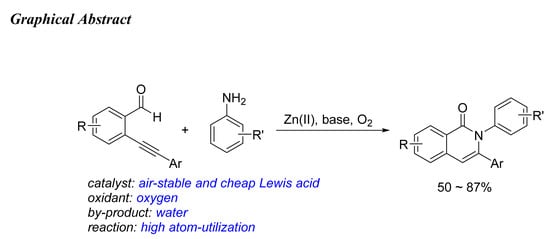
Isoquinolone Synthesis via Zn(OTf)2-Catalyzed Aerobic Cyclocondensation of 2-(1-Alkynyl)-benzaldehydes with Arylamines
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Procedure for the Synthesis of 2,3-Diphenylisoquinolin-1(2H)-one (3aa)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nakamura, I.; Yamamoto, Y. Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev. 2004, 104, 2127–2198. [Google Scholar] [CrossRef] [PubMed]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium π-olefin and π-alkyne Chemistry. Chem. Rev. 2004, 104, 2285–2309. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Abrenica, M.V.A.; Wang, P. Cycloaddition of alkynes: Atom-economic protocols for constructing six-membered cycles. Curr. Org. Chem. 2011, 15, 712–729. [Google Scholar] [CrossRef]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef]
- Nizami, T.A.; Hua, R. Cycloaddition of 1,3-butadiynes: Efficient synthesis of carbo- and heterocycles. Molecules 2014, 19, 13788–13802. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-catalysed reactions of alkynes: Recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef]
- Vessally, E.; Soleimani-Amiri, S.; Hosseinian, A.; Edjlalid, L.; Bekhradnia, A. New protocols to access imidazoles and their ring fused analogues: Synthesis from N-propargylamines. RSC Adv. 2017, 7, 7079–7091. [Google Scholar] [CrossRef]
- Huang, M.-H.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Recent advances in radical transformations of internal alkynes. Chem. Commun. 2018, 54, 10791–10811. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: Towards high step economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef]
- Hua, R.; Nizami, T.A. Synthesis of heterocycles by using propargyl compounds as versatile synthons. Mini-Rev. Org. Chem. 2018, 15, 198–207. [Google Scholar] [CrossRef]
- Glushkov, V.A.; Shklyaev, Y.V. Synthesis of 1(2H)-isoquinolones. Chem. Heterocycl. Compd. 2001, 37, 663–687. [Google Scholar] [CrossRef]
- Mayo, M.S.; Yu, X.; Feng, X.; Yamamoto, Y.; Bao, M. Isoquinolone synthesis through SNAr reaction of 2-halobenzonitriles with ketones followed by cyclization. J. Org. Chem. 2015, 80, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Yamauchi, M.; Murakami, M. Synthesis of 1(2H)-isoquinolones by the nickel-catalyzed denitrogenative alkyne insertion of 1,2,3-benzotriazin-4(3H)-ones. Org. Lett. 2008, 10, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Fenner, S. Ruthenium-catalyzed C-H/N-O bond functionalization: Green isoquinolone syntheses in water. Org. Lett. 2011, 13, 6548–6551. [Google Scholar] [CrossRef] [PubMed]
- Too, P.C.; Chiba, S. A CuBr-mediated aerobic reaction of 2-alkynylbenzaldehydes and primary amines: Synthesis of 4-bromoisoquinolones. Chem. Commun. 2012, 48, 7634–7636. [Google Scholar] [CrossRef]
- Zhang, N.; Li, B.; Zhong, H.; Huang, J. Synthesis of N-alkyl and N-aryl isoquinolones and derivatives via Pd-catalysed C-H activation and cyclization reactions. Org. Biomol. Chem. 2012, 10, 9429–9439. [Google Scholar] [CrossRef]
- Antczak, M.I.; Ready, J.M. Two-, three- and four-component coupling to form isoquinolones based on directed metalation. Chem. Sci. 2012, 3, 1450–1454. [Google Scholar] [CrossRef]
- Allu, S.; Swamy, K.C.K. Ruthenium-catalyzed synthesis of isoquinolones with 8-aminoquinoline as a bidentate directing group in C−H functionalization. J. Org. Chem. 2014, 79, 3963–3972. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Zhu, Y.-Z.; Ou, J.-W.; Wang, Y.-P.; Zheng, J.-Y. Metal-free iodine(III)-promoted synthesis of isoquinolones. J. Org. Chem. 2014, 79, 10988–10998. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Y.; Hong, M.; Duan, P.; Gan, S.; Chao, H.; Zhao, Z.; Zhao, J. Rhodium-catalyzed C-H activation ofhydrazines leads to isoquinolones with tunable aggregation-induced emission properties. Chem. Commun. 2015, 51, 14365–14368. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.-J.; Ruan, W.; Wen, T.-B. Construction of isoquinolin-(2H)-ones by copper-catalyzed tandem reactions of 2-(1-alkynyl) benzaldimines with water. Eur. J. Org. Chem. 2015, 27, 5914–5918. [Google Scholar] [CrossRef]
- Hao, X.-Q.; Du, C.; Zhu, X.; Li, P.-X.; Zhang, J.-H.; Niu, J.-L.; Song, M.-P. Cobalt(II)-catalyzed decarboxylative C-H activation/annulations cascades: Regioselective access to isoquinolones and isoindolinones. Org. Lett. 2016, 18, 3610–3613. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Saha, R.; Parveen, N.; Sekar, G. Palladium-nanoparticles-catalyzed oxidative annulation of benzamides with alkynes for the synthesis of isoquinolones. Adv. Synth. Catal. 2017, 359, 1947–1958. [Google Scholar] [CrossRef]
- Shu, Z.; Guo, Y.; Li, W.; Wang, B. Pd/C-catalyzed synthesis of N-aryl and N-alkyl isoquinolones via C-H/N-H activation. Catal. Today 2017, 297, 292–297. [Google Scholar] [CrossRef]
- Yang, J.; Wu, L.; Xu, H.; Gao, H.; Zhou, Z.; Yi, W. Redox-neutral [4+2] annulation of N-methoxybenzamides with alkynes enabled by an osmium(II)/HOAc catalytic system. Org. Lett. 2019, 21, 9904–9908. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Niu, J.-L.; Yang, D.; Song, M.-P. Development of a traceless directing group: Cp*-free cobalt-catalyzed C-H activation/annulations to access isoquinolinones. J. Org. Chem. 2020, 85, 4067–4078. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.-J. Water-triggered and gold(I)-catalyzed cascade addition/cyclization of terminal alkynes with ortho-alkynylaryl aldehyde. Org. Lett. 2006, 8, 1953–1955. [Google Scholar] [CrossRef]
- Ju, J.; Hua, R. Copper-catalyzed synthesis of isoquinolines by the cyclocondensation of ortho-alkynyl aromatic aldehydes or ketones with urea. Curr. Org. Synth. 2013, 10, 328–332. [Google Scholar] [CrossRef]
- Dell’Acqua, M.; Castano, B.; Cecchini, C.; Pedrazzini, T.; Pirovano, V.; Rossi, E.; Caselli, A.; Abbiati, G. Mild regiospecific synthesis of 1-alkoxy-isochromenes catalyzed by well-defined [silver(I)(pyridine-containing ligand)] complexes. J. Org. Chem. 2014, 79, 3494–3505. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, Y.; Kong, Q.; Jiang, H.; Liu, H.; Li, J. “One-pot” synthesis of dihydrobenzo [4,5][1,3]oxazino[2,3-a]isoquinolines via a silver(I)-catalyzed cascade approach. Molecules 2013, 18, 814–831. [Google Scholar] [CrossRef]
- Mariaule, G.; Newsome, G.; Toullec, P.Y.; Belmont, P.; Michelet, V. Silver-catalyzed domino hydroarylation/cycloisomerization reactions of ortho-alkynylbenzaldehydes: An entry to functionalized isochromene derivatives. Org. Lett. 2014, 16, 4570–4573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Peng, P.; Xie, Y.; Wang, Z.-Y.; Zhou, L. Ag(I)-catalyzed three-component reaction of 2-alkynylbenzaldehydes, amines, and diazo compounds. Org. Lett. 2015, 17, 4332–4335. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, L.; Hua, R. [Cu(maloNHC)]-catalyzed synthesis of 2-aryl pyrazolo[5,1-a]isoquinolines by annulation of N′-(2-((trimethylsilyl)ethynyl)benzylidene)hydrazides with terminal aromatic alkynes. Tetrahedron 2017, 73, 6428–6435. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.-X.; Tang, R.-Y.; Deng, C.-L.; Li, J.-H. ZnI2-catalyzed benzannulation of o-alkynylbenzaldehydes with alkenes leading to 1-acyl-2-substituted naphthalenes. Eur. J. Org. Chem. 2010, 22, 4211–4217. [Google Scholar] [CrossRef]
- Malhotra, D.; Liu, L.-P.; Mashuta, M.S.; Hammond, G.B. Gold-catalyzed annulations of 2-alkynyl benzaldehydes with vinyl ethers: Synthesis of dihydronaphthalene, isochromene, and bicyclo [2.2.2] octane derivatives. Chem. Eur. J. 2013, 19, 4043–4050. [Google Scholar] [CrossRef]
- Sakthivel, K.; Srinivasan, K. Indium(III) triflate-catalysed [4+2] benzannulation reactions of o-alkynylbenzaldehydes with enolisable carbonyl compounds: Selective synthesis of naphthyl ketones. Org. Biomol. Chem. 2014, 12, 269–277. [Google Scholar] [CrossRef]
- Manojveer, S.; Balamurugan, R. A facile access to substituted benzo[a]fluorenes from o-alkynylbenzaldehydes via in situ formed acetals. Chem. Commun. 2014, 50, 9925–9928. [Google Scholar] [CrossRef]
- Manojveer, S.; Balamurugan, R. A cascade approach to naphthalene derivatives from o-alkynylbenzaldehydes and enolizable ketones via in-situ-formed acetals. Eur. J. Org. Chem. 2015, 19, 4254–4260. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, Y.; Zhang, L.; Hua, R. Brønsted acid-promoted one-pot synthesis of chrysene derivatives via isochromenylium intermediate formed in situ. J. Org. Chem. 2015, 80, 7635–7641. [Google Scholar] [CrossRef]
- Sun, H.-B.; Hua, R.; Yin, Y. An efficient synthesis of diarylmetnanes via InCl3.4H2O-catalyzed dehydration of electron-rich arenas with trioxane. Tetrahedron Lett. 2006, 47, 2291–2294. [Google Scholar] [CrossRef]
- Sun, H.-B.; Hua, R.; Li, B.; Yin, Y. An efficient and selective hydroarylation of styrenes with electron-rich arenes catalyzed by bismuth(III) chloride and affording Markovnikov adducts. Eur. J. Org. Chem. 2006, 18, 4231–4236. [Google Scholar] [CrossRef]
- Sun, H.-B.; Hua, R.; Chen, S.; Yin, Y. An efficient bismuth(III) chloride-catalyzed synthesis of 1,1-diarylalkenes via Friedel-Craft reaction of acyl chloride or vinyl chloride with arenes. Adv. Synth. Catal. 2006, 348, 1919–1925. [Google Scholar] [CrossRef]
- Sun, H.-B.; Li, B.; Chen, S.; Hua, R. An efficient synthesis of unsymmetrical diarylmethanes from the dehydration of arenes with benzyl alcohols using InCl3·4H2O/acetylacetone catalyst system. Tetrahedron 2007, 63, 10185–10188. [Google Scholar] [CrossRef]
- Hua, R. Recent advances in bismuth-catalyzed organic synthesis. Curr. Org. Synth. 2008, 5, 1–27. [Google Scholar] [CrossRef]
- Zheng, L.; Ju, J.; Bin, Y.; Hua, R. Synthesis of isoquinolines and heterocycle-fused pyridines via three-component cascade reaction of aryl ketones, hydroxylamine, and alkynes. J. Org. Chem. 2012, 77, 5794–5800. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. Rhodium(III)-catalyzed C-H activation and indole synthesis with hydrazone as an auto-formed and auto-cleavable directing group. Chem. Eur. J. 2014, 20, 2352–2356. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. Modular assembly of ring-fused and π-extended phenanthroimidazoles via C−H activation and alkyne annulation. J. Org. Chem. 2014, 79, 3930–3936. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, L.; Guo, B.; Hua, R. One-pot synthesis of multisubstituted 2-aminoquinolines from annulation of 1-aryl tetrazoles with internal alkynes via double C-H activation and denitrogenation. J. Org. Chem. 2014, 79, 11541–11548. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R.; Jiang, J.; Zhang, L. A general approach to arylated furans, pyrroles, and thiophenes. Tetrahedron 2014, 70, 8252–8256. [Google Scholar] [CrossRef]
- Su, J.; Liu, H.; Hua, R. Au(I)-catalyzed annulation of propargyl amine with aldehydes: One-pot cascade synthesis of 2,5-dimethylpyrazines. Int. J. Mol. Sci. 2015, 16, 3599–3608. [Google Scholar] [CrossRef]
- Zheng, L.; Bin, Y.; Wang, Y.; Hua, R. Synthesis of natural product-like polyheterocycles via one-pot cascade oximation, C–H activation, and alkyne annulation. J. Org. Chem. 2016, 81, 8911–8919. [Google Scholar] [CrossRef] [PubMed]
- Nizami, T.A.; Hua, R. Silver-catalyzed chemoselective annulation of propargyl amines with alkynes for access to pyridines and pyrroles. Tetrahedron 2017, 73, 6080–6084. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Hua, R. Base-promoted chemodivergent formation of 1,4-benzoxazepin-5(4H)-ones and 1,3-benzoxazin-4(4H)-ones switched by solvents. Molecules 2019, 24, 3773. [Google Scholar] [CrossRef] [PubMed]


 | ||||
| Entry | Catalyst (mol%) | Base (2 equiv) | Solvent | Yield of 3aa (%)b |
| 1c | − | − | DMSO | trace |
| 2d | − | K2CO3 | DMSO | 51 |
| 3 | Fe(OTf)2 (4.0) | K2CO3 | DMSO | 45 |
| 4 | ZnCl2 (4.0) | K2CO3 | DMSO | 50 |
| 5 | Zn(OTf)2 (4.0) | K2CO3 | DMSO | 87 |
| 6 | Zn(OTf)2 (3.0) | K2CO3 | DMSO | 87 |
| 7 | Zn(OTf)2 (2.0) | K2CO3 | DMSO | 78 |
| 8 | Zn(OTf)2 (4.0) | KHCO3 | DMSO | 40 |
| 9 | Zn(OTf)2 (4.0) | KOtBu | DMSO | 50 |
| 10 | Zn(OTf)2 (4.0) | K2CO3 | DMF | 60 |
| 11 | Zn(OTf)2 (4.0) | K2CO3 | Dioxane | 55 |
| a Reactions were carried out using 1.0 mmol of 1a, 1.2 mmol of 2a, and 2.0 mmol of base in 5.0 mL of solvent under oxygen atmosphere at 120 °C for 24 h. b Isolated yields. c 20% of 3aa’. d 33% of 3aa’. | ||||
 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, D.M.; Hua, R. Isoquinolone Synthesis via Zn(OTf)2-Catalyzed Aerobic Cyclocondensation of 2-(1-Alkynyl)-benzaldehydes with Arylamines. Catalysts 2020, 10, 683. https://doi.org/10.3390/catal10060683
Khan DM, Hua R. Isoquinolone Synthesis via Zn(OTf)2-Catalyzed Aerobic Cyclocondensation of 2-(1-Alkynyl)-benzaldehydes with Arylamines. Catalysts. 2020; 10(6):683. https://doi.org/10.3390/catal10060683
Chicago/Turabian StyleKhan, Dost Muhammad, and Ruimao Hua. 2020. "Isoquinolone Synthesis via Zn(OTf)2-Catalyzed Aerobic Cyclocondensation of 2-(1-Alkynyl)-benzaldehydes with Arylamines" Catalysts 10, no. 6: 683. https://doi.org/10.3390/catal10060683
APA StyleKhan, D. M., & Hua, R. (2020). Isoquinolone Synthesis via Zn(OTf)2-Catalyzed Aerobic Cyclocondensation of 2-(1-Alkynyl)-benzaldehydes with Arylamines. Catalysts, 10(6), 683. https://doi.org/10.3390/catal10060683