Asymmetric Ring-Opening of Epoxides Catalyzed by Metal–Salen Complexes
Abstract
1. Introduction
2. Desymmetrization of meso-Epoxides
2.1. With Azides
2.2. With Anilines, Amines, and Carbamates
2.3. With Oxygen-Containing Nucleophiles
2.4. With Halogens
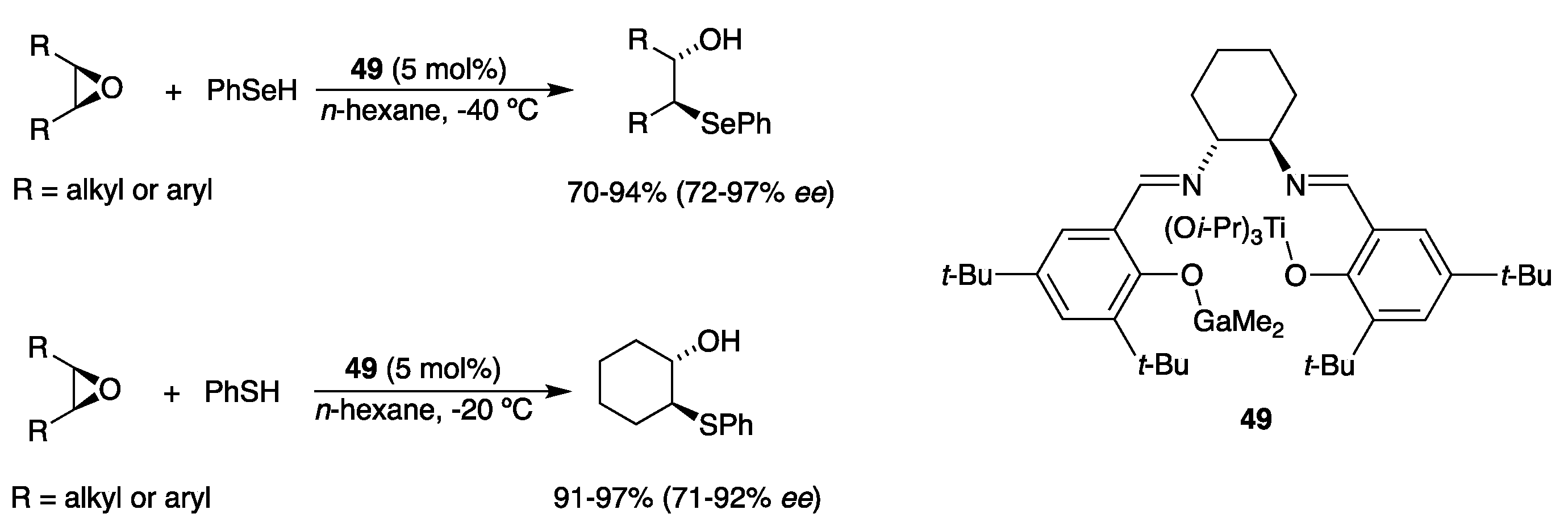
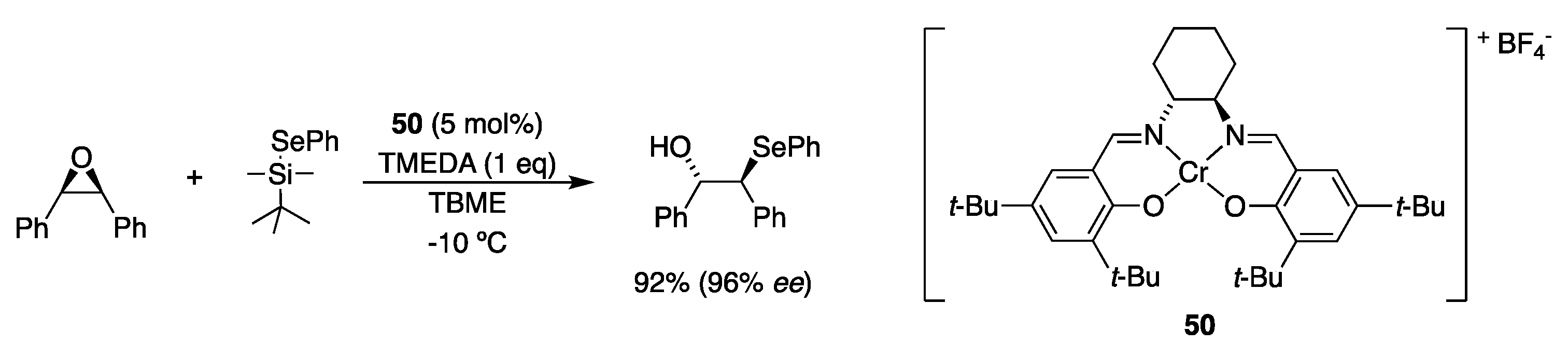
2.5. With Thiols and Selenols
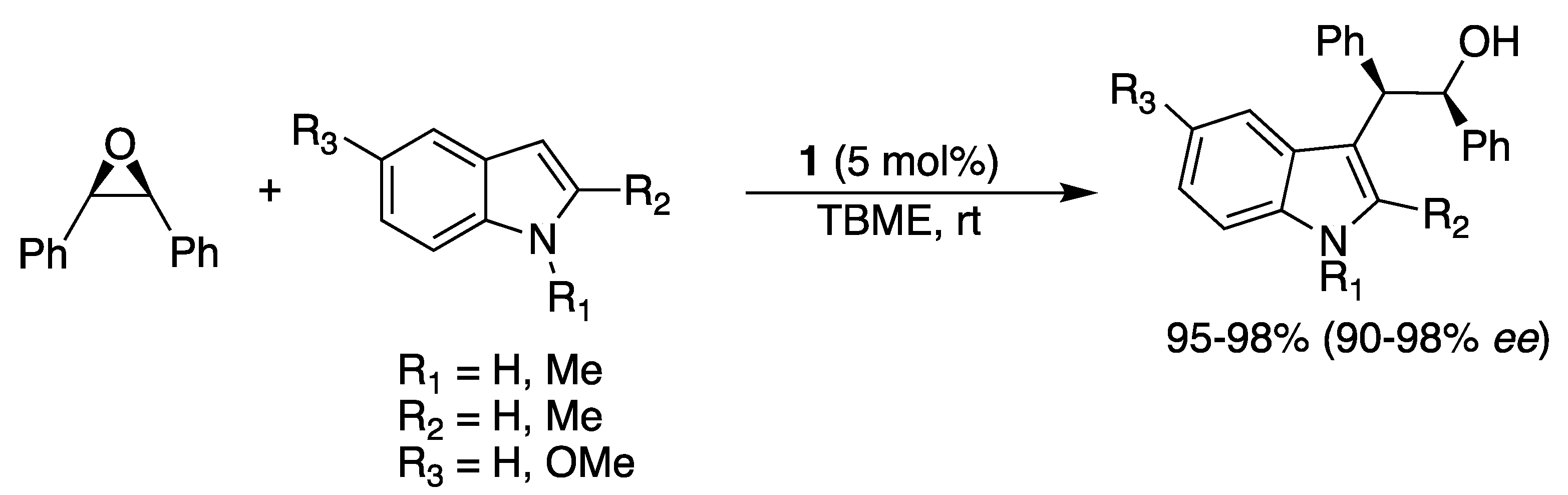
2.6. With Carbon-Containing Nucleophiles
3. Kinetic Resolution of Epoxides
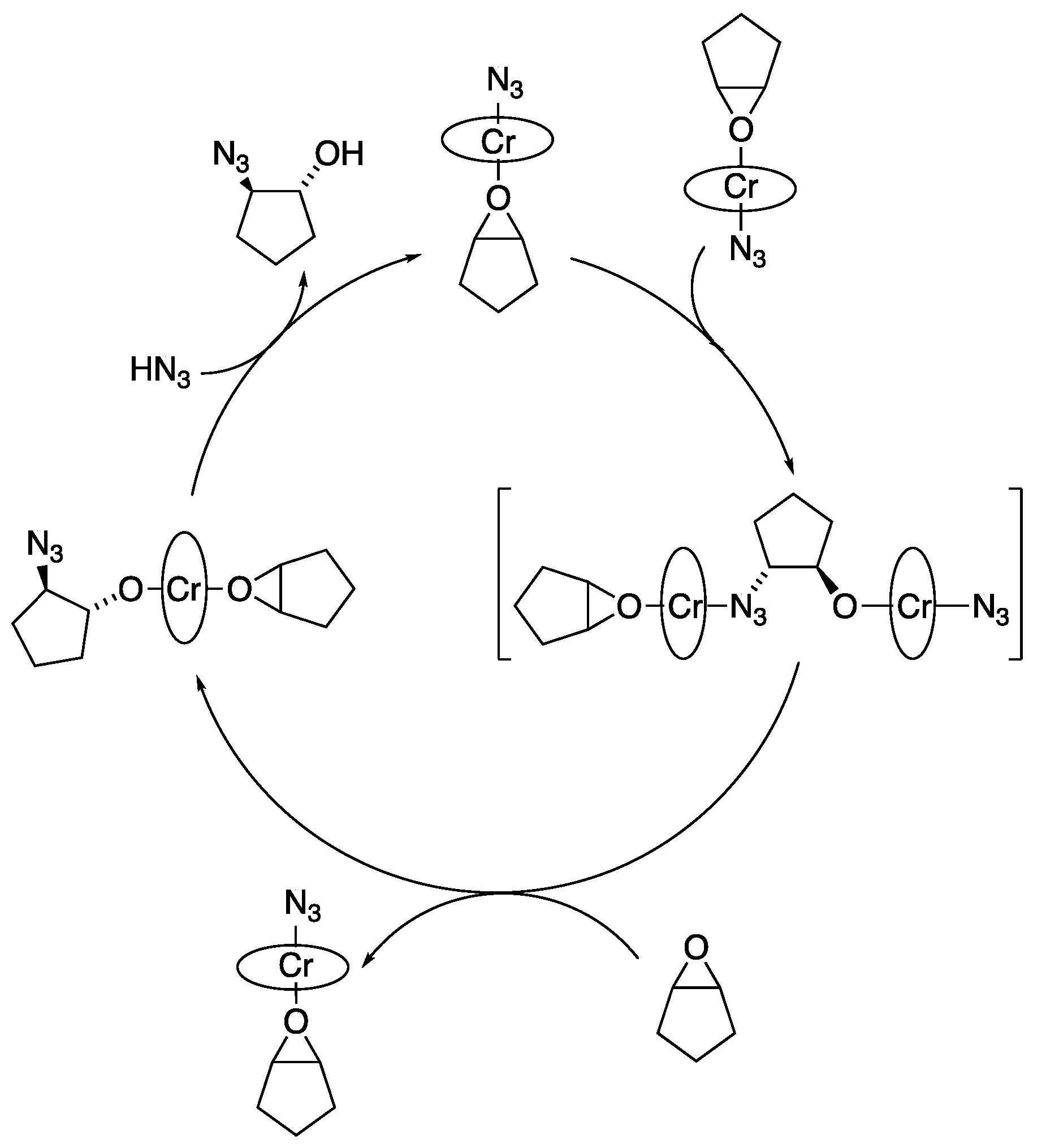
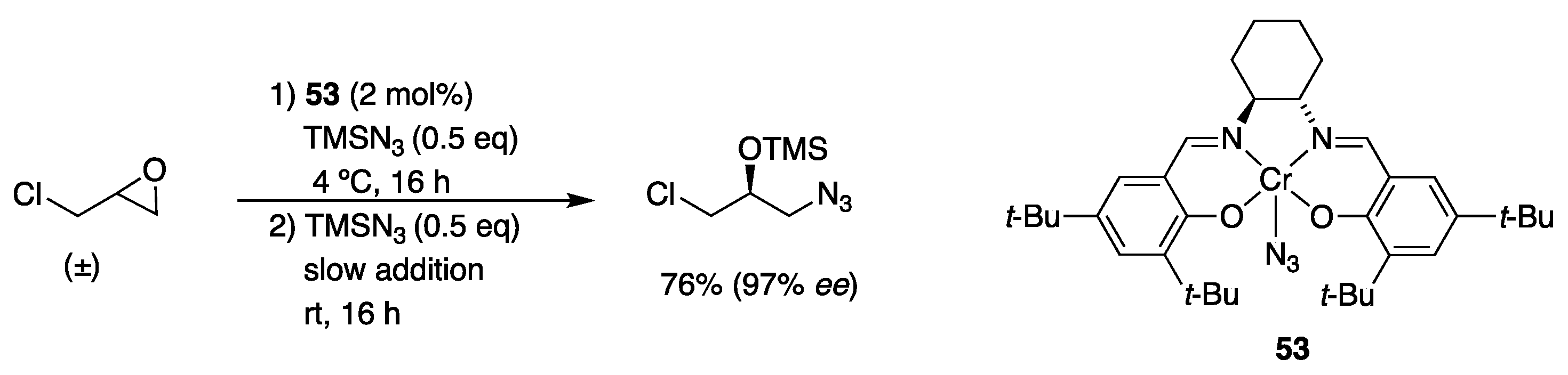
3.1. With Azides
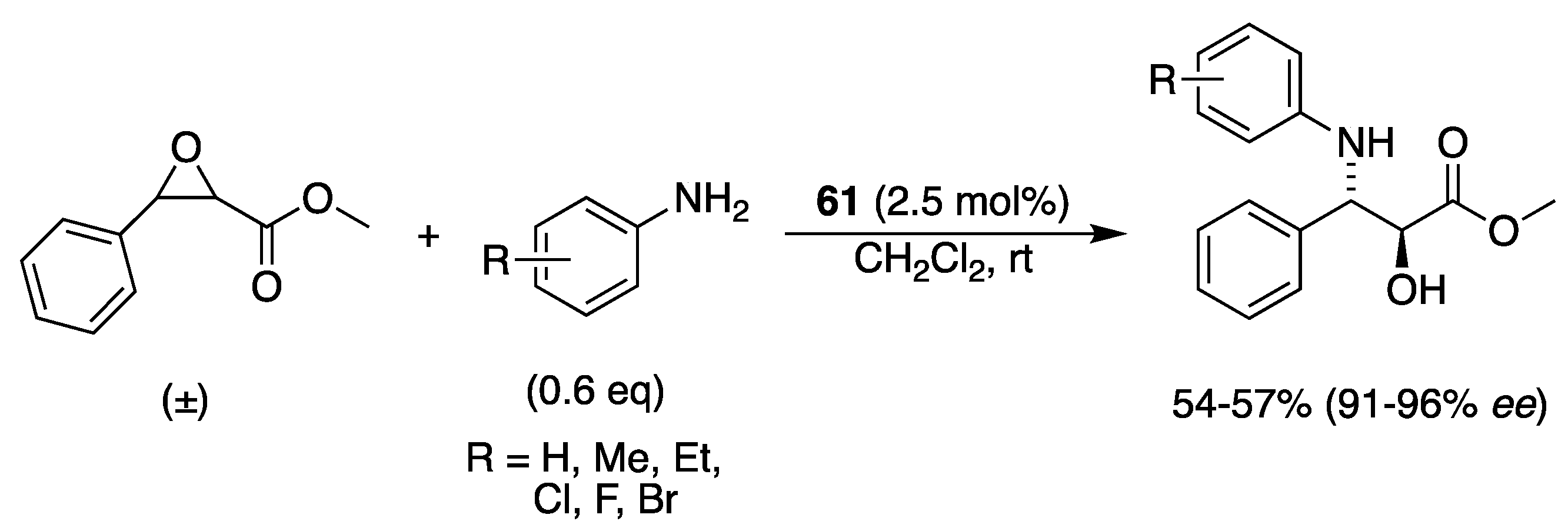
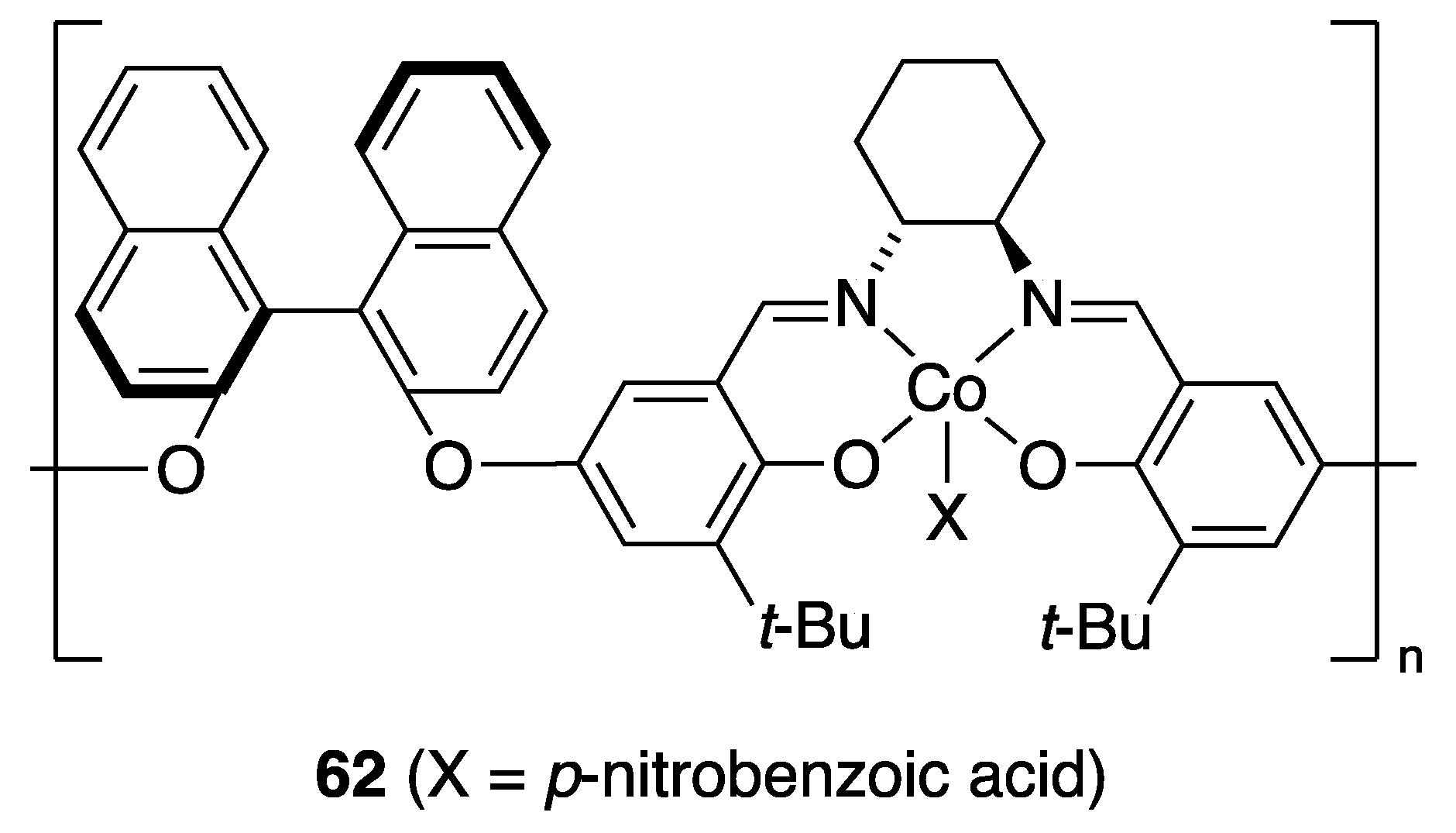
3.2. With Anilines, Amines and Carbamates
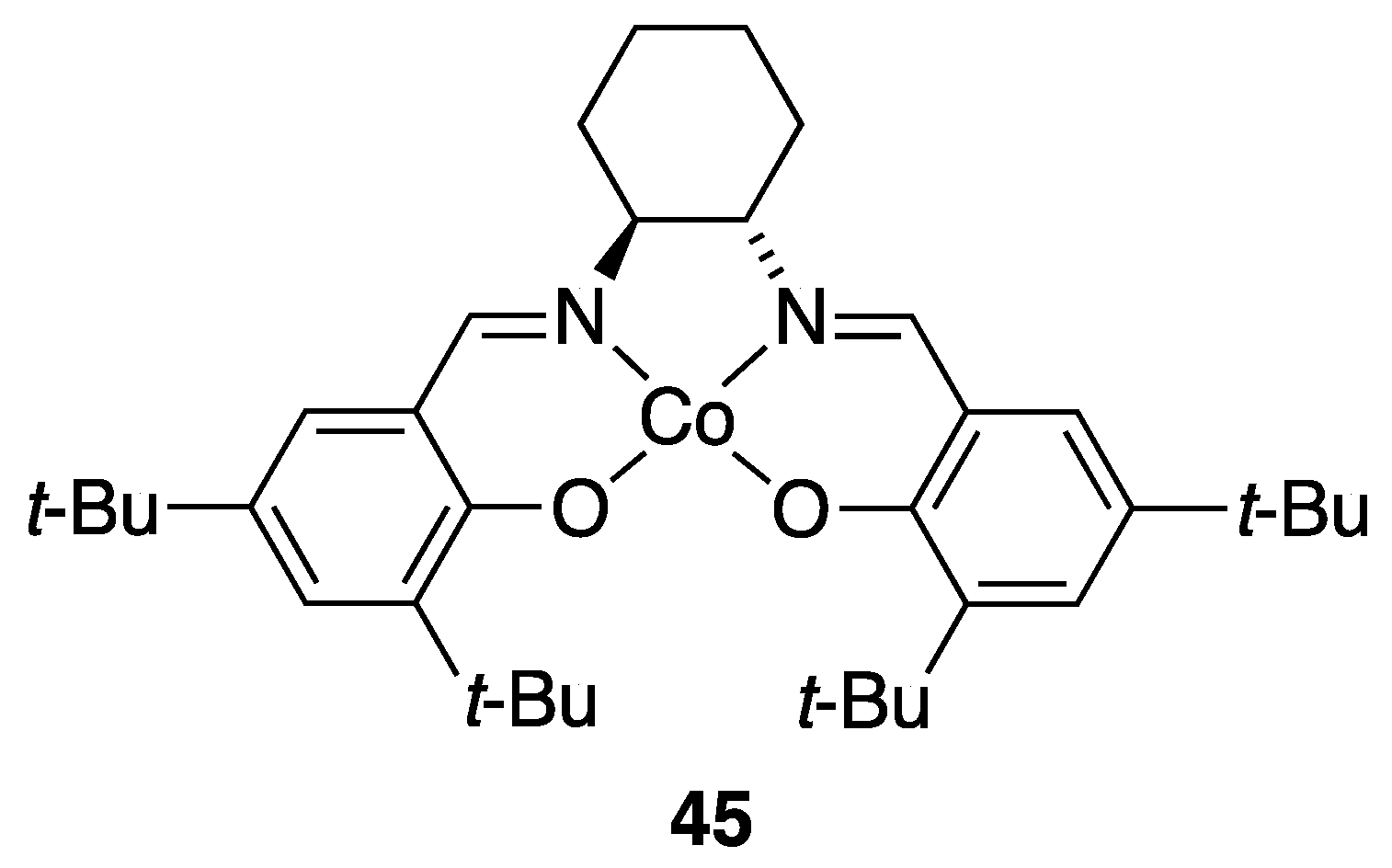
3.3. With Water (Hydrolytic Kinetic Resolution)
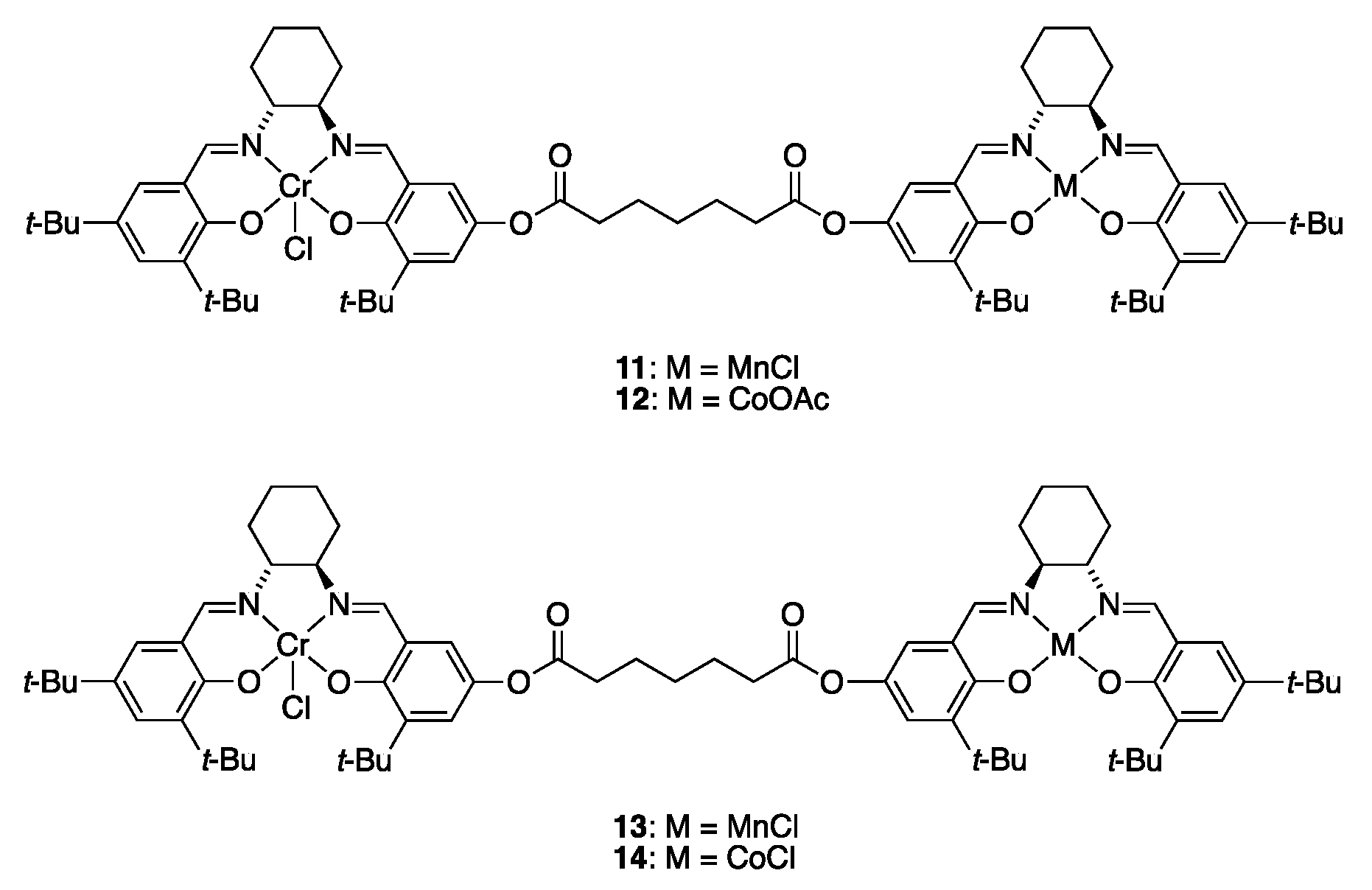
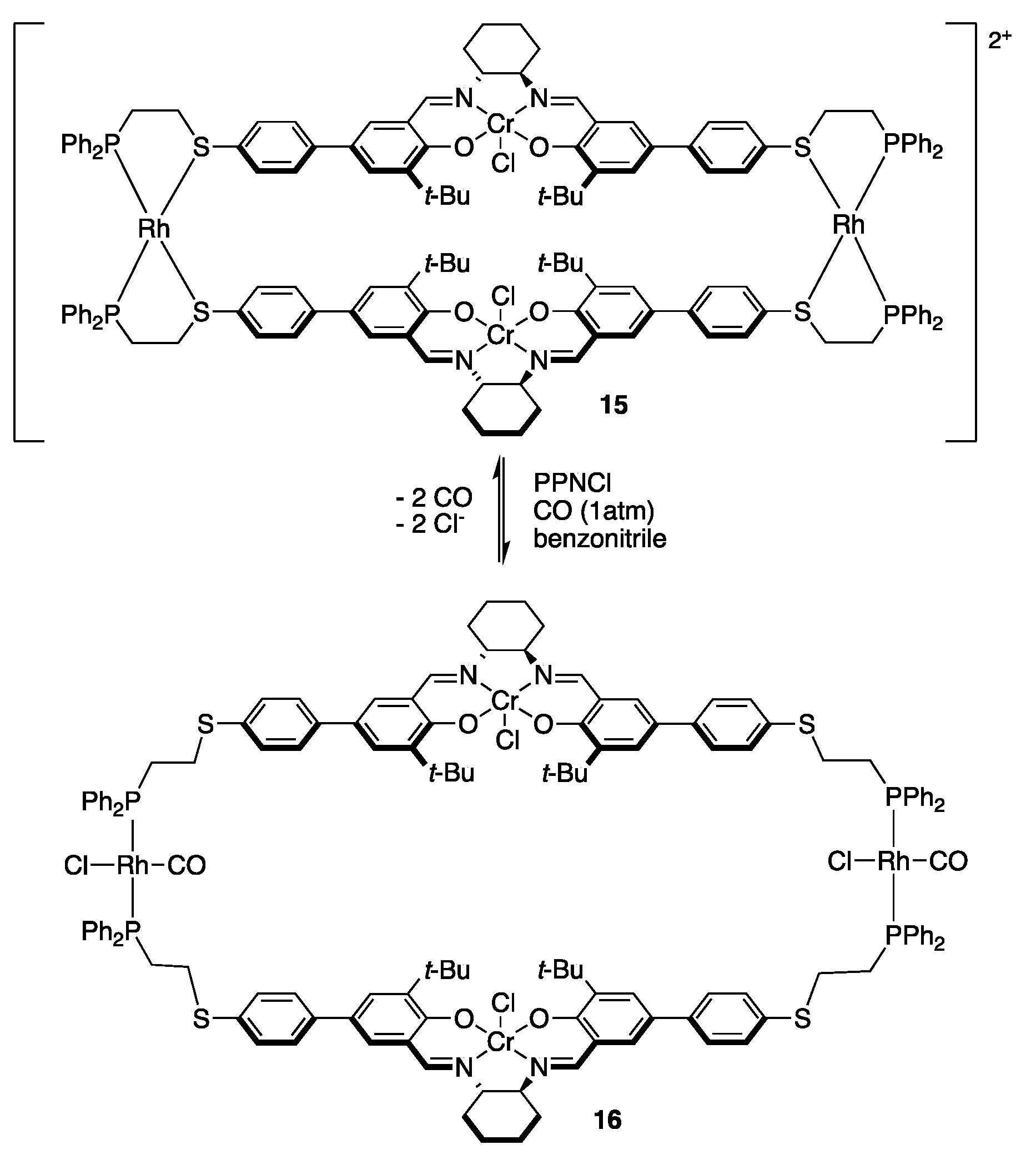
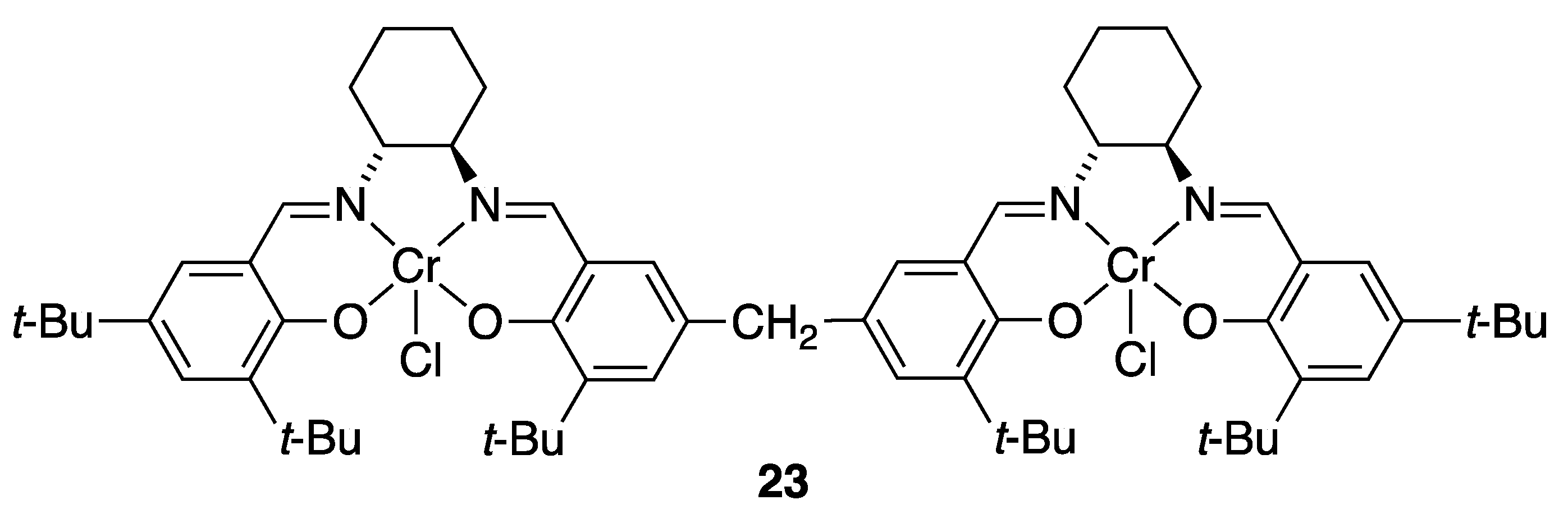
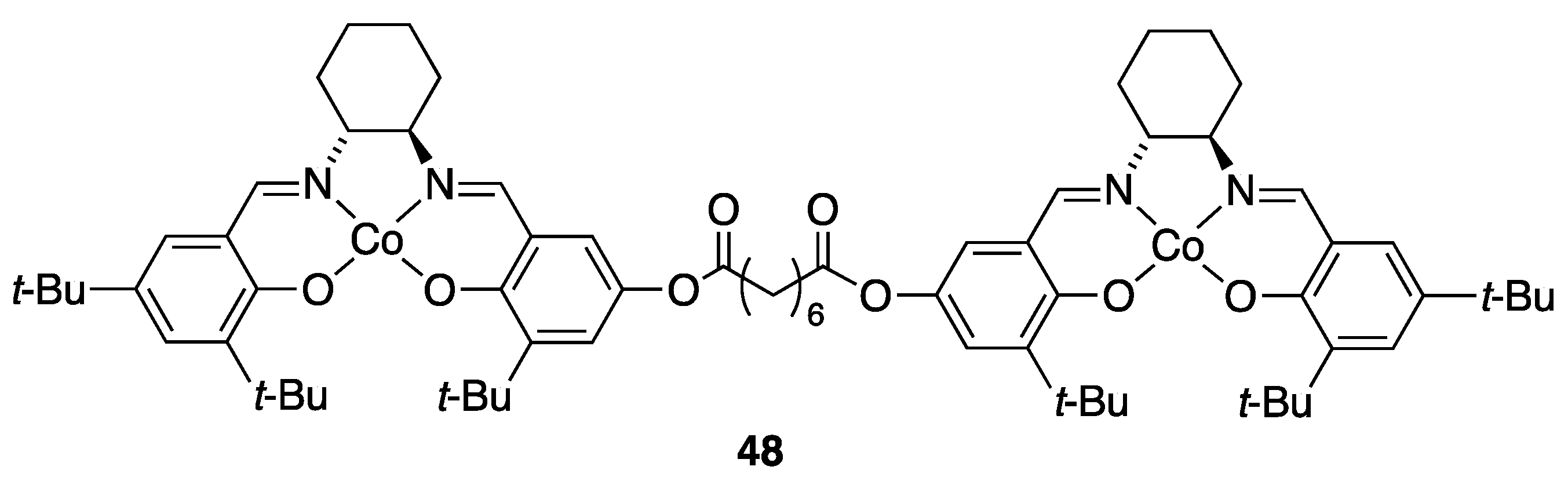
3.3.1. Multi-Metallic Catalysts
3.3.2. Immobilized Catalysts
3.3.3. Mechanistic Studies
3.3.4. Other Studies
3.4. With Alcohols, Phenols, and Carboxylic Acids
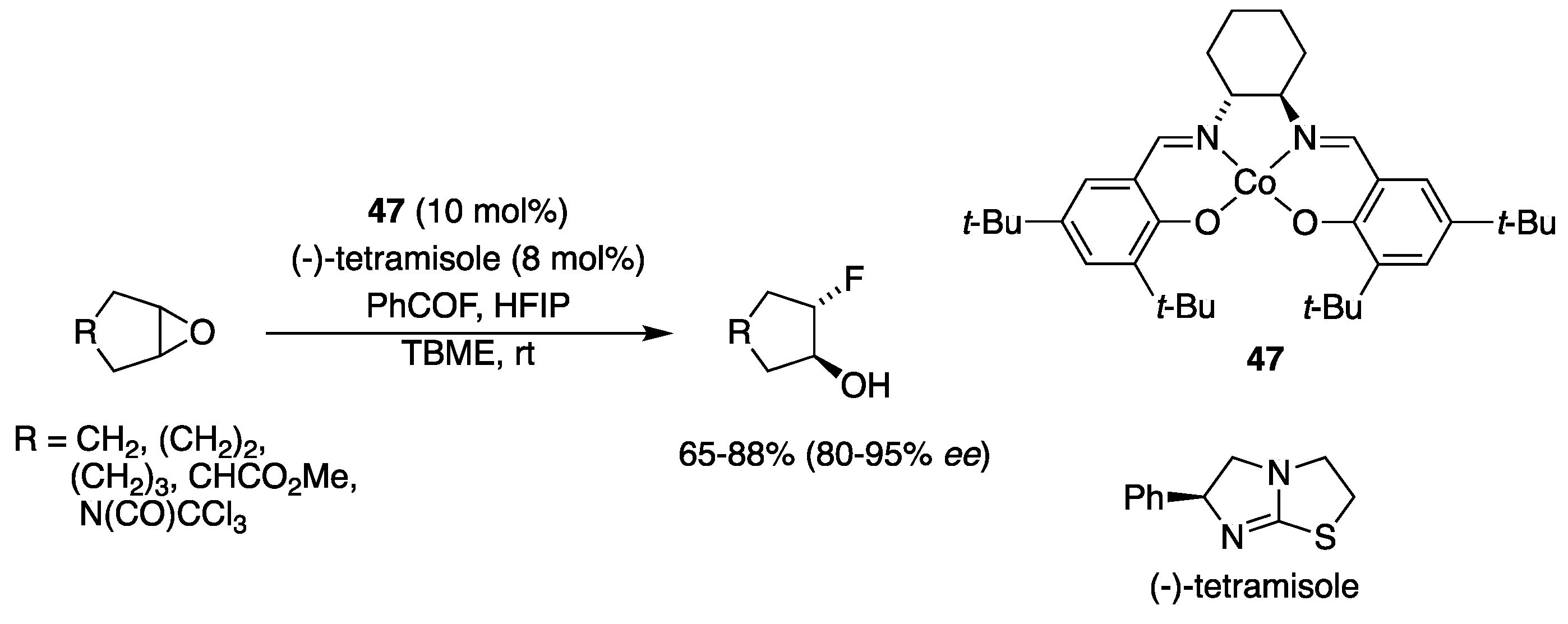
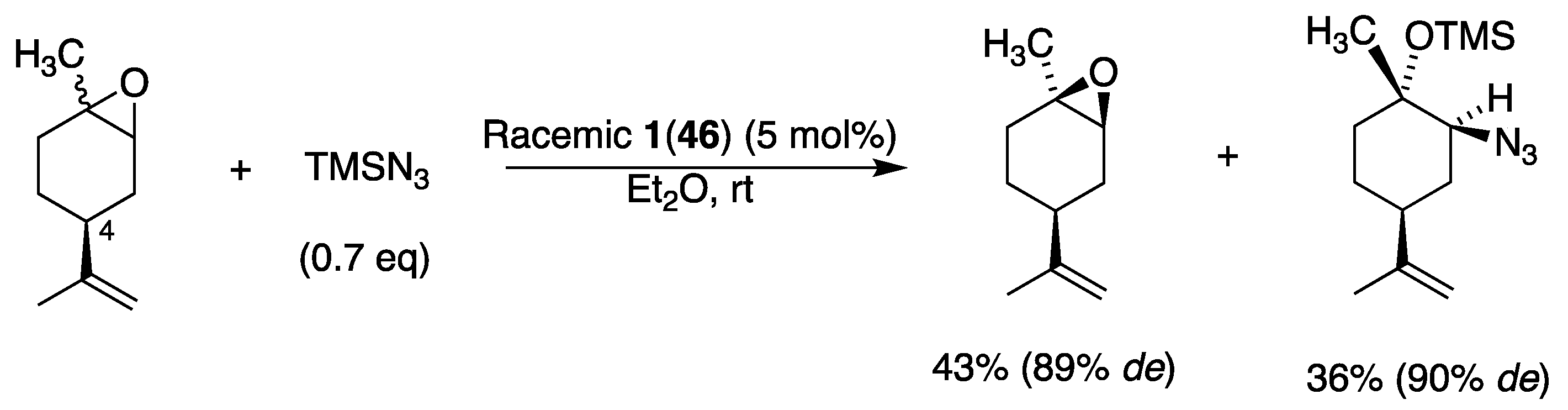
3.5. With Halogens
3.6. With Carbon-Containing Nucleophiles
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Walsh, P.J.; Kozlowski, M.C. Fundamentals of Asymmetric Catalysis; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Halpern, J.; Trost, B.M. Asymmetric Catalysis. Proc. Natl. Acad. Sci. USA 2004, 101, 5347. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysts. Science 2003, 299, 1691. [Google Scholar] [CrossRef]
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal–Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Zhang, W.-Z.; Lu, X.-B. Chiral Salen Complexes. In Privileged Chiral Ligands and Catalysts; Zhou, Q.L., Ed.; Wiley: Weinheim, Germany, 2011; pp. 257–293. [Google Scholar]
- Trost, B.; Fleming, I. Comprehensive Organic Synthesis; Pergamon: Oxford, UK, 1991. [Google Scholar]
- Xia, Q.H.; Ge, H.Q.; Ye, C.P.; Liu, Z.M.; Su, K.X. Advances in Homogeneous and Heterogeneous Catalytic Asymmetric Epoxidation. Chem. Rev. 2005, 105, 1603–1662. [Google Scholar] [CrossRef]
- Pastor, I.M.; Yus, M. Asymmetric Ring Opening of Epoxides. Curr. Org. Chem. 2005, 9, 1–29. [Google Scholar] [CrossRef]
- Schneider, C. Synthesis of 1, 2-difunctionalized fine chemicals through catalytic, enantioselective ring-opening reactions of epoxides. Synthesis 2006, 2006, 3919–3944. [Google Scholar] [CrossRef]
- Clarke, R.M.; Storr, T. The chemistry and applications of multimetallic salen complexes. Dalton Trans. 2014, 43, 9380–9391. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shibasaki, M. Multimetallic Schiff Base Complexes as Cooperative Asymmetric Catalysts. Synthesis 2013, 45, 421–437. [Google Scholar] [CrossRef]
- Haak, R.M.; Wezenberg, S.J.; Kleij, A.W. Cooperative multimetallic catalysis using metallosalens. Chem. Commun. 2010, 46, 2713–2723. [Google Scholar] [CrossRef]
- Abd El Sater, M.; Jaber, N.; Schulz, E. Chiral Salen Complexes for Asymmetric Heterogeneous Catalysis: Recent Examples for Recycling and Cooperativity. ChemCatChem 2019, 11, 3662–3687. [Google Scholar] [CrossRef]
- Baleizão, C.; Garcia, H. Chiral Salen Complexes: An Overview to Recoverable and Reusable Homogeneous and Heterogeneous Catalysts. Chem. Rev. 2006, 106, 3987–4043. [Google Scholar] [CrossRef]
- Shioiri, T.; Hamada, Y. Natural product syntheses utilizing 4-alkoxycarbonyloxazoles as β-hydroxy-α-amino acid synthons. Heterocycles 1988, 27, 1035–1050. [Google Scholar] [CrossRef]
- Kikelj, D.; Kidrič, J.; Pristovšek, P.; Pečar, S.; Urleb, U.; Krbavčič, A.; Hönig, H. Preparation of diastereomerically pure immunologically active carbocyclic nor-muramyldipeptide analogues. Tetrahedron 1992, 48, 5915–5932. [Google Scholar] [CrossRef]
- Vacca, J.P.; Dorsey, B.; Schleif, W.; Levin, R.; McDaniel, S.; Darke, P.; Zugay, J.; Quintero, J.; Blahy, O.; Roth, E. L-735,524: An orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc. Natl. Acad. Sci. USA 1994, 91, 4096–4100. [Google Scholar] [CrossRef]
- Wang, R.; Wong, C.-H. Synthesis of sialyl Lewis X mimetics: Use of O-α-fucosyl-(1R, 2R)-2-aminocyclohexanol as core structure. Tetrahedron Lett. 1996, 37, 5427–5430. [Google Scholar] [CrossRef]
- Martinez, L.E.; Leighton, J.L.; Carsten, D.H.; Jacobsen, E.N. Highly enantioselective ring opening of epoxides catalyzed by (salen) Cr (III) complexes. J. Am. Chem. Soc. 1995, 117, 5897–5898. [Google Scholar] [CrossRef]
- Jacobsen, E.N. Asymmetric catalysis of epoxide ring-opening reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef]
- Pakulski, Z.; Pietrusiewicz, K.M. Enantioselective desymmetrization of phospholene meso-epoxide by nucleophilic opening of the epoxide. Tetrahedron Asymmetry 2004, 15, 41–45. [Google Scholar] [CrossRef]
- Saha, B.; Lin, M.-H.; RajanBabu, T. Exceptionally Active Yttrium−Salen Complexes for the Catalyzed Ring Opening of Epoxides by TMSCN and TMSN3. J. Org. Chem. 2007, 72, 8648–8655. [Google Scholar] [CrossRef]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Zhang, W.; Muci, A.R.; Ecker, J.R.; Deng, L. Highly enantioselective epoxidation catalysts derived from 1, 2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar] [CrossRef]
- Brandes, B.D.; Jacobsen, E.N. Highly enantioselective, catalytic epoxidation of trisubstituted olefins. J. Org. Chem. 1994, 59, 4378–4380. [Google Scholar] [CrossRef]
- Schaus, S.E.; Larrow, J.F.; Jacobsen, E.N. Practical synthesis of enantiopure cyclic 1, 2-amino alcohols via catalytic asymmetric ring opening of meso epoxides. J. Org. Chem. 1997, 62, 4197–4199. [Google Scholar] [CrossRef]
- Hansen, K.B.; Leighton, J.L.; Jacobsen, E.N. On the mechanism of asymmetric nucleophilic ring-opening of epoxides catalyzed by (salen) CrIII complexes. J. Am. Chem. Soc. 1996, 118, 10924–10925. [Google Scholar] [CrossRef]
- Guillaneux, D.; Zhao, S.-H.; Samuel, O.; Rainford, D.; Kagan, H.B. Nonlinear effects in asymmetric catalysis. J. Am. Chem. Soc. 1994, 116, 9430–9439. [Google Scholar] [CrossRef]
- Konsler, R.G.; Karl, J.; Jacobsen, E.N. Cooperative asymmetric catalysis with dimeric salen complexes. J. Am. Chem. Soc. 1998, 120, 10780–10781. [Google Scholar] [CrossRef]
- Ma, D.Y.; Xiao, Z.Y.; Etxabe, J.; Wärnmark, K. Pseudo-C2-Symmetric Bimetallic Bissalen Catalysts for Efficient and Enantioselective Ring-Opening of meso-Epoxides. ChemCatChem 2012, 4, 1321–1329. [Google Scholar] [CrossRef]
- Li, Y.; Lidskog, A.; Armengol-Relats, H.; Pham, T.H.; Favraud, A.; Nicolas, M.; Dawaigher, S.; Xiao, Z.; Ma, D.; Lindbäck, E.; et al. Enantiotopic Discrimination by Coordination-Desymmetrized meso-Ligands. ChemCatChem 2020, 12, 1575–1579. [Google Scholar] [CrossRef]
- Gianneschi, N.C.; Bertin, P.A.; Nguyen, S.T.; Mirkin, C.A.; Zakharov, L.N.; Rheingold, A.L. A supramolecular approach to an allosteric catalyst. J. Am. Chem. Soc. 2003, 125, 10508–10509. [Google Scholar] [CrossRef]
- Gianneschi, N.C.; Cho, S.H.; Nguyen, S.T.; Mirkin, C.A. Reversibly addressing an allosteric catalyst in situ: Catalytic molecular tweezers. Angew. Chem. Int. Ed. 2004, 43, 5503–5507. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.Y.; Norouzi-Arasi, H.; Sheibani, E.; Wärnmark, K. Dynamic Supramolecular [(Salen) CrCl] Complexes as Efficient Catalysts for Ring Opening of Epoxides. ChemCatChem 2010, 2, 629–632. [Google Scholar] [CrossRef]
- Lindbäck, E.; Norouzi-Arasi, H.; Sheibani, E.; Ma, D.; Dawaigher, S.; Wärnmark, K. Synthesis of Cr (III) Salen Complexes as Supramolecular Catalytic Systems for Ring-Opening Reactions of Epoxides. ChemistrySelect 2016, 1, 1789–1794. [Google Scholar] [CrossRef]
- Odille, F.G.J.; Jónsson, S.; Stjernqvist, S.; Rydén, T.; Wärnmark, K. On the Characterization of Dynamic Supramolecular Systems: A General Mathematical Association Model for Linear Supramolecular Copolymers and Application on a Complex Two-Component Hydrogen-Bonding System. Chem. Eur. J. 2007, 13, 9617–9636. [Google Scholar] [CrossRef] [PubMed]
- Zulauf, A.; Mellah, M.; Schulz, E. New Chiral Calixsalen Chromium Complexes: Recyclable Asymmetric Catalysts. Chem. Eur. J. 2010, 16, 11108–11114. [Google Scholar] [CrossRef]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal (salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Angelino, M.D.; Laibinis, P.E. Polymer-supported salen complexes for heterogeneous asymmetric synthesis: Stability and selectivity. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3888–3898. [Google Scholar] [CrossRef]
- Gigante, B.; Corma, A.; García, H.; Sabater, M.J. Assessment of the negative factors responsible for the decrease in the enantioselectivity for the ring opening of epoxides catalyzed by chiral supported Cr (III)-salen complexes. Catal. Lett. 2000, 68, 113–119. [Google Scholar] [CrossRef]
- Baleizão, C.; Gigante, B.; Sabater, M.J.; Garcia, H.; Corma, A. On the activity of chiral chromium salen complexes covalently bound to solid silicates for the enantioselective epoxide ring opening. Appl. Catal. A 2002, 228, 279–288. [Google Scholar] [CrossRef]
- Dioos, B.M.; Geurts, W.A.; Jacobs, P.A. Coordination of Cr III (salen) on functionalised silica for asymmetric ring opening reactions of epoxides. Catal. Lett. 2004, 97, 125–129. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Jacobs, P.A. Impregnation of dimeric CrIII(salen) on silica and its application in epoxide asymmetric ring opening reactions. Appl. Catal. A 2005, 282, 181–188. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Jacobs, P.A. CrIII(salen) impregnated on silica for asymmetric ring opening reactions and its recovery via desorption/re-impregnation. Tetrahedron Lett. 2003, 44, 8815–8817. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Jacobs, P.A. Heterogenisation of dimeric Cr(salen) with supported ionic liquids. J. Catal. 2006, 243, 217–219. [Google Scholar] [CrossRef]
- Keilitz, J.; Haag, R. Intramolecular Acceleration of Asymmetric Epoxide Ring-Opening by Dendritic Polyglycerol Salen–CrIII Complexes. Eur. J. Org. Chem. 2009, 2009, 3272–3278. [Google Scholar] [CrossRef]
- Zheng, X.; Jones, C.W.; Weck, M. Ring-Expanding Olefin Metathesis: A Route to Highly Active Unsymmetrical Macrocyclic Oligomeric Co-Salen Catalysts for the Hydrolytic Kinetic Resolution of Epoxides. J. Am. Chem. Soc. 2007, 129, 1105–1112. [Google Scholar] [CrossRef]
- Kinslow, K.; Sevde, A.M.; Liang, J.; Liu, Y. Enantioselective ring opening of epoxides with TMSN3 by macrocyclic oligomer-supported Cr (III)–salen catalysts. Tetrahedron Asymmetry 2015, 26, 385–392. [Google Scholar] [CrossRef]
- Song, C.E.; Oh, C.R.; Roh, E.J.; Choo, D.J. Cr (salen) catalysed asymmetric ring opening reactions of epoxides in room temperature ionic liquids. Chem. Commun. 2000, 1743–1744. [Google Scholar] [CrossRef]
- Jiao, J.; Tan, C.; Li, Z.; Liu, Y.; Han, X.; Cui, Y. Design and assembly of chiral coordination cages for asymmetric sequential reactions. J. Am. Chem. Soc. 2018, 140, 2251–2259. [Google Scholar] [CrossRef]
- Tan, C.; Han, X.; Li, Z.; Liu, Y.; Cui, Y. Controlled exchange of achiral linkers with chiral linkers in Zr-based UiO-68 metal–organic framework. J. Am. Chem. Soc. 2018, 140, 16229–16236. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Ettelaie, R.; Guan, R.; Zhang, M.; Zhang, Y.; Yang, H. Pickering Emulsion-Derived Liquid–Solid Hybrid Catalyst for Bridging Homogeneous and Heterogeneous Catalysis. J. Am. Chem. Soc. 2019, 141, 5220–5230. [Google Scholar] [CrossRef]
- Bartoli, G.; Bosco, M.; Carlone, A.; Locatelli, M.; Massaccesi, M.; Melchiorre, P.; Sambri, L. Asymmetric Aminolysis of Aromatic Epoxides: A Facile Catalytic Enantioselective Synthesis of anti-β-Amino Alcohols. Org. Lett. 2004, 6, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Kureshy, R.I.; Kumar, M.; Agrawal, S.; Khan, N.U.H.; Dangi, B.; Abdi, S.H.; Bajaj, H.C. Enantioselective desymmetrization of meso-epoxides with anilines catalyzed by polymeric and monomeric Ti (IV) salen complexes. Chirality 2011, 23, 76–83. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Prathap, K.J.; Kumar, M.; Bera, P.K.; Khan, N.-u.H.; Abdi, S.H.R.; Bajaj, H.C. Synthesis of enantiopure β-amino alcohols via AKR/ARO of epoxides using recyclable macrocyclic Cr (III) salen complexes. Tetrahedron 2011, 67, 8300–8307. [Google Scholar] [CrossRef]
- Birrell, J.A.; Jacobsen, E.N. A practical method for the synthesis of highly enantioenriched trans-1, 2-amino alcohols. Org. Lett. 2013, 15, 2895–2897. [Google Scholar] [CrossRef] [PubMed]
- White, D.E.; Tadross, P.M.; Lu, Z.; Jacobsen, E.N. A broadly applicable and practical oligomeric (salen)Co catalyst for enantioselective epoxide ring-opening reactions. Tetrahedron 2014, 70, 4165–4180. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Agarwal, J.; Peddinti, R.K. Direct access to the optically active VAChT inhibitor vesamicol and its analogues via the asymmetric aminolysis of meso-epoxides with secondary aliphatic amines. Org. Biomol. Chem. 2017, 15, 1913–1920. [Google Scholar] [CrossRef]
- Roy, S.; Bhanja, P.; Islam, S.S.; Bhaumik, A.; Islam, S.M. A new chiral Fe (iii)–salen grafted mesoporous catalyst for enantioselective asymmetric ring opening of racemic epoxides at room temperature under solvent-free conditions. Chem. Commun. 2016, 52, 1871–1874. [Google Scholar] [CrossRef]
- Islam, M.M.; Bhanja, P.; Halder, M.; Kundu, S.K.; Bhaumik, A.; Islam, S.M. Chiral Co(iii)–salen complex supported over highly ordered functionalized mesoporous silica for enantioselective aminolysis of racemic epoxides. RSC Adv. 2016, 6, 109315–109321. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, J.; Liu, Y.; Tu, T. Chiral Titanium Coordination Assemblies: Robust Cooperative Self-Supported Catalysts for Asymmetric Ring Opening of meso-Epoxides with Aliphatic Amines. Adv. Synth. Catal. 2017, 359, 494–505. [Google Scholar] [CrossRef]
- Xi, W.; Liu, Y.; Xia, Q.; Li, Z.; Cui, Y. Direct and Post-Synthesis Incorporation of Chiral Metallosalen Catalysts into Metal–Organic Frameworks for Asymmetric Organic Transformations. Chem. Eur. J. 2015, 21, 12581–12585. [Google Scholar] [CrossRef]
- Kolb, H.C.; VanNieuwenhze, M.S.; Sharpless, K.B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547. [Google Scholar] [CrossRef]
- Kim, G.-J.; Park, D.-W. The catalytic activity of new chiral salen complexes immobilized on MCM-41 in the asymmetric hydrolysis of epoxides to diols. Catal. Today 2000, 63, 537–547. [Google Scholar] [CrossRef]
- Haak, R.M.; Martinez Belmonte, M.; Escudero-Adan, E.C.; Benet-Buchholz, J.; Kleij, A.W. Olefin metathesis as a tool for multinuclear Co(III)salen catalyst construction: Access to cooperative catalysts. Dalton Trans. 2010, 39, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Mellah, M.; Schulz, E. Heterobimetallic dual-catalyst systems for the hydrolytic kinetic resolution of terminal epoxides. Catal. Sci. Technol. 2014, 4, 2608–2617. [Google Scholar] [CrossRef]
- Dandachi, H.; Zaborova, E.; Kolodziej, E.; David, O.R.P.; Hannedouche, J.; Mellah, M.; Jaber, N.; Schulz, E. Mixing and matching chiral cobalt- and manganese-based calix-salen catalysts for the asymmetric hydrolytic ring opening of epoxides. Tetrahedron Asymmetry 2016, 27, 246–253. [Google Scholar] [CrossRef]
- Ready, J.M.; Jacobsen, E.N. Highly active oligomeric (salen)Co catalysts for asymmetric epoxide ring-opening reactions. J. Am. Chem. Soc. 2001, 123, 2687–2688. [Google Scholar] [CrossRef]
- Liang, J.; Soucie, L.N.; Blechschmidt, D.R.; Yoder, A.; Gustafson, A.; Liu, Y. Aromatic Donor-Acceptor Interaction-Based Co(III)-salen Self-Assemblies and Their Applications in Asymmetric Ring Opening of Epoxides. Org. Lett. 2019, 21, 513–518. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Kakiuchi, F.; Konsler, R.G.; Larrow, J.F.; Tokunaga, M. Enantioselective catalytic ring opening of epoxides with carboxylic acids. Tetrahedron Lett. 1997, 38, 773–776. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Banks, R.E.; Smart, B.E.; Tatlow, J. Organofluorine Chemistry: Principles and Commercial Applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Bruns, S.; Haufe, G. Enantioselective introduction of fluoride into organic compounds: First asymmetric ring opening of epoxides by hydrofluorinating reagents. J. Fluor. Chem. 2000, 104, 247–254. [Google Scholar] [CrossRef]
- Haufe, G.; Bruns, S.; Runge, M. Enantioselective ring-opening of epoxides by HF-reagents: Asymmetric synthesis of fluoro lactones. J. Fluor. Chem. 2001, 112, 55–61. [Google Scholar] [CrossRef]
- Haufe, G.; Bruns, S. (Salen)chromium Complex Mediated Asymmetric Ring Opening of meso- and Racemic Epoxides with Different Fluoride Sources. Adv. Synth. Catal. 2002, 344, 165–171. [Google Scholar] [CrossRef]
- Kalow, J.A.; Doyle, A.G. Enantioselective ring opening of epoxides by fluoride anion promoted by a cooperative dual-catalyst system. J. Am. Chem. Soc. 2010, 132, 3268–3269. [Google Scholar] [CrossRef]
- Kalow, J.A.; Doyle, A.G. Mechanistic Investigations of Cooperative Catalysis in the Enantioselective Fluorination of Epoxides. J. Am. Chem. Soc. 2011, 133, 16001–16012. [Google Scholar] [CrossRef]
- Wu, M.H.; Jacobsen, E.N. Asymmetric ring opening of meso epoxides with thiols: Enantiomeric enrichment using a bifunctional nucleophile. J. Org. Chem. 1998, 63, 5252–5254. [Google Scholar] [CrossRef]
- Wu, J.; Hou, X.-L.; Dai, L.-X.; Xia, L.-J.; Tang, M.-H. Enantioselective ring opening of meso-epoxides with thiols catalyzed by a chiral (salen) Ti (IV) complex. Tetrahedron Asymmetry 1998, 9, 3431–3436. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Li, K.; Wang, L.; Zhou, Q.; Tang, C. Regio-and stereoselective ring-opening of epoxides using organic dithiophosphorus acids as nucleophiles. Tetrahedron Lett. 2002, 43, 7609–7611. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Quanyong, W.; Liu, B.; Li, K.; Zhao, G.; Zhou, Q.; Tang, C. (Salen) Ti (IV) complex catalyzed asymmetric ring-opening of epoxides using dithiophosphorus acid as the nucleophile. J. Organomet. Chem. 2006, 691, 5790–5797. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, C.; Yuan, F.; Huang, Y.; Pan, Y. Enantioselective Ring-Opening Reaction of m eso-Epoxides with ArSeH Catalyzed by Heterometallic Ti−Ga−Salen System. Org. Lett. 2005, 7, 1927–1930. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Yuan, F.; Jia, X.; Yang, X.; Pan, Y.; Zhu, C. Catalytic Asymmetric Ring-Opening Reaction of meso-Epoxides with Aryl Selenols and Thiols Catalyzed by a Heterobimetallic Gallium-Titanium-Salen Complex. Adv. Synth. Catal. 2009, 351, 920–930. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, F.; Yang, M.; Pan, Y.; Zhu, C. Enantioselective ring-opening reaction of meso-epoxides with ArSH catalyzed by heterobimetallic Ti–Ga–Salen system. Tetrahedron Lett. 2009, 50, 548–551. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Marini, F.; Sternativo, S.; Del Verme, F.; Santi, C.; Bagnoli, L.; Temperini, A. Synthesis of enantiomerically enriched β-hydroxy selenides by catalytic asymmetric ring opening of meso-epoxides with (phenylseleno) silanes. Tetrahedron 2008, 64, 3337–3342. [Google Scholar] [CrossRef]
- Scheffler, U.; Mahrwald, R. Recent Advances in Organocatalytic Methods for Asymmetric C–C Bond Formation. Chem. Eur. J. 2013, 19, 14346–14396. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G. Design for carbon–carbon bond forming reactions under ambient conditions. RSC Adv. 2016, 6, 64676–64725. [Google Scholar] [CrossRef]
- Bandini, M.; Cozzi, P.G.; Melchiorre, P.; Umani-Ronchi, A. Kinetic Resolution of Epoxides by a C-C Bond-Forming Reaction: Highly Enantioselective Addition of Indoles to cis, trans, and meso Aromatic Epoxides Catalyzed by [Cr (salen)] Complexes. Angew. Chem. Int. Ed. 2004, 43, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.M.; Larrow, J.F.; Jacobsen, E.N. Practical considerations in kinetic resolution reactions. Adv. Synth. Catal. 2001, 343, 5–26. [Google Scholar] [CrossRef]
- Larrow, J.F.; Schaus, S.E.; Jacobsen, E.N. Kinetic resolution of terminal epoxides via highly regioselective and enantioselective ring opening with TMSN3. An efficient, catalytic route to 1, 2-amino alcohols. J. Am. Chem. Soc. 1996, 118, 7420–7421. [Google Scholar] [CrossRef]
- Schaus, S.E.; Jacobsen, E.N. Dynamic kinetic resolution of epichlorohydrin via enantioselective catalytic ring opening with TMSN3. Practical synthesis of aryl oxazolidinone antibacterial agents. Tetrahedron Lett. 1996, 37, 7937–7940. [Google Scholar] [CrossRef]
- Lebel, H.; Jacobsen, E.N. Chromium catalyzed kinetic resolution of 2,2-disubstituted epoxides. Tetrahedron Lett. 1999, 40, 7303–7306. [Google Scholar] [CrossRef]
- Lebel, H.; Jacobsen, E.N. Enantioselective Total Synthesis of Taurospongin A. J. Org. Chem. 1998, 63, 9624–9625. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Jacobs, P.A. CrIII(salen) catalysed asymmetric ring opening of monocyclic terpene-epoxides. Tetrahedron Lett. 2003, 44, 4715–4717. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Jacobs, P.A. Microwave-assisted Cr(salen)-catalysed asymmetric ring opening of epoxides. J. Catal. 2005, 235, 428–430. [Google Scholar] [CrossRef]
- Chen, S.-W.; Thakur, S.S.; Li, W.; Shin, C.-K.; Kawthekar, R.B.; Kim, G.-J. Efficient catalytic synthesis of optically pure 1,2-azido alcohols through enantioselective epoxide ring opening with HN3. J. Mol. Catal. A Chem. 2006, 259, 116–120. [Google Scholar] [CrossRef]
- Bai, S.; Li, B.; Peng, J.; Zhang, X.; Yang, Q.; Li, C. Promoted activity of Cr(Salen) in a nanoreactor for kinetic resolution of terminal epoxides. Chem. Sci. 2012, 3, 2864–2867. [Google Scholar] [CrossRef]
- Bartoli, G.; Bosco, M.; Carlone, A.; Locatelli, M.; Melchiorre, P.; Sambri, L. Asymmetric Catalytic Synthesis of Enantiopure N-Protected 1,2-Amino Alcohols. Org. Lett. 2004, 6, 3973–3975. [Google Scholar] [CrossRef]
- Baudequin, C.; Baudoux, J.; Levillain, J.; Cahard, D.; Gaumont, A.-C.; Plaquevent, J.-C. Ionic liquids and chirality: Opportunities and challenges. Tetrahedron Asymmetry 2003, 14, 3081–3093. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Prathap, K.J.; Agrawal, S.; Kumar, M.; Khan, N.-u.H.; Abdi, S.H.R.; Bajaj, H.C. Highly Efficient Recyclable CoIII–salen Complexes in the Catalyzed Asymmetric Aminolytic Kinetic Resolution of Aryloxy/Terminal Epoxides for the Simultaneous Production of N-Protected 1,2-Amino Alcohols and the Corresponding Epoxides in High Optical Purity. Eur. J. Org. Chem. 2009, 2009, 2863–2871. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Kumar, M.; Agrawal, S.; Khan, N.-u.H.; Abdi, S.H.R.; Bajaj, H.C. Aminolytic kinetic resolution of trans epoxides for the simultaneous production of chiral trans β-amino alcohols in the presence of chiral Cr(III) salen complex using an ionic liquid as a green reaction media. Tetrahedron Asymmetry 2010, 21, 451–456. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Singh, S.; Khan, N.-u.H.; Abdi, S.H.R.; Agrawal, S.; Jasra, R.V. Enantioselective aminolytic kinetic resolution (AKR) of epoxides catalyzed by recyclable polymeric Cr(III) salen complexes. Tetrahedron Asymmetry 2006, 17, 1638–1643. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Prathap, K.J.; Singh, S.; Agrawal, S.; Khan, N.-U.H.; Abdi, S.H.R.; Jasra, R.V. Chiral recyclable dimeric and polymeric Cr(III) salen complexes catalyzed aminolytic kinetic resolution of trans-aromatic epoxides under microwave irradiation. Chirality 2007, 19, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kureshy, R.I.; Prathap, K.J.; Roy, T.; Maity, N.C.; Khan, N.-u.H.; Abdi, S.H.R.; Bajaj, H.C. Reusable Chiral Dicationic Chromium(III) Salen Catalysts for Aminolytic Kinetic Resolution of trans-Epoxides. Adv. Synth. Catal. 2010, 352, 3053–3060. [Google Scholar] [CrossRef]
- Tak, R.; Kumar, M.; Menapara, T.; Choudhary, M.K.; Kureshy, R.I.; Khan, N.-u.H. Asymmetric Catalytic Syntheses of Pharmaceutically Important β-Amino-α-Hydroxyl Esters by Enantioselective Aminolysis of Methyl Phenylglycidate. ChemCatChem 2017, 9, 322–328. [Google Scholar] [CrossRef]
- Kumar, M.; Kureshy, R.I.; Shah, A.K.; Das, A.; Khan, N.-u.H.; Abdi, S.H.R.; Bajaj, H.C. Asymmetric Aminolytic Kinetic Resolution of Racemic Epoxides Using Recyclable Chiral Polymeric Co(III)-Salen Complexes: A Protocol for Total Utilization of Racemic Epoxide in the Synthesis of (R)-Naftopidil and (S)-Propranolol. J. Org. Chem. 2013, 78, 9076–9084. [Google Scholar] [CrossRef]
- Tokunaga, M.; Larrow, J.F.; Kakiuchi, F.; Jacobsen, E.N. Asymmetric Catalysis with Water: Efficient Kinetic Resolution of Terminal Epoxides by Means of Catalytic Hydrolysis. Science 1997, 277, 936–938. [Google Scholar] [CrossRef]
- Schaus, S.E.; Brandes, B.D.; Larrow, J.F.; Tokunaga, M.; Hansen, K.B.; Gould, A.E.; Furrow, M.E.; Jacobsen, E.N. Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)CoIII Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc. 2002, 124, 1307–1315. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Khan, N.H.; Abdi, S.H.R.; Patel, S.T.; Jasra, R.V. Simultaneous production of chirally enriched epoxides and 1,2-diols from racemic epoxides via hydrolytic kinetic resolution (HKR). J. Mol. Catal. A Chem. 2002, 179, 73–77. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Singh, S.; Khan, N.U.H.; Abdi, S.H.R.; Ahmad, I.; Bhatt, A.; Jasra, R.V. Improved catalytic activity of homochiral dimeric cobalt-salen complex in hydrolytic kinetic resolution of terminal racemic epoxides. Chirality 2005, 17, 590–594. [Google Scholar] [CrossRef]
- Venkatasubbaiah, K.; Gill, C.S.; Takatani, T.; Sherrill, C.D.; Jones, C.W. A Versatile Co(bisalen) Unit for Homogeneous and Heterogeneous Cooperative Catalysis in the Hydrolytic Kinetic Resolution of Epoxides. Chem. Eur. J. 2009, 15, 3951–3955. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Kleij, A.W. Cooperative Activation in the Hydrolytic Kinetic Resolution of Epoxides by a Bis-Cobalt(III)salen-Calix 4 arene Hybrid. Adv. Synth. Catal. 2010, 352, 85–91. [Google Scholar] [CrossRef]
- Ready, J.M.; Jacobsen, E.N. A practical oligomeric (salen)Co catalyst for asymmetric epoxide ring-opening reactions. Angew. Chem. Int. Ed. 2002, 41, 1374–1377. [Google Scholar] [CrossRef]
- Kahn, M.G.C.; Weck, M. Highly crosslinked polycyclooctyl-salen cobalt (III) for the hydrolytic kinetic resolution of terminal epoxides. Catal. Sci. Technol. 2012, 2, 386–389. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Khan, N.-u.H.; Roy, T.; Kureshy, R.I.; Abdi, S.H.R.; Bajaj, H.C. Asymmetric Hydrolytic Kinetic Resolution with Recyclable Macrocyclic CoIII-Salen Complexes: A Practical Strategy in the Preparation of (R)-Mexiletine and (S)-Propranolol. Chem. Eur. J. 2012, 18, 5256–5260. [Google Scholar] [CrossRef]
- Roy, T.; Barik, S.; Kumar, M.; Kureshy, R.I.; Ganguly, B.; Khan, N.-u.H.; Abdi, S.H.R.; Bajaj, H.C. Asymmetric hydrolytic kinetic resolution with recyclable polymeric Co(III)-salen complexes: A practical strategy in the preparation of (S)-metoprolol, (S)-toliprolol and (S)-alprenolol: Computational rationale for enantioselectivity. Catal. Sci. Technol. 2014, 4, 3899–3908. [Google Scholar] [CrossRef]
- Hong, X.; Mellah, M.; Bordier, F.; Guillot, R.; Schulz, E. Electrogenerated Polymers as Efficient and Robust Heterogeneous Catalysts for the Hydrolytic Kinetic Resolution of Terminal Epoxides. ChemCatChem 2012, 4, 1115–1121. [Google Scholar] [CrossRef]
- Dandachi, H.; Nasrallah, H.; Ibrahim, F.; Hong, X.; Mellah, M.; Jaber, N.; Schulz, E. Chiral calix-salen cobalt complexes, catalysts for the enantioselective dynamic hydrolytic kinetic resolution of epibromohydrin. J. Mol. Catal. A Chem. 2014, 395, 457–462. [Google Scholar] [CrossRef]
- Breinbauer, R.; Jacobsen, E.N. Cooperative Asymmetric Catalysis with Dendrimeric [Co(salen)] Complexes. Angew. Chem. Int. Ed. 2000, 39, 3604–3607. [Google Scholar] [CrossRef]
- Song, Y.; Yao, X.; Chen, H.; Bai, C.; Hu, X.; Zheng, Z. Highly enantioselective resolution of terminal epoxides using polymeric catalysts. Tetrahedron Lett. 2002, 43, 6625–6627. [Google Scholar] [CrossRef]
- Song, Y.M.; Chen, H.L.; Hu, X.Q.; Bai, C.M.; Zheng, Z. Highly enantioselective resolution of terminal epoxides with cross-linked polymeric salen-Co(III) complexes. Tetrahedron Lett. 2003, 44, 7081–7085. [Google Scholar] [CrossRef]
- Zheng, X.; Jones, C.W.; Weck, M. Poly(styrene)-Supported Co–Salen Complexes as Efficient Recyclable Catalysts for the Hydrolytic Kinetic Resolution of Epichlorohydrin. Chem. Eur. J. 2006, 12, 576–583. [Google Scholar] [CrossRef]
- Zheng, X.; Jones, C.W.; Weck, M. Engineering Polymer-Enhanced Bimetallic Cooperative Interactions in the Hydrolytic Kinetic Resolution of Epoxides. Adv. Synth. Catal. 2008, 350, 255–261. [Google Scholar] [CrossRef]
- Rossbach, B.M.; Leopold, K.; Weberskirch, R. Self-Assembled Nanoreactors as Highly Active Catalysts in the Hydrolytic Kinetic Resolution (HKR) of Epoxides in Water. Angew. Chem. Int. Ed. 2006, 45, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.S.; Li, W.J.; Kim, S.J.; Kim, G.J. Highly reactive and enantioselective kinetic resolution of terminal epoxides with H2O and HCl catalyzed by new chiral (salen)Co complex linked with Al. Tetrahedron Lett. 2005, 46, 2263–2266. [Google Scholar] [CrossRef]
- Thakur, S.S.; Chen, S.W.; Li, W.J.; Shin, C.K.; Kim, S.J.; Koo, Y.M.; Kim, G.J. A new dinuclear chiral salen complexes for asymmetric ring opening and closing reactions: Synthesis of valuable chiral intermediates. J. Organomet. Chem. 2006, 691, 1862–1872. [Google Scholar] [CrossRef]
- Jiang, C.J.; Chen, Z.R. Chiral cobalt salen complexes containing Lewis acid: A highly reactive and enantioselective catalyst for the hydrolytic kinetic resolution of terminal epoxides. Kinet. Catal. 2008, 49, 474–478. [Google Scholar] [CrossRef]
- Kawthekar, R.B.; Bi, W.-t.; Kim, G.-J. Asymmetric ring opening of epoxides catalyzed by novel heterobimetallic Schiff-bases containing transition metal salts. Bull. Korean Chem. Soc. 2008, 29, 313–318. [Google Scholar] [CrossRef]
- Jiang, C. Asymmetric ring opening of terminal epoxides via kinetic resolution catalyzed by chiral (salen)Co mixture. Kinet. Catal. 2011, 52, 691–696. [Google Scholar] [CrossRef]
- Park, J.; Lang, K.; Abboud, K.A.; Hong, S. Self-Assembly Approach toward Chiral Bimetallic Catalysts: Bis-Urea-Functionalized (Salen)Cobalt Complexes for the Hydrolytic Kinetic Resolution of Epoxides. Chem. Eur. J. 2011, 17, 2236–2245. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, L.; Su, W.; Yang, Q.; Li, C. Asymmetric ring-opening of epoxides on chiral Co(Salen) catalyst synthesized in SBA-16 through the “ship in a bottle” strategy. J. Catal. 2007, 248, 204–212. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, L.; Zhong, L.; Yang, Q.; Li, C. Enhanced Cooperative Activation Effect in the Hydrolytic Kinetic Resolution of Epoxides on [Co(salen)] Catalysts Confined in Nanocages. Angew. Chem. Int. Ed. 2007, 46, 6861–6865. [Google Scholar] [CrossRef]
- Cavazzini, M.; Quici, S.; Pozzi, G. Hydrolytic kinetic resolution of terminal epoxides catalyzed by fluorous chiral Co(salen) complexes. Tetrahedron 2002, 58, 3943–3949. [Google Scholar] [CrossRef]
- Shepperson, I.; Cavazzini, M.; Pozzi, G.; Quici, S. Fluorous biphasic hydrolytic kinetic resolution of terminal epoxides. J. Fluorine Chem. 2004, 125, 175–180. [Google Scholar] [CrossRef]
- Rim Oh, C.; Joon Choo, D.; Ho Shim, W.; Hoon Lee, D.; Joo Roh, E.; Lee, S.-g.; Eui Song, C. Chiral Co(iii)(salen)-catalysed hydrolytic kinetic resolution of racemic epoxides in ionic liquids. Chem. Commun. 2003, 1100–1101. [Google Scholar] [CrossRef] [PubMed]
- Aerts, S.; Weyten, H.; Buekenhoudt, A.; Gevers, L.E.M.; Vankelecom, I.F.J.; Jacobs, P.A. Recycling of the homogeneous Co-Jacobsen catalyst through solvent-resistent nanofiltration (SRNF). Chem. Commun. 2004, 710–711. [Google Scholar] [CrossRef]
- Annis, D.A.; Jacobsen, E.N. Polymer-supported chiral Co(salen) complexes: Synthetic applications and mechanistic investigations in the hydrolytic kinetic resolution of terminal epoxides. J. Am. Chem. Soc. 1999, 121, 4147–4154. [Google Scholar] [CrossRef]
- Solodenko, W.; Jas, G.; Kunz, U.; Kirschning, A. Continuous enantioselective kinetic resolution of terminal epoxides using immobilized chiral cobalt-salen complexes. Synthesis 2007, 583–589. [Google Scholar] [CrossRef]
- Kunz, U.; Schönfeld, H.; Solodenko, W.; Jas, G.; Kirschning, A. Manufacturing and Construction of PASSflow Flow Reactors and Their Utilization in Suzuki−Miyaura Cross-Coupling Reactions. Ind. Eng. Chem. Res. 2005, 44, 8458–8467. [Google Scholar] [CrossRef]
- Goyal, P.; Zheng, X.; Weck, M. Enhanced Cooperativity in Hydrolytic Kinetic Resolution of Epoxides using Poly(styrene) Resin-Supported Dendronized Co-(Salen) Catalysts. Adv. Synth. Catal. 2008, 350, 1816–1822. [Google Scholar] [CrossRef]
- Kahn, M.G.C.; Stenlid, J.H.; Weck, M. Poly(styrene) Resin-Supported Cobalt(III) Salen Cyclic Oligomers as Active Heterogeneous HKR Catalysts. Adv. Synth. Catal. 2012, 354, 3016–3024. [Google Scholar] [CrossRef]
- Gill, C.S.; Venkatasubbaiah, K.; Phan, N.T.S.; Weck, M.; Jones, C.W. Enhanced Cooperativity through Design: Pendant CoIII-Salen Polymer Brush Catalysts for the Hydrolytic Kinetic Resolution of Epichlorohydrin (Salen=N,N′-Bis(salicylidene)ethylenediamine Dianion). Chem. Eur. J. 2008, 14, 7306–7313. [Google Scholar] [CrossRef]
- Belser, T.; Jacobsen, E.N. Cooperative catalysis in the hydrolytic kinetic resolution of epoxides by chiral (salen)Co(III) complexes immobilized on gold colloids. Adv. Synth. Catal. 2008, 350, 967–971. [Google Scholar] [CrossRef]
- Kawthekar, R.B.; Lee, Y.-H.; Kim, G.-J. Asymmetric catalysis by chiral salen complexes immobilized on mesoporous materials having modified pore channel system by dimethylcarbonate. J. Porous Mater. 2009, 16, 367–378. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Guo, X.-F.; Kim, G.-J. Highly active new chiral Co(III) salen catalysts immobilized by electrostatic interaction with sulfonic acid linkages on ordered mesoporous SBA-16 silica. Chem. Commun. 2009, 4296–4298. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, C.-Y.; Kim, G.-J. Asymmetric Ring Opening of Terminal Epoxides Catalyzed by Chiral Co(III)-BF3 Salen Complex Immobilized on SBA-16. Bull. Korean Chem. Soc. 2009, 30, 1771–1777. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, C.-Y.; Kim, G.-J. Synthesis of New Bimetallic Chiral Salen Catalyst Bearing Co(BF4)(2) Salt and Its Application in Asymmetric Ring Opening of Epoxide. Bull. Korean Chem. Soc. 2010, 31, 2973–2979. [Google Scholar] [CrossRef][Green Version]
- Kim, G.-J.; Lee, H.; Kim, S.-J. Catalytic activity and recyclability of new enantioselective chiral Co–salen complexes in the hydrolytic kinetic resolution of epichlorohydrine. Tetrahedron Lett. 2003, 44, 5005–5008. [Google Scholar] [CrossRef]
- Nielsen, L.P.C.; Stevenson, C.P.; Blackmond, D.G.; Jacobsen, E.N. Mechanistic investigation leads to a synthetic improvement in the hydrolytic kinetic resolution of terminal epoxides. J. Am. Chem. Soc. 2004, 126, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, W.-X.; Feng, Z.; Li, C. Cooperative activation in ring-opening hydrolysis of epoxides by Co-salen complexes: A first principle study. Chem. Phys. Lett. 2009, 470, 259–263. [Google Scholar] [CrossRef]
- Kennedy, M.R.; Burns, L.A.; Sherrill, C.D. Counterion and Substrate Effects on Barrier Heights of the Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Co(III)-salen. J. Phys. Chem. A 2015, 119, 403–409. [Google Scholar] [CrossRef]
- Jain, S.; Zheng, X.; Jones, C.W.; Weck, M.; Davis, R.J. Importance of counterion reactivity on the deactivation of co-salen catalysts in the hydrolytic kinetic resolution of epichlorohydrin. Inorg. Chem. 2007, 46, 8887–8896. [Google Scholar] [CrossRef]
- Jain, S.; Venkatasubbaiah, K.; Jones, C.W.; Davis, R.J. Factors influencing recyclability of Co(III)-salen catalysts in the hydrolytic kinetic resolution of epichlorohydrin. J. Mol. Catal. A Chem. 2010, 316, 8–15. [Google Scholar] [CrossRef]
- Ford, D.D.; Nielsen, L.P.C.; Zuend, S.J.; Musgrave, C.B.; Jacobsen, E.N. Mechanistic Basis for High Stereoselectivity and Broad Substrate Scope in the (salen)Co(III)-Catalyzed Hydrolytic Kinetic Resolution. J. Am. Chem. Soc. 2013, 135, 15595–15608. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Ertuerk, E. Hydrolytic kinetic resolution of epoxides catalyzed by chromium(III)-endo,endo-2,5-diaminonorbornane-salen Cr(III)-DIANANE-salen complexes. Improved activity, low catalyst loading. Adv. Synth. Catal. 2006, 348, 2619–2625. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, J.; Park, K.; Kim, G.J. Superior Effect of Ultrasonic Homogenization to Mechanical Agitation on Accelerating Reaction Rates in Asymmetric Ring Opening of Epoxides. Bull. Korean Chem. Soc. 2017, 38, 795–803. [Google Scholar] [CrossRef]
- Wright, J.L.; Gregory, T.F.; Heffner, T.G.; MacKenzie, R.G.; Pugsley, T.A.; Meulen, S.V.; Wise, L.D. Discovery of selective dopamine D4 receptor antagonists: 1-Aryloxy-3-(4-aryloxypiperidinyl)-2-propanols. Bioorgan. Med. Chem. Lett. 1997, 7, 1377–1380. [Google Scholar] [CrossRef]
- Ready, J.M.; Jacobsen, E.N. Asymmetric Catalytic Synthesis of α-Aryloxy Alcohols: Kinetic Resolution of Terminal Epoxides via Highly Enantioselective Ring-Opening with Phenols. J. Am. Chem. Soc. 1999, 121, 6086–6087. [Google Scholar] [CrossRef]
- Peukert, S.; Jacobsen, E.N. Enantioselective Parallel Synthesis Using Polymer-Supported Chiral Co(salen) Complexes. Org. Lett. 1999, 1, 1245–1248. [Google Scholar] [CrossRef]
- Zhu, X.; Venkatasubbaiah, K.; Weck, M.; Jones, C.W. Highly active oligomeric Co(salen) catalysts for the asymmetric synthesis of alpha-aryloxy or alpha-alkoxy alcohols via kinetic resolution of terminal epoxides. J. Mol. Catal. A Chem. 2010, 329, 1–6. [Google Scholar] [CrossRef]
- Kawthekar, R.B.; Ahn, C.-H.; Kim, G.-J. Kinetic resolution of terminal epoxides with phenols promoted by heterometallic Co-Al and Co-Ga salen complexes. Catal. Lett. 2007, 115, 62–69. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, C.-Y.; Kim, G.-J. Asymmetric synthesis of alpha-aryloxy alcohols via kinetic resolution of terminal epoxides catalyzed by (R,R)-N,N ′-bis(2-hydroxy-5-tert-butylsalisilidine)-1,2-cyclohexenediamino cobalt. React. Kinet. Catal. Lett. 2008, 93, 75–83. [Google Scholar] [CrossRef]
- Guo, X.-F.; Kim, Y.-S.; Kim, G.-J. Chiral (Salen) Cobalt Complexes Encapsulated in Mesoporous MFI as an Enantioselective Catalyst for Asymmetric Ring Opening of Terminal Epoxides. Top. Catal. 2009, 52, 153–160. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, C.-Y.; Kim, G.-J. Non-Covalent Immobilization of Chiral (Salen) Complexes on HF-treated Mesoporous MFI-type Zeolite for Asymmetric Catalysis. Bull. Korean Chem. Soc. 2009, 30, 389–396. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Guo, X.-f.; Kim, G.-J. Asymmetric Ring Opening Reaction of Catalyst Immobilized on Silica Monolith with Bimodal Meso/Macroscopic Pore Structure. Top. Catal. 2009, 52, 197–204. [Google Scholar] [CrossRef]
- Jeon, H.-G.; Choi, S.D.; Jeon, S.K.; Park, G.W.; Yang, J.Y.; Kim, G.-J. Application of Mesoporous Silica Foam for Immobilization of Salen Complexes in Chiral Intermediates Synthesis. Bull. Korean Chem. Soc. 2015, 36, 1396–1404. [Google Scholar] [CrossRef]
- Li, W.J.; Thakur, S.S.; Chen, S.W.; Shin, C.K.; Kawthekar, R.B.; Kim, G.J. Synthesis of optically active 2-hydroxy monoesters via-kinetic resolution and asymmetric cyclization catalyzed by heterometallic chiral (salen) Co complex. Tetrahedron Lett. 2006, 47, 3453–3457. [Google Scholar] [CrossRef]
- Lee, Y.W.; Yang, H.C.; Kim, G.-J. Synthesis of Highly Enantiomerically Enriched Arenesulfonic Acid 2-Hydroxy Esters via Kinetic Resolution of Terminal Epoxides. Appl. Chem. Eng. 2016, 27, 490–494. [Google Scholar] [CrossRef][Green Version]
- Hajra, S.; Roy, S. Feedback Inhibition in Chemical Catalysis Leads the Dynamic Kinetic to Kinetic Resolution in C3-Indolylation of Spiro-epoxyoxindoles. Org. Lett. 2020, 22, 1458–1463. [Google Scholar] [CrossRef]
- Hajra, S.; Maity, S.; Maity, R. Efficient Synthesis of 3,3′-Mixed Bisindoles via Lewis Acid Catalyzed Reaction of Spiro-epoxyoxindoles and Indoles. Org. Lett. 2015, 17, 3430–3433. [Google Scholar] [CrossRef]
- Linker, T. The Jacobsen–Katsuki Epoxidation and Its Controversial Mechanism. Angew. Chem. Int. Ed. 1997, 36, 2060–2062. [Google Scholar] [CrossRef]








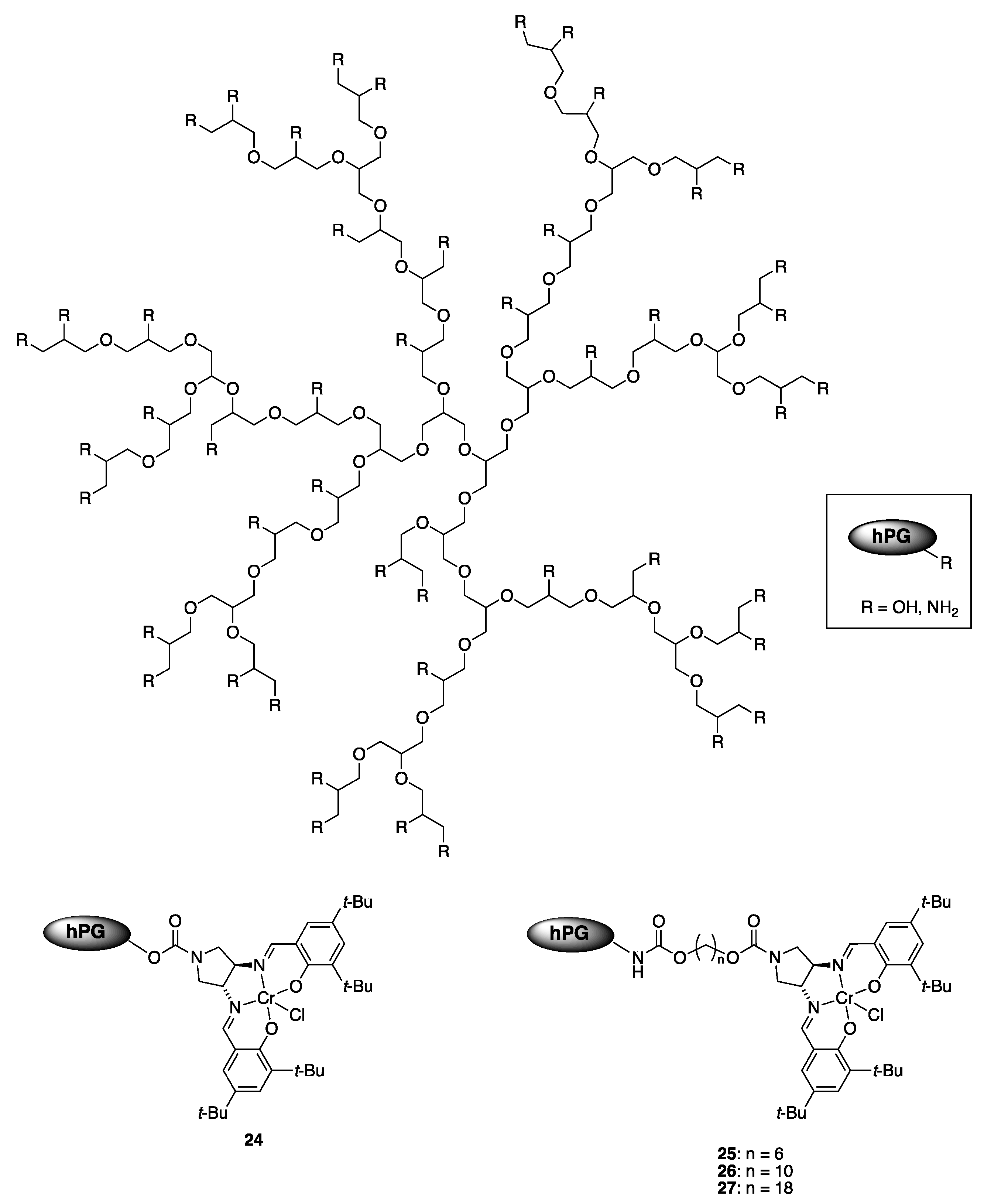
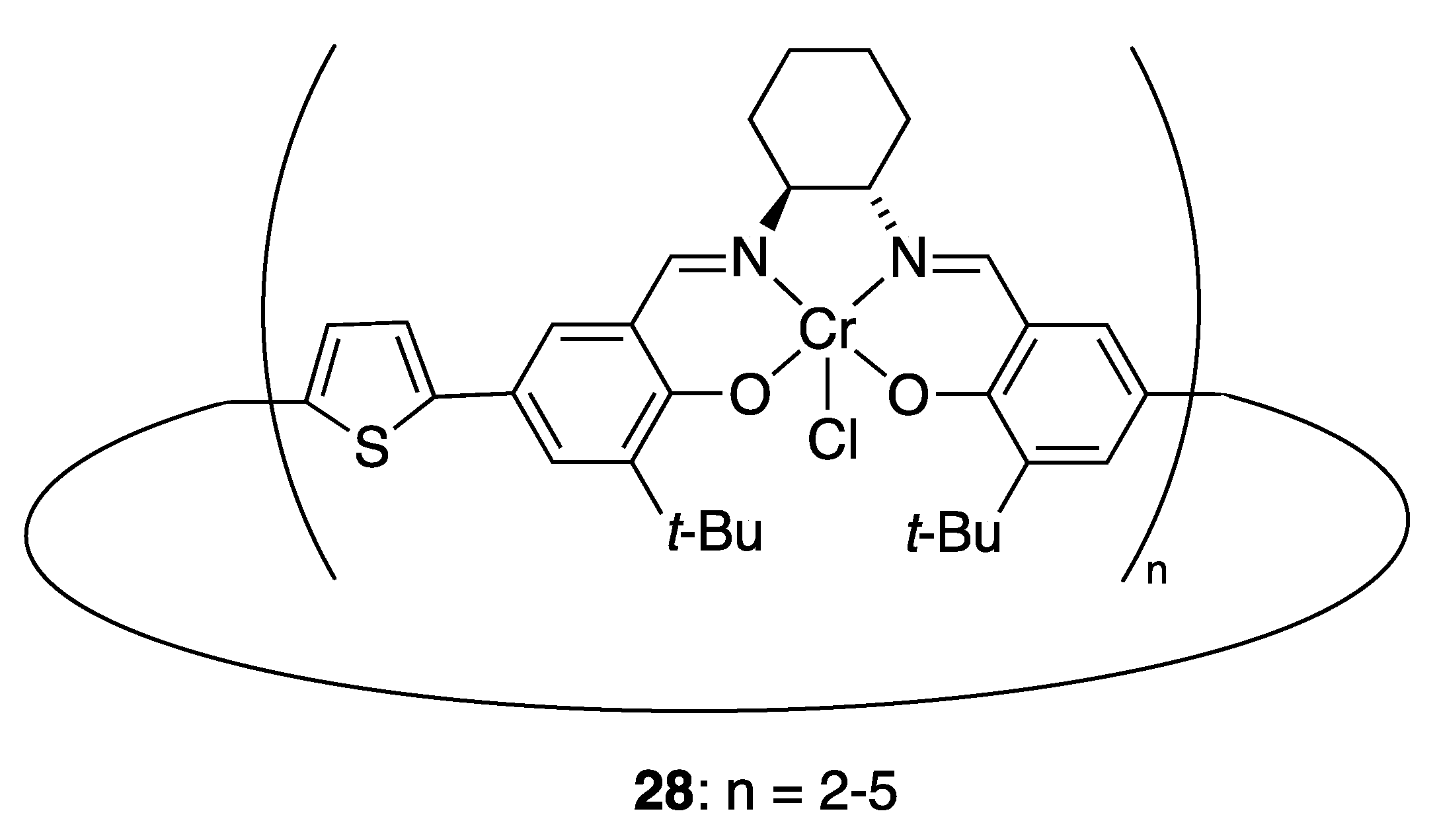







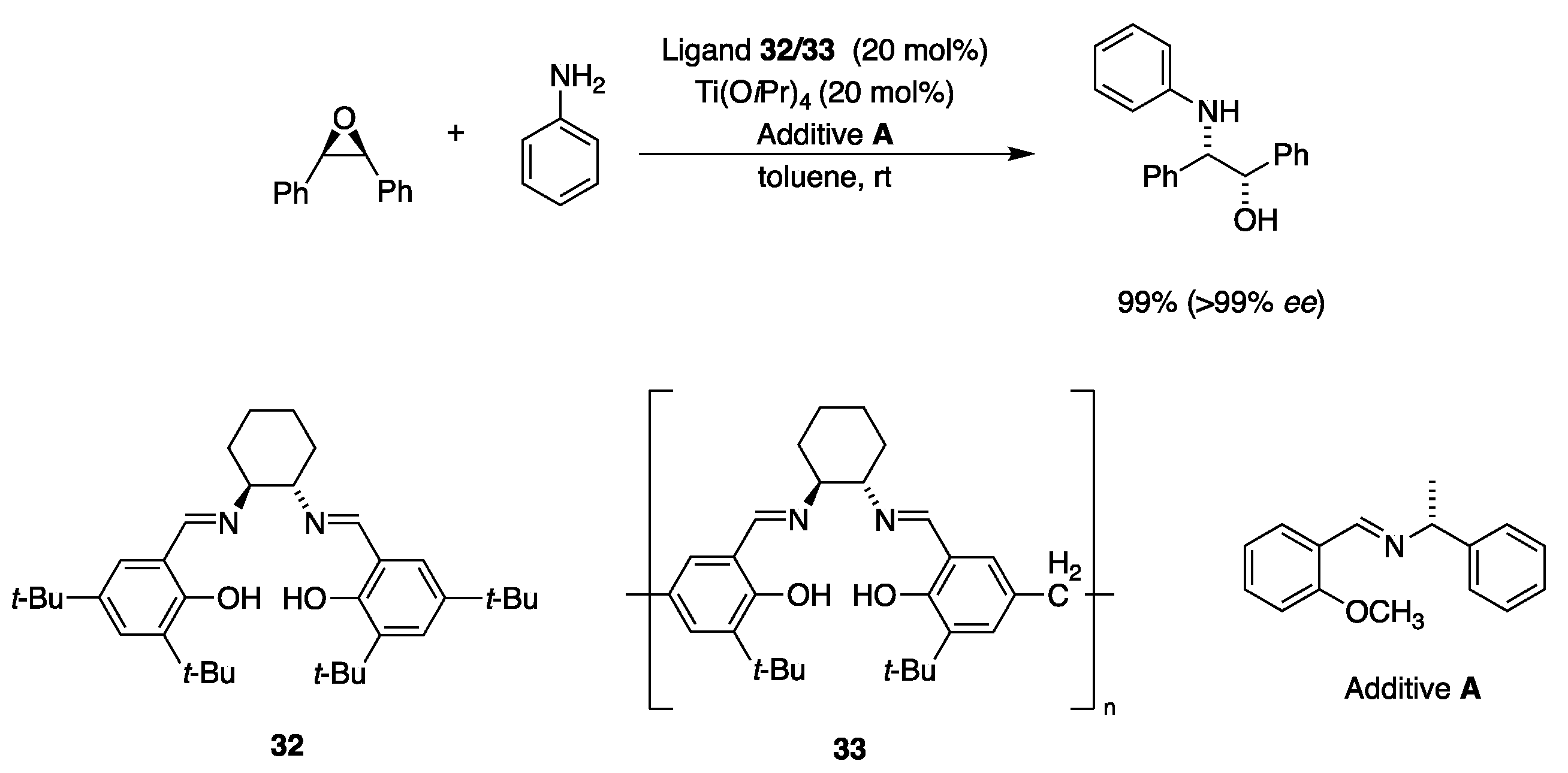
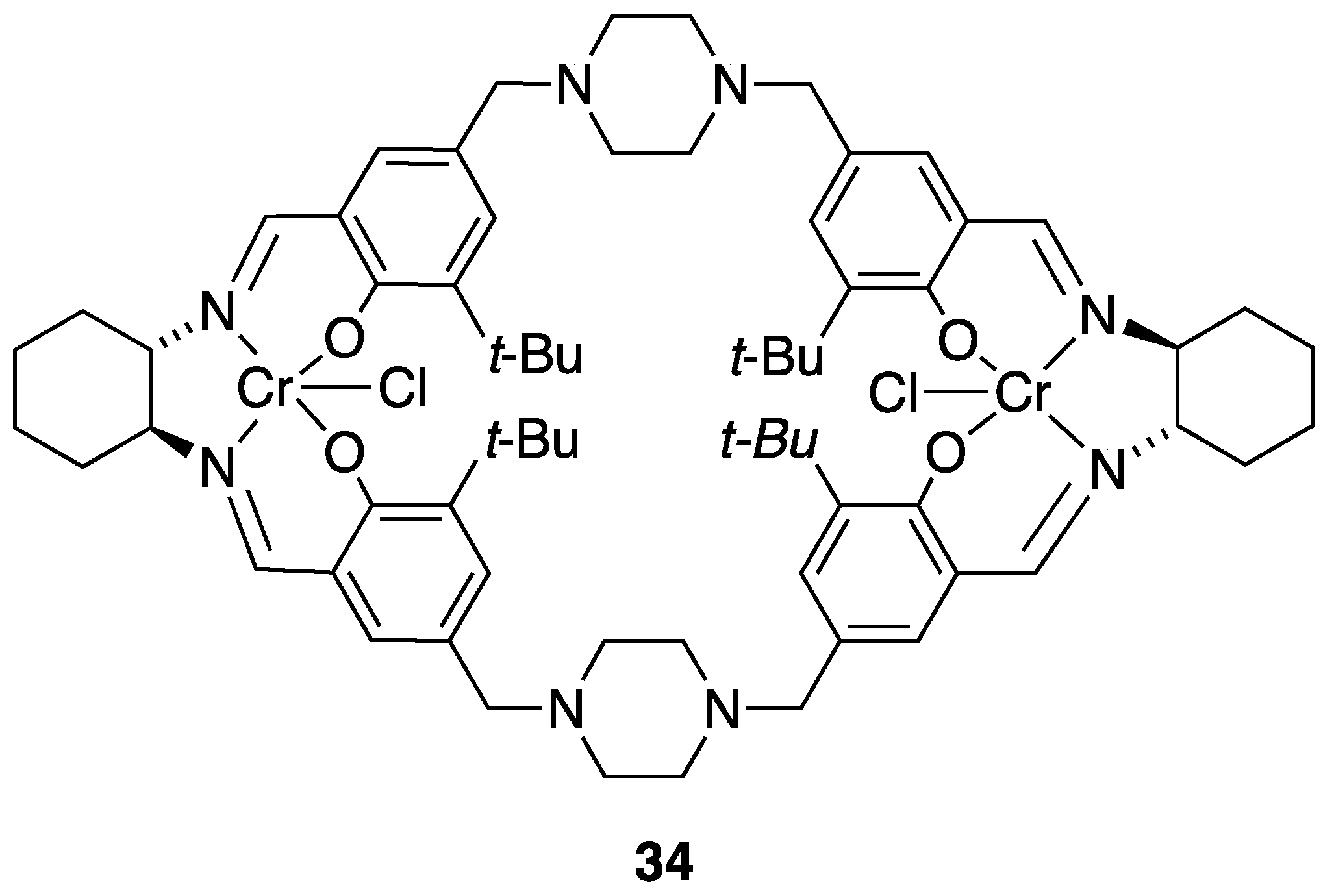
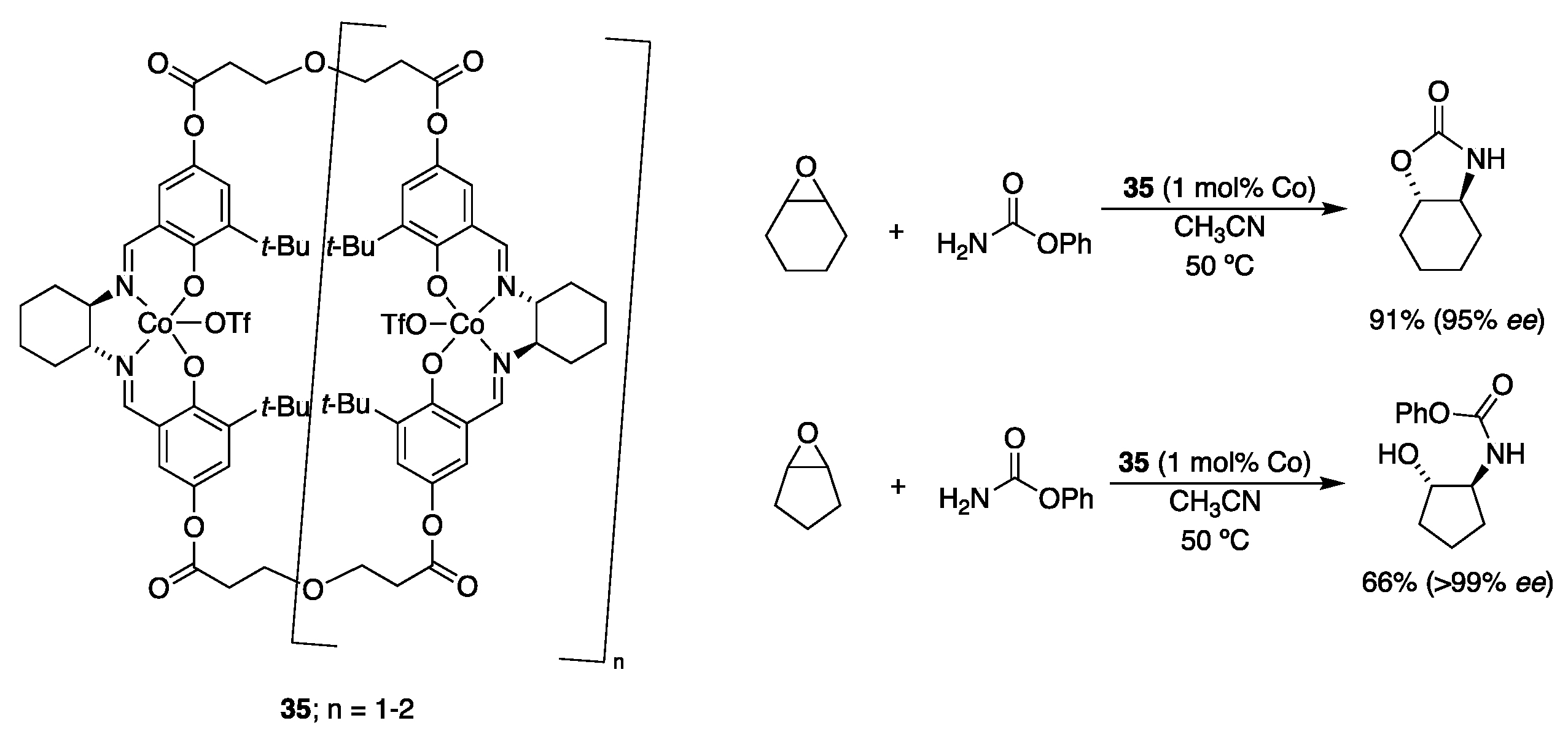

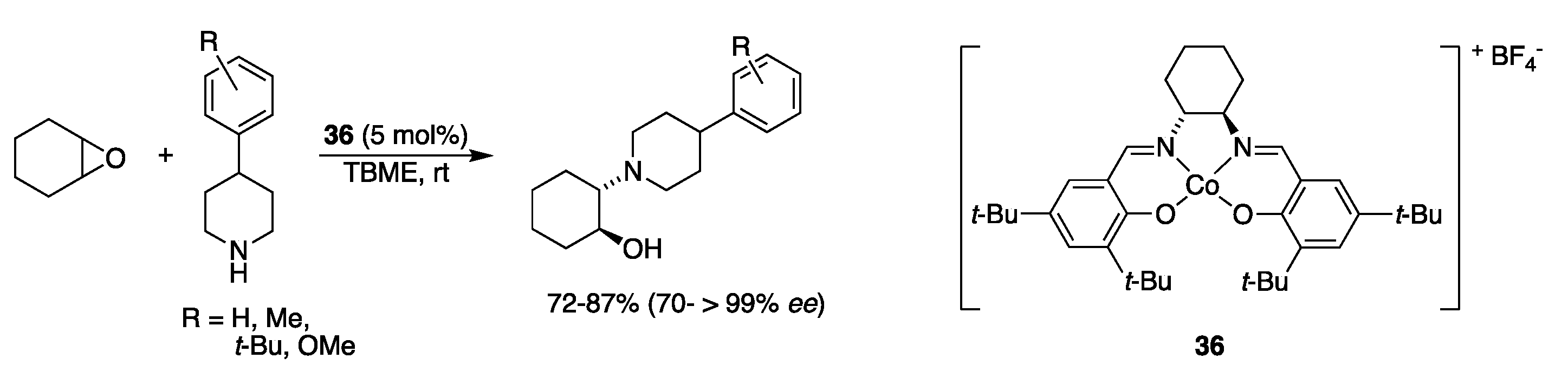
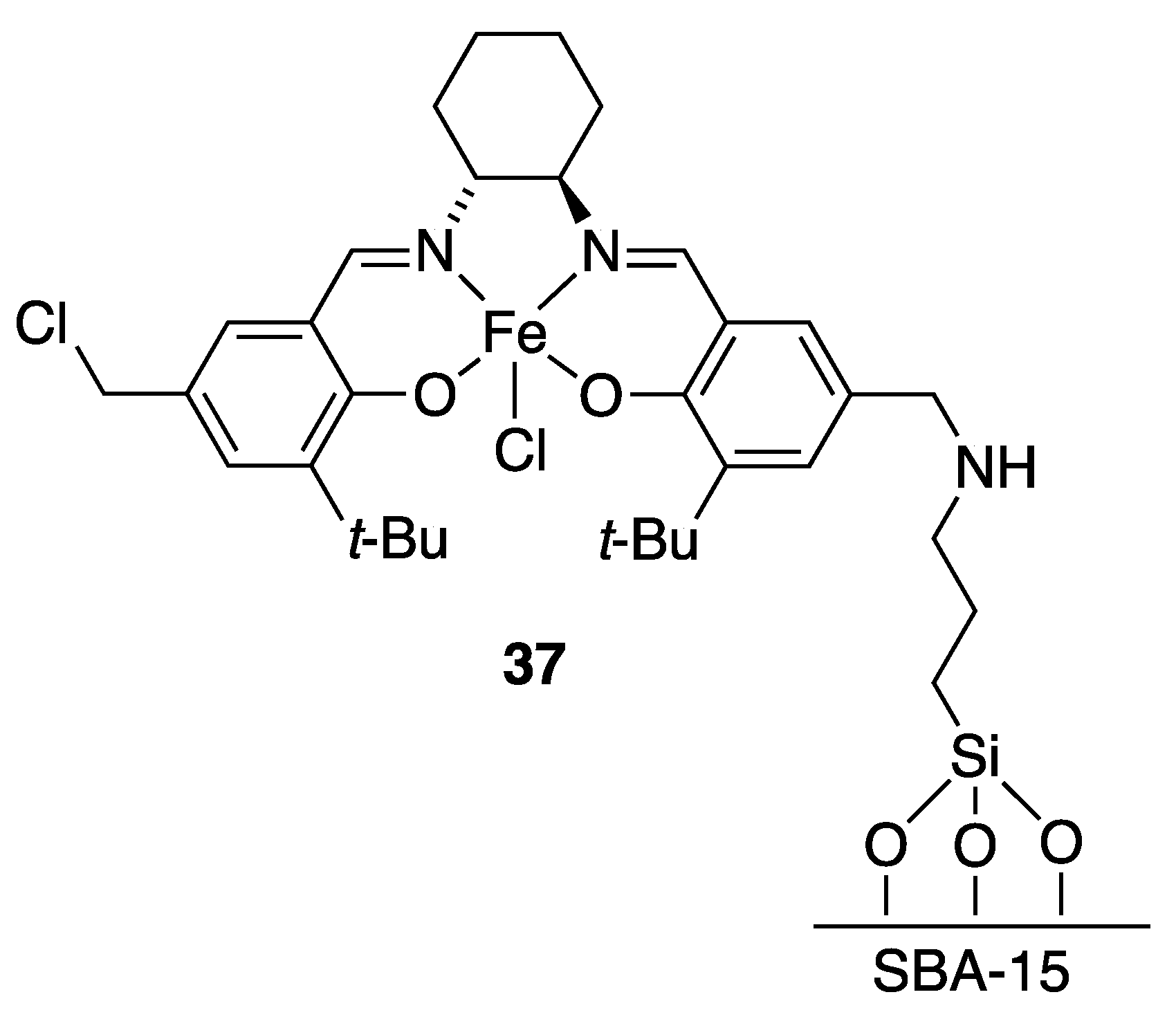
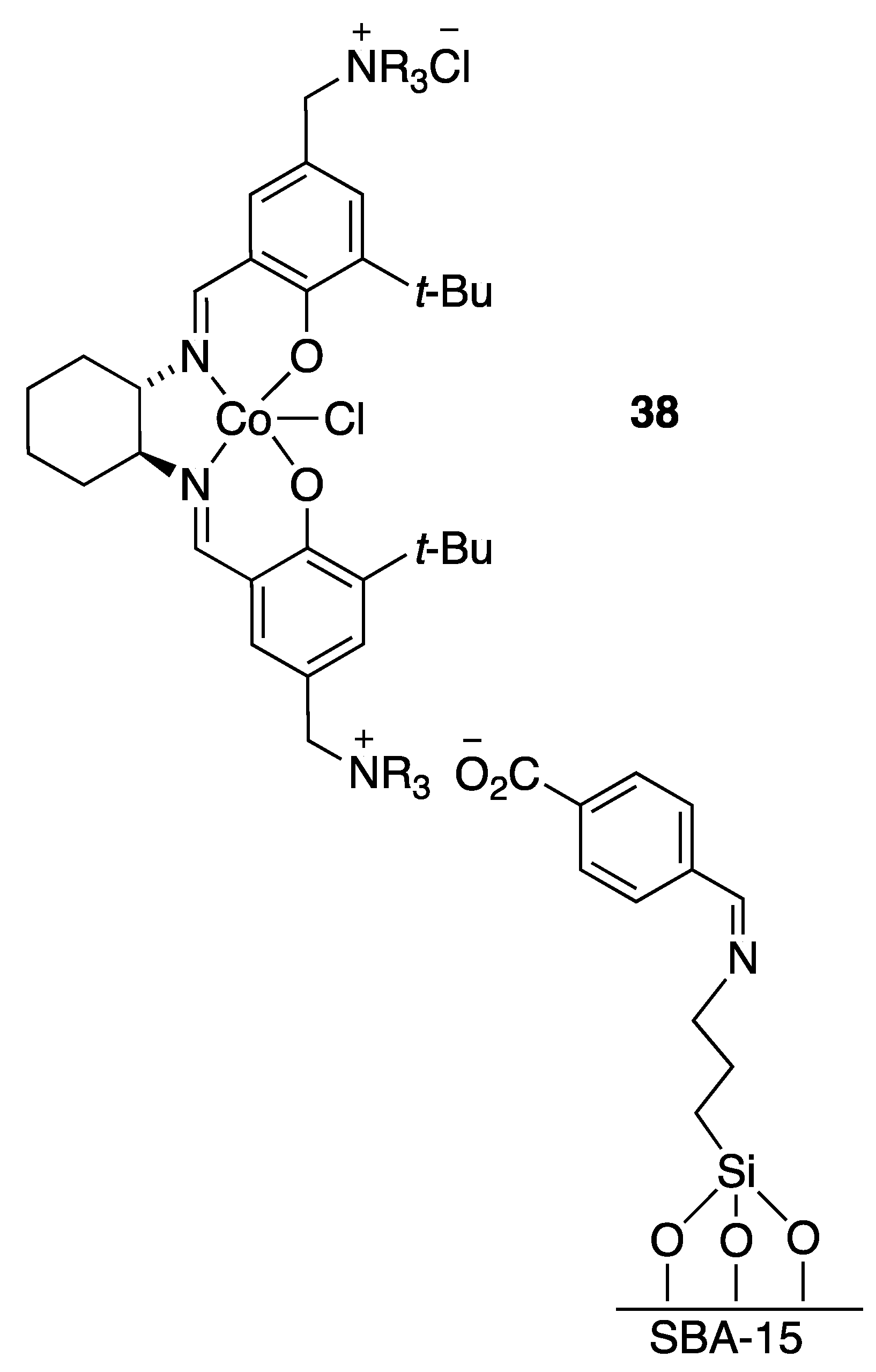

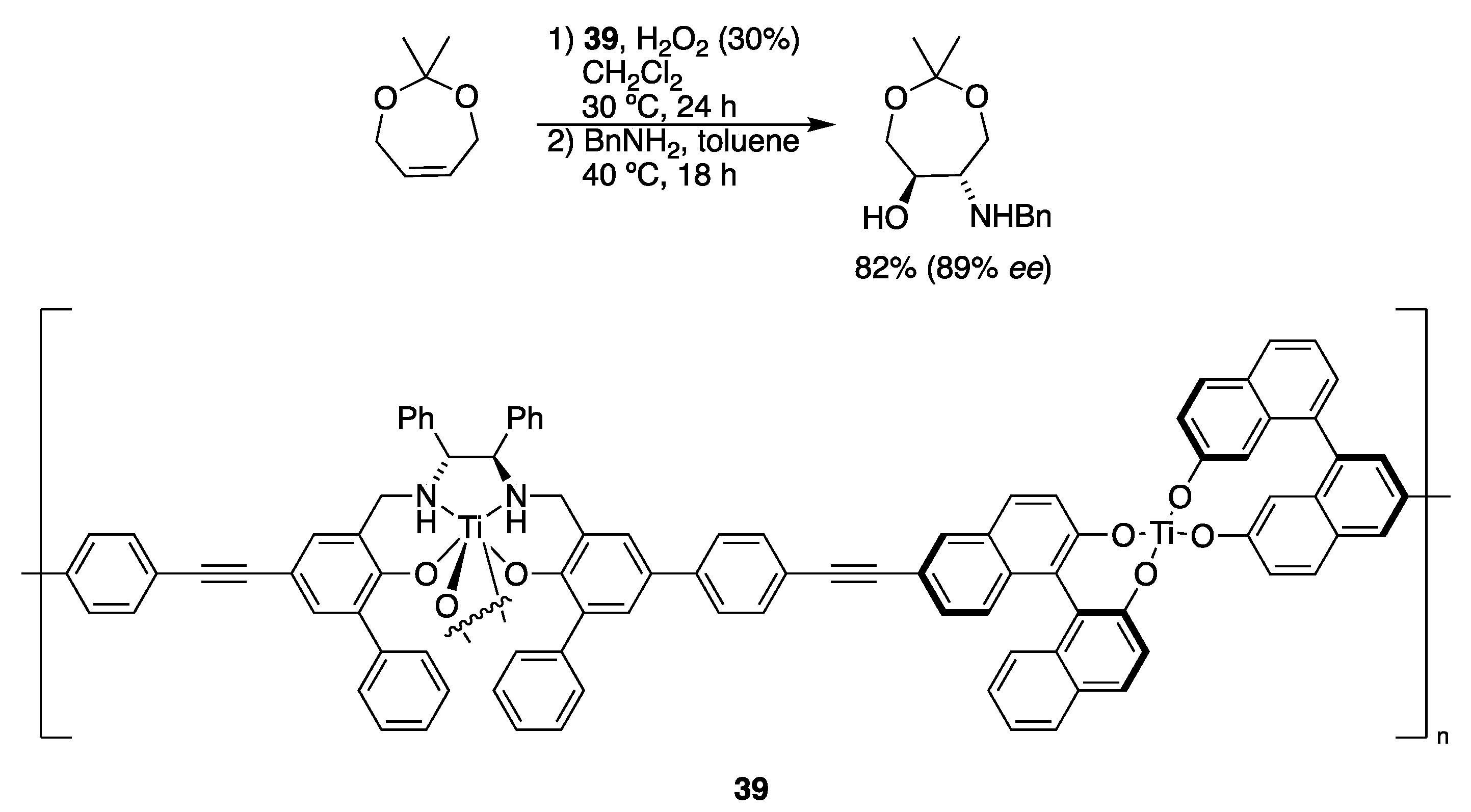
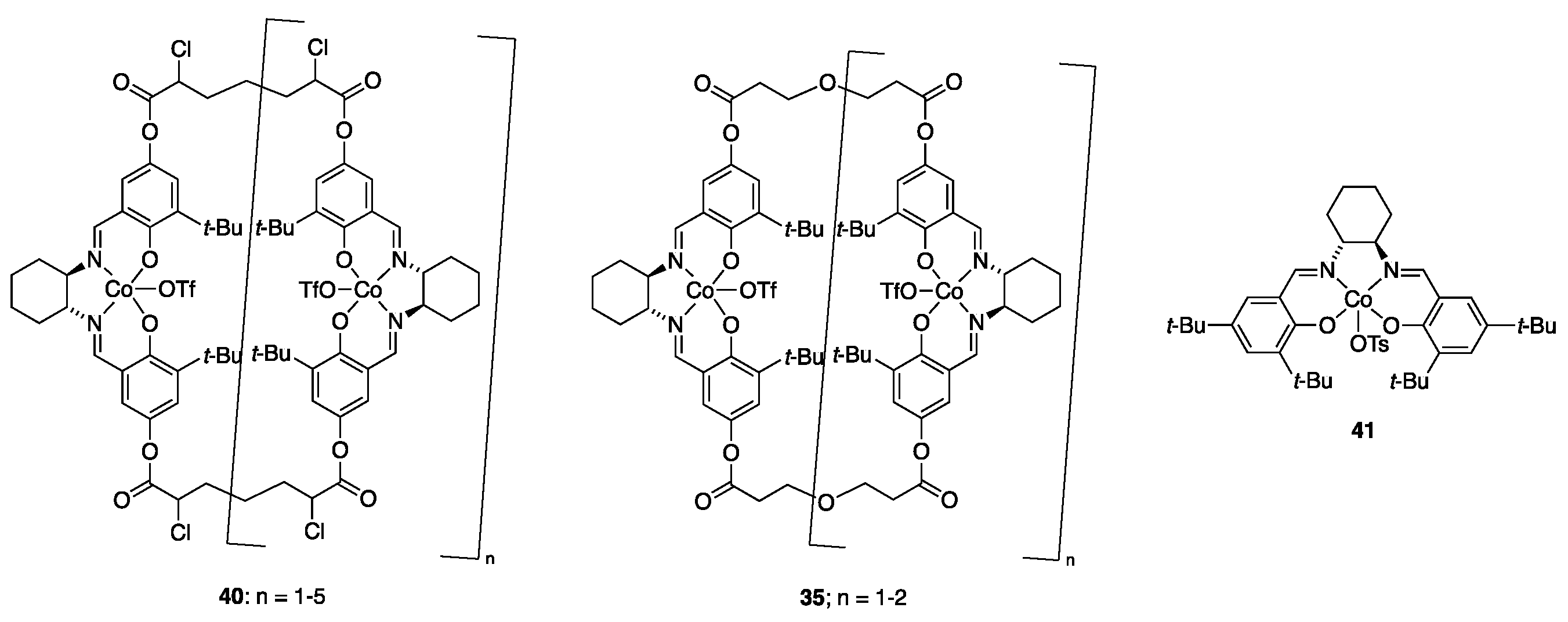
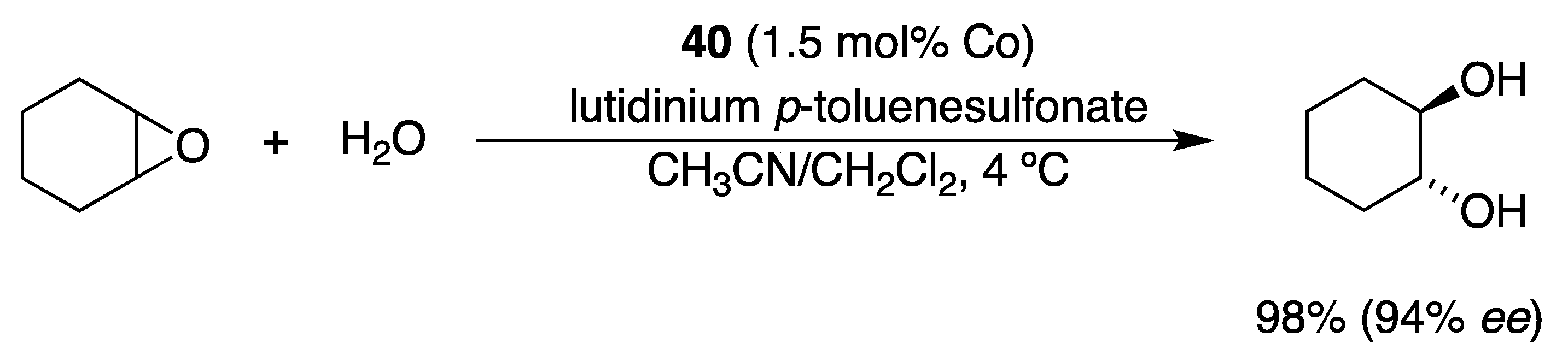

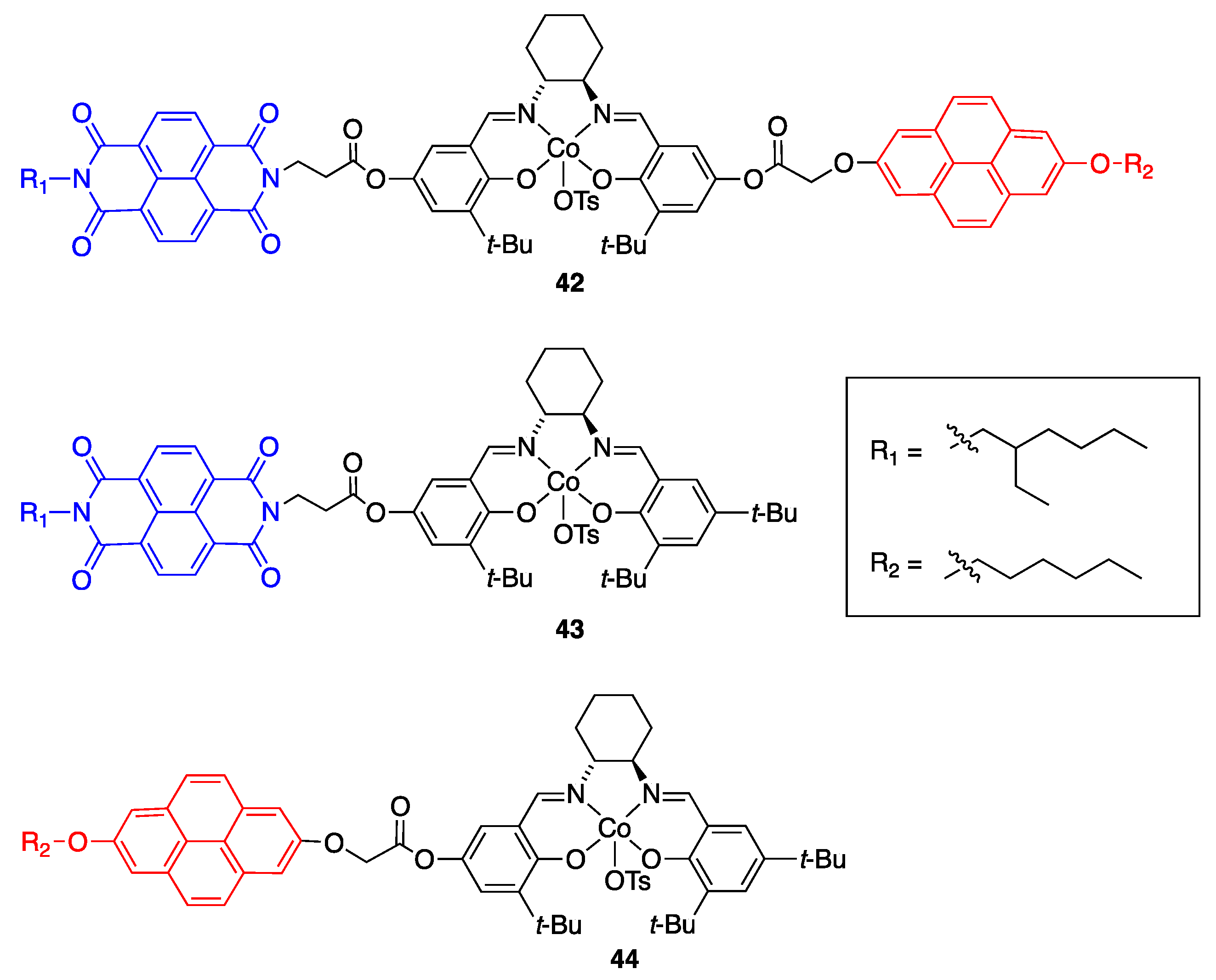
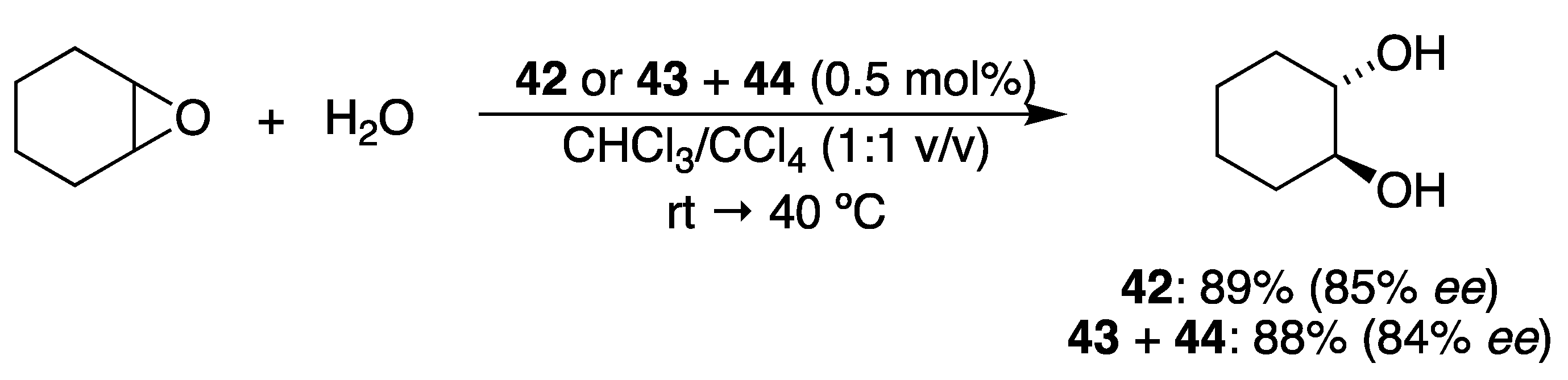



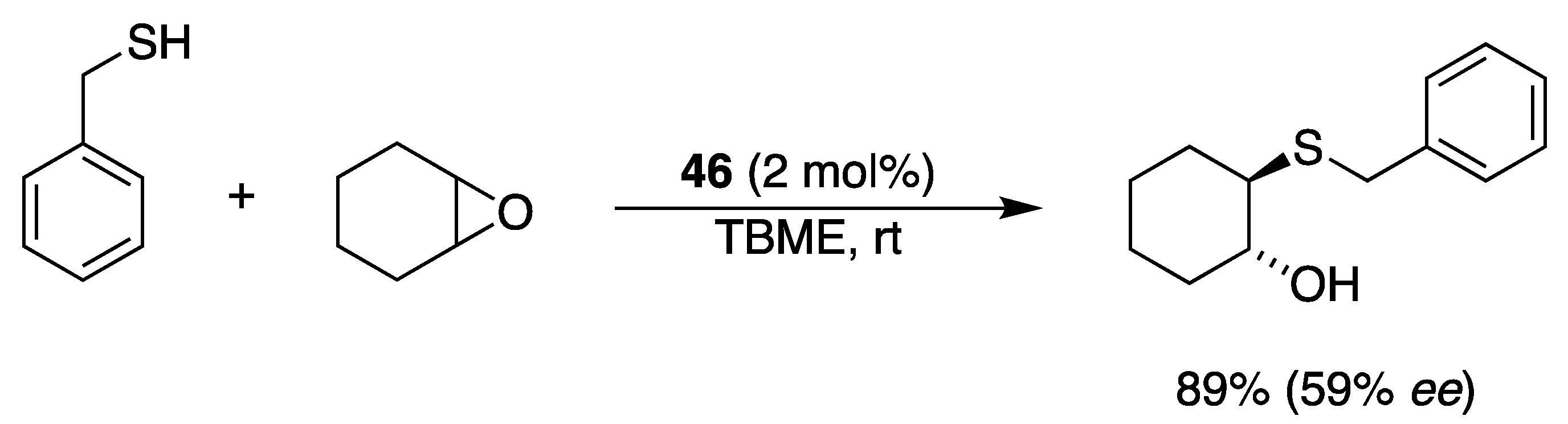








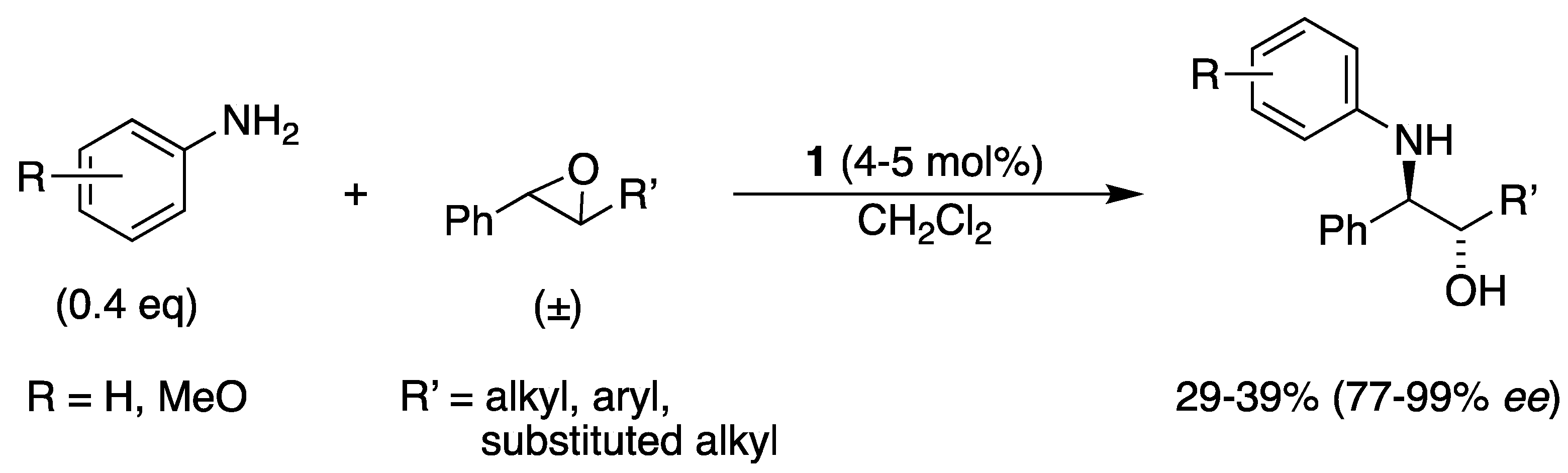
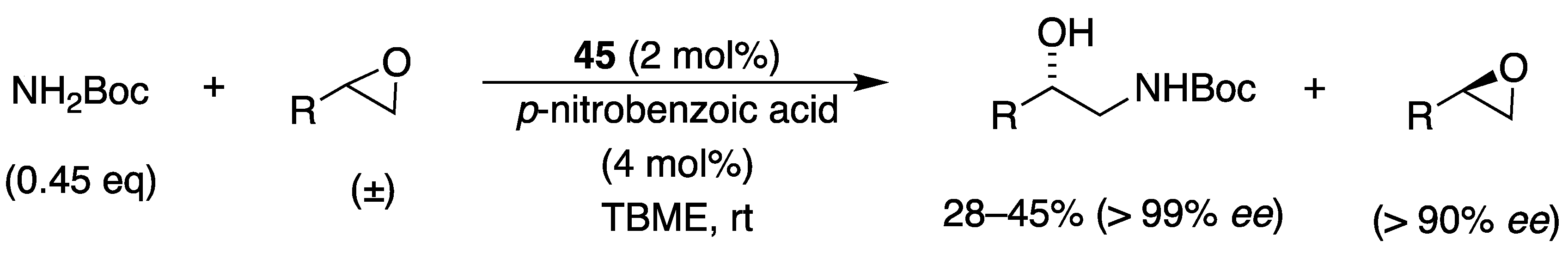
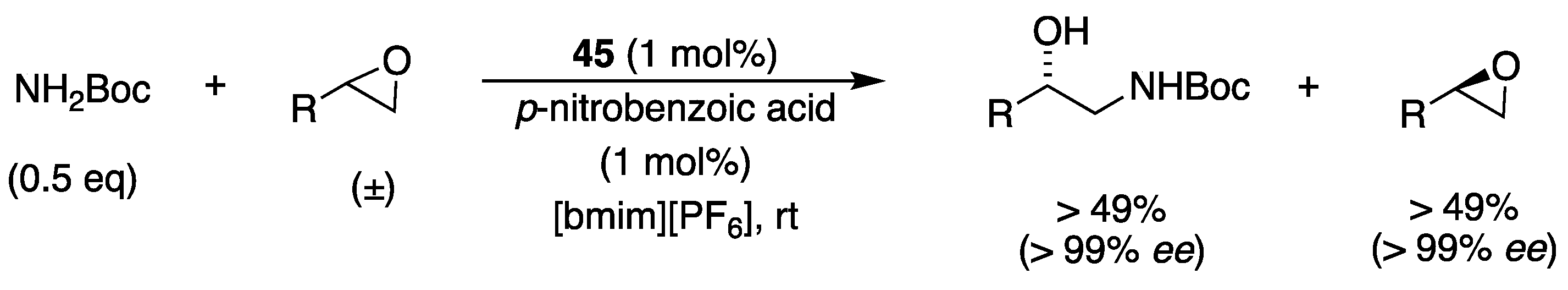
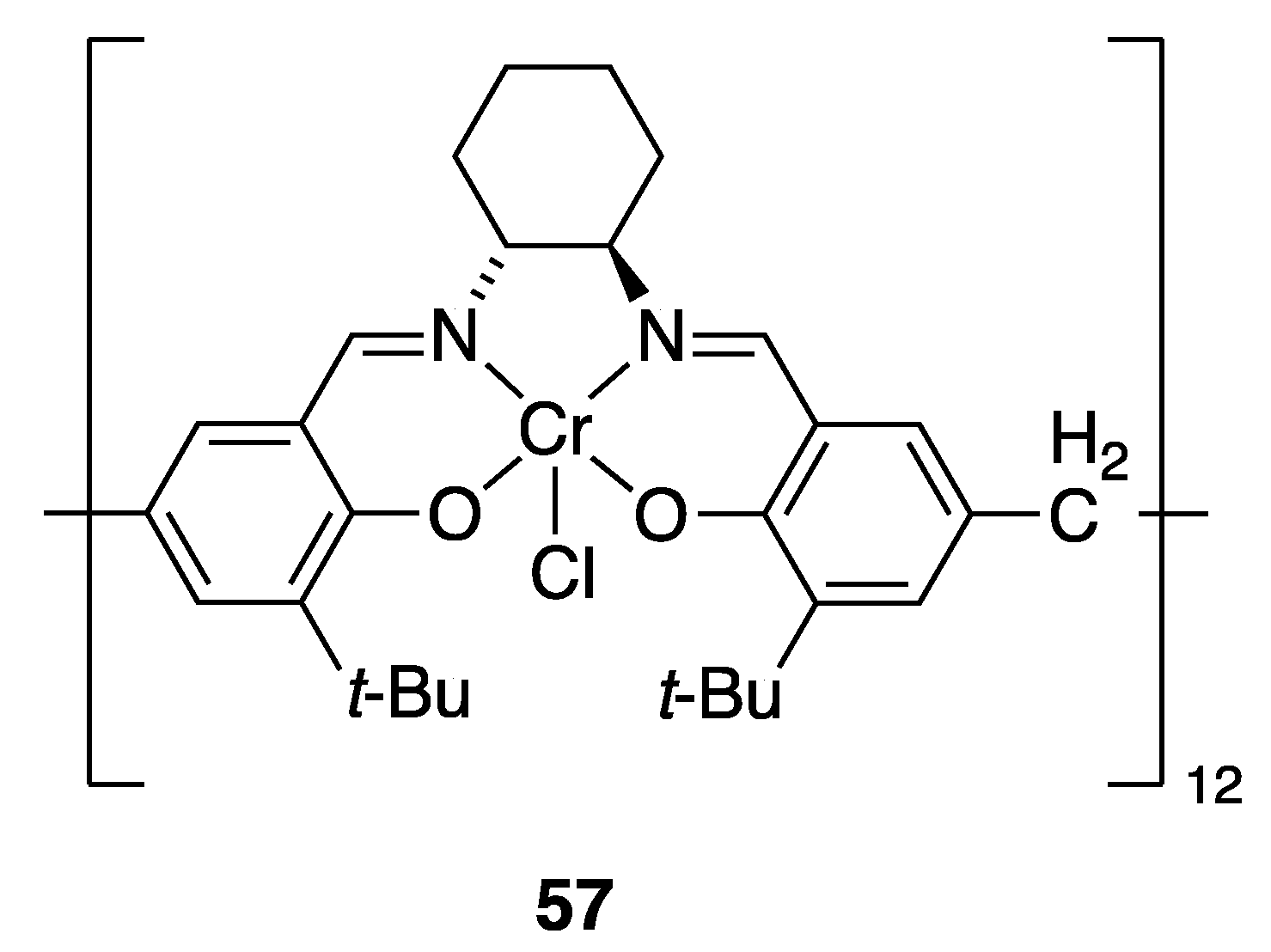
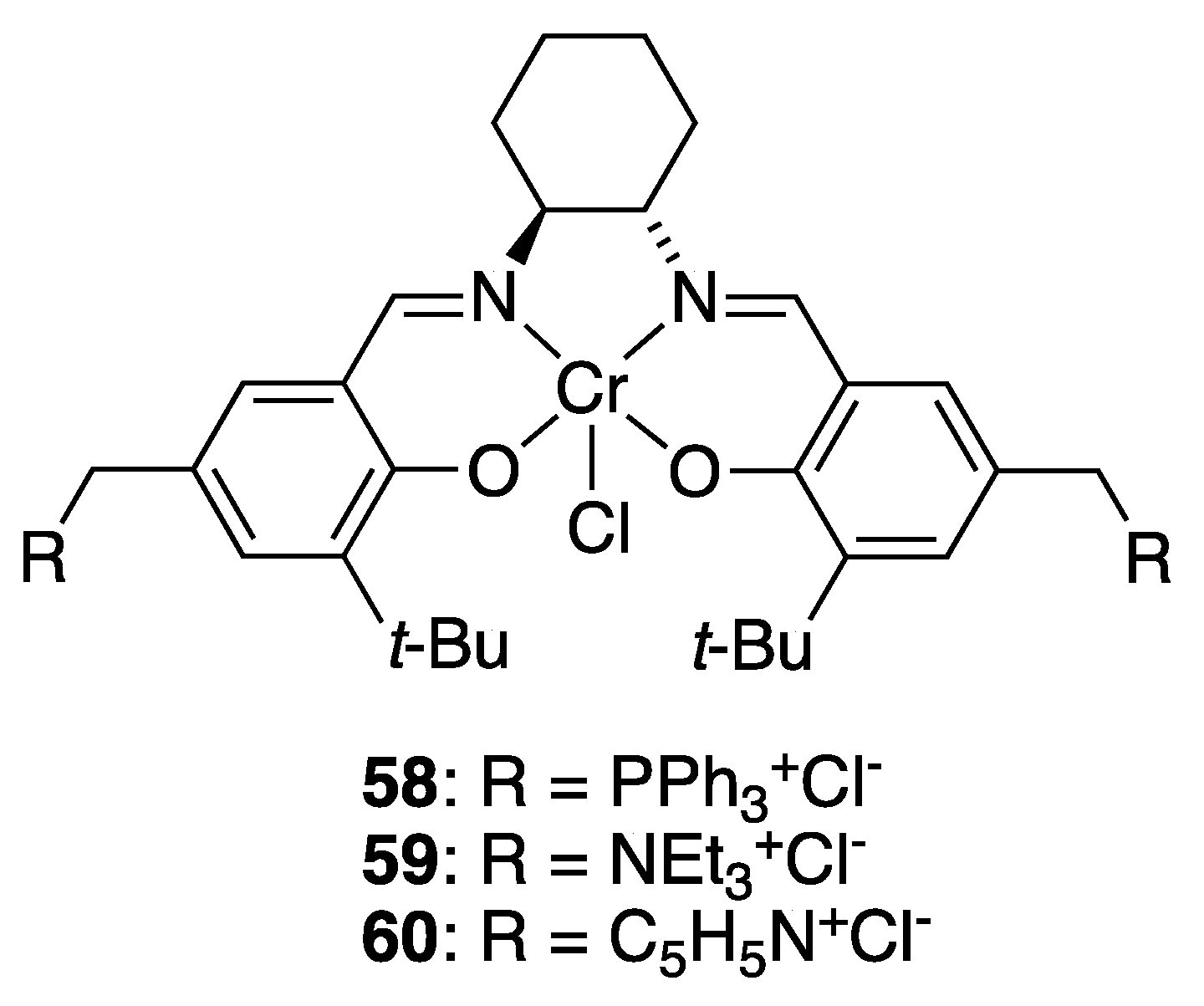
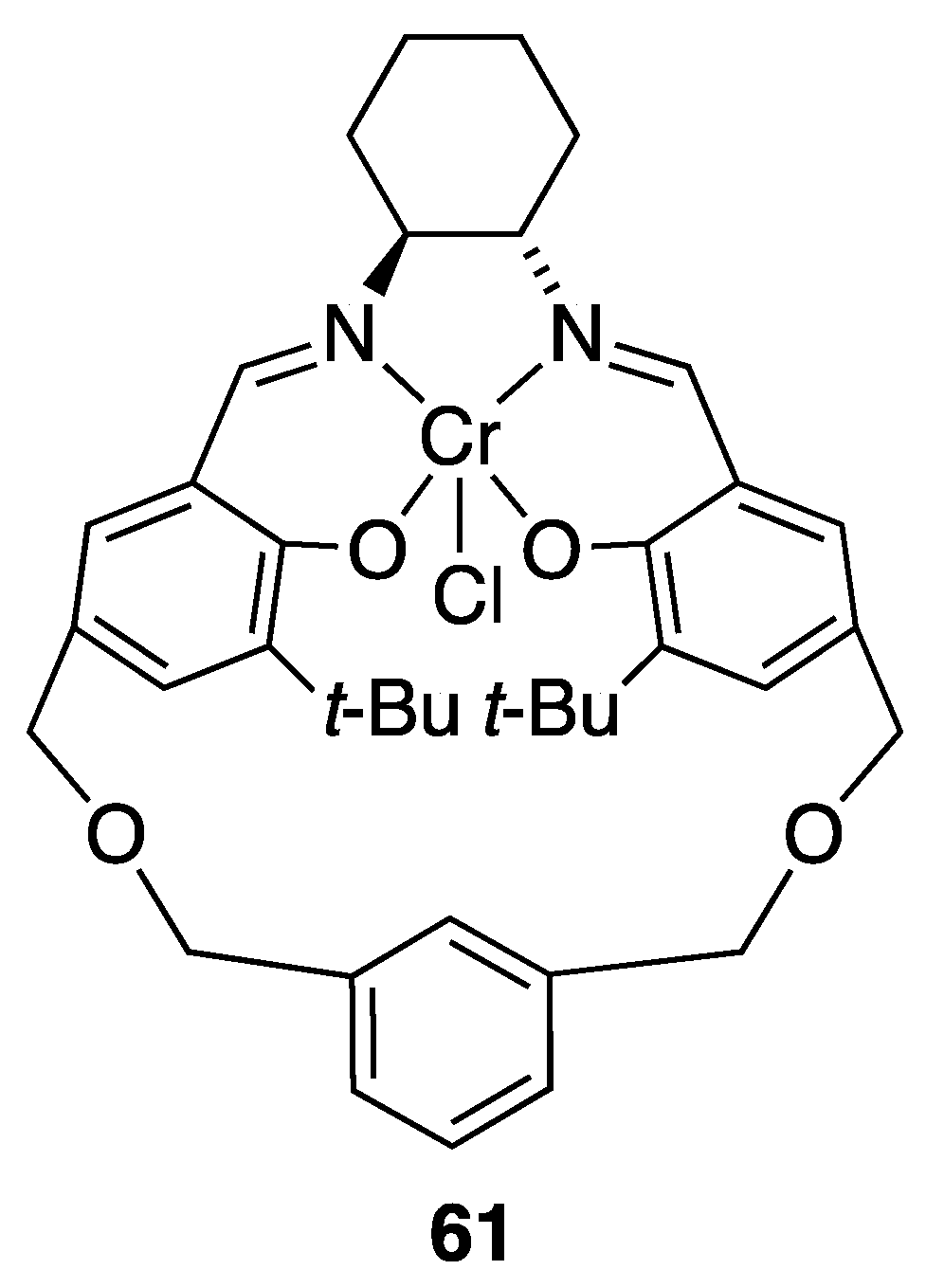
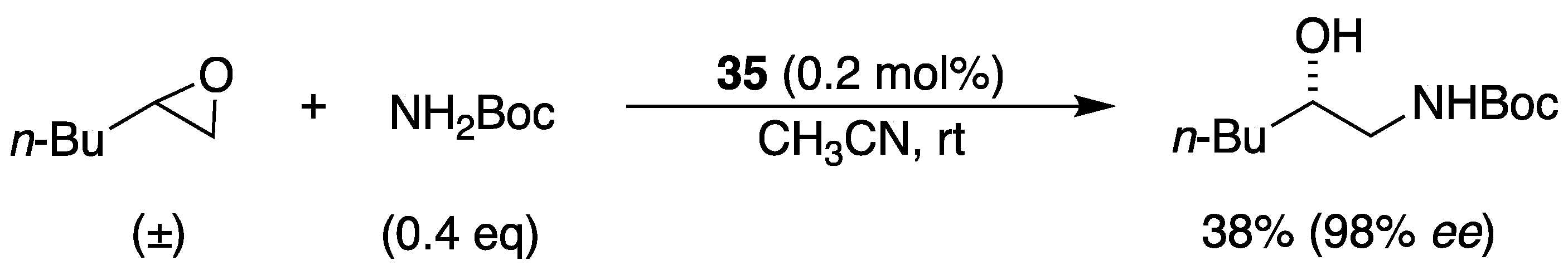
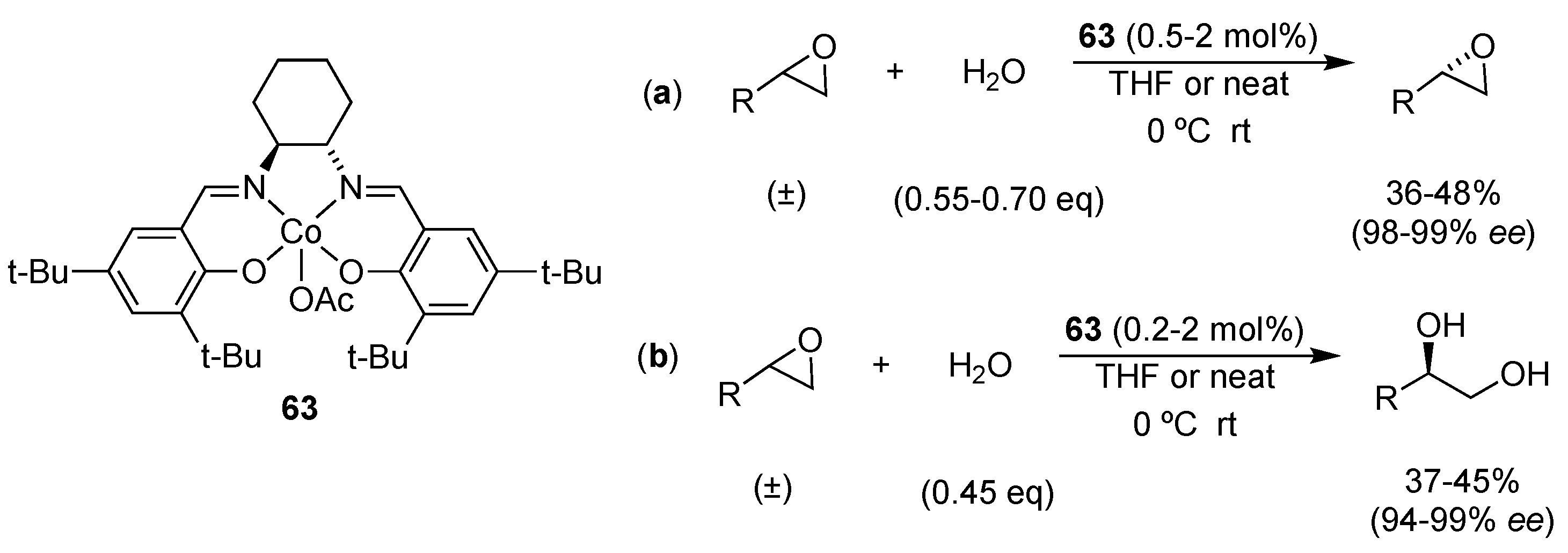

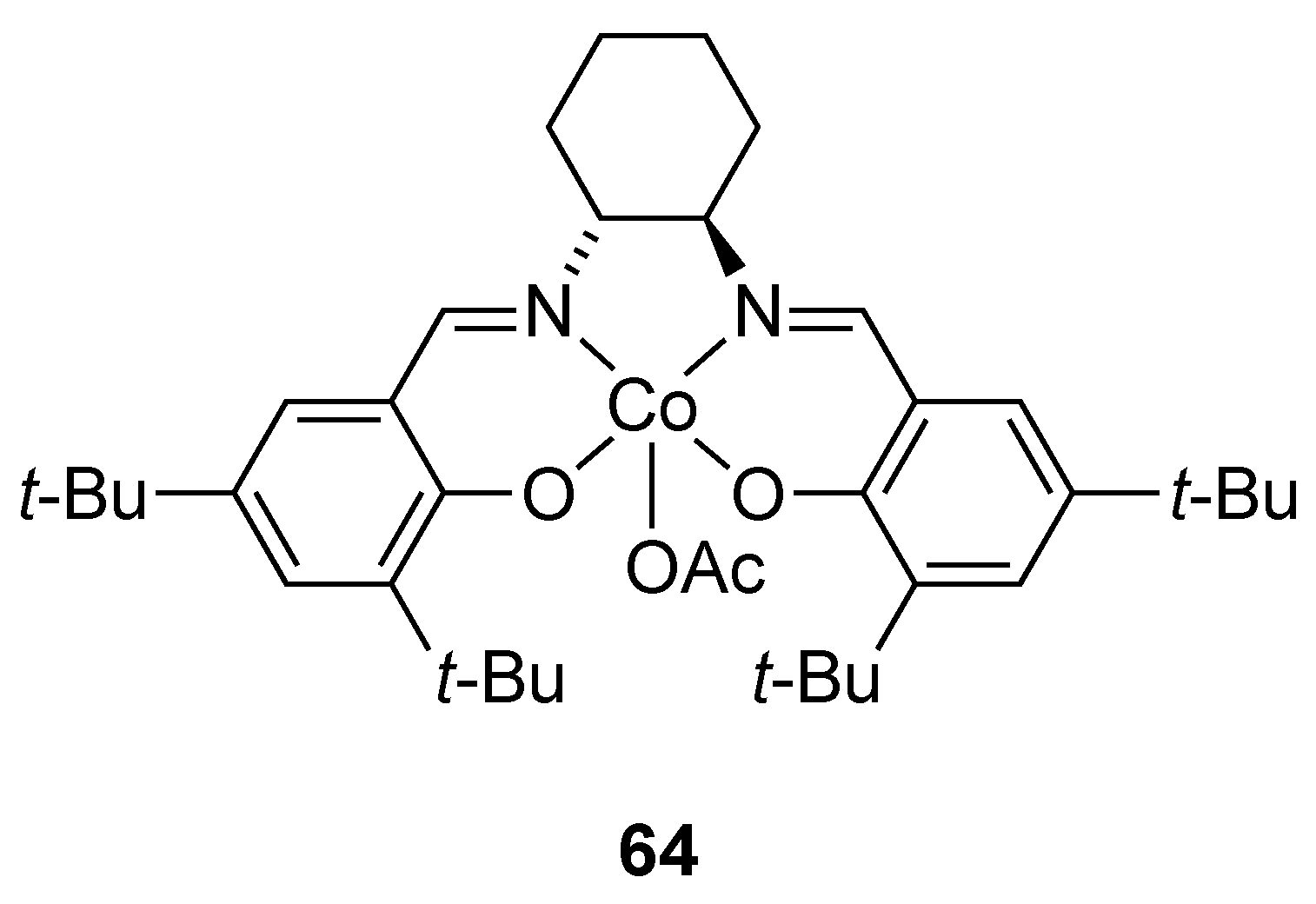
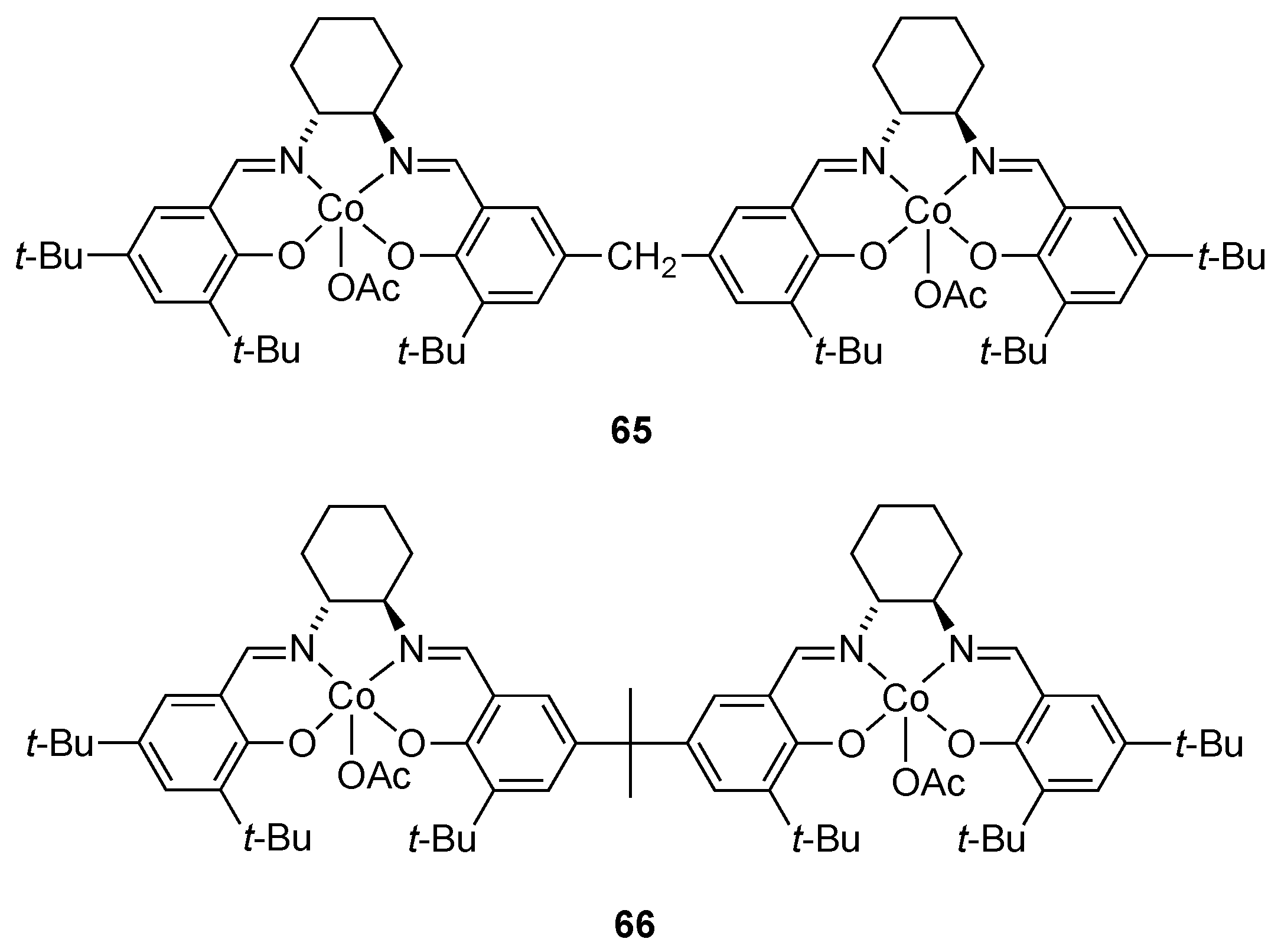
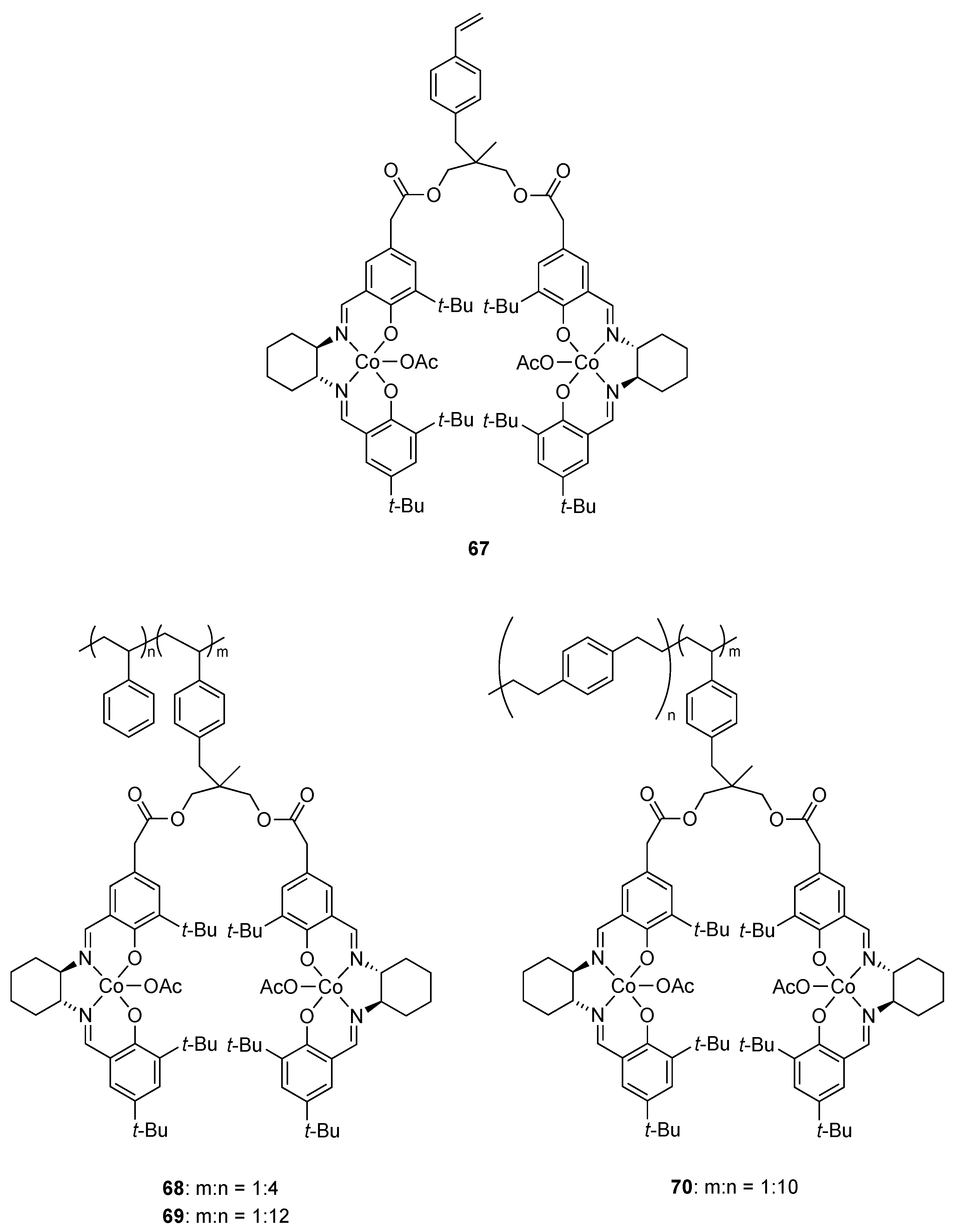

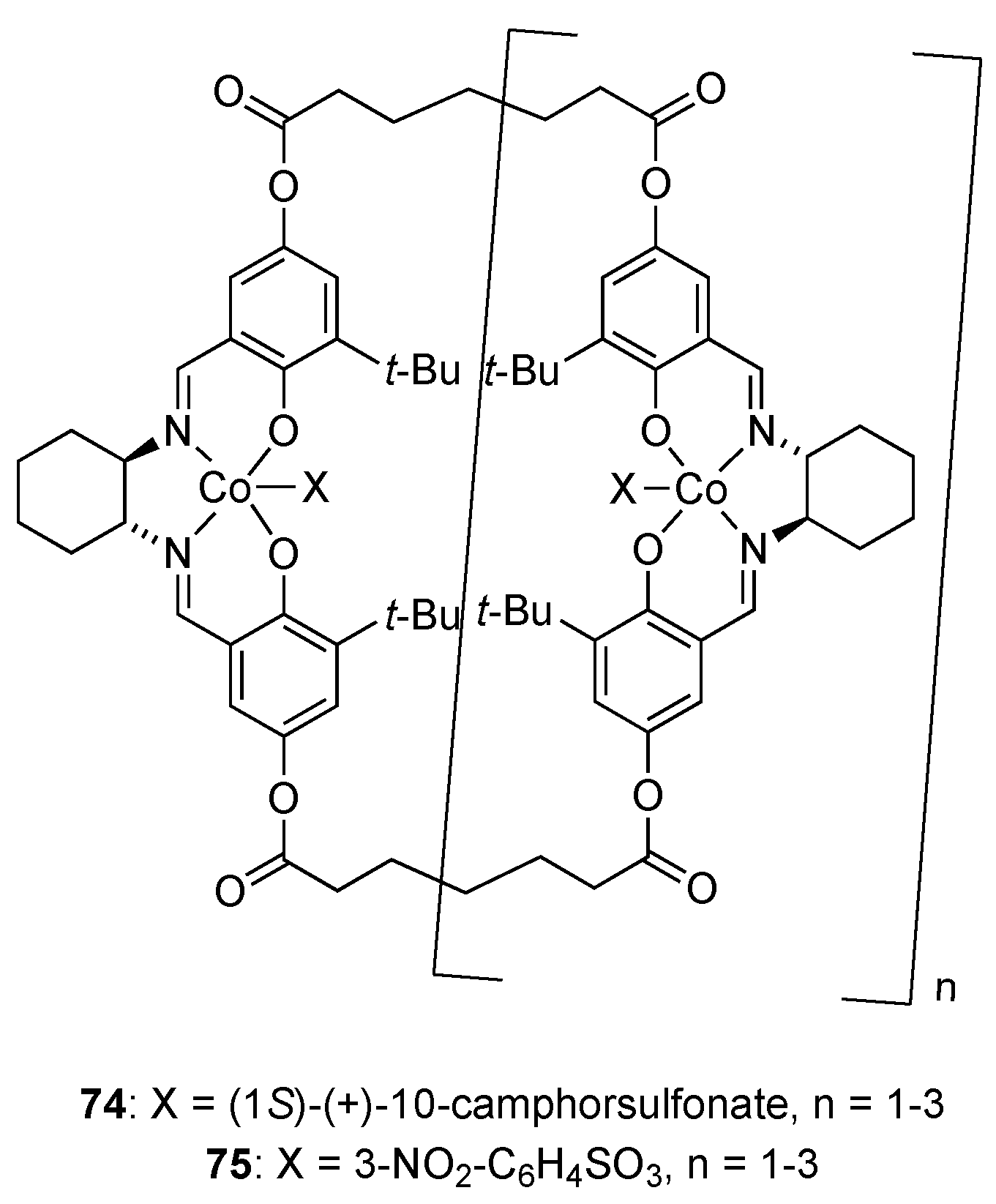
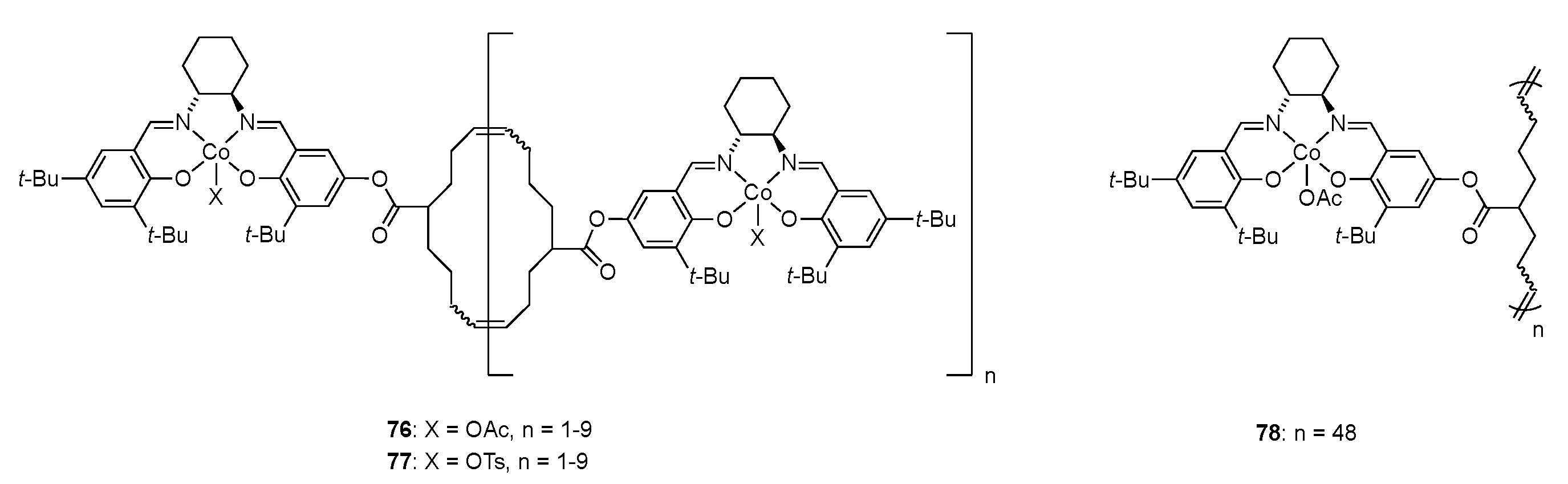
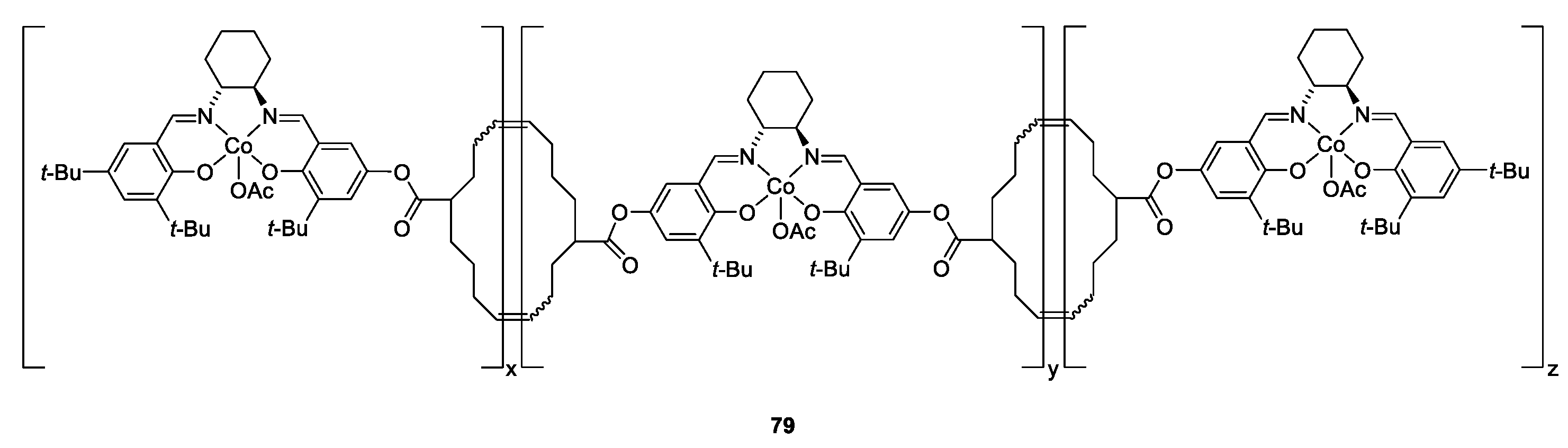
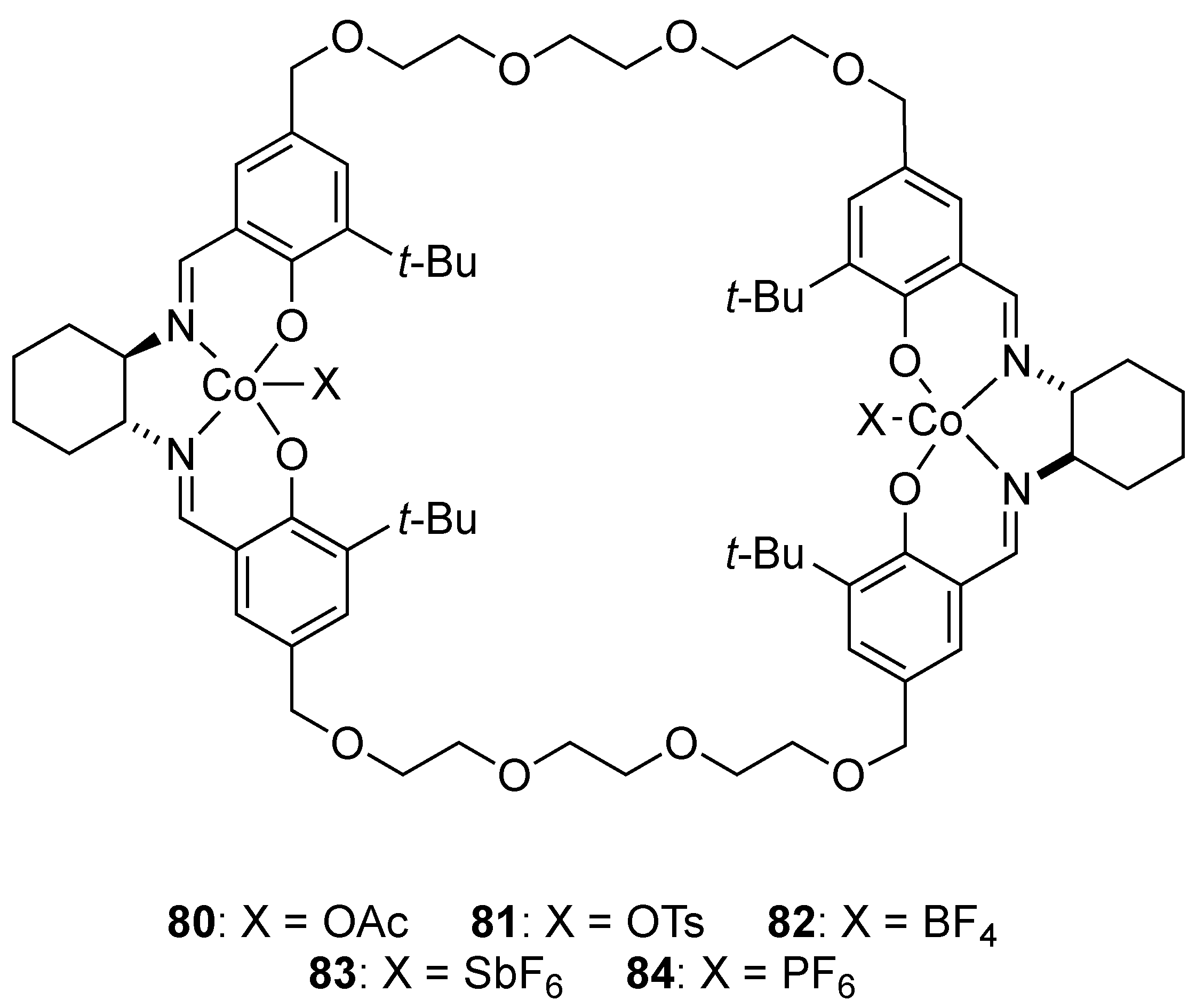

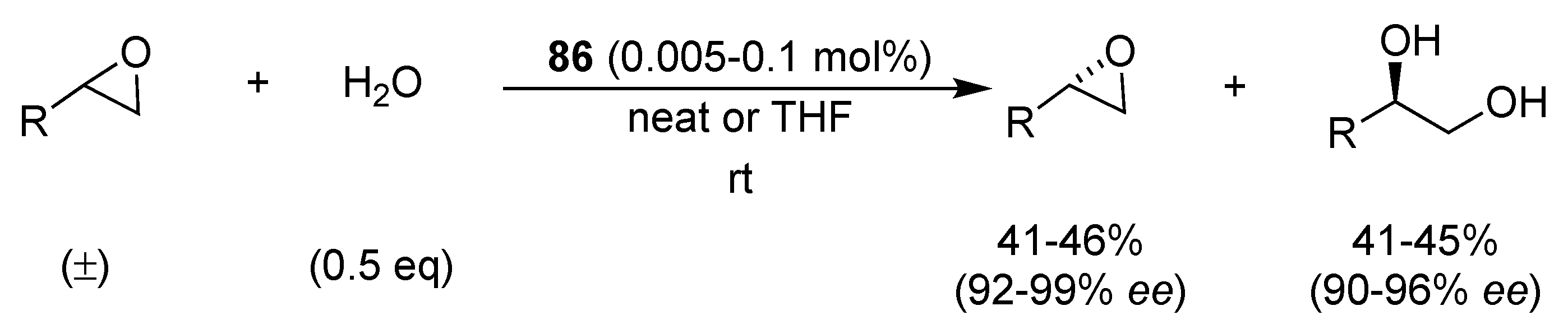
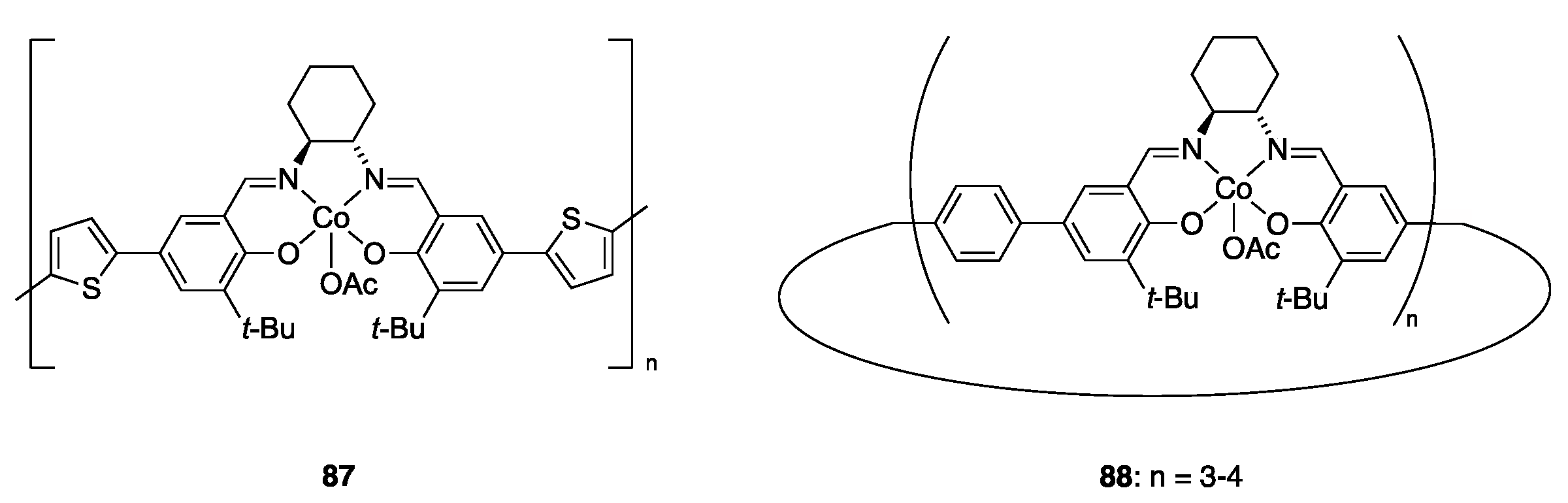
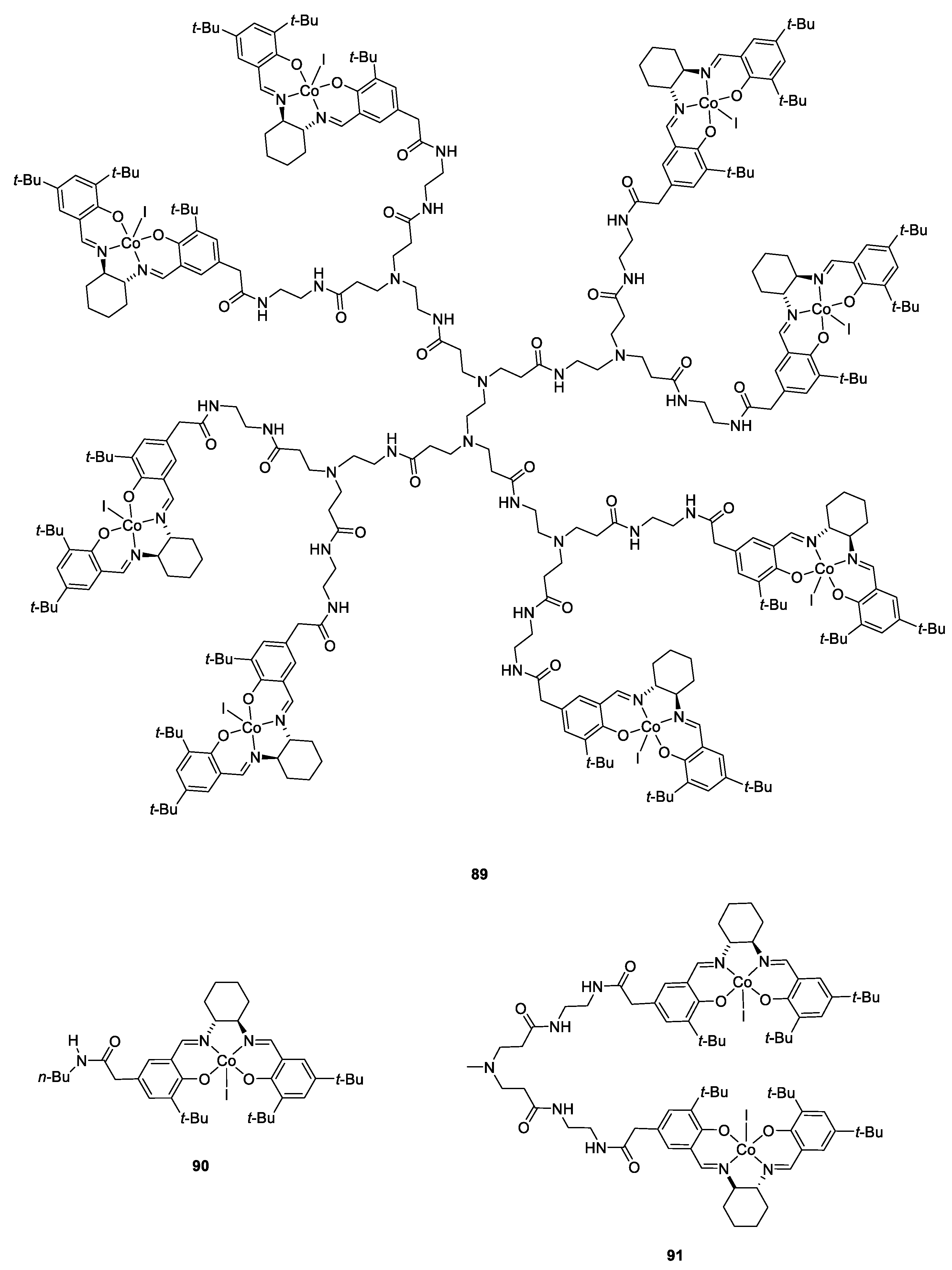
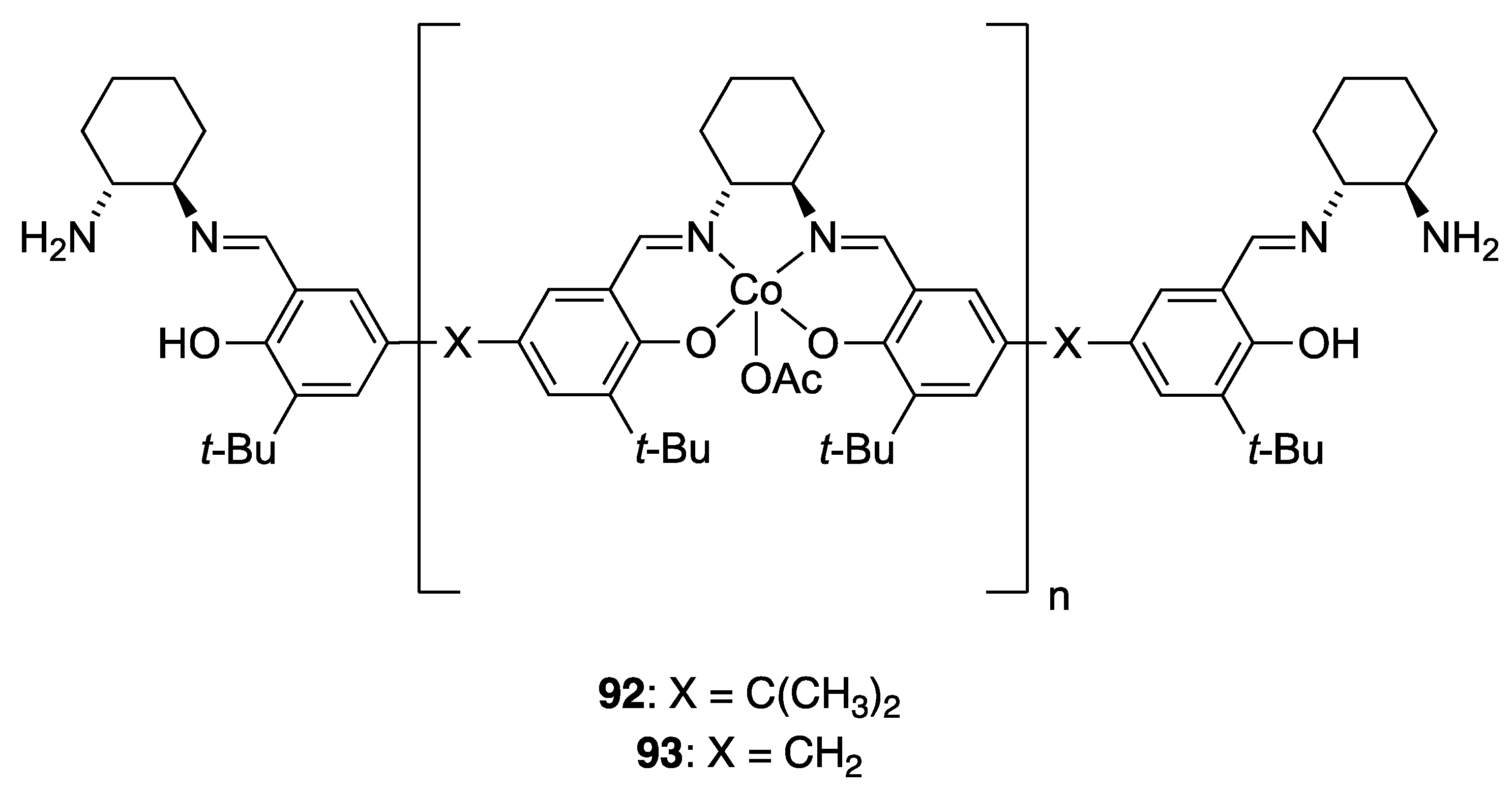
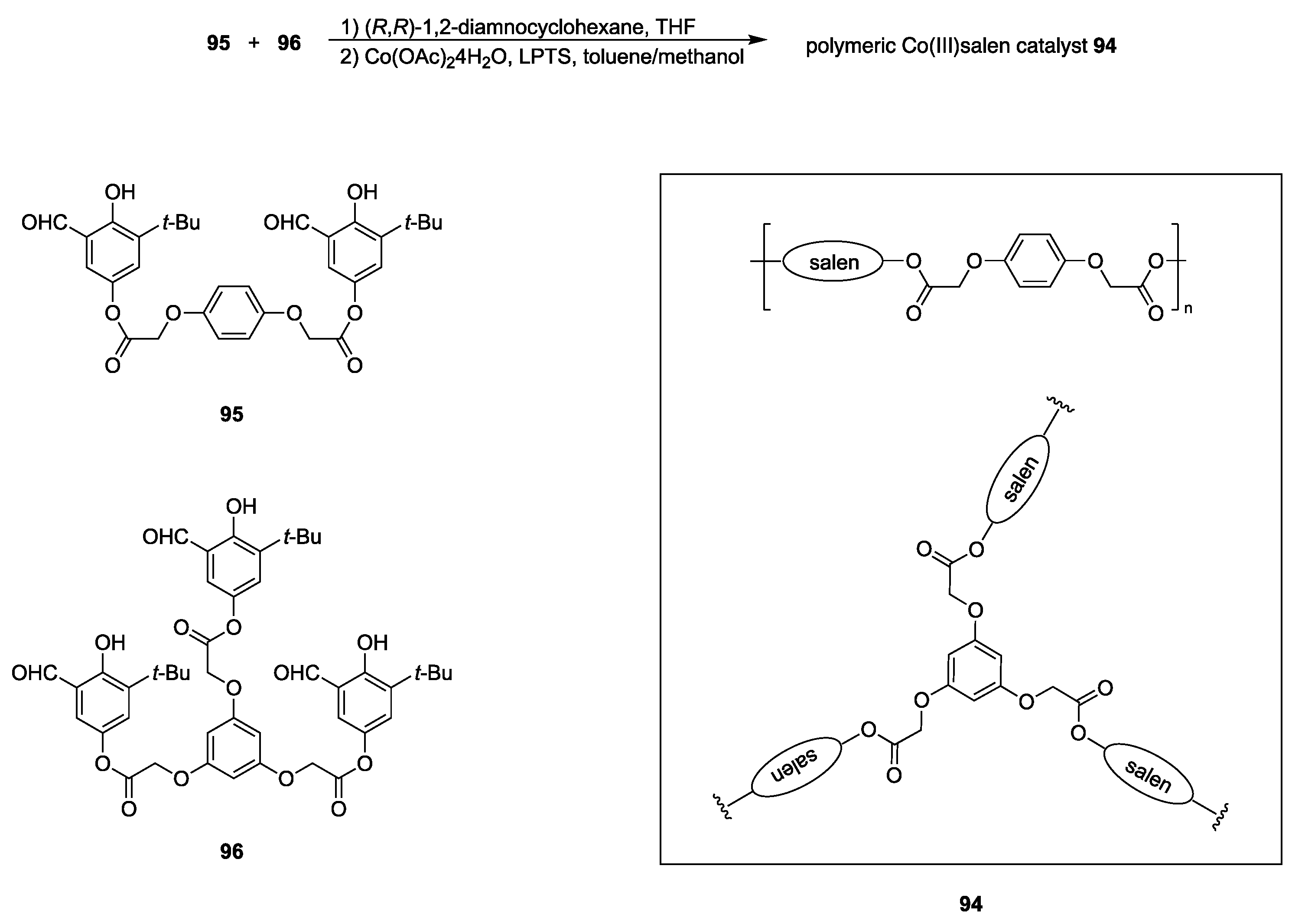
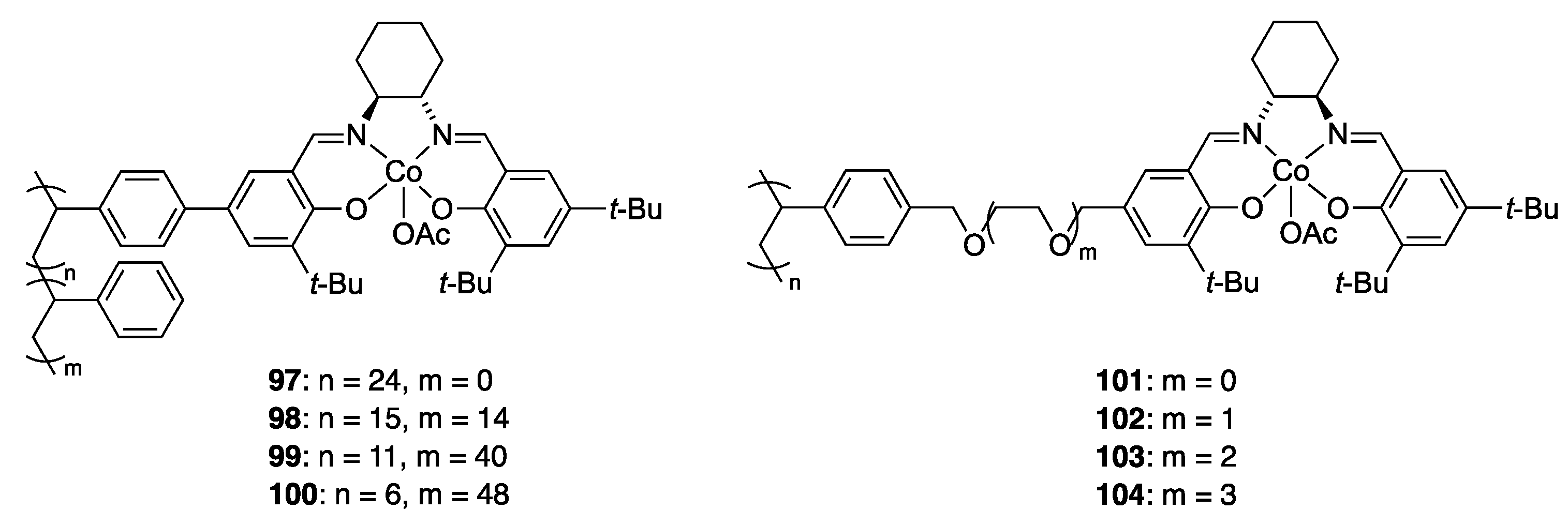
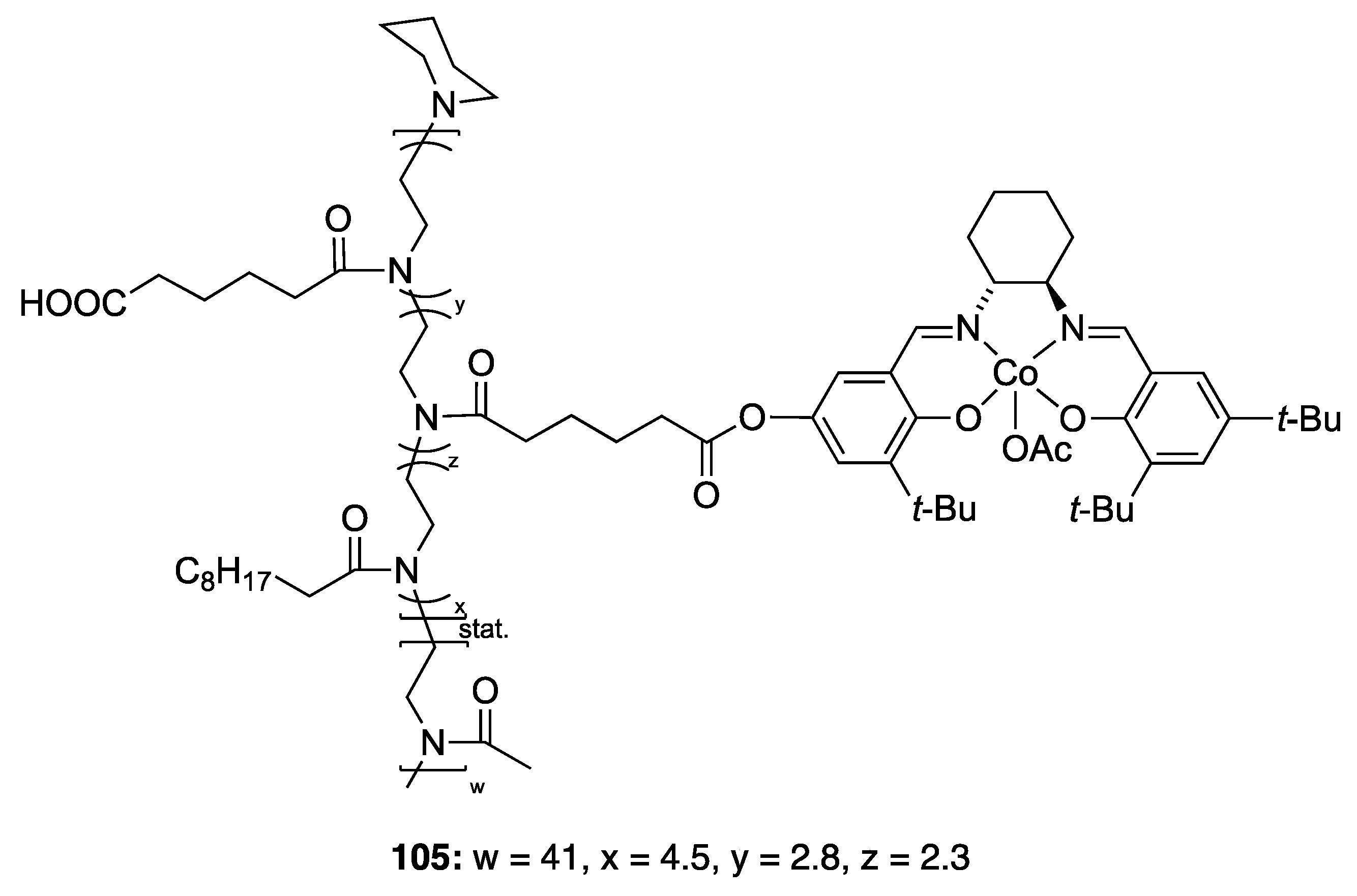


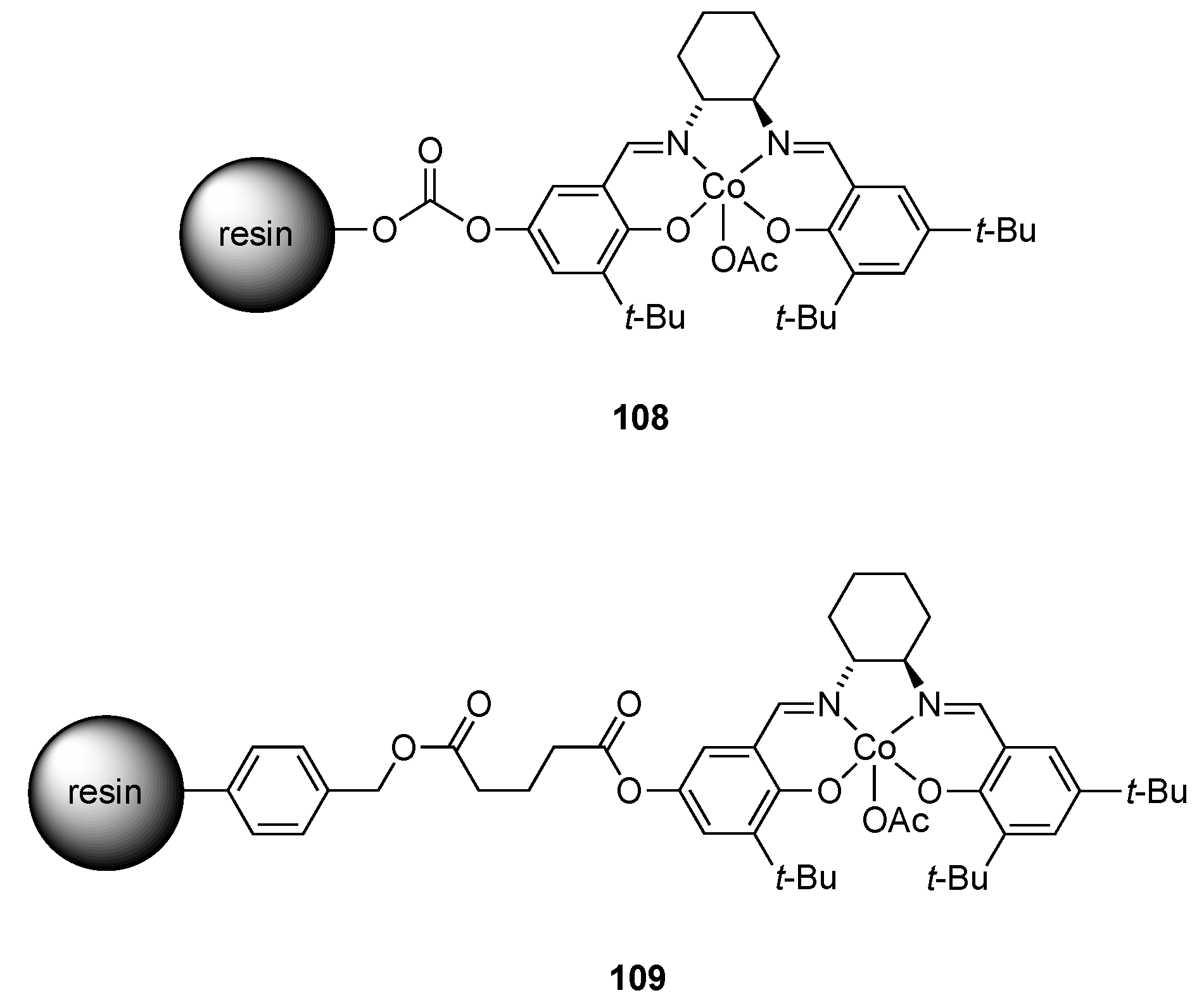
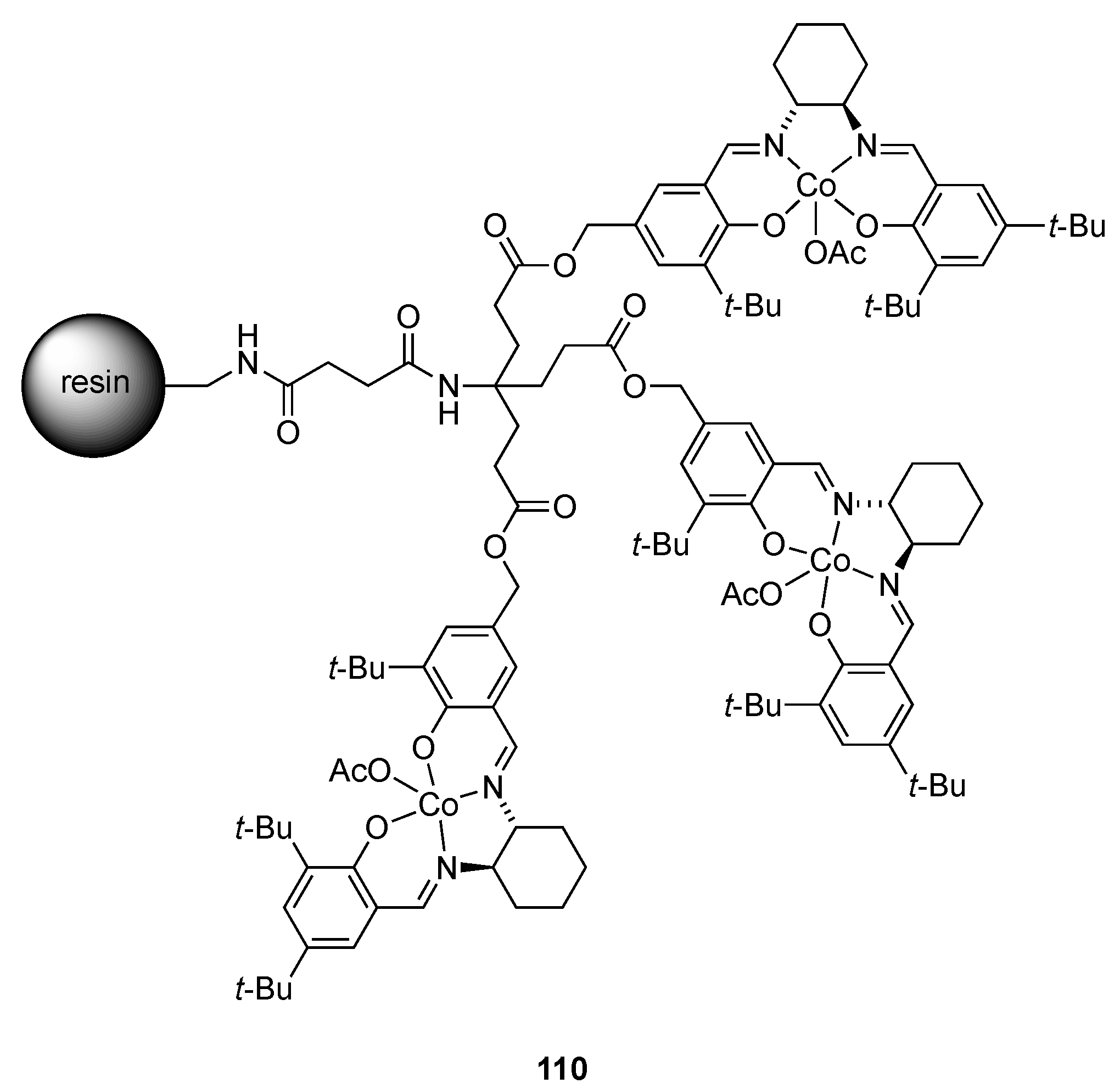

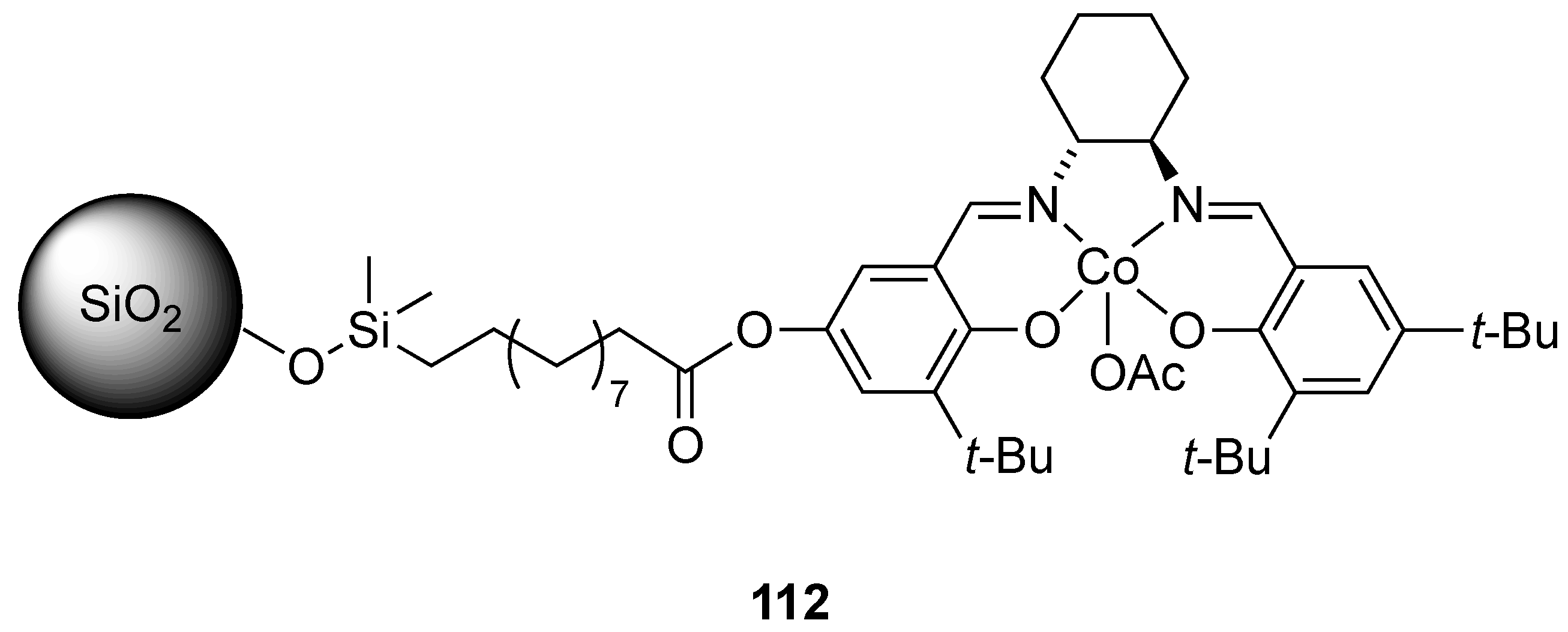
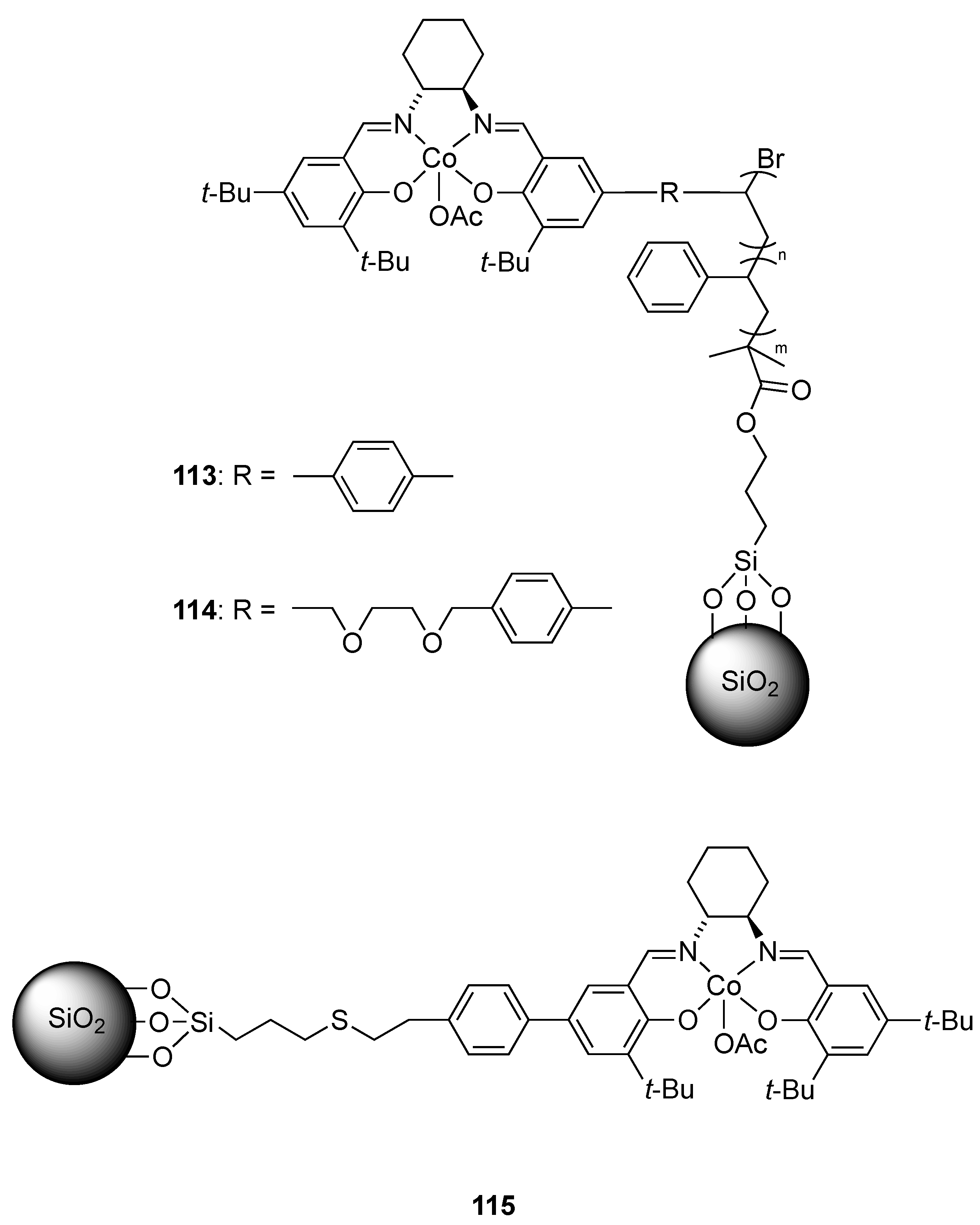
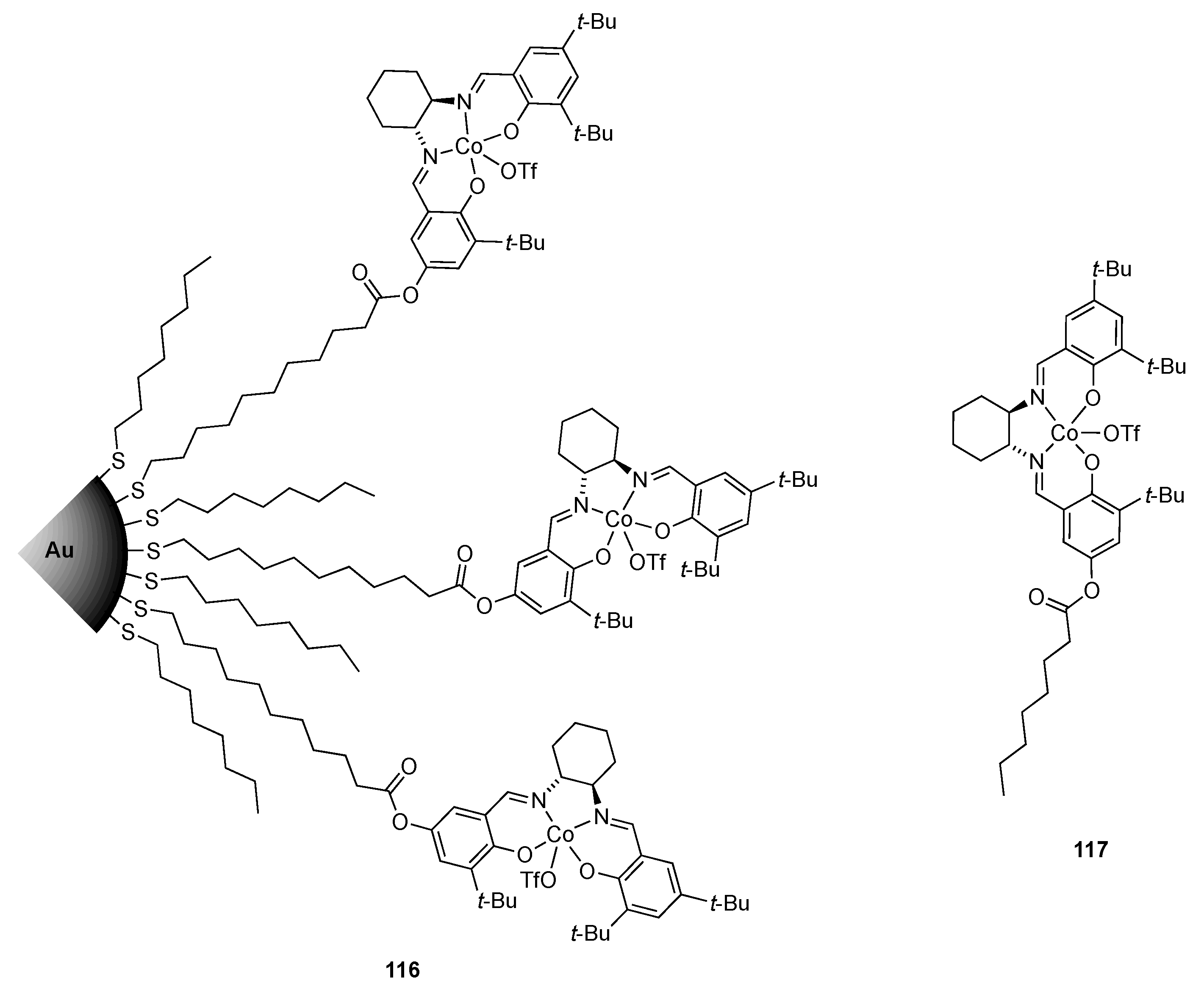


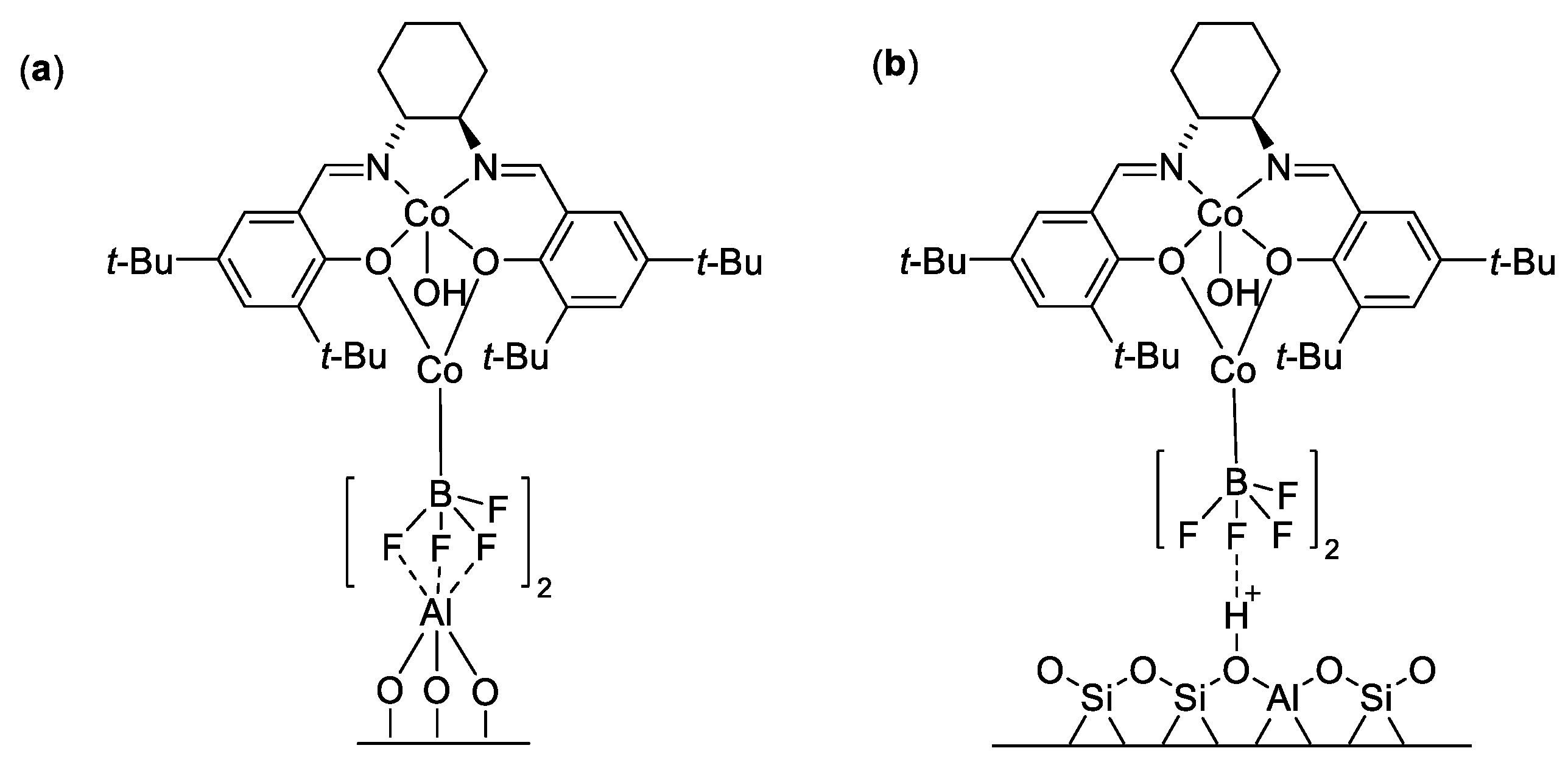


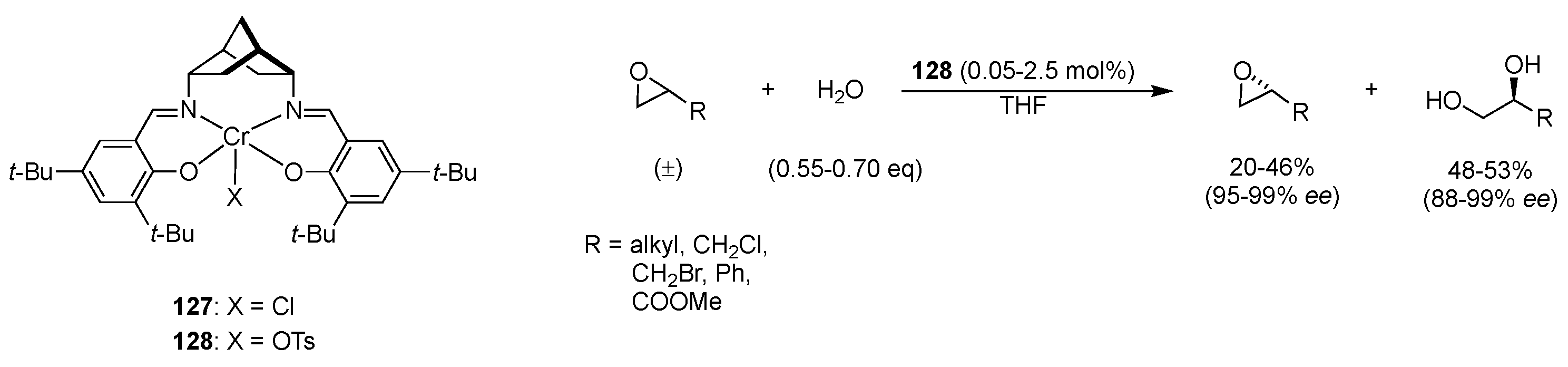
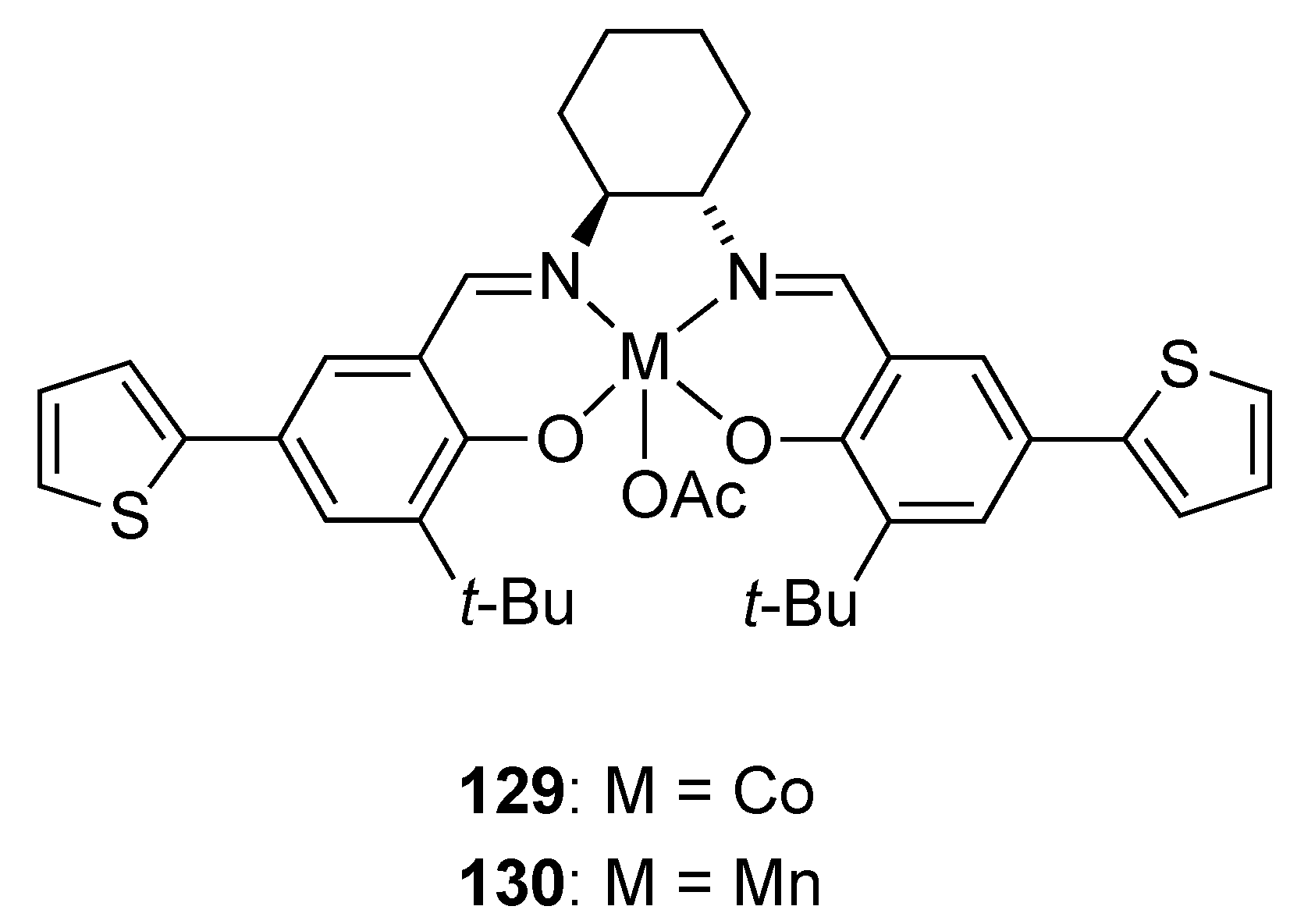


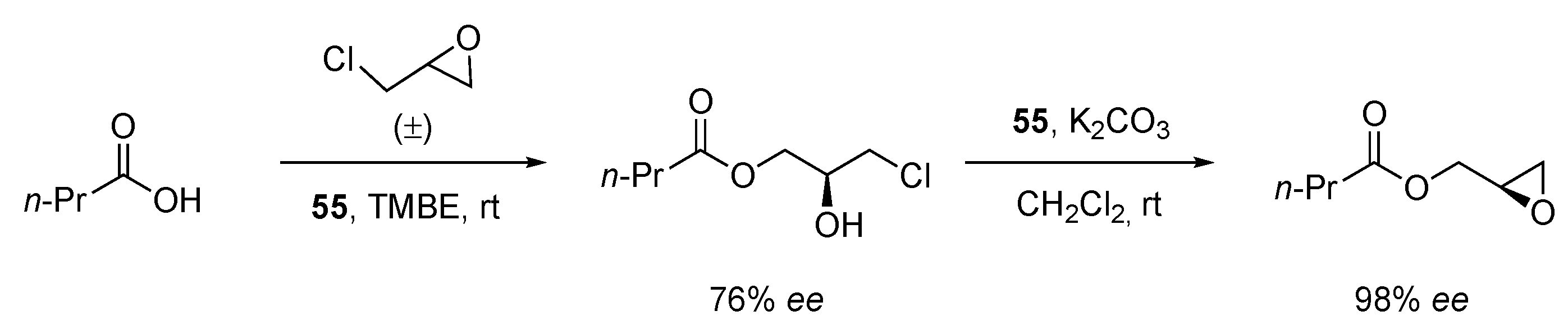
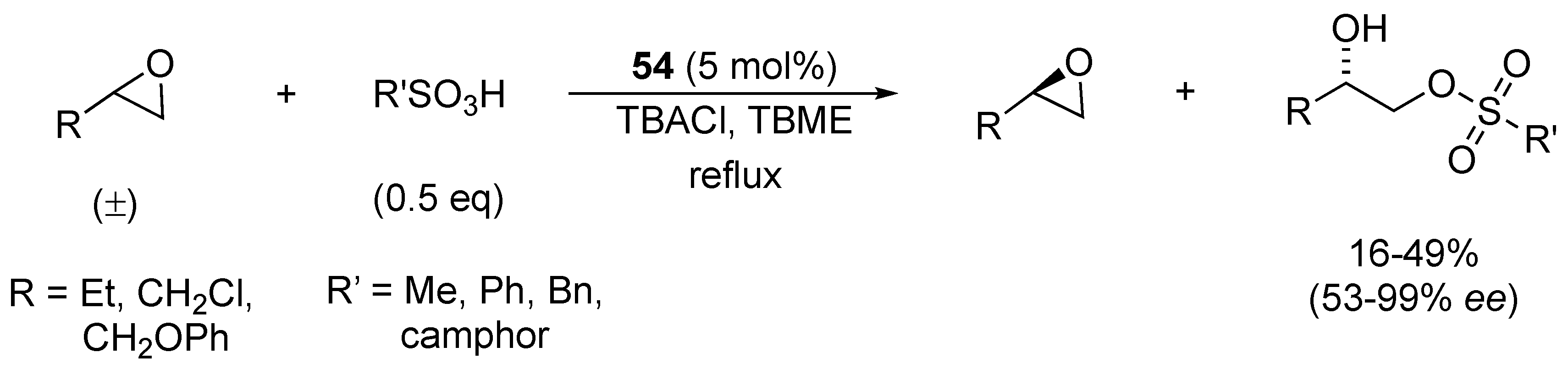


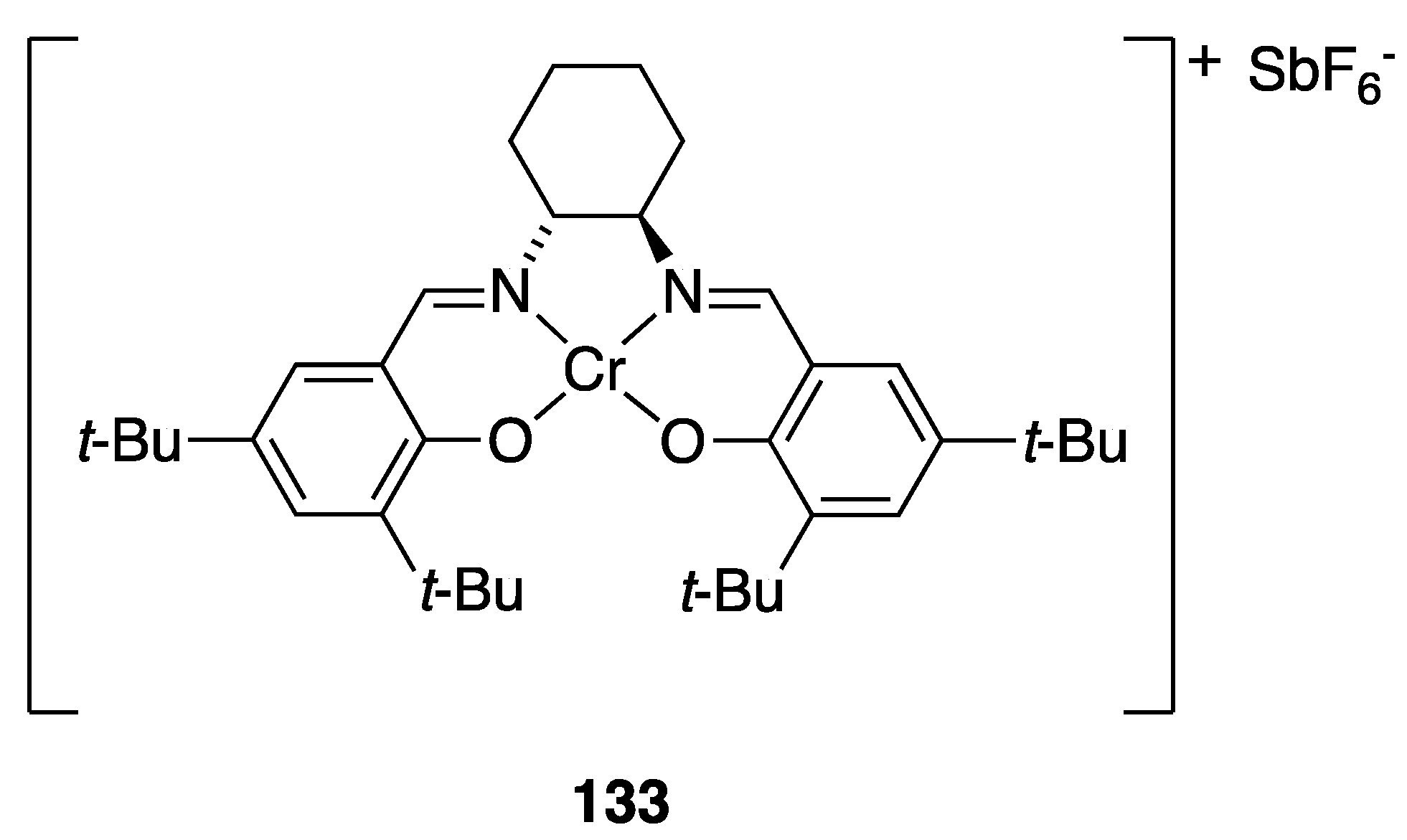

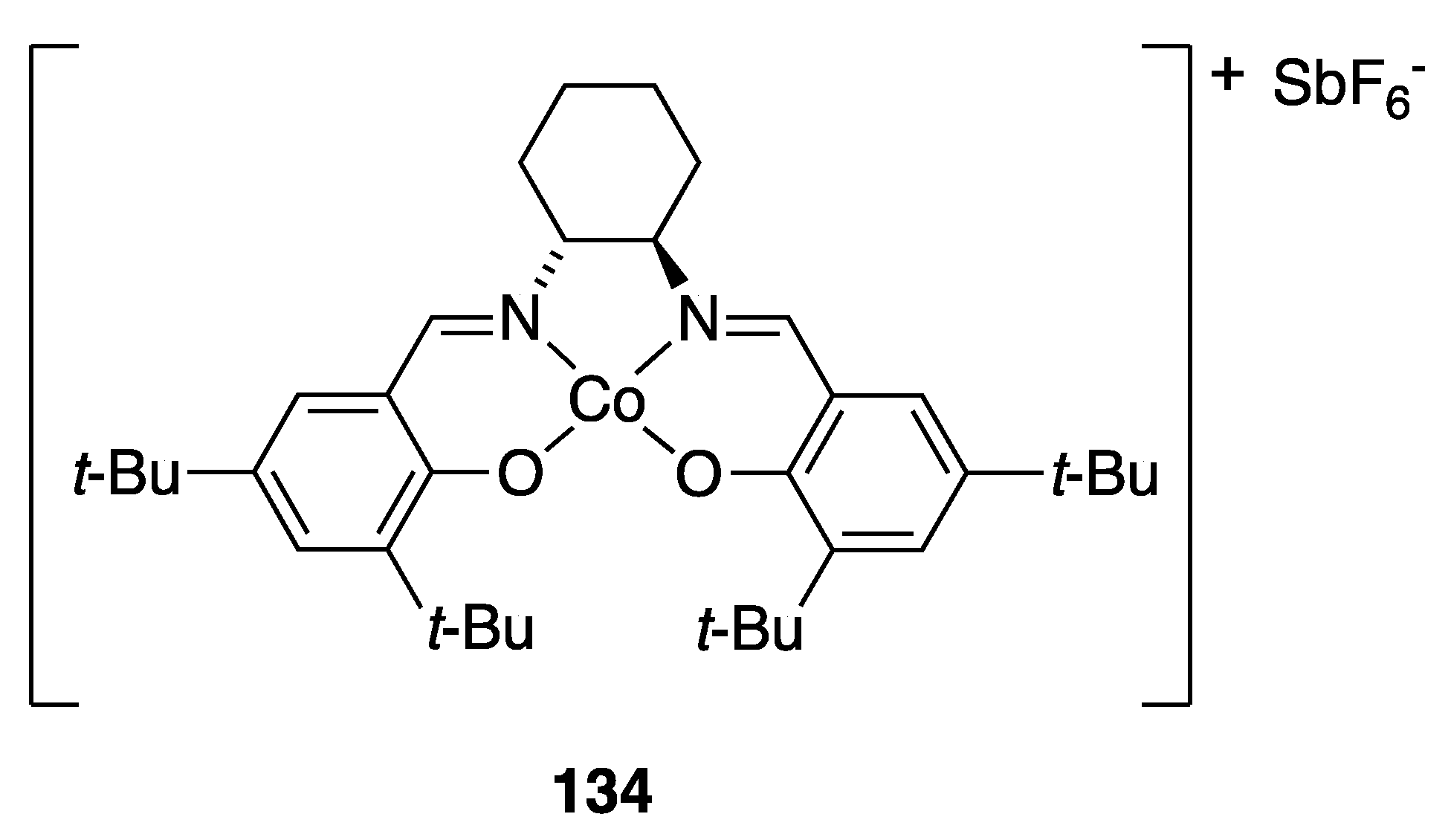

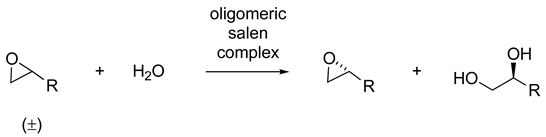
| Catalyst | Lowest mol% Co | Epoxide | Diol | Solvent |
|---|---|---|---|---|
| 63 or 64 | 0.5 | 36–48% (98–99% ee) | 37–45% (94–99% ee) | solvent-free or THF |
| 40 | 0.01 | 36–45% (99% ee) | 40–50% (94–98% ee) | solvent-free |
| 74 or 75 | 0.0004 | 44–45% (>99% ee) | 44–51 (97% ee) | MeCN or MeCN/CH2Cl2 |
| 35 | 0.0003 | 35–44% (>99% ee) | - | solvent-free |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidskog, A.; Li, Y.; Wärnmark, K. Asymmetric Ring-Opening of Epoxides Catalyzed by Metal–Salen Complexes. Catalysts 2020, 10, 705. https://doi.org/10.3390/catal10060705
Lidskog A, Li Y, Wärnmark K. Asymmetric Ring-Opening of Epoxides Catalyzed by Metal–Salen Complexes. Catalysts. 2020; 10(6):705. https://doi.org/10.3390/catal10060705
Chicago/Turabian StyleLidskog, Anna, Yutang Li, and Kenneth Wärnmark. 2020. "Asymmetric Ring-Opening of Epoxides Catalyzed by Metal–Salen Complexes" Catalysts 10, no. 6: 705. https://doi.org/10.3390/catal10060705
APA StyleLidskog, A., Li, Y., & Wärnmark, K. (2020). Asymmetric Ring-Opening of Epoxides Catalyzed by Metal–Salen Complexes. Catalysts, 10(6), 705. https://doi.org/10.3390/catal10060705






