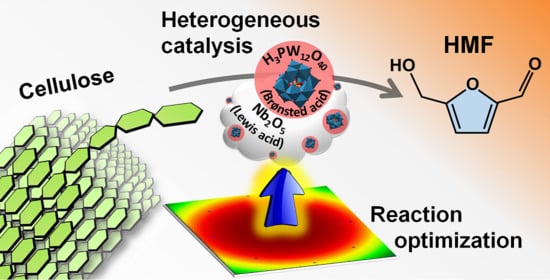
Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Evaluation of Reaction Conditions
2.3. Reaction Conditions Optimization
2.4. Kinetic Modeling Results
2.5. HMF Production from Different Sources of Cellulose
3. Materials and Methods
3.1. Catalyst Preparation (HPW Supported on Nb2O5)
3.2. HMF Production
- A: cellulose;
- B: HMF;
- C: other products.
- AIC: Akaike information criterion;
- AICC: Akaike information criterion corrected for small samples;
- : smallest AICC among all candidate models;
- N: number of data points;
- K: number of model parameters;
- SSE: error sum of squares;
- : AICC differences.
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atanda, L.; Shrotri, A.; Mukundan, S.; Ma, Q.; Konarova, M.; Beltramini, J. Direct production of 5-hydroxymethylfurfural via catalytic conversion of simple and complex sugars over phosphated TiO2. ChemSusChem 2015, 8, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ma, R.; Wei, H.; Li, L.; Zou, B.; Xu, Y. Ruthenium trichloride catalyzed conversion of cellulose into 5-hydroxymethylfurfural in biphasic system. Bioresour. Technol. 2019, 279, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sweygers, N.; Alewaters, N.; Dewil, R.; Appels, L. Microwave effects in the dilute acid hydrolysis of cellulose to 5-hydroxymethylfurfural. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Candu, N.; Fergani, M.E.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Lopes, M.; Dussan, K.; Leahy, J.J.; Da Silva, V.T. Conversion of d-glucose to 5-hydroxymethylfurfural using Al2O3-promoted sulphated tin oxide as catalyst. Catal. Today 2017, 279, 233–243. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithofer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the conversion of glucose and xylose catalyzed by a combination of Lewis and BrØnsted acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Fang, J.; Zheng, W.; Liu, K.; Li, H.; Li, C. Molecular design and experimental study on the synergistic catalysis of cellulose into 5-hydroxymethylfurfural with Brønsted–Lewis acidic ionic liquids. Chem. Eng. J. 2020, 385, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B Environ. 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2,5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Xia, Q.; Liu, X.; Wang, Y. Efficient conversion of cellulose into 5-hydroxymethylfurfural over niobia/carbon composites. Chem. Eng. J. 2018, 332, 528–536. [Google Scholar] [CrossRef]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and BrØnsted acid catalysts in water in a biphasic reactor with and alkylphenol solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Eminov, S.; Filippousi, P.; Brandt, A.; Wilton-Ely, J.D.E.T.; Hallett, J.P. Direct catalytic conversion of cellulose to 5-hydroxymethylfurfural using ionic liquids. Inorganics 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Yang, J.; Chou, L. Catalytic hydrolysis of cellulose into furans in MnCl2–ionic liquid system. Carbohydr. Polym. 2011, 85, 363–368. [Google Scholar] [CrossRef]
- Jing, S.; Cao, X.; Zhong, L.; Peng, X.; Sun, R.; Liu, J. Effectively enhancing conversion of cellulose to HMF by combining in-situ carbonic acid from CO2 and metal oxides. Ind. Crops Prod. 2018, 126, 151–157. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.; Wu, K.; Liu, Y.; Liu, C.; Zhu, Y.; Liang, B. Hydrolysis of mechanically pre-treated cellulose catalyzed by solid acid SO42−-TiO2 in water–ethanol solvent. Chin. J. Chem. Eng. 2020, 28, 136–142. [Google Scholar] [CrossRef]
- Tang, Z.; Su, J. Direct conversion of cellulose to 5-hydroxymethylfurfural (HMF) using an efficient and inexpensive boehmite catalyst. Carbohydr. Res. 2019, 481, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Nandiwale, K.Y.; Galande, N.D.; Thakur, P.; Sawant, S.D.; Zambre, V.P.; Bokade, V.V. One-pot synthesis of 5‑hydroxymethylfurfural by cellulose hydrolysis over highly active bimodal micro/mesoporous H‑ZSM‑5 catalyst. ACS Sustain. Chem. Eng. 2014, 2, 1928–1932. [Google Scholar] [CrossRef]
- Kawamura, K.; Yasuda, T.; Hatanaka, T.; Hamahiga, K.; Matsuda, N.; Ueshima, M.; Nakai, K. In situ UV–VIS spectrophotometry within the second time scale as a research tool for solid-state catalyst and liquid-phase reactions at high temperatures: Its application to the formation of HMF from glucose and cellulose. Chem. Eng. J. 2017, 307, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Li, Y.; Zhang, Q.; Sun, X.; Fan, H.; Xu, N.; Li, G. Selective conversion of cotton cellulose to glucose and 5-hydroxymethyl furfural with SO42-/MxOy solid superacid catalyst. Carbohydr. Polym. 2015, 131, 9–14. [Google Scholar] [CrossRef]
- Tokarz-Sobieraj, R.; Niemiec, P. Cu2+ in Keggin anion—Influence of copper position on electronic structure/redox properties of heteropolyacids. DFT cluster model study. J. Mol. Struct. 2017, 1135, 20–25. [Google Scholar] [CrossRef]
- Caicedo, A.M.E.; Rengifo-Herrera, J.A.; Florian, P.; Blanco, M.N.; Romanelli, G.P.; Pizzio, L.R. Valorization of biomass derivatives: Keggin heteropolyacids supported on titania as catalysts in the suitable synthesis of 2-phenoxyethyl-2-furoate. J. Mol. Catal. A Chem. 2016, 425, 266–274. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Reis, M.O.; Pires, M.S.; Ruotolo, L.A.M.; Ramalho, T.C.; Oliveira, C.R.; Lacerda, L.C.T.; Nogueira, F.G.E. Zn-doped Nb2O5 photocatalysts driven by visible-light: An experimental and theoretical study. Mater. Chem. Phys. 2019, 228, 160–167. [Google Scholar] [CrossRef]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5.nH2O as a heterogeneous catalyst with water-tolerant lewis acid sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef]
- Gomes, F.N.D.C.; Pereira, L.R.; Riberiro, N.F.P.; Souza, M.M.V.M. Production of 5-hydroxymethylfurfural (HMF) via fructose dehydration: Effect of solvent and salting-out. Braz. J. Chem. Eng. 2015, 32, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Perez, G.P.; Mukherjee, A.; Dumont, M.J. Insights into HMF catalysis. J. Ind. Eng. Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Bicker, M.; Kaiser, D.; Ott, L.; Vogel, H. Dehydration of d-fructose to hydroxymethylfurfural in sub- and supercritical fluids. J. Supercrit. Fluids 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Cao, Z.; Fan, Z.; Chen, Y.; Li, M.; Shen, T.; Zhu, C.; Ying, H. Efficient preparation of 5-hydroxymethylfurfural from cellulose in a biphasic system over hafnyl phosphates. Appl. Catal. B Environ. 2019, 244, 170–177. [Google Scholar] [CrossRef]
- Caliman, E.; Dias, J.A.; Dias, S.C.L.; Garcia, F.A.C.; Macedo, J.L.D.; Almeida, L.S. Preparation and characterization of H3PW12O40 supported on niobia. Microporous Mesoporous Mater. 2010, 132, 103–111. [Google Scholar] [CrossRef]
- Liao, X.; Huang, Y.; Zhou, Y.; Liu, H.; Cai, Y.; Lu, S.; Yao, Y. Homogeneously dispersed HPW/graphene for high efficient catalytic oxidative desulfurization prepared by electrochemical deposition. Appl. Surf. Sci. 2019, 484, 917–924. [Google Scholar] [CrossRef]
- Shen, H.; Li, Y.; Huang, S.; Cai, K.; Cheng, Z.; Lv, J.; Ma, X. The carbonylation of dimethyl ether catalyzed by supported heteropoly acids: The role of BrØnsted acid properties. Catal. Today 2019, 330, 117–123. [Google Scholar] [CrossRef]
- Nogueira, J.S.M.; Silva, J.P.A.; Mussatto, S.I.; Carneiro, L.M. Synthesis and application of heterogeneous catalysts based on heteropolyacids for 5-hydroxymethylfurfural production from glucose. Energies 2020, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Al-Rubaie, K.S.; Godefroid, L.B.; Lopes, J.A.M. Statistical modeling of fatigue crack growth rate Inconel alloy 600. Int. J. Fatigue 2007, 29, 931–940. [Google Scholar] [CrossRef]
- Lacerda, V.S.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hérnandez-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef]
- Kerssemakers, A.A.J.; Doménech, P.; Cassano, M.; Yamakawa, C.K.; Dragone, G.; Mussatto, S.I. Production of itaconic acid from cellulose pulp: Feedstock feasibility and process strategies for an efficient microbial performance. Energies 2020, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I.; Rocha, G.J.M.; Roberto, I.C. Hydrogen peroxide bleaching of cellulose pulps obtained from brewer’s spent grain. Cellulose 2008, 15, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Ozel, B.K.; Ozturk, D.; Nis, B. One-pot hydrothermal conversion of different residues to value-added chemicals usıng new acidic carbonaceous catalyst. Bioresour. Technol. 2019, 289, 1–6. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Zhang, L.; Yu, X.; Liu, H.; Yagoub, A.E.A.; Zhou, C. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020, 149, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Zhang, J.; Yu, H.; Wang, X. Efficient catalytic system for the direct transformation of lignocellulosic biomass to furfural and 5-hydroxymethylfurfural. Bioresour. Technol. 2017, 224, 656–661. [Google Scholar] [CrossRef]
- Gouveia, E.R.; Nascimento, R.T.; Souto-Maior, A.M.; Rocha, G.J.M. Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quim. Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]








| Experimental Conditions (Independent Variables and Interactions) | Response Variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | AT (°C) | B Acetone: Water (v/v) | AB | C 1 CCell (% w/v) | AC | BC | CE | D Catalyst (% w/v) | AD | BD | CE | CD | BE | AE | 2 E | 3 CHMF (g/L) | 4 YHMF (%) |
| 1 | 160 | 50:50 | 1 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.0 | 0.0 |
| 2 | 160 | 50:50 | 1 | 5 | 1 | 1 | 1 | 5 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0.7 | 1.8 |
| 3 | 160 | 50:50 | 1 | 10 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 0.0 | 0.0 |
| 4 | 160 | 50:50 | 1 | 10 | 2 | 2 | 2 | 5 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1.0 | 1.3 |
| 5 | 160 | 75:25 | 2 | 5 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 0.0 | 0.0 |
| 6 | 160 | 75:25 | 2 | 5 | 1 | 2 | 2 | 5 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2.0 | 5.0 |
| 7 | 160 | 75:25 | 2 | 10 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 0.0 | 0.0 |
| 8 | 160 | 75:25 | 2 | 10 | 2 | 1 | 1 | 5 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2.6 | 3.3 |
| 9 | 200 | 50:50 | 2 | 5 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2.1 | 5.3 |
| 10 | 200 | 50:50 | 2 | 5 | 2 | 1 | 2 | 5 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 3.6 | 9.3 |
| 11 | 200 | 50:50 | 2 | 10 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 3.2 | 4.2 |
| 12 | 200 | 50:50 | 2 | 10 | 1 | 2 | 1 | 5 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 6.2 | 8.0 |
| 13 | 200 | 75:25 | 1 | 5 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 6.8 | 17.5 |
| 14 | 200 | 75:25 | 1 | 5 | 2 | 2 | 1 | 5 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 7.3 | 18.8 |
| 15 | 200 | 75:25 | 1 | 10 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 8.4 | 10.8 |
| 16 | 200 | 75:25 | 1 | 10 | 1 | 1 | 2 | 5 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 16.0 | 20.6 |
| Source of Variation | Response Variables | |
|---|---|---|
| p-Value for CHMF | p-Value for YHMF | |
| Temperature (A) | 0.0004 * | 0.0001 * |
| Acetone:water (B) | 0.0055 * | 0.0014 * |
| CCel (C) | 0.0436 * | 0.2515 |
| CCat (D) | 0.0202 * | 0.0085 * |
| AB | 0.0146 * | 0.0043 * |
| AC | 0.0662 | 0.5148 |
| AD | 0.3073 | 0.3478 |
| BC | 0.2808 | 0.6262 |
| BD | 0.3097 | 0.2940 |
| CD | 0.1537 | 0.4378 |
| R2 | 0.9648 | 0.9778 |
| Experimental Conditions (Independent Variables) | Response Variables | ||||
|---|---|---|---|---|---|
| Exp. | A: T (°C) | B: Acetone:Water (v/v) | YHMF 1 (%) | SHMF 2 (%) | XCel 3 (%) |
| 1 | 160 | 60:40 | 2.2 | 9.3 | 24.2 |
| 2 | 240 | 60:40 | 2.1 | 2.1 | 99.6 |
| 3 | 160 | 90:10 | 4.2 | 14.3 | 29.4 |
| 4 | 240 | 90:10 | 1.2 | 1.2 | 100.0 |
| 5 | 143 | 75:25 | 0.0 | 0.0 | 47.6 |
| 6 | 257 | 75:25 | 0.0 | 0.0 | 100.0 |
| 7 | 200 | 54:46 | 10.0 | 18.9 | 53.0 |
| 8 | 200 | 96:4 | 5.0 | 9.1 | 55.2 |
| 9 | 200 | 75:25 | 21.5 | 24.8 | 86.5 |
| 10 | 200 | 75:25 | 15.2 | 19.6 | 77.7 |
| 11 | 200 | 75:25 | 17.7 | 20.7 | 85.7 |
| Source of Variation | Response Variables | ||
|---|---|---|---|
| p-Value for YHMF | p-Value for SHMF | p-Value for XCel | |
| Temperature (A) | 0.6672 | 0.1533 | 0.0002 * |
| Acetone:Water (B) | 0.4175 | 0.4619 | 0.7864 |
| AA | 0.0001 * | 0.0010 * | - |
| BB | 0.0015 * | 0.0782 | 0.0192 * |
| Lack of fit | 0.815 | 0.243 | 0.135 |
| R2 adjust. | 0.9005 | 0.7746 | 0.8505 |
| Parameter | PL Model | LHHW Model | ||
|---|---|---|---|---|
| T = 200 °C | T = 180 °C | T = 200 °C | T = 180 °C | |
| k1 (min−1) | 0.110 | 0.180 | 0.120 | 0.016 |
| k2 (min−1) | 0.017 | 0.100 | 2.100 | 0.120 |
| kc (L mol−1) | - | - | 66.000 | 2.300 |
| Model | AICC | Δ |
|---|---|---|
| PL | −111.03 | 4.44 |
| LHHW | −115.47 | 0.00 |
| Feedstock | Purity (%) | CHMF 1 (g/L) | YHMF 2 (%) | SHMF 3 (%) | XCel 4 (%) |
|---|---|---|---|---|---|
| Microcrystalline cellulose | >99 | 14.1 | 18.1 | 24.0 | 75.2 |
| Commercial eucalyptus cellulose pulp | 89.7 | 9.8 | 14.1 | 17.6 | 80.4 |
| Brewer’s spent grain cellulose pulp | 90.4 | 8.2 | 11.7 | 15.1 | 77.4 |
| Feedstock | Cellulose (%) | Catalyst | Solvent | CCel 1 (%w/v) | T (°C) | t (min) | CHMF (g/L) | YHMF (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Raw sugarcane bagasse | 42.62 | D001-cc ion-exchange resin | Water | 5 | 140 | 25 (MWH) 2 | 3.42 | 8.8 | [39] |
| Ball-milling cellulose | - | SO42−/TiO2—450 °C | Water: ethanol (1:1 v/v) | 3.33 | 200 | 30 | 3.37 | 13.0 | [15] |
| Corncob | 30.2 | SPTPA—porous polytriphenylamine–SO3H solid acid | γ-valerolactone (GVL) | 1.25 | 175 | 85 | 0.97 | 10 | [40] |
| Corn straw | 49.9 | BT300S—sulfonated solid acid carbonaceous catalyst | Water | 0.2 | 200 | 60 | 0.4 | - | [38] |
| Cotton linter | 82.2 | BT300S—sulfonated solid acid carbonaceous catalyst | Water | 0.2 | 200 | 240 | 0.23 | - | |
| Commercial eucalyptus cellulose pulp | 89.7 | HPW/Nb2O5—300 °C | Acetone: water (3:1 v/v) | 10 | 200 | 10 | 9.8 | 14.1 | Present study |
| Brewer’s spent grain cellulose pulp | 90.4 | HPW/Nb2O5—300 °C | Acetone: water (3:1 v/v) | 10 | 200 | 10 | 8.2 | 11.7 |
| Model | Rate Equations |
|---|---|
| Power law | |
| LHHW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, J.S.M.; Santana, V.T.; Henrique, P.V.; Aguiar, L.G.d.; Silva, J.P.A.; Mussatto, S.I.; Carneiro, L.M. Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst. Catalysts 2020, 10, 1417. https://doi.org/10.3390/catal10121417
Nogueira JSM, Santana VT, Henrique PV, Aguiar LGd, Silva JPA, Mussatto SI, Carneiro LM. Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst. Catalysts. 2020; 10(12):1417. https://doi.org/10.3390/catal10121417
Chicago/Turabian StyleNogueira, Jéssica Siqueira Mancilha, Vinícius Tomaz Santana, Paulo Vitor Henrique, Leandro Gonçalves de Aguiar, João Paulo Alves Silva, Solange I. Mussatto, and Livia Melo Carneiro. 2020. "Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst" Catalysts 10, no. 12: 1417. https://doi.org/10.3390/catal10121417
APA StyleNogueira, J. S. M., Santana, V. T., Henrique, P. V., Aguiar, L. G. d., Silva, J. P. A., Mussatto, S. I., & Carneiro, L. M. (2020). Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst. Catalysts, 10(12), 1417. https://doi.org/10.3390/catal10121417