Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature
Abstract
:1. Introduction
2. Results and Discussion
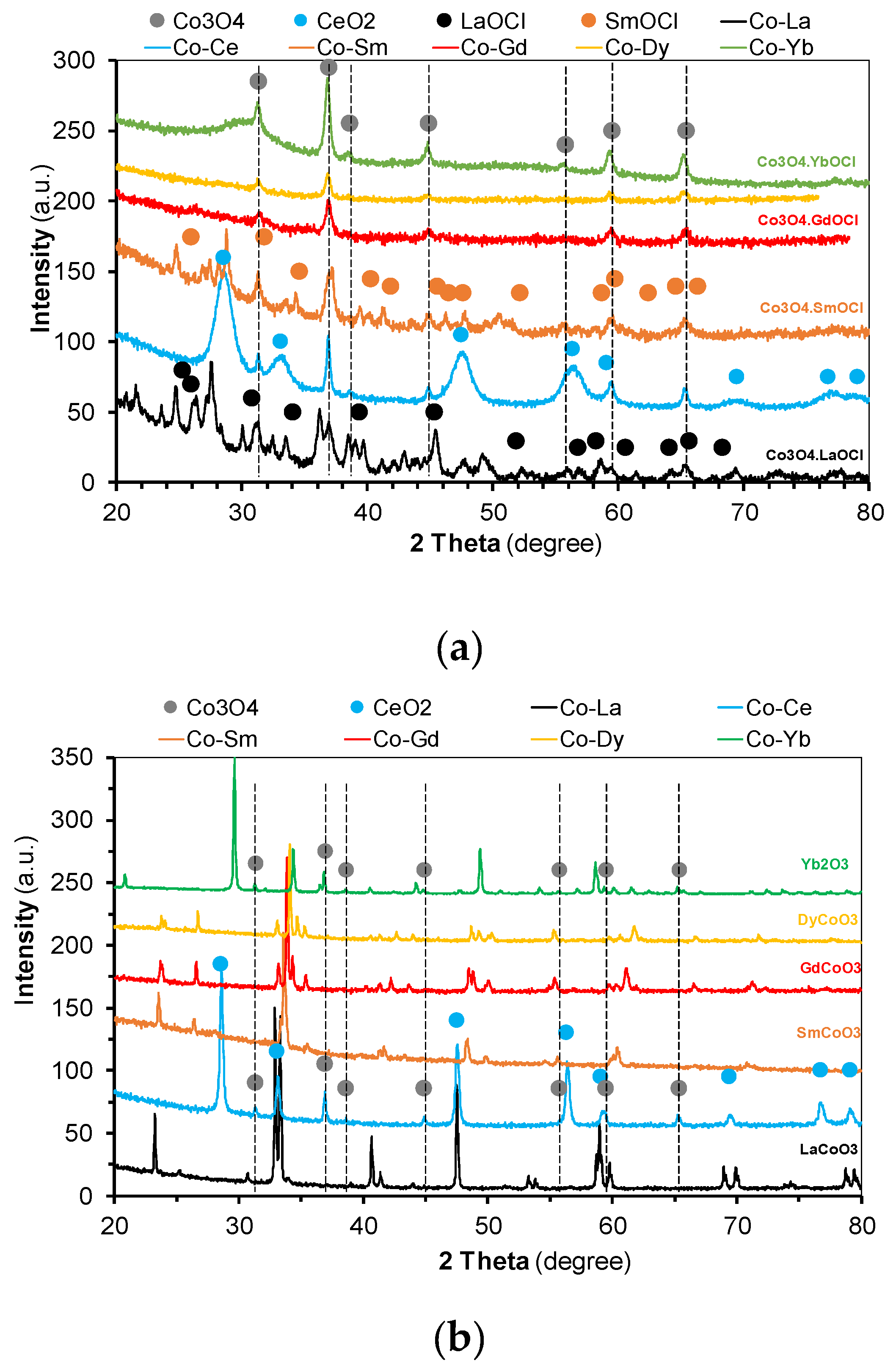
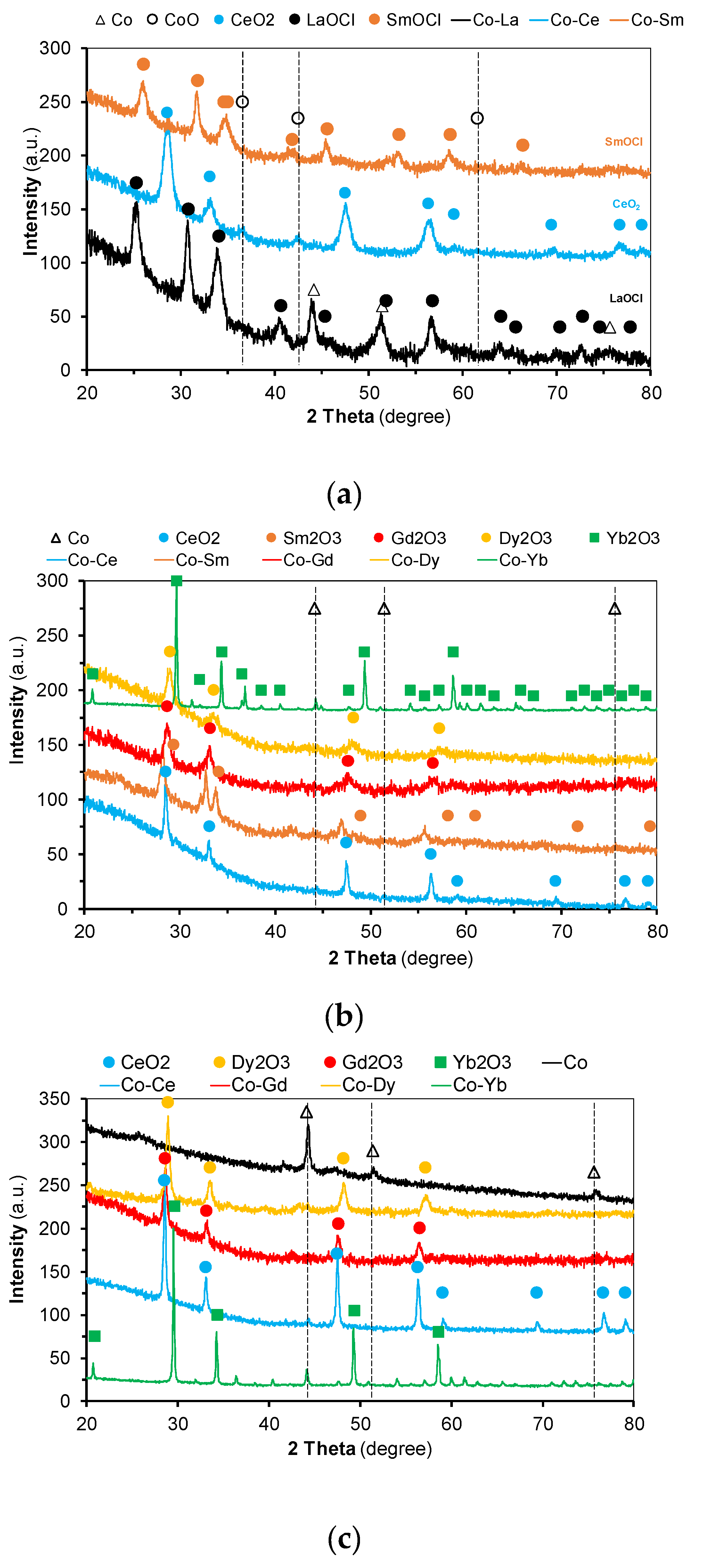
2.1. Catalysts Characterization
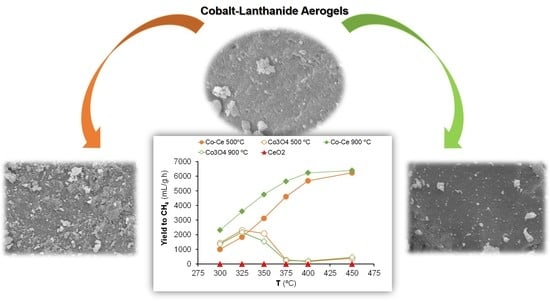
2.2. Hydrogenation of CO2
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.H.; Wang, H.Z.; Jiang, X.; Zhu, J.; Liu, Z.M.; Guo, X.W.; Song, C.S. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [Green Version]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Perez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Ronsch, S.; Schneider, J.; Matthischke, S.; Schluter, M.; Gotz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.Y.; Chu, W.; Zhou, Y.L.; Kohler, K. CO2 methanation over supported Ru/Al2O3 catalysts: Mechanistic studies by in situ infrared spectroscopy. Chemistryselect 2016, 1, 3197–3203. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.S.; Jun, K.W.; Choi, M.J.; Kishan, G.; Lee, K.W. Comparative study of Fischer-Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe- and Co-based catalysts. Appl. Catal. A Gen. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Davis, B.H.; Willauer, H.D. Influence of Gas Feed Composition and Pressure on the Catalytic Conversion of CO2 to Hydrocarbons using a traditional cobalt-based fischer-tropsch catalyst. Energy Fuels 2009, 23, 4190–4195. [Google Scholar] [CrossRef]
- Zhang, J.L.; Su, X.J.; Wang, X.; Ma, Q.X.; Fan, S.B.; Zhao, T.S. Promotion effects of Ce added Fe-Zr-K on CO2 hydrogenation to light olefins. React. Kinet. Mech. Cat. 2018, 124, 575–585. [Google Scholar] [CrossRef]
- Zeng, S.H.; Du, Y.; Su, H.Q.; Zhang, Y.L. Promotion effect of single or mixed rare earths on cobalt-based catalysts for Fischer-Tropsch synthesis. Catal. Commun. 2011, 13, 6–9. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Park, E.D. CO and CO2 methanation over supported cobalt catalysts. Top. Catal. 2017, 60, 714–720. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Jiang, Y.X.; Qin, Z.Z.; Xie, Q.R.; Ji, H.B. Influence of Zr, Ce, and La on Co3O4 catalyst for CO2 methanation at low temperature. Chin. J. Chem. Eng. 2018, 26, 768–774. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ferraria, A.M.; do Rego, A.M.B.; Goncalves, A.P.; Correia, M.R.; Gasche, T.A.; Branco, J.B. Partial oxidation of methane over bimetallic nickel-lanthanide oxides. J. Alloys Compd. 2010, 489, 316–323. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ferraria, A.M.; do Rego, A.M.B.; Goncalves, A.P.; Girao, A.V.; Correia, R.; Gasche, T.A.; Branco, J.B. Partial oxidation of methane over bimetallic copper-cerium oxide catalysts. J. Mol. Catal. A Chem. 2010, 320, 47–55. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Goncalves, A.P.; Gasche, T.A.; Ferraria, A.M.; do Rego, A.M.B.; Correia, M.R.; Bola, A.M.; Branco, J.B. Partial oxidation of methane over bimetallic copper- and nickel-actinide oxides (Th, U). J. Alloys Compd. 2010, 497, 249–258. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C.; Gasche, T.A.; Pimenta, G.; Leal, J.P. Low temperature partial oxidation of methane over bimetallic nickel-f block element oxide nanocatalysts. Adv. Synth. Catal. 2014, 356, 3048–3058. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C.; Leal, J.P. Light hydrocarbons production over bimetallic calcium-actinide oxide catalysts using N2O as oxidant. J. Mol. Catal. A Chem. 2014, 390, 45–51. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Gasche, T.A.; Leal, J.P.; Branco, J.B. Methane activation with nitrous oxide over bimetallic oxide Ca-lanthanide nanocatalysts. Mol. Catal. 2017, 443, 155–164. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C. Methanation of CO2 over bimetallic Ni-5f block element (Th, U) oxides. Eur. J. Inorg. Chem. 2019, 1039–1045. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Branco, J.B. Methanation of CO2 over nanostructured nickel-4f block element bimetallic oxides. Int. J. Hydrog. Energy 2019, 44, 6505–6513. [Google Scholar] [CrossRef]
- Zhou, G.L.; Liu, H.R.; Cui, K.K.; Xie, H.M.; Jiao, Z.J.; Zhang, G.Z.; Xiong, K.; Zheng, X.X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrog. Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Clapsaddle, B.J.; Neumann, B.; Wittstock, A.; Sprehn, D.W.; Gash, A.E.; Satcher, J.H.; Simpson, R.L.; Baumer, M. A sol-gel methodology for the preparation of lanthanide-oxide aerogels: Preparation and characterization. J. Solgel Sci. Technol. 2012, 64, 381–389. [Google Scholar] [CrossRef]
- Cho, Y.J.; Yang, H.C.; Eun, H.C.; Kim, E.H.; Kim, I.T. Characteristics of oxidation reaction of rare-earth chlorides for precipitation in LiCl-KCl molten salt by oxygen sparging. J. Nucl. Sci. Technol. 2006, 43, 1280–1286. [Google Scholar] [CrossRef]
- Ben Farhat, L.; Ben Hassen, R.; Dammak, L. Rietveld refinement of YbCoO3 prepared from aqueous solution-gel precursor. Powder Diffr. 2007, 22, 35–39. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhao, M.; Song, Z.X.; Zhao, H.; Liu, W.; Zhao, J.G.; Ma, Z.A.; Xing, Y. The effect of different metal oxides on the catalytic activity of a Co3O4 catalyst for toluene combustion: Importance of the structure-property relationship and surface active species. New J. Chem. 2019, 43, 10868–10877. [Google Scholar] [CrossRef]
- Lago, R.; Bini, G.; Pena, M.A.; Fierro, J.L.G. Partial oxidation of methane to synthesis gas using LnCoO3 perovskites as catalyst precursors. J. Catal. 1997, 167, 198–209. [Google Scholar] [CrossRef]
- Arakawa, T.; Ohara, N.; Shiokawa, J. Reduction of Perovskite Oxide La-EuCoO3 in a Hydrogen Atmosphere. J. Mater. Sci. 1986, 21, 1824–1827. [Google Scholar] [CrossRef]
- Hattori, H. Heterogeneous Basic Catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. New Acids and Bases-Their catalytic Properties; Studies in Surface Science and Catalysis; Delmon, B., Yates, J.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 51, pp. 200–211. [Google Scholar]
- Williams, J.; Jones, R.H.; Thomas, J.M.; Kent, J. A comparison of the catalytic performance of the layered oxychlorides of bismuth, lanthanum and samarium in the conversion of methane to ethylene. Catal. Lett. 1989, 3, 247–255. [Google Scholar] [CrossRef]
- Ueda, W.; Sakyu, F.; Isozaki, T.; Morikawa, Y.; Thomas, J.M. Catalytic oxidative dimerization of methane to form C2-Compounds over Arppe phase oxychlorides of Bi, La and Sm. Catal. Lett. 1991, 10, 83–90. [Google Scholar] [CrossRef]
- Chanaud, P.; Julbe, A.; Larbot, A.; Guizard, C.; Cot, L.; Borges, H.; Fendler, A.G.; Mirodatos, C. Catalytic membrane reactor for oxidative coupling of methane. 1. preparation and characterization of LaOCl membranes. Catal. Today 1995, 25, 225–230. [Google Scholar] [CrossRef]
- Kienneman, A.; Kieffer, R.; Kaddouri, A.; Poix, P.; Rehspringer, J.L. Oxidative coupling of methane over LnLiO2, LnNaO2 and LnOX catalysts (Ln = Sm, Nd, La; X = Cl, Br). Promoting effect of MgO, CaO and SrO. Catal. Today 1990, 6, 409–416. [Google Scholar] [CrossRef]
- Molander, G.A. Application of lanthanide reagents in organic-synthesis. Chem. Rev. 1992, 92, 29–68. [Google Scholar] [CrossRef]
- Yasuda, H.; He, L.N.; Sakakura, T. Cyclic carbonate synthesis from supercritical carbon dioxide and epoxide over lanthanide oxychloride. J. Catal. 2002, 209, 547–550. [Google Scholar] [CrossRef]
- Xu, J.H.; Su, X.; Duan, H.M.; Hou, B.L.; Lin, Q.Q.; Liu, X.Y.; Pan, X.L.; Pei, G.X.; Geng, H.R.; Huang, Y.Q.; et al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R.; Urasaki, K.; Satokawa, S. Effect of reduction pretreatment and support materials on selective CO methanation over supported Ru catalysts. Appl. Catal. A Gen. 2011, 404, 149–154. [Google Scholar] [CrossRef]
- Ahn, C.I.; Koo, H.M.; Jin, M.; Kim, J.M.; Kim, T.; Suh, Y.W.; Yoon, K.J.; Bae, J.W. Catalyst deactivation by carbon formation during CO hydrogenation to hydrocarbons on mesoporous Co3O4. Microporous Mesoporous Mater. 2014, 188, 196–202. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chang, K.H.; Lu, S.Y.; Hu, C.C. Cobalt oxide aerogels of ideal supercapacitive properties prepared with an epoxide synthetic route. Chem. Mater. 2009, 21, 3228–3233. [Google Scholar] [CrossRef]
- Ren, L.L.; Cui, S.M.; Cao, F.C.; Guo, Q.H. An easy way to prepare monolithic inorganic oxide aerogels. Angew. Chem. Int. Ed. 2014, 53, 10147–10149. [Google Scholar] [CrossRef]
- JCPDS. The Powder Diffraction File; JCPDS: Swarthmore, PA, USA, 1981. [Google Scholar]
- Dekker, F.H.M.; Bliek, A.; Kapteijn, F.; Moulijn, J.A. Analyisis of mass and heat transfer in trasient experiments over heterogeneous catalysts. Chem. Eng. Sci. 1995, 50, 3573–3580. [Google Scholar] [CrossRef] [Green Version]
- Oyama, S.T.; Zhang, X.; Lu, J.; Gu, Y.; Fujitani, T. Epoxidation of propylene with H2 and O2 in the explosive regime in a packed-bed catalytic membrane reactor. J. Catal. 2008, 257, 1–4. [Google Scholar] [CrossRef]
- Froment, G.F.; Bischoff, K.B.; De Wilde, J. Chemical Reactor Analysis and Design; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]









| Catalyst | Conversion CO2 (%) | Yield CH4 (%) | Selectivity CH4 (%) |
|---|---|---|---|
| Co-Ce a,b | 37.0 | 31.7 | 85.7 |
| NiO/CeO2 ref1 a,c | 35.0 | 33.7 | 96.3 |
| 5%Rh/Al2O3 ref2 a,d | 35.6 | 35.5 | 99.8 |
| Co-Ce e | 26.1 | 24.9 | 95.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, J.B.; da Silva, R.P.; Ferreira, A.C. Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature. Catalysts 2020, 10, 704. https://doi.org/10.3390/catal10060704
Branco JB, da Silva RP, Ferreira AC. Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature. Catalysts. 2020; 10(6):704. https://doi.org/10.3390/catal10060704
Chicago/Turabian StyleBranco, Joaquim Badalo, Ricardo Pinto da Silva, and Ana Cristina Ferreira. 2020. "Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature" Catalysts 10, no. 6: 704. https://doi.org/10.3390/catal10060704