Photocatalytic Degradation of Chlorpyrifos with Mn-WO3/SnS2 Heterostructure
Abstract
:1. Introduction
2. Results and Discussion
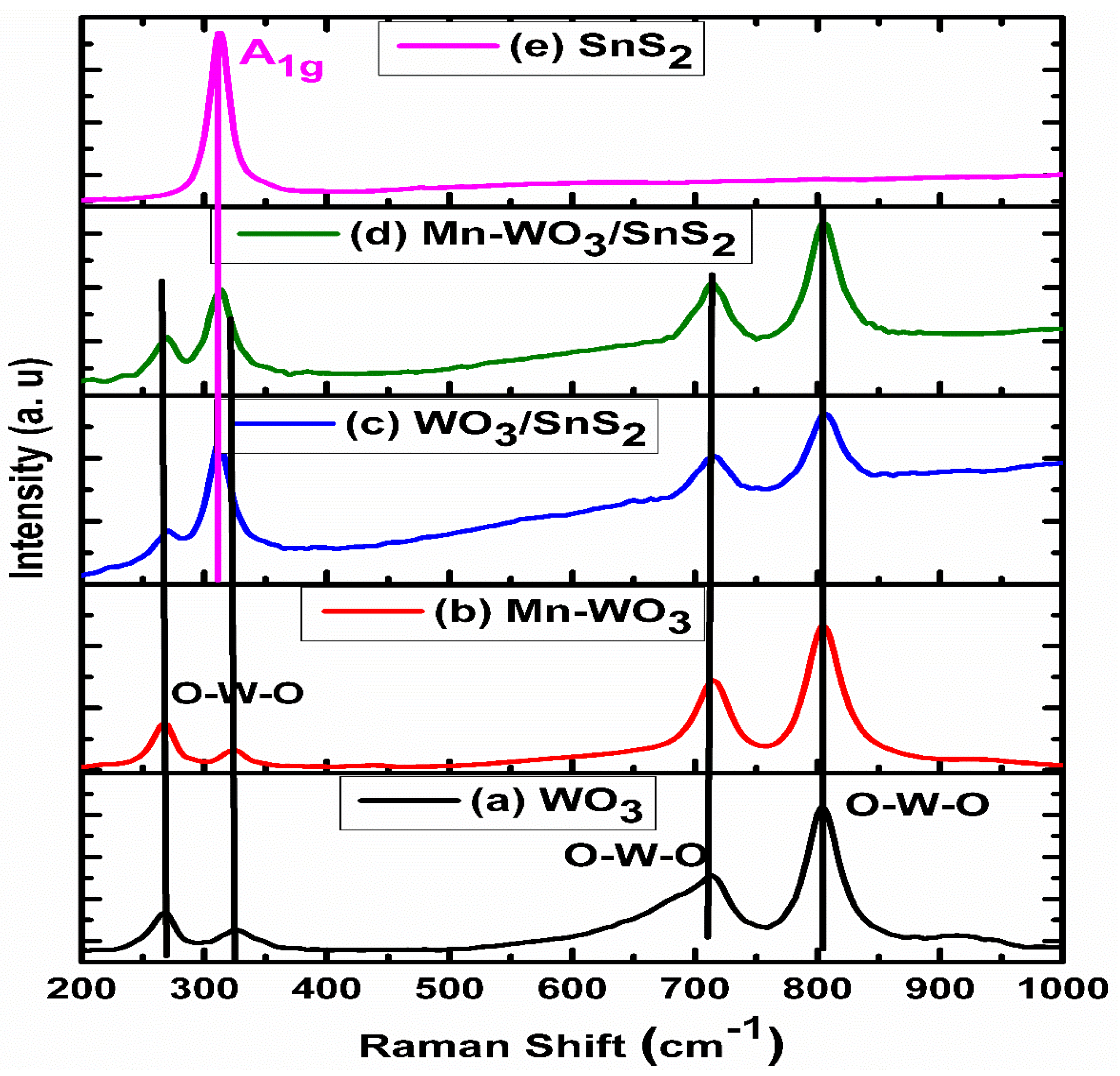
2.1. X-ray Diffraction and Raman Analyses
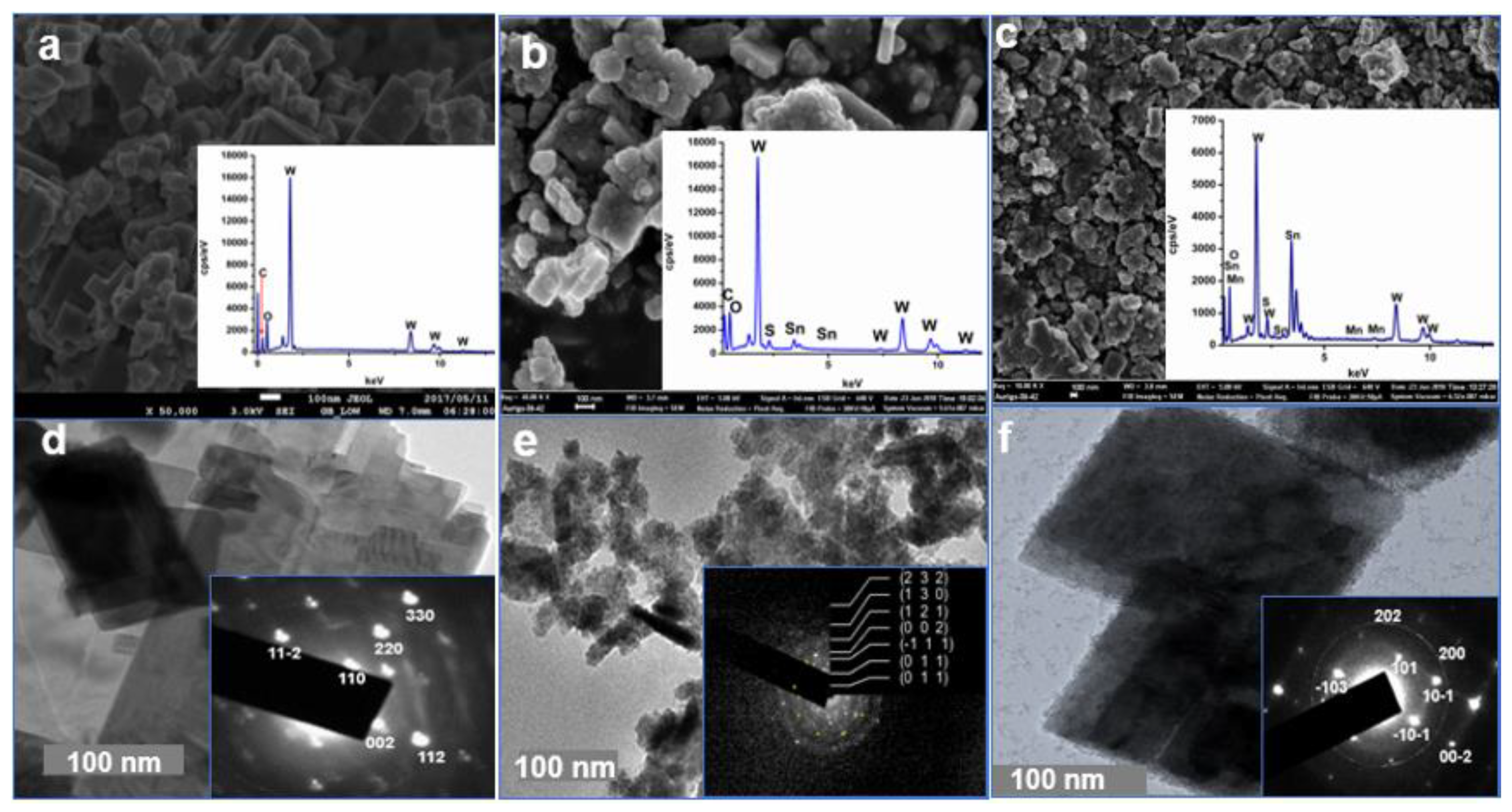
2.2. Morphological Studies
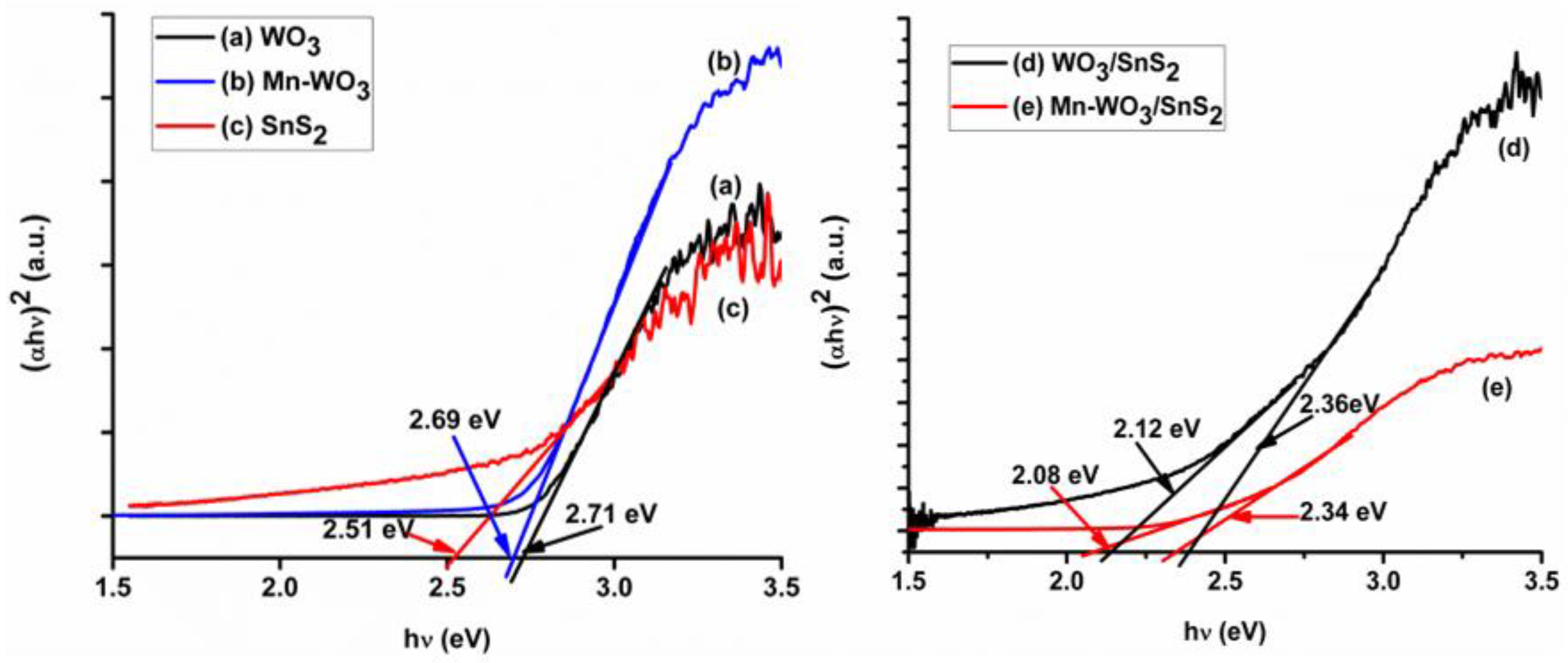
2.3. Optical Properties
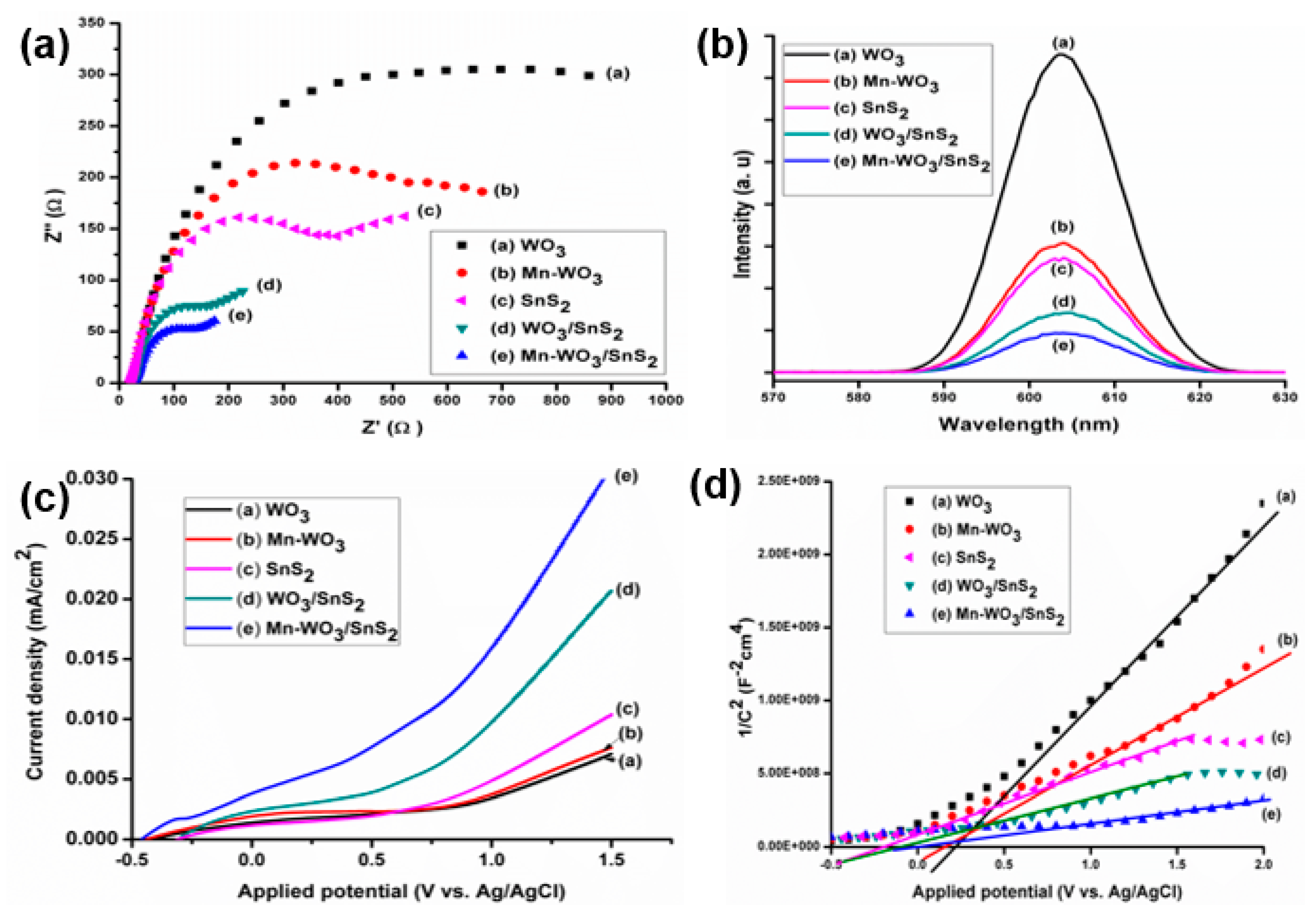
2.4. Electrochemical and Photoluminescence Measurements
2.5. BET Analysis
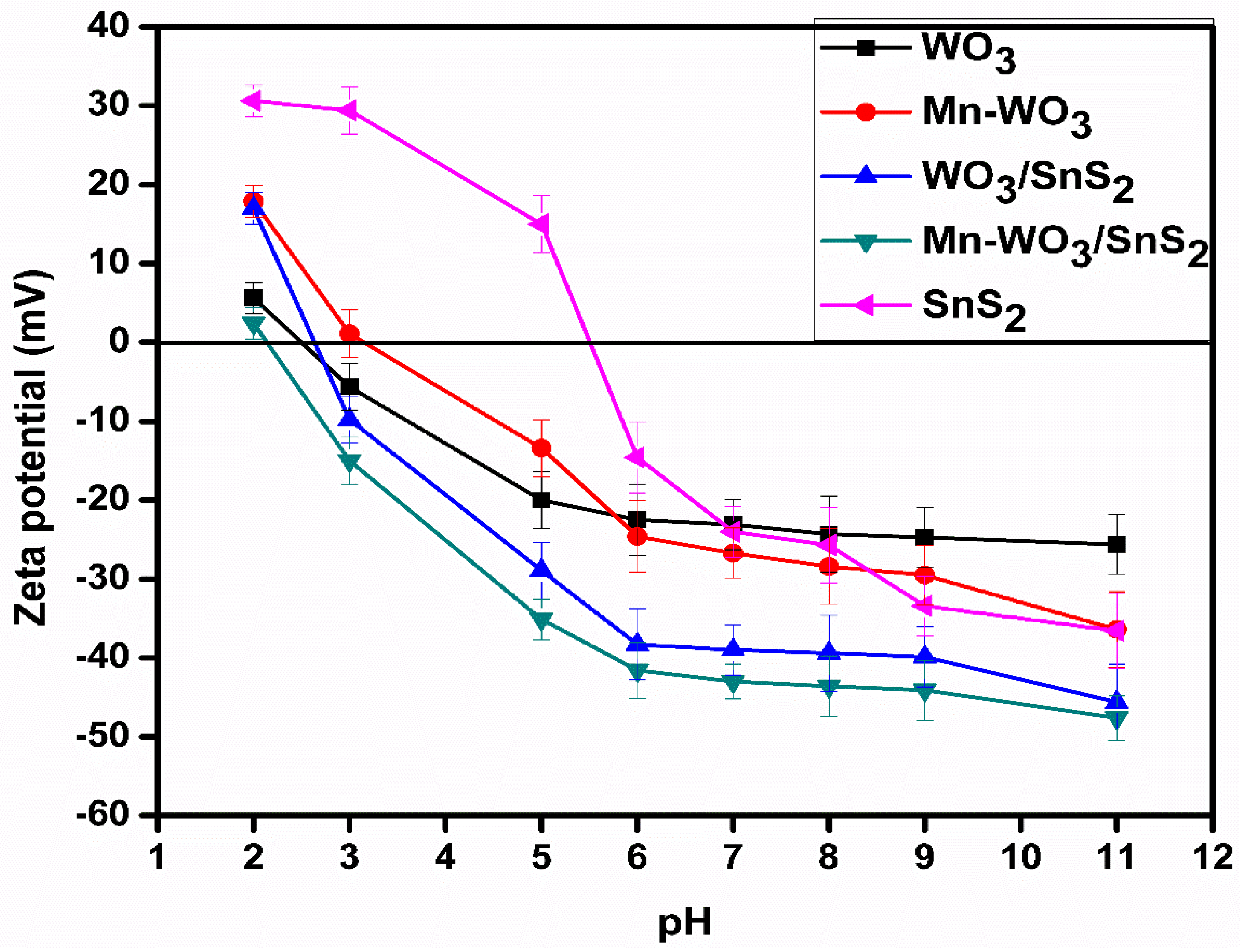
2.6. Surface Charge of Nanoparticles
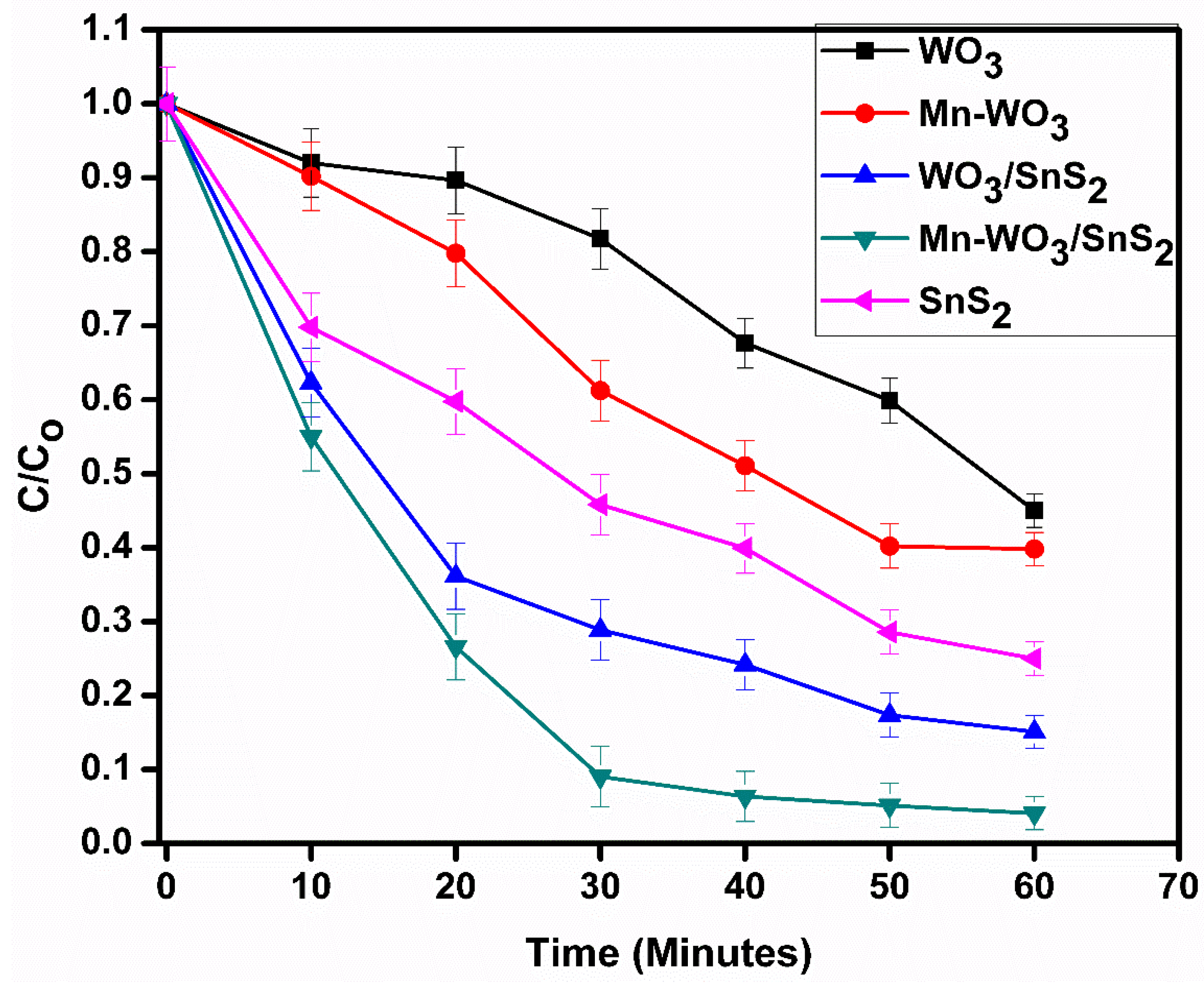
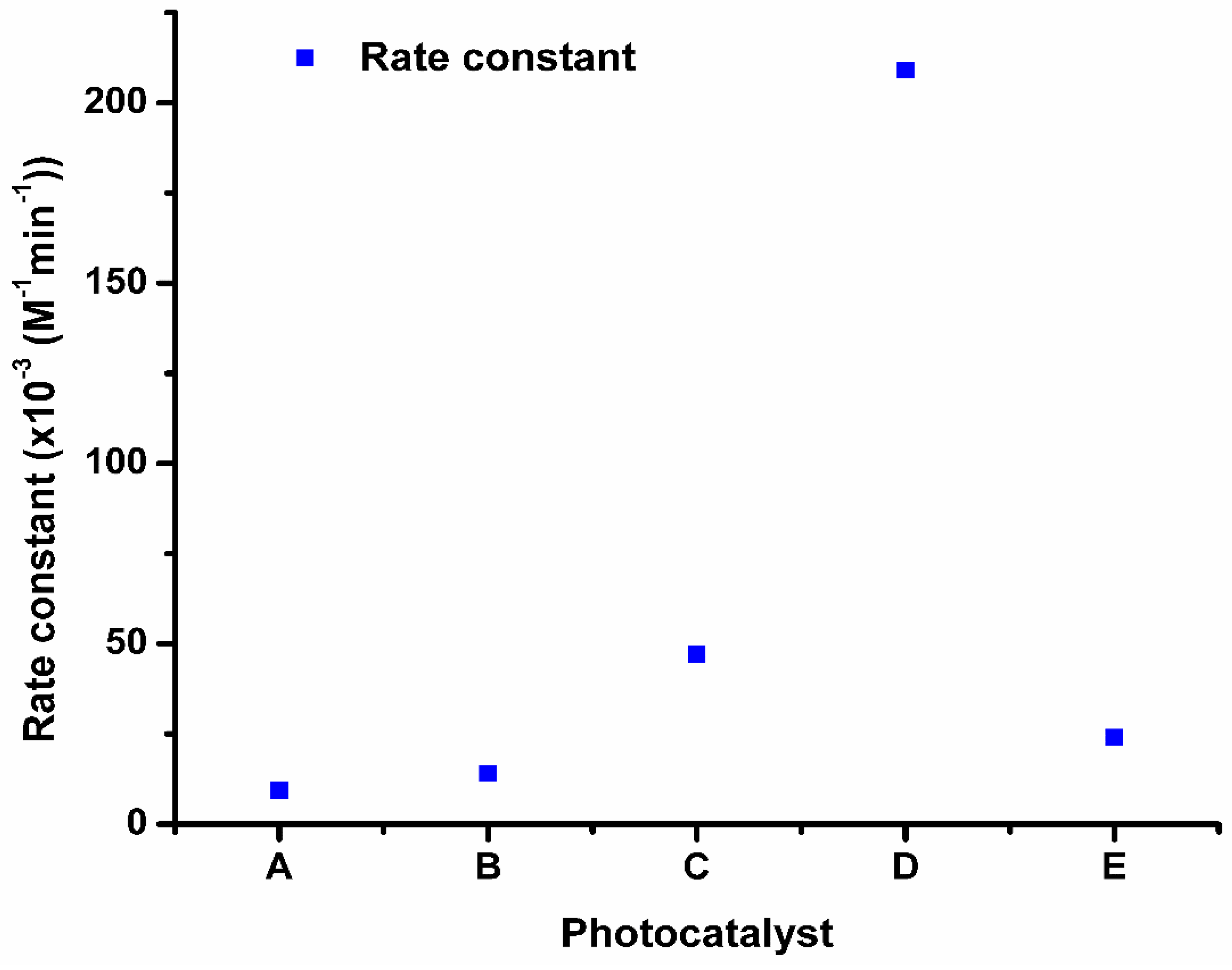
2.7. Degradation of Chlorpyrifos
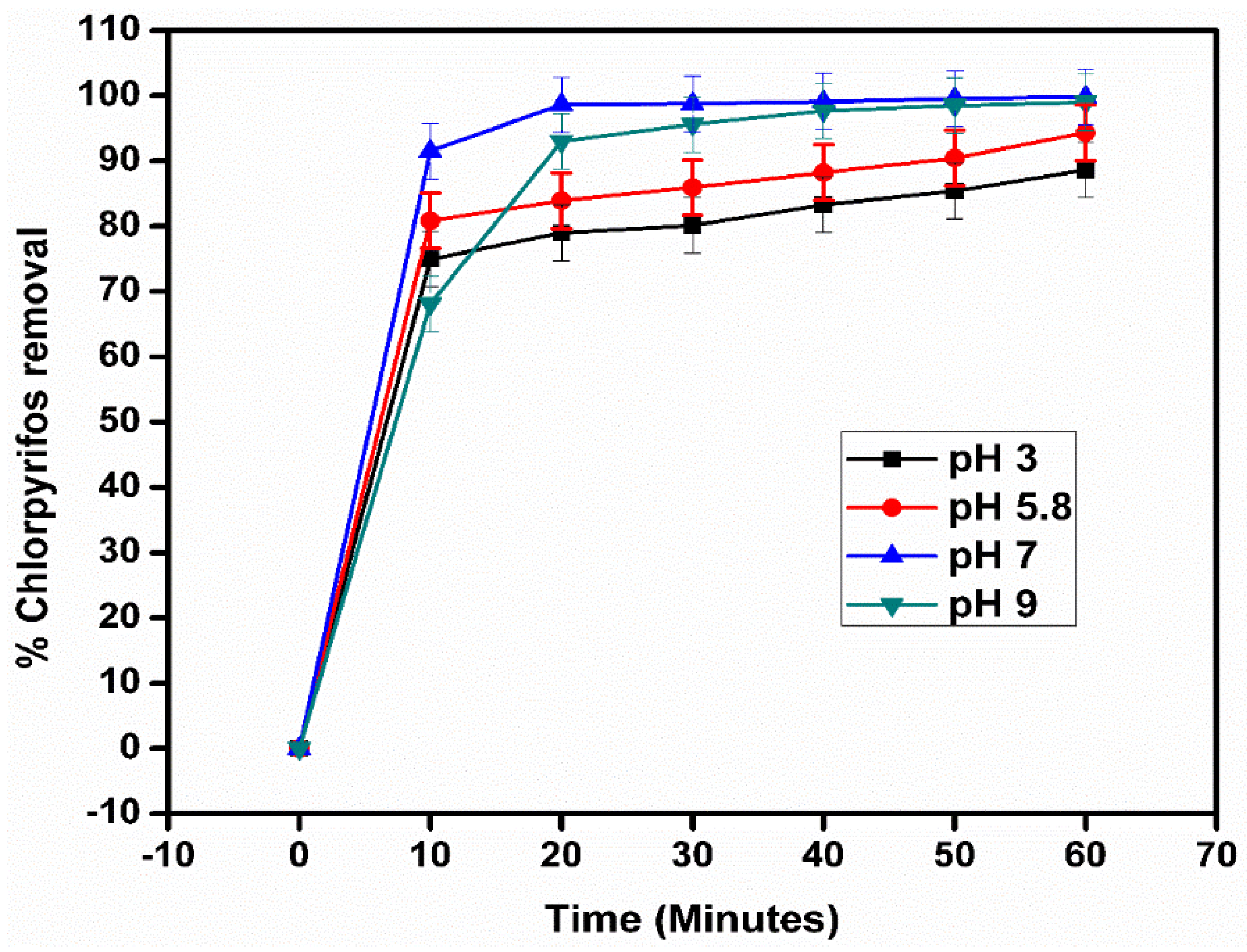
2.8. Effect of pH on the Photocatalytic Activity
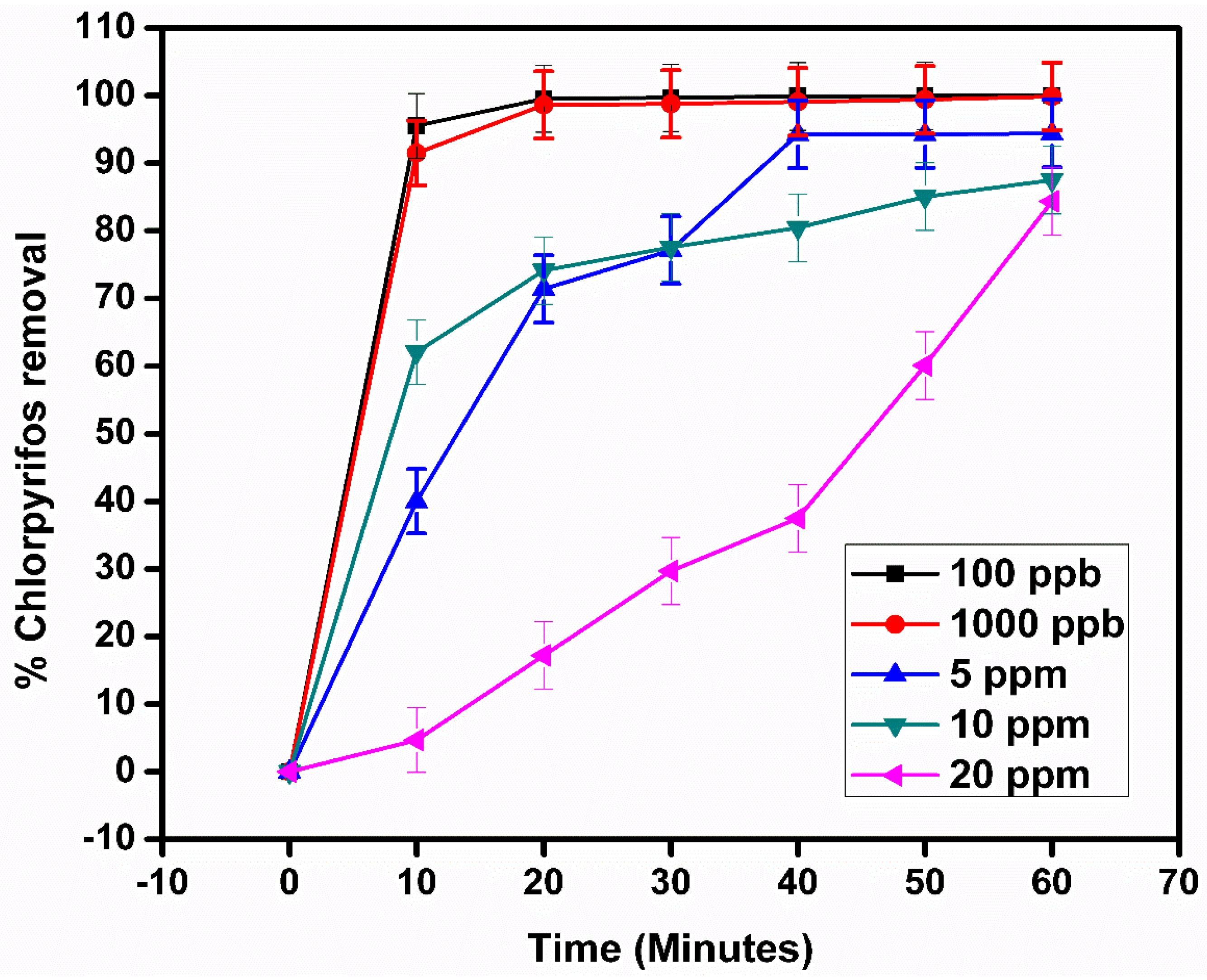
2.9. Effect of Initial Concentration
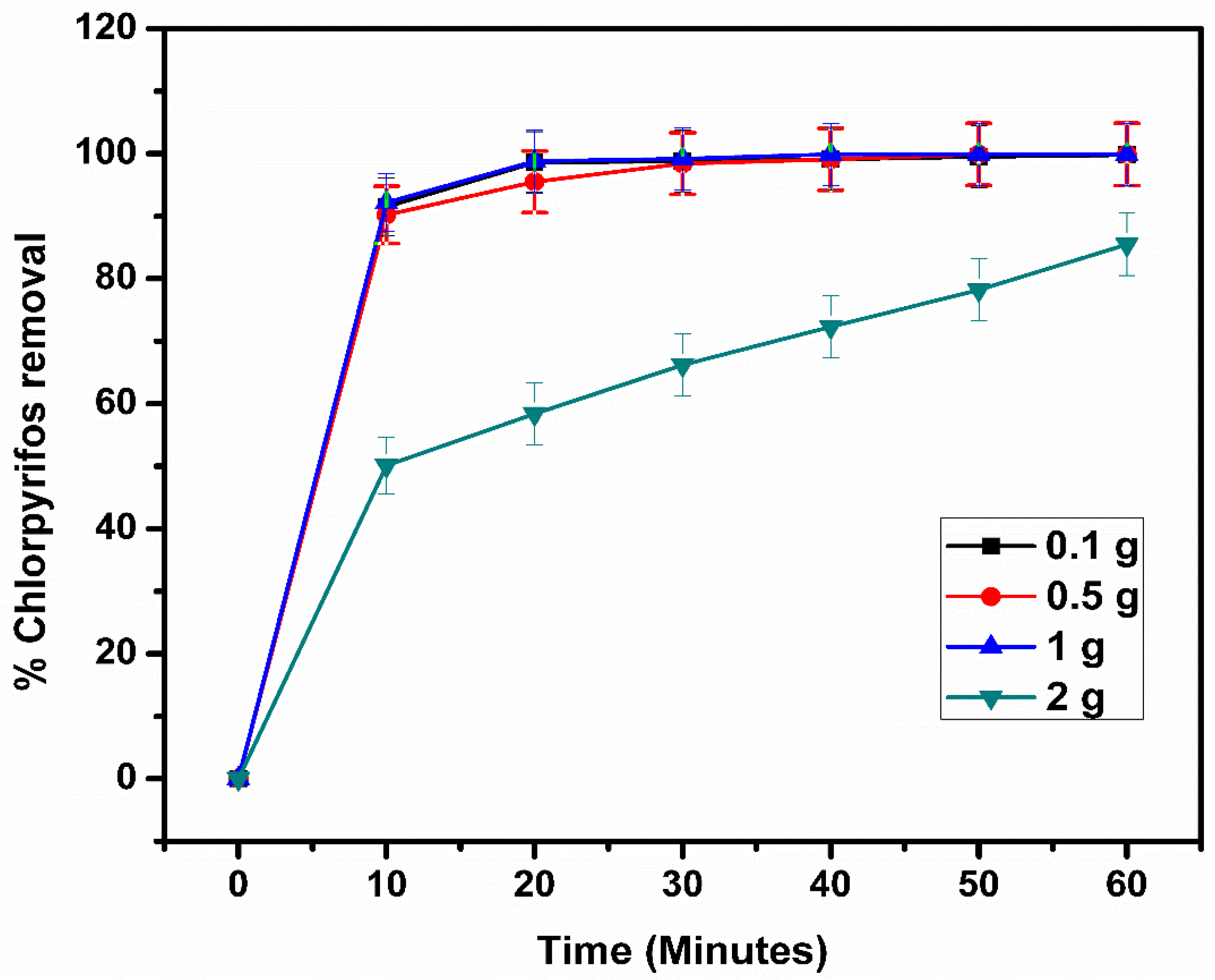
2.10. Effect of Initial Photocatalyst Loading
2.11. Mechanistic Pathway
3. Materials and Methods
3.1. Materials
Synthesis of Nanomaterial
3.2. Characterization Techniques
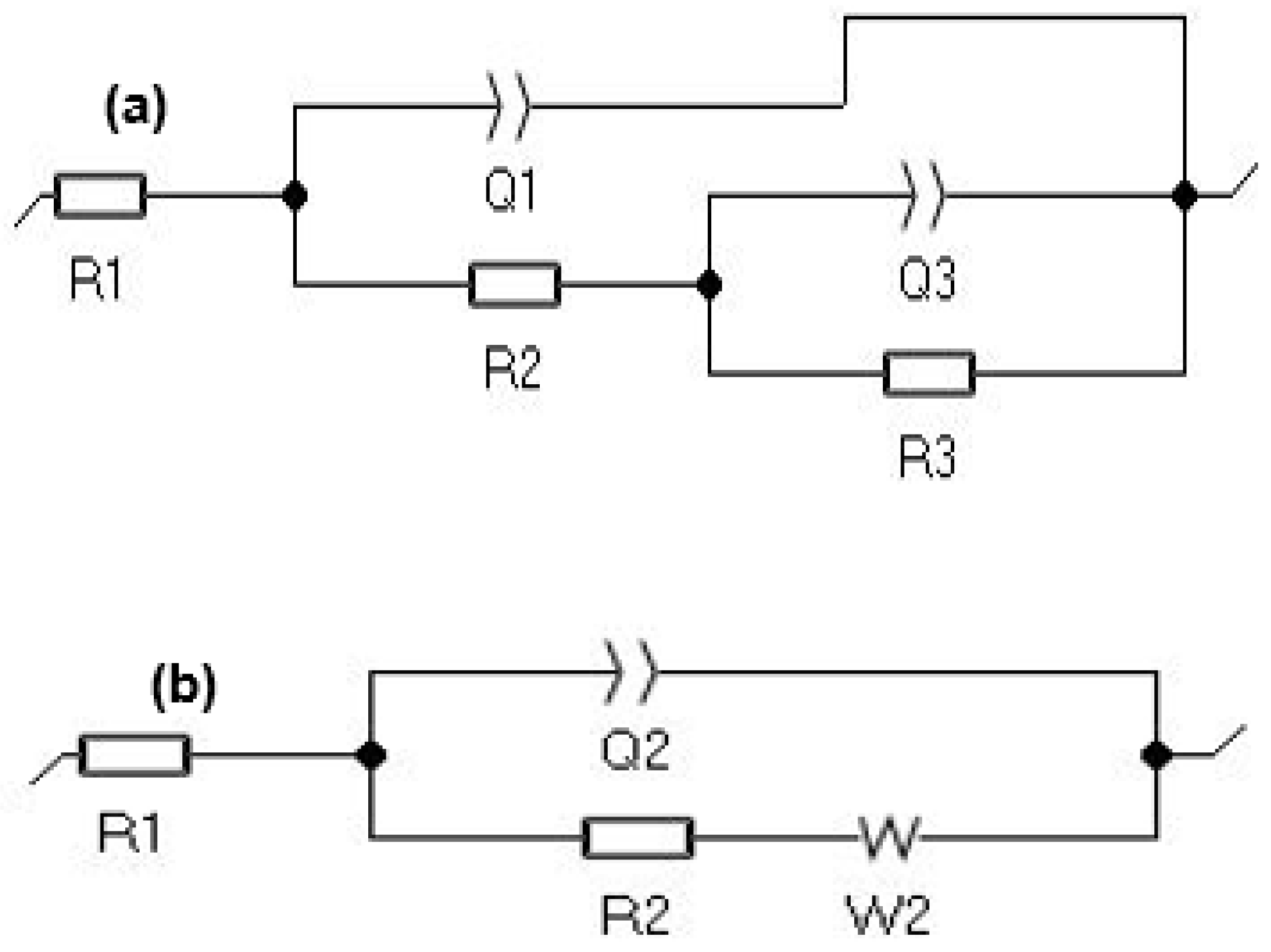
3.3. Electrochemical Measurements
3.4. Surface Charge
3.5. Degradation of Chlorpyrifos
3.5.1. Chlorpyrifos Standard Preparations
3.5.2. Photocatalytic Degradation of Chlorpyrifos
3.6. LC-MS Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. J. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Xie, Y.P.; Liu, G.; Yin, L.; Cheng, H.M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO3 for solar energy conversion. J. Mater. Chem. 2012, 22, 6746. [Google Scholar] [CrossRef]
- Mahlalela, L.C.; Ngila, J.C.; Dlamini, L.N. Characterization and stability of TiO2 nanoparticles in industrial dye stuff effluent. J. Dispers. Sci. Technol. 2017, 38, 584–593. [Google Scholar] [CrossRef]
- Batzill, M. Fundamental aspects of surface engineering of transition metal oxide photocatalysts. Int. J. Energy Environ. Sci. 2011, 4, 3275. [Google Scholar] [CrossRef]
- Harshulkhan, S.M.; Velraj, K.J.G. Structural and optical properties of Mg doped tungsten oxide prepared by microwave irradiation method. J. Mater. Sci. Mater. Electron. 2017, 28, 11794–11799. [Google Scholar] [CrossRef]
- Parthibavarman, M.K.M.; Prabhakaran, A.K.S. One-step microwave synthesis of pure and Mn doped WO3 nanoparticles and its structural, optical and electrochemical properties. J. Mater. Sci. Mater. Electron. 2017, 28, 6635–6642. [Google Scholar]
- Lu, M.W.; Wang, Q.F.; Miao, J.; Huang, Y. Synthesis and electrochemical performances of cotton ball-like SnS2 compound as anode material for lithium ion batteries. J. Mater. Sci. Technol. 2016, 31, 281–285. [Google Scholar]
- Yamamoto, T.; Teramachi, A.; Orita, A.; Kurimoto, A.; Motoi, T.; Tanaka, T. Generation of strong acid sites on yttrium-doped tetragonal ZrO2-supported tungsten oxides: Effects of dopant amounts on acidity, crystalline phase, kinds of tungsten species, and their dispersion. J. Phys. Chem. C 2016, 120, 19705–19713. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, C.S.; Jung, H.; Min, B.K.; Kim, J.H.; Hwang, Y.J. Insight into charge separation in WO3/BiVO4 heterojunction for solar water splitting. ACS Appl. Mater. Interfaces 2017, 9, 19780–19790. [Google Scholar] [CrossRef]
- Velanganni, S.; Pravinraj, S.; Immanuel, P.; Thiruneelakandan, R. Nanostructure CdS/ZnO heterojunction configuration for photocatalytic degradation of Methylene blue. Physics B 2018, 534, 56–62. [Google Scholar] [CrossRef]
- Afuyoni, M.; Nashed, G.; Mohammed, I. TiO2 doped with SnO2 and studing its structural and electrical properties. Energy Procedia 2011, 6, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Shaposhnik, D.; Pavelko, R.; Llobet, E.; Gispert-guirado, F.; Vilanova, X. Hydrogen sensors on the basis of SnO2-TiO2 systems. Procedia Eng. 2011, 25, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.J.; Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar]
- Simelane, S.; Ngila, J.C.; Dlamini, L.N. The effect of humic acid on the stability and aggregation kinetics of WO3 nanoparticles. Part Sci. Technol. 2017, 35, 632–642. [Google Scholar] [CrossRef]
- Georgaki, I.V.I.; Kenanakis, D.V.G.; Katsarakis, N. Synthesis of WO3 catalytic powders: Evaluation of photocatalytic activity under NUV/visible light irradiation and alkaline reaction pH. J. Sol-Gel Sci. Technol. 2015, 76, 120–128. [Google Scholar]
- Székely, I.; Kovács, G.; Baia, L.; Danciu, V.; Pap, Z. Synthesis of shape-tailored WO3 micro-/nanocrystals and the photocatalytic activity of WO3/TiO2 composites. Materials 2016, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, U.A.; Darwent, J.R.; Yiu, H.H.P.; Rosseinsky, M.J. The effect of platinum on the performance of WO3 nanocrystal photocatalysts for the oxidation of Methyl Orange and iso-propanol. J. Chem. Technol. Biotechnol. 2011, 86, 1018–1023. [Google Scholar] [CrossRef]
- Cai, J.; Wu, X.; Li, S.; Zheng, F. Synthesis of TiO2@WO3/Au nanocomposite hollow spheres with controllable size and high visible-light-driven photocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 1581–1590. [Google Scholar] [CrossRef]
- Dalvie, M.A.; Sosan, M.B.; Africa, A.; Cairncross, E.; London, L. Environmental monitoring of pesticide residues from farms at a neighbouring primary and pre-school in the Western Cape in South Africa. Sci. Total Environ. 2014, 466–467, 1078–1084. [Google Scholar] [CrossRef]
- Glynnis, R.; Perry, M.; Lee, M.M.; Hoffman, E.; Delport, S.; Dalvie, M.A. Farm residence and reproductive health among boys in rural South Africa. Environ. Int. 2012, 47, 73–79. [Google Scholar]
- Gao, J.; Naughton, S.X.; Beck, W.D.; Hernandez, C.M.; Wu, G.; Wei, Z.; Yang, X.; Bartlett, M.G.; Terry, A.V., Jr. Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. Neurotoxicology 2017, 62, 111–123. [Google Scholar] [CrossRef]
- Michael, J.; Madimetja, J.V.; Wepener, V. Prioritizing agricultural pesticides used in South Africa based on their environmental mobility and potential human health effects. Environ. Int. 2014, 62, 31–40. [Google Scholar]
- Ismail, M.; Khan, H.M.; Sayed, M.; Cooper, W.J. Advanced oxidation for the treatment of chlorpyrifos in aqueous solution. Chemosphere 2013, 93, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Silvia, M.D. Highly selective sample preparation and gas chromatographic—Mass spectrometric analysis of chlorpyrifos, diazinon and their major metabolites in sludge and sludge-fertilized agricultural soils. J. Chromatogr. A 2006, 1132, 21–27. [Google Scholar]
- Sharma, B.; Saxena, S.; Datta, A.; Arora, S. Spectrophotometric analysis of degradation of chlorpyrifos pesticide by indigenous microorganisms isolated from affected soil. Int. J. Curr. Microb. Appl. Sci. 2016, 5, 742–749. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Peng, C.; Liu, H.; Hu, M.; Zhong, G. Biodegradation of Chlorpyrifos and Its Hydrolysis Product 3,5,6-Trichloro-2-Pyridinol by a New Fungal Strain Cladosporium cladosporioides Hu-01. PLoS ONE 2012, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fadaei, A.; Kargar, M. Photocatalytic degradation of chlorpyrifos in water using titanium dioxide and zinc oxide. Fres. Environ. Bull. 2013, 22, 2442–2447. [Google Scholar]
- Ma, C.; Xu, J.; Alvarado, J.; Qu, B.; Somerville, J.; Lee, J.Y.; Meng, Y.S. Investigating the energy storage mechanism of SnS2-rGO composite anode for advanced Na-ion batteries. Chem. Mater. 2015, 27, 5633–5640. [Google Scholar] [CrossRef]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystallogr. 2017, 50, 1–9. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, F.; Wang, P.; Wang, C.; Chen, J.; Xu, Y.; You, G.; Zhou, Q.; Li, Z. Enhanced anaerobic biological treatment of chlorpyrifos in farmland drainage with zero valent iron. J. Chem. Eng. 2018, 336, 352–360. [Google Scholar] [CrossRef]





| Material | SBET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| WO3 | 6.01 | 0.0276 |
| Mn-WO3 | 4.41 | 0.0294 |
| WO3/SnS2 | 44.36 | 0.0514 |
| Mn-WO3/SnS2 | 77.14 | 0.0641 |
| Pristine SnS2 | 99.72 | 0.0748 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgoetlana, C.M.; Malinga, S.P.; Dlamini, L.N. Photocatalytic Degradation of Chlorpyrifos with Mn-WO3/SnS2 Heterostructure. Catalysts 2020, 10, 699. https://doi.org/10.3390/catal10060699
Kgoetlana CM, Malinga SP, Dlamini LN. Photocatalytic Degradation of Chlorpyrifos with Mn-WO3/SnS2 Heterostructure. Catalysts. 2020; 10(6):699. https://doi.org/10.3390/catal10060699
Chicago/Turabian StyleKgoetlana, Charlie M., Soraya P. Malinga, and Langelihle N. Dlamini. 2020. "Photocatalytic Degradation of Chlorpyrifos with Mn-WO3/SnS2 Heterostructure" Catalysts 10, no. 6: 699. https://doi.org/10.3390/catal10060699
APA StyleKgoetlana, C. M., Malinga, S. P., & Dlamini, L. N. (2020). Photocatalytic Degradation of Chlorpyrifos with Mn-WO3/SnS2 Heterostructure. Catalysts, 10(6), 699. https://doi.org/10.3390/catal10060699






