Google Scholar]
- Jung, H.; Kittelson, D.B.; Zachariah, M.R. The influence of a cerium additive on ultrafine diesel particle emissions and kinetics of oxidation. Combust. Flame 2005, 142, 276–288. [Google Scholar] [CrossRef]
- Brewer, G.J. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef]
- Khair, M.K. A Review of Diesel Particulate Filter Technologies; 0148-7191; SAE Technical Paper; SAE: Warrendale, PA, USA, 2003. [Google Scholar]
- Guo, X.; Du, D.; Wang, F.; Ma, Y.; Yang, C.; Zhang, H.-Z. Study on test instrument and filtration theory of the carbonized micron wood fiber DPF. Microporous Mesoporous Mater. 2011, 142, 655–660. [Google Scholar] [CrossRef]
- Du, D.; Guo, X.; Xu, Y.; Yang, X. Performance study about a new kind wood fiber filter element utilized in capturing diesel particulate material. J. Wood Sci. 2019, 65, 12. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Z.; Shangguan, W. Simultaneous catalytic removal of NOx and soot particulate over Co–Al mixed oxide catalysts derived from hydrotalcites. Catal. Commun. 2007, 8, 1659–1664. [Google Scholar] [CrossRef]
- Wang, Z.; Shangguan, W.; Su, J.; Jiang, Z. Catalytic oxidation of diesel soot on mixed oxides derived from hydrotalcites. Catal. Lett. 2006, 112, 149–154. [Google Scholar] [CrossRef]
- Makshina, E.; Sirotin, S.; Yushchenko, V.; Mazo, G.; van den Berg, M.; Klements’ev, K.; Grünert, W.; Romanovskii, B. Nanocomposites based on LaCoO 3 and mesoporous molecular sieves: Preparation and physicochemical and catalytic properties. Kinet. Catal. 2006, 47, 49–53. [Google Scholar] [CrossRef]
- Zou, G.; Chen, M.; Shangguan, W. Promotion effects of LaCoO3 formation on the catalytic performance of Co–La oxides for soot combustion in air. Catal. Commun. 2014, 51, 68–71. [Google Scholar] [CrossRef]
- Kirienko, P.; Solov’ev, S.; Orlik, S. Effect of CeO 2 on the properties of the Pd/Co 3 O 4/cordierite catalyst in the conversion of CO, NO, and hydrocarbons. Theor. Exp. Chem. 2010, 46, 39–44. [Google Scholar] [CrossRef]
- Apostolescu, N.; Geiger, B.; Hizbullah, K.; Jan, M.; Kureti, S.; Reichert, D.; Schott, F.; Weisweiler, W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts. Appl. Catal. B Environ. 2006, 62, 104–114. [Google Scholar] [CrossRef]
- Yoshida, K.; Okubo, M.; Yamamoto, T. Distinction between nonthermal plasma and thermal desorptions for NOx and CO2. Appl. Phys. Lett. 2007, 90, 131501. [Google Scholar] [CrossRef]
- Shangguan, W.; Zou, G.; Jiang, Z. Simultaneous Catalytic Removal of Diesel Soot and NOx; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Miessner, H.; Francke, K.-P.; Rudolph, R.; Hammer, T. NOx removal in excess oxygen by plasma-enhanced selective catalytic reduction. Catal. Today 2002, 75, 325–330. [Google Scholar] [CrossRef]
- Sato, S.; Kimura, M.; Aki, T.; Koyamotor, I.; Takashima, K.; Katsura, S.; Mizuno, A. A removal system of diesel particulate using electrostatic precipitator with discharge plasma. In Proceedings of the Fourtieth IAS Annual Meeting. Conference Record of the 2005 Industry Applications Conference, Kowloon, HK, China, 2–6 October 2005; pp. 2203–2206. [Google Scholar]
- Yezerets, A.; Currier, N.W.; Eadler, H.A.; Suresh, A.; Madden, P.F.; Branigin, M.A. Investigation of the oxidation behavior of diesel particulate matter. Catal. Today 2003, 88, 17–25. [Google Scholar] [CrossRef]
- Gomez, E.; Rani, D.A.; Cheeseman, C.; Deegan, D.; Wise, M.; Boccaccini, A. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Lu, W.; Abbas, Y.; Mustafa, M.F.; Pan, C.; Wang, H. A review on application of dielectric barrier discharge plasma technology on the abatement of volatile organic compounds. Front. Environ. Sci. Eng. 2019, 13, 30. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, W.; Yu, Z.; Li, S.; Zhu, J.; Yan, K. Comparison of styrene removal in air by positive and negative DC corona discharges. Int. J. Environ. Sci. Technol. 2013, 10, 1377–1382. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Li, K.; Li, L.; Liu, L.; Meng, X.; Sun, T.; Jia, J.; Fan, M. High efficient styrene mineralization through novel NiO-TiO2-Al2O3 packed pre-treatment/treatment/post-treatment dielectric barrier discharge plasma. Chem. Eng. J. 2018, 343, 759–769. [Google Scholar] [CrossRef]
- Raju, B.R.; Reddy, E.L.; Karuppiah, J.; Reddy, P.M.K.; Subrahmanyam, C. Catalytic non-thermal plasma reactor for the decomposition of a mixture of volatile organic compounds. J. Chem. Sci. 2013, 125, 673–678. [Google Scholar] [CrossRef]
- Ondarts, M.; Hajji, W.; Outin, J.; Bejat, T.; Gonze, E. Non-Thermal Plasma for indoor air treatment: Toluene degradation in a corona discharge at ppbv levels. Chem. Eng. Res. Des. 2017, 118, 194–205. [Google Scholar] [CrossRef]
- Chong-Lin, S.; Feng, B.; Ze-Min, T.; Fang-Cheng, L.; Qi-Fei, H. Simultaneous removals of NOx, HC and PM from diesel exhaust emissions by dielectric barrier discharges. J. Hazard. Mater. 2009, 166, 523–530. [Google Scholar]
- Mok, Y.S.; Huh, Y.J. Simultaneous Removal of Nitrogen Oxides and Particulate Matters from Diesel Engine Exhaust using Dielectric Barrier Discharge and Catalysis Hybrid System. Plasma Chem. Plasma Process. 2005, 25, 625–639. [Google Scholar] [CrossRef]
- Okubo, M.; Yamada, H.; Yoshida, K.; Kuroki, T. Simultaneous Reduction of Diesel Particulate and NOx Using a Catalysis-Combined Nonthermal Plasma Reactor. IEEE Trans. Ind. Appl. 2010, 53, 5875–5882. [Google Scholar] [CrossRef]
- Babaie, M.; Kishi, T.; Arai, M.; Zama, Y.; Furuhata, T.; Ristovski, Z.; Rahimzadeh, H.; Brown, R. Influence of non-thermal plasma after-treatment technology on diesel engine particulate matter composition and NOx concentration. Int. J. Environ. Sci. Technol. 2016, 13, 221–230. [Google Scholar] [CrossRef]
- Guo, X.; Du, D.; Qi, Z.; Peng, W.; Song, H. Microstructure characterization of wood fiber PIT adsorbingultrafine particles emitted by diesel engine and simulation of its influence factors. Wood Res. 2016, 61, 175–186. [Google Scholar]
- Yao, S. Plasma reactors for diesel particulate matter removal. Recent Pat. Chem. Eng. 2009, 2, 67–75. [Google Scholar] [CrossRef]
- Wang, H.Y.; Du, G.B.; Li, Q.; Xu, R.Y.; Yuan, S.F. Bonding Performance of Wood Treatment by Oxygen and Nitrogen Cold Plasma. Appl. Mech. Mater. 2014, 633–634, 583–588. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, J.; Zhong, K.; Wang, L.; Guan, Z. Synergy study for plasma-facilitated C2H4 selective catalytic reduction of NOΧ over ag/γ-Al2O3 catalyst. IEEE Trans. Plasma Sci. 2007, 35, 663–669. [Google Scholar] [CrossRef]
- Nolte, J.; Grussdorf, J.; Temps, F.; Wagner, H.G. Kinetics of the reaction HOCO+ O2 in the gas phase. Z. Für Nat. A 1993, 48, 1234–1238. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Ha, K.H.; Du, D. New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma–Wood Fiber Combination. Catalysts 2020, 10, 577. https://doi.org/10.3390/catal10050577
Guo X, Ha KH, Du D. New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma–Wood Fiber Combination. Catalysts. 2020; 10(5):577. https://doi.org/10.3390/catal10050577
Chicago/Turabian StyleGuo, Xiurong, Khanh Hop Ha, and Danfeng Du. 2020. "New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma–Wood Fiber Combination" Catalysts 10, no. 5: 577. https://doi.org/10.3390/catal10050577
APA StyleGuo, X., Ha, K. H., & Du, D. (2020). New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma–Wood Fiber Combination. Catalysts, 10(5), 577. https://doi.org/10.3390/catal10050577



