Modeling of Urea Decomposition in Selective Catalytic Reduction (SCR) for Systems of Diesel Exhaust Gases Aftertreatment by Finite Volume Method
Abstract
:1. Introduction
2. Results and Discussion
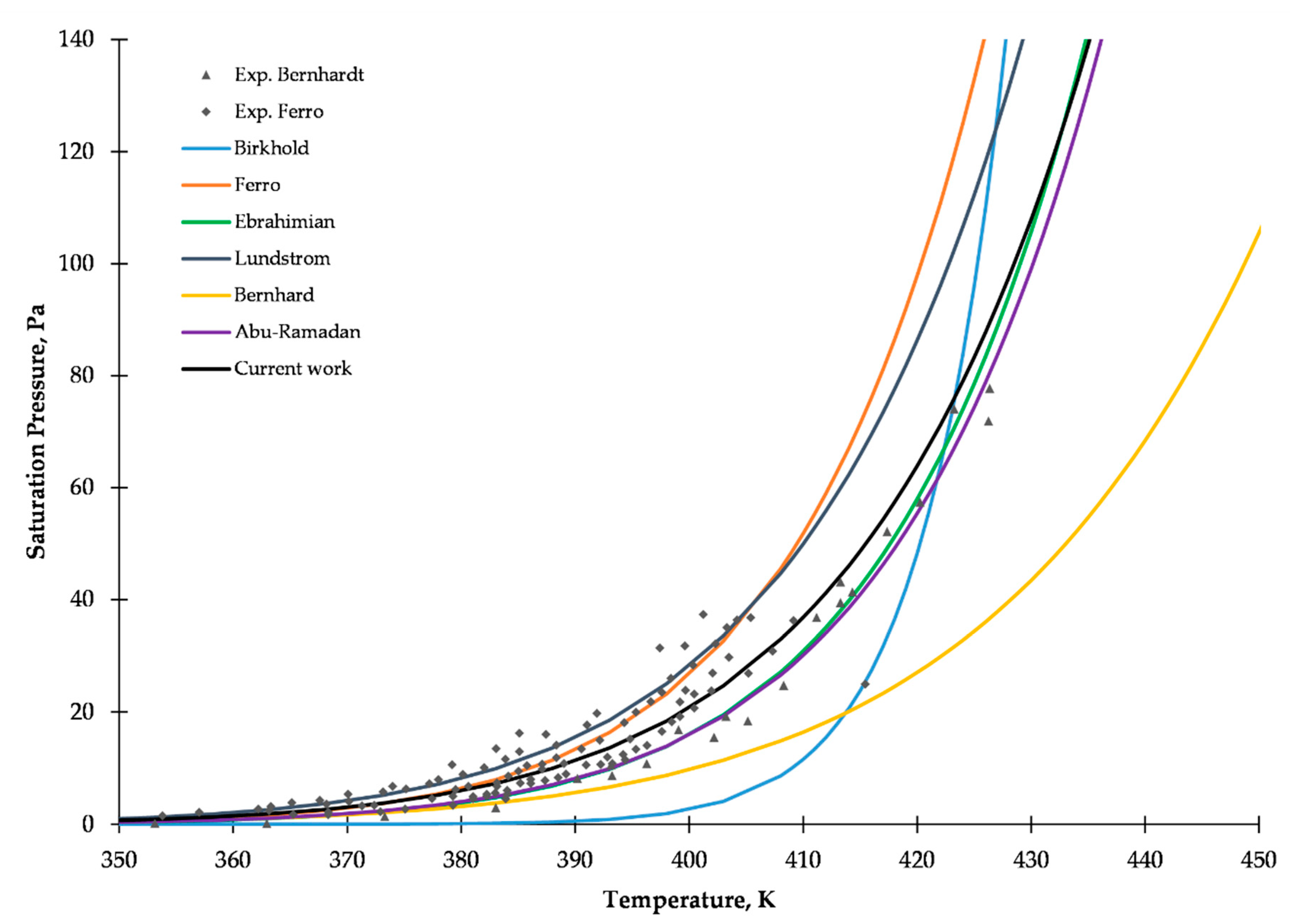
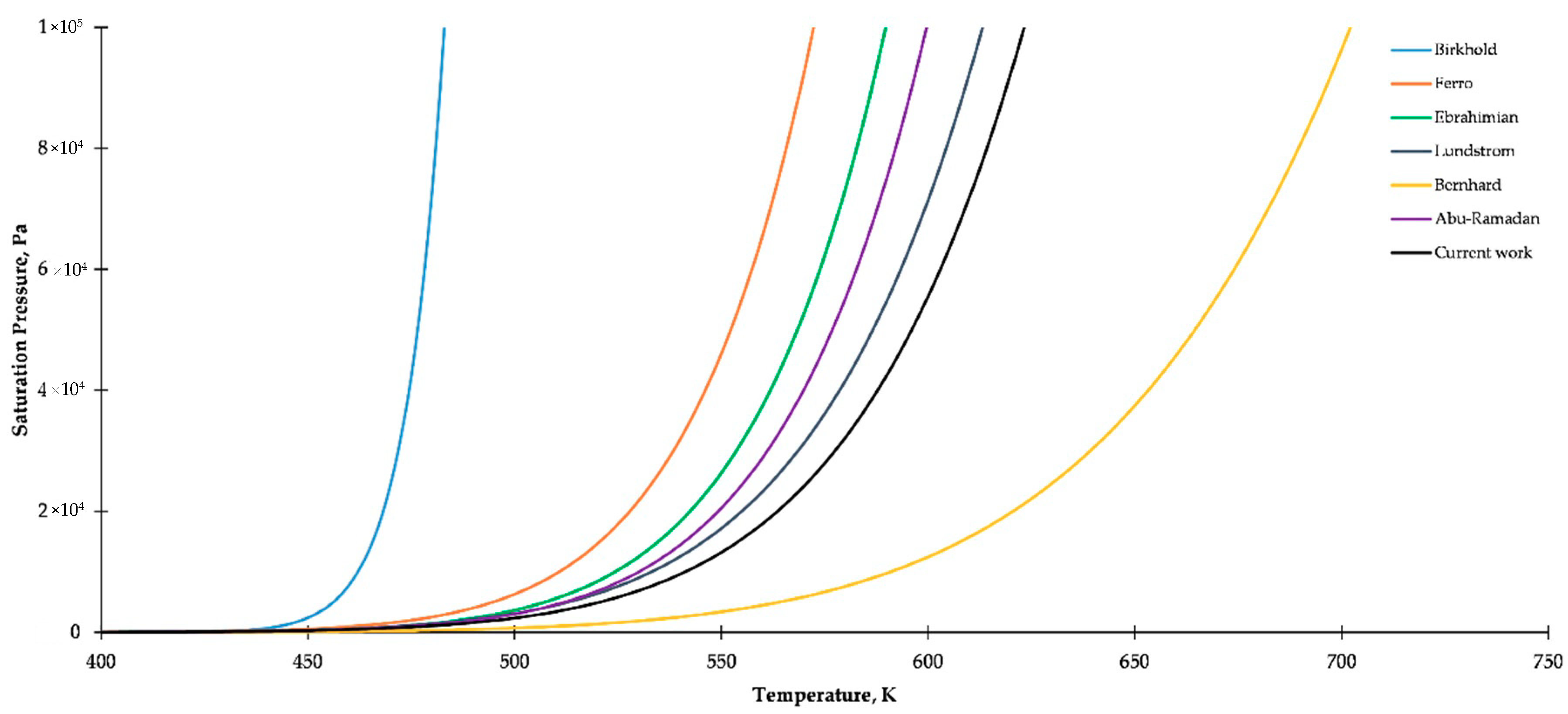
2.1. Saturated Vapor Pressure
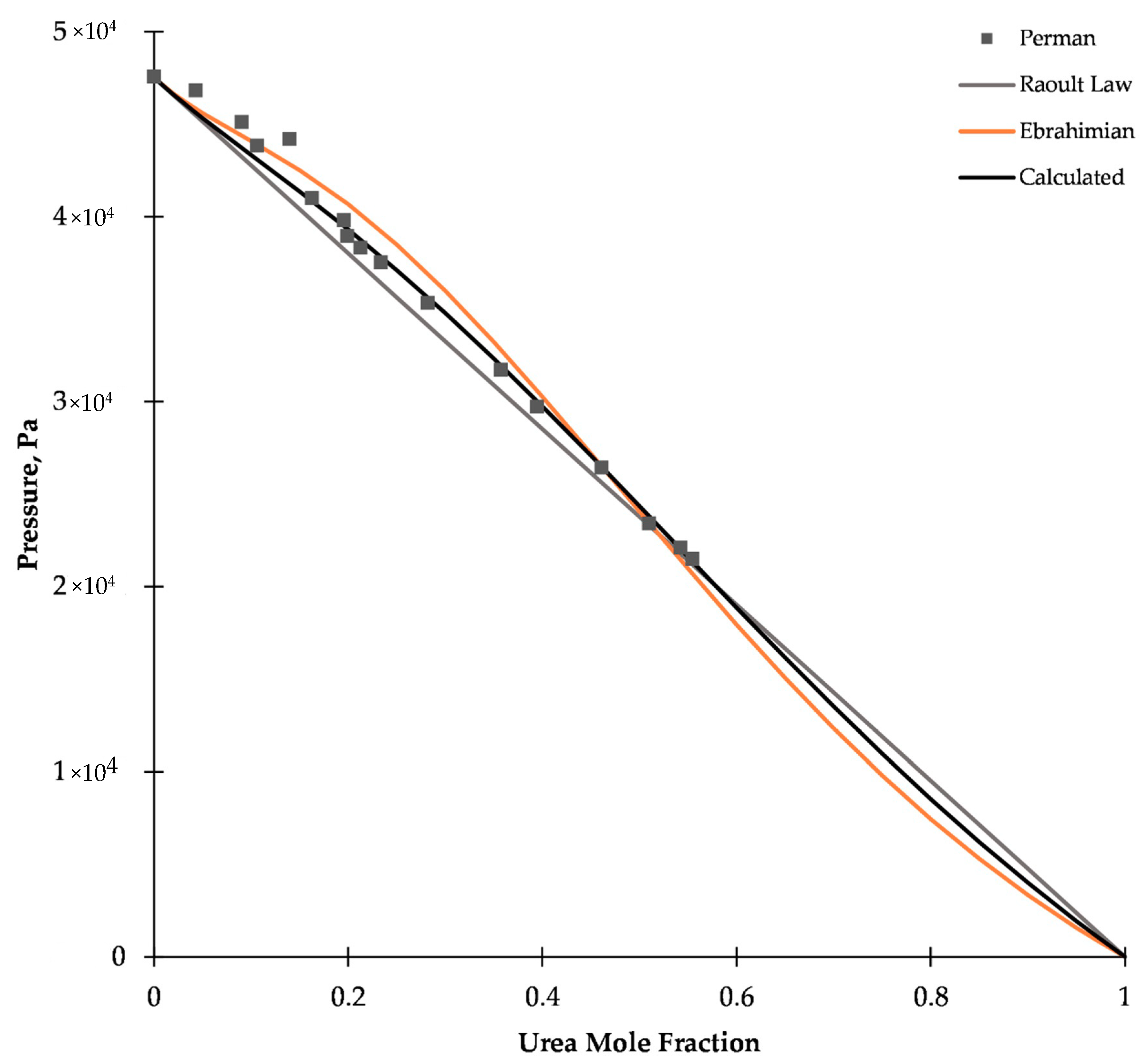
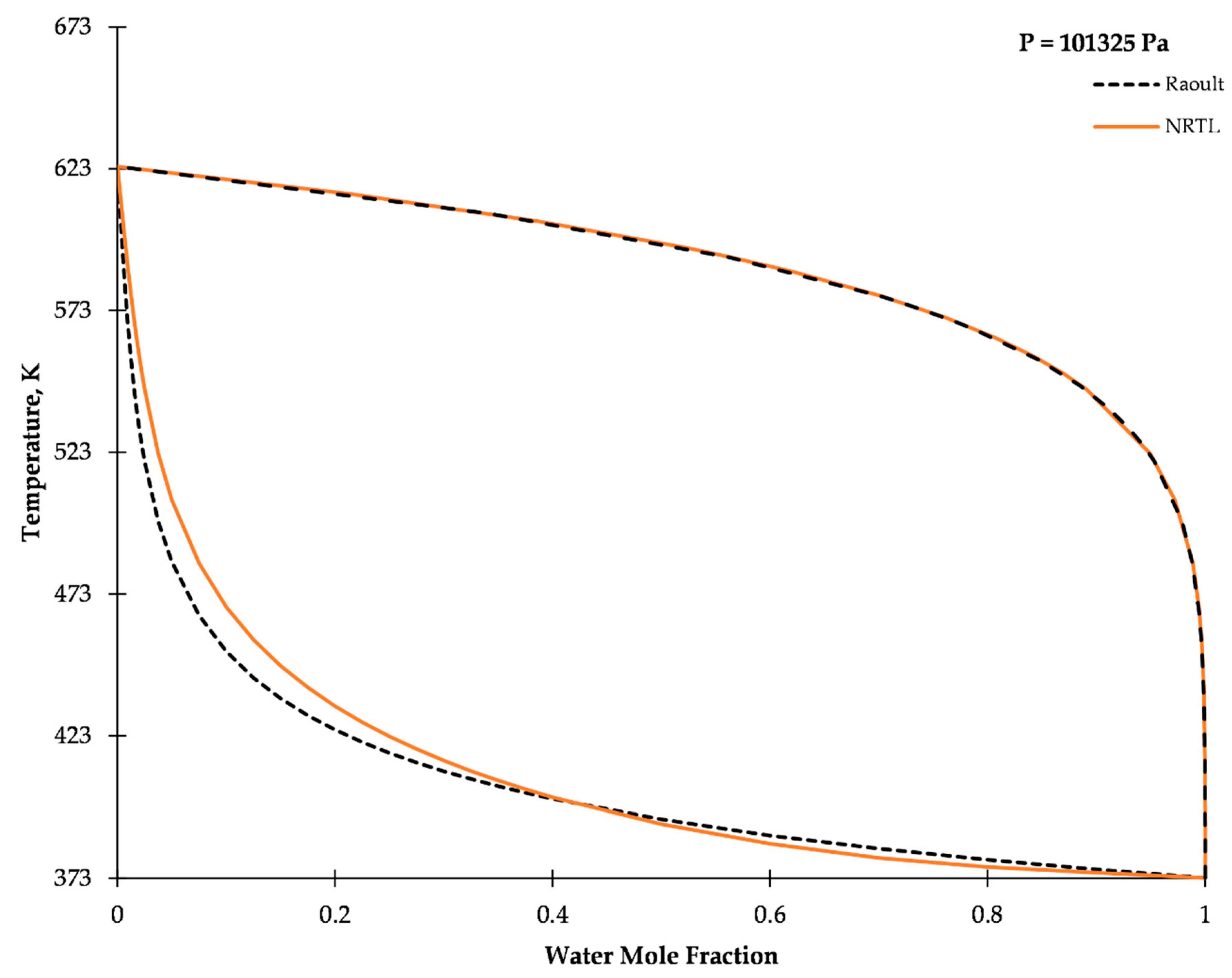
2.2. Vapor–Liquid Equilibrium
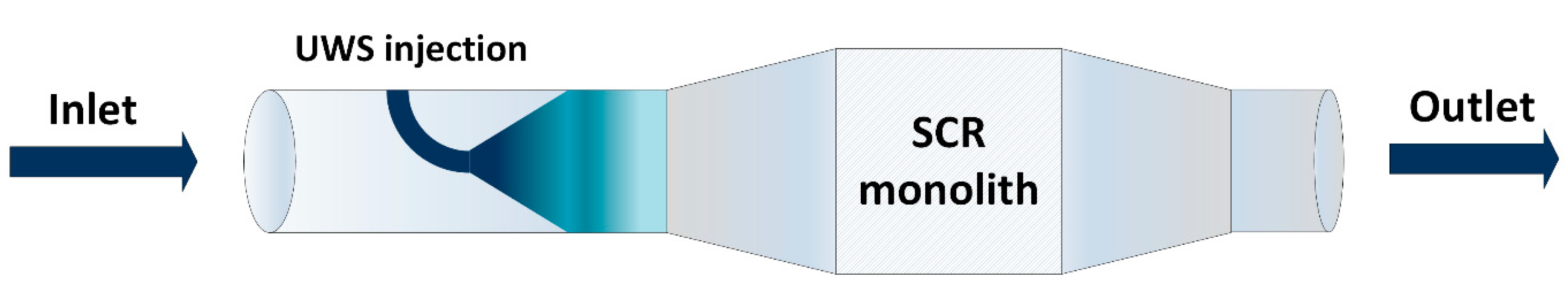
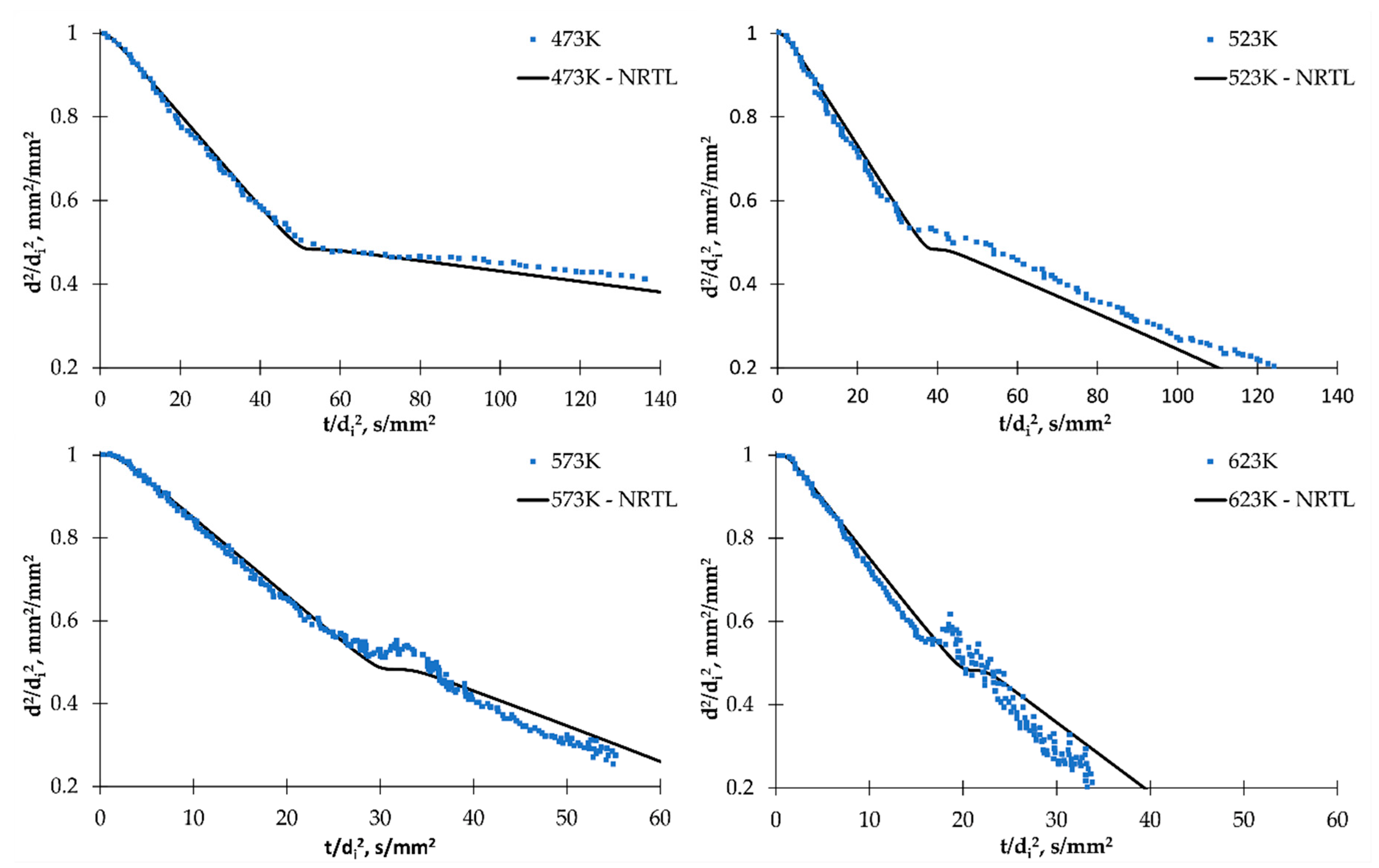
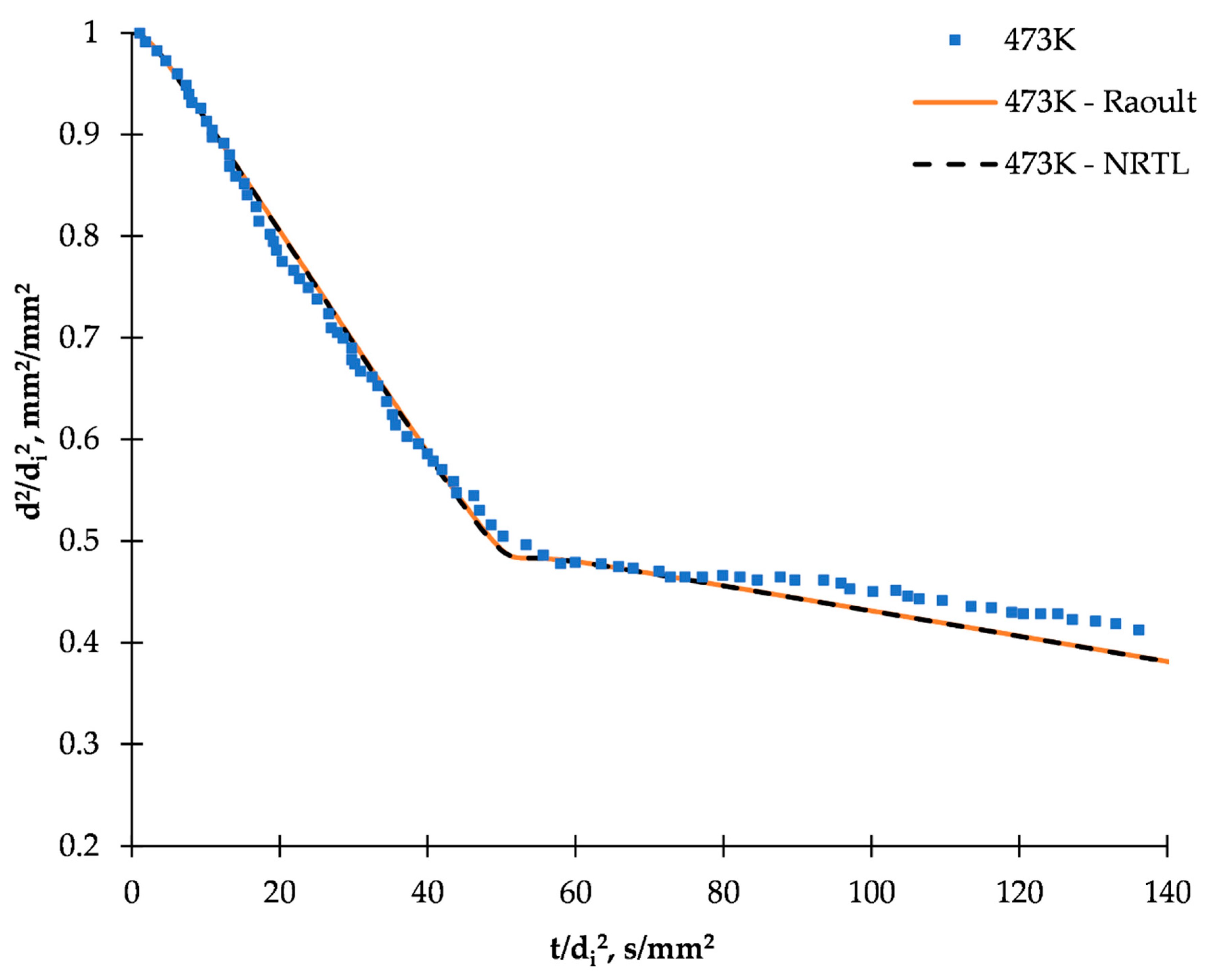
2.3. Simulation and Model Validation
3. Materials
3.1. Material Definition
3.2. Reaction Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Notation
| m | mass (kg) |
| Ad | droplet surface area (m2) |
| ρ | density (kg/m3) |
| Mw | molecular weight (kg/kmol) |
| Sh | Schmidt number |
| Nu | Nusselt number |
| D | diffusion coefficient (m2/s) |
| cp | specific heat (J/kg) |
| μ | kinematic viscosity (Pa·s) |
| k | thermal conductivity (W/(m·K)) |
| d | diameter (m) |
| Bm | Spalding mass transfer number |
| BT | Spalding heat transfer number |
| Y | mass fraction |
| x | molar fraction |
| P | pressure (Pa) |
| ΩD | diffusion collision integral |
| σ | Lennard–Jones length (Angstrom) |
| T | temperature (K) |
| hf | latent heat (J/kg) |
| A | pre-exponential factor (1/s) |
| Ea | activation energy (kcal/kmol) |
| a, b, c | binary interaction coefficients |
| A, B, C | Antoine equation parameters |
| α | nonrandomness constant for binary interactions |
| γ | activity coefficient |
| τ | dimensionless interaction parameter |
| G | dimensionless interaction parameter |
Subscripts
| g | gas |
| d | droplet |
| i, j | indices of species |
| s | surface |
| ∞ | ambient |
| abs | absolute |
| sat | saturation |
| bp | boiling point |
| ref | reference |
| v | vapor |
| H2O | water |
| T | temperature |
| Y | mass fraction |
| 0 | initial |
References
- Final Rule for Control of Emissions of Air Pollution from 2004 and Later Model Year Heavy-Duty Highway Engines and Vehicles; Revision of Light-Duty On-Board Diagnostics Requirements. Federal Register; Volume 65, No. 195; 6 October 2000; Rules and Regulations. Available online: https://www.epa.gov/regulations-emissions-vehicles-and-engines/final-rule-control-emissions-air-pollution-2004-and-later (accessed on 13 May 2020).
- Kröcher, O.; Elsener, M.; Jacob, E. A model gas study of ammonium formate, methanamide and guanidinium formate as alternative ammonia precursor compounds for the selective catalytic reduction of nitrogen oxides in diesel exhaust gas. Appl. Catal. B Environ. 2009, 88, 66–82. [Google Scholar] [CrossRef]
- Johnson, T. Review of diesel emissions and control. SAE Int. J. Fuels Lubr. 2010, 3, 16–29. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K. Energy Industry. Phys. Sci. Rev. 2018, 3, 20180023. [Google Scholar]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Ström, H.; Lundström, A.; Andersson, B. Choice of urea-spray models in CFD simulations of urea-SCR systems. Chem. Eng. J. 2009, 150, 69–82. [Google Scholar] [CrossRef]
- Ström, L.; Ström, H.; Darnell, A.; Carlsson, P.; Skoglundh, M.; Härelind, H. Quantification of Urea-spray Non-uniformity Effects on the H2-assistedNO Reduction and NH3 Slip over an Ag/Al2O3 Catalyst. Energy Procedia 2015, 75, 2317–2322. [Google Scholar] [CrossRef] [Green Version]
- Brack, W.; Heine, B.; Birkhold, F.; Kruse, M.; Schoch, G.; Tischer, S.; Deutschmann, O. Kinetic modeling of urea decomposition based on systematic thermogravimetric analyses of urea and its most important by-products. Chem. Eng. Sci. 2014, 106, 1–8. [Google Scholar] [CrossRef]
- Birkhold, F.; Meingast, U.; Wassermann, P.; Deutschmann, O. Analysis of the Injection of Urea-Water-Solution for Automotive SCR DeNOx-Systems: Modeling of Two-Phase Flow and Spray/Wall-Interaction. SAE Tech. Paper 2006, 1, 0643. [Google Scholar]
- Birkhold, F. Selektive Katalytische Reduktion von Stickoxiden: Untersuchung der Einspritzung von Harnstoffwasserlösung. Ph.D. Thesis, Fakultät für Maschinenbau, Universität Karlsruhe, Karlsruhe, Germany, 2007. [Google Scholar]
- Abu-Ramadan, E.; Saha, K.; Li, X. Modeling the Depleting Mechanism of Urea-Water-Solution Droplet for Automotive Selective Catalytic Reduction System. AIChE J. 2011, 57, 3210–3225. [Google Scholar] [CrossRef]
- Lundström, A.; Waldheim, B.; Ström, H.; Westerberg, B. Modelling of urea gas phase thermolysis and theoretical details on urea evaporation. Proc. Inst. Mech. Eng. D-J. Automob. 2011, 225, 1392–1398. [Google Scholar] [CrossRef]
- Ebrahimian, V.; Nicolle, A.; Habchi, C. Detailed modeling of the evaporation and thermal decomposition of urea-water solution in SCR Systems. AIChE J. 2012, 58, 1998–2009. [Google Scholar] [CrossRef] [Green Version]
- Ferro, D.; Barone, G.; Della Gatta, G.; Piacente, V. Vapour pressures and sublimation enthalpies of urea and some of its derivatives. J. Chem. Thermodyn. 1987, 19, 915–923. [Google Scholar] [CrossRef]
- Bernhard, M.; Czekaj, I.; Elsener, M.; Wokaun, A.; Krӧcher, O. Evaporation of urea at atmospheric pressure. J. Phys. Chem. A 2011, 115, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Perman, E.P.; Lovett, T. Vapour pressure and heat dilution of aqueous solutions. Trans. Faraday Soc. 1926, 22, 1–19. [Google Scholar] [CrossRef]
- Wang, T.J.; Baek, S.W.; Lee, S.Y.; Kang, D.H.; Yeo, G.K. Experimental investigation on evaporation of urea–water-solution droplet for SCR applications. AIChE J. 2009, 55, 3267–3276. [Google Scholar] [CrossRef]
- Yaws, C.L. Yaws’ Critical Property Data for Chemical Engineers and Chemists; Knovel: New York, NY, USA, 2012. [Google Scholar]
- Yaws, C.L. Yaws’ Thermophysical Properties of Chemicals and Hydrocarbons; Knovel: New York, NY, USA, 2010. [Google Scholar]
- Yaws, C.L. Yaws’ Handbook of Properties of the Chemical Elements; Knovel: New York, NY, USA, 2011. [Google Scholar]
- Available online: http://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe?component=Water (accessed on 13 May 2020).
- Emelyaneko, V.N.; Kabo, G.J.; Verevkin, S.P. Measurement and prediction of thermochemical properties: Improved increments for the estimation of enthalpies of sublimation and standard enthalpies of formation of akryl derivatives of urea. J. Chem. Eng. Data 2006, 51, 79–87. [Google Scholar] [CrossRef]
- Yim, S.D.; Kim, S.J.; Baik, J.H.; Nam, I.; Mok, Y.S.; Lee, J.H.; Cho, B.K.; Oh, S.H. Decomposition of urea into NH3 for the SCR process. Ind. Eng. Chem. Res. 2004, 43, 4856–4863. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kasipandi, S.; Kim, J.; Kang, S.-H.; Kim, J.-H.; Ryu, J.-H.; Bae, J.-W. Current Catalyst Technology of Selective Catalytic Reduction (SCR) for NOx Removal in South Korea. Catalysts 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]

| Parameter | A | B | C |
|---|---|---|---|
| Value | 4.603 | 4115 | 0 |
| Parameter | aij | aji | bij | bji | α | cij |
|---|---|---|---|---|---|---|
| Value | 2.538 | −1.430 | −2.891 | 0.539 | 0.3 | 0 |
| Parameter | Gas Phase | Droplet (Liquid Phase) | ||
|---|---|---|---|---|
| Density | (19) | (20) | ||
| Viscosity | (21) | (22) | ||
| Thermal conductivity | (23) | (24) | ||
| Specific heat | (25) | (26) | ||
| Diffusion coefficient | (27) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuternowski, F.; Staszak, M.; Staszak, K. Modeling of Urea Decomposition in Selective Catalytic Reduction (SCR) for Systems of Diesel Exhaust Gases Aftertreatment by Finite Volume Method. Catalysts 2020, 10, 749. https://doi.org/10.3390/catal10070749
Kuternowski F, Staszak M, Staszak K. Modeling of Urea Decomposition in Selective Catalytic Reduction (SCR) for Systems of Diesel Exhaust Gases Aftertreatment by Finite Volume Method. Catalysts. 2020; 10(7):749. https://doi.org/10.3390/catal10070749
Chicago/Turabian StyleKuternowski, Filip, Maciej Staszak, and Katarzyna Staszak. 2020. "Modeling of Urea Decomposition in Selective Catalytic Reduction (SCR) for Systems of Diesel Exhaust Gases Aftertreatment by Finite Volume Method" Catalysts 10, no. 7: 749. https://doi.org/10.3390/catal10070749






