High Active PdSn Binary Alloyed Catalysts Supported on B and N Codoped Graphene for Formic Acid Electro-Oxidation
Abstract
:1. Introduction
2. Results and Discussion
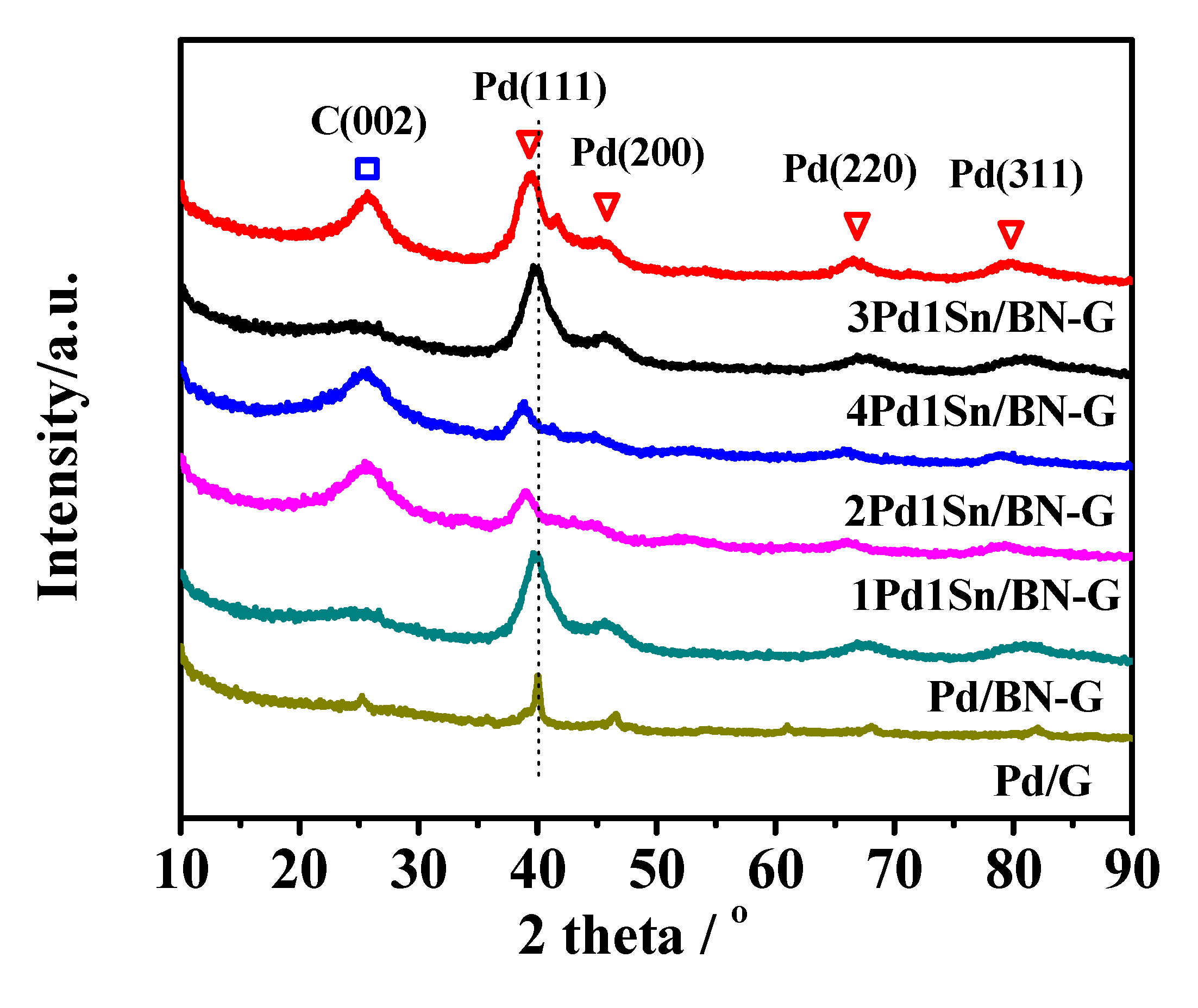
2.1. Structrural Analysis
2.2. Electrochemical Characterization
2.3. XPS Analysis
3. Materials and Methods
3.1. Synthesis of BN-G Supporting Materials
3.2. Synthesis of PdSn/BN-G Binary Catalysts
3.3. Materials Characterization
3.4. Electrocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Sources 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Meng, H.; Zeng, D.R.; Xie, F.Y. Recent development of Pd-based electrocatalysts for proton exchange membrane fuel cells. Catalysts 2015, 5, 1221–1274. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Zhang, H.Y.; Dai, Y.X.; Zhang, F.Q.; Wu, P.S.; Wu, P.; Tang, Y.W. Sacrificial template-based synthesis of unified hollow porous palladium nanospheres for formic acid electro-oxidation. Catalysts 2015, 5, 992–1002. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Liu, Z.; Liu, X.R.; Liu, B.; Liu, J.; Deng, Y.D.; Han, X.P.; Hu, W.B.; Zhong, C. Tunable periodically ordered mesoporosity in palladium membranes enables exceptional enhancement of intrinsic electrocatalytic activity for formic acid oxidation. Angew. Chem. Int. Ed. 2020, 132, 5130–5139. [Google Scholar] [CrossRef]
- Mondal, S.; Raj, C.R. Electrochemical dealloying-assissted surface-engineered Pd-based bifunctional electrocatalyst for formic acid oxidation and oxygen reduction. ACS Appl. Mater. Interfaces 2019, 11, 14110–14119. [Google Scholar] [CrossRef]
- Cai, B.F.; Ma, Y.R.; Wang, S.Z.; Yi, N.; Zheng, Y.; Qiu, X.Y.; Tang, Y.W.; Bao, J.C. Facile synthesis of PdFe alloy tetrahedrons for boosting electrocatalytic properties towards formic acid oxidation. Nanoscale 2019, 11, 18015–18020. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Gong, Y.Y.; Wu, D.B.; Wu, G.L.; Xu, B.H.; Bi, L.; Yuan, W.Y.; Cui, Z.M. Twisted palladium-copper nanochains toward efficient electrocatalytic oxidation of formic acid. J. Colloid Interface Sci. 2019, 537, 366–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.L.; Shao, Q.; Feng, Y.G.; Xiong, L.K.; Peng, Y.; Huang, X.Q. Defect engineering of palladium-tin nanowires enables efficient electrocatalysts for fuel cell reactions. Nano Lett. 2019, 19, 6894–6903. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Yan, B.; Wang, J.; Wang, C.Q.; Li, S.M.; Gu, Z.L.; Du, Y.K.; Yang, P. Ultra-uniform PdBi nanodots with high activity towards formic acid oxidation. J. Power Sources 2017, 356, 27–35. [Google Scholar] [CrossRef]
- Du, W.X.; Mackenzie, K.E.; Milano, D.F.; Deskins, N.A.; Su, D.; Teng, X.W. Palladium-tinalloyed catalysts for the ethanol oxidation reaction in an alkaline medium. ACS Catal. 2012, 2, 287–297. [Google Scholar] [CrossRef]
- Adam, A.M.M.; Zhu, A.M.; Ning, L.N.; Deng, M.; Zhang, Q.G.; Liu, Q.L. Carbon supported PdSn nanocatalysts with enhanced performance for ethanol electrooxidation in alkaline medium. Int. J. Hydrog. Energy 2019, 44, 20368–20378. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.; Tsiakaras, P. Carbon-supported PdSn and Pd3Sn2 anodes for glucose electrooxidation in alkaline media. Appl. Catal. B Environ. 2014, 158–159, 209–216. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ge, J.J.; Ma, L.; Liao, J.H.; Lu, T.H.; Xing, W. Highly active carbon-supported PdSn catalysts for formic acid electrooxidation. Fuel Cells 2009, 9, 114–120. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, X.H. Carbon-supported PdSn nanoparticles as catalysts for formic acid oxidation. Electrochem. Commun. 2009, 11, 1667–1670. [Google Scholar] [CrossRef]
- Tu, D.D.; Wu, B.; Wang, B.X.; Deng, C.; Gao, Y. A highly active carbon-supported PdSn catalyst for formic acid electrooxidation. Appl. Catal. B Environ. 2011, 103, 163–168. [Google Scholar]
- Asgardi, J.; Calderón, J.C.; Alcaide, F.; Querejeta, A.; Calvillo, L.; Lázaro, M.J.; García, G.; Pastor, E. Carbon monoxide and ethanol oxidation on PtSn supported catalysts: Effect of the nature of the carbon support and Pt:Sn composition. Appl. Catal. B Environ. 2015, 168–169, 33–41. [Google Scholar] [CrossRef]
- Juárez-Marmolejo, L.; Pérez-Rodríguez, S.; Montes de Oca-Yemha, M.G.; Palomar-Pardavé, M.; Romero-Romo, M.; Ezeta-Mejía, A.; Morales-Gil, P.; Martínez-Huerta, M.V.; Lázaro, M.J. Carbon supported PdM (M=Fe, Co) electrocatalysts for formic acid oxidation. Influence of the Fe and Co precursors. Int. J. Hydrog. Energy 2019, 44, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Mikolajczuk-Zychora, A.; Mierzwa, B.; Borodzinski, A.; Stobinski, L. A simple method for enhancing the catalytic activity of Pd deposited on carbon nanotubes used in direct formic acid fuel cells. Appl. Surf. Sci. 2019, 476, 806–814. [Google Scholar] [CrossRef]
- Kankla, P.; Limtrakul, J.; Green, M.L.H.; Chanlek, N.; Luksirikul, P. Electrooxidation of formic acid enhanced by surfactant-free palladium nanocubes on surface modified graphene catalyst. Appl. Surf. Sci. 2019, 471, 176–184. [Google Scholar] [CrossRef]
- Liu, M.M.; Zhang, R.Z.; Chen, W. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef]
- Li, F.; Shu, H.B.; Liu, X.T.; Shi, Z.Y. Electrocatalytic activity and design principles of heteroatom-doped graphene catalysts for oxygen-reduction reaction. J. Phys. Chem. C 2017, 121, 14434–14442. [Google Scholar] [CrossRef]
- Chai, G.L.; Qiu, K.P.; Qiao, M.; Titirici, M.M.; Shang, C.X.; Guo, Z.X. Active sites engineering leads to exceptional ORR and OER bifunctionality in P, N codoped graphene frameworks. Energy Environ. Sci. 2017, 10, 1186–1195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Zhu, J.X.; Tiwary, C.S.; Ma, Z.Y.; Huang, H.J.; Zhang, J.F.; Lu, Z.Y.; Huang, W.; Wu, Y.P. Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interf. 2016, 8, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Li, S.W.; Feng, F.; Ou, S.Y.; Li, F.; Yang, M.; Qian, K.L.; Jin, J.; Ma, J.T. Palladium nanoparticles with surface enrichment of palladium oxide species immobilized on the aniline-functionalized graphene as an advanced electrocatalyst of ethanol oxidation. ACS Sustain. Chem. Eng. 2019, 7, 14621–14628. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Maiyalagan, T. Enhanced electro-catalytic activity of nitrogen-doped reduced graphene oxide supported PdCu nanoparticles for formic acid electro-oxidation. Int. J. Hydrog. Energy 2019, 44, 14808–14819. [Google Scholar] [CrossRef]
- Zhu, F.C.; Wang, M.; He, Y.W.; Ma, G.S.; Zhang, Z.H.; Wang, X.G. High activity of carbon nanotubes supported binary and ternary Pd-based catalysts for methanol, ethanol and formic acid electro-oxidation. J. Power Sources 2013, 242, 610–620. [Google Scholar] [CrossRef]
- Fontes, E.H.; Ramos, C.E.D.; Nandenha, J.; Piasentin, R.M.; Neto, A.O.; Landers, R. Structural analysis of PdRh/C and PdSn/C and its use as electrocatalysts for ethanol oxidation in alkaline medium. Int. J. Hydrog. Energy 2019, 44, 937–951. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, H.J.; Shen, B.F.; Jin, L.; Jiang, Q.G.; Yang, L.; He, H.Y. Anchoring nanosized Pd on three-dimensional boron- and nitrogen-codoped graphene aerogels as a highly active multifunctional electrocatalyst for formic acid and methanol oxidation reactions. Inorg. Chem. Front. 2020, 7, 700–708. [Google Scholar] [CrossRef]
- Geraldes, A.N.; Silva, D.F.; Silva, J.C.M.; Sá, O.A.; Spinacé, E.V.; Neto, A.O.; Santos, M.C. Palladium and palladium-tin supported on multi wall carbon nanotubes or carbon for alkaline direct ethanol fuel cell. J. Power Sources 2015, 275, 189–199. [Google Scholar] [CrossRef]
- Wang, W.; Kang, Y.M.; Yang, Y.; Liu, Y.Q.; Chai, D.; Lei, Z.Q. PdSn alloy supported on phenanthroline-functionalized carbon as highly active electrocatalysts for glycerol oxidation. Int. J. Hydrog. Energy 2016, 41, 1272–1280. [Google Scholar] [CrossRef]
- Suo, Y.G.; Zhuang, L.; Lu, J.T. First-principles considerations in design of Pd-alloy catalysts for oxygen reduction. Angew. Chem. Int. Ed. 2007, 46, 2862–2864. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.R.; Du, C.Y.; Han, G.K.; Qu, Y.T.; Du, L.; Wang, Y.J.; Chen, G.Y.; Gao, Y.Z.; Yin, G.P. Boron, nitrogen co-doped graphene: A superior electrocatalyst support and enhancing mechanism for methanol electrooxidation. Electrochim. Acta 2016, 212, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.C.; Wang, M.; He, Y.W.; Ma, G.S.; Zhang, Z.H.; Wang, X.G. A comparative study of elemental additives (Ni, Co and Ag) on electrocatalytic activity improvement of PdSn-based catalysts for ethanol and formic acid electro-oxidation. Electrochim. Acta 2014, 148, 291–301. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, C.; Wang, J.Y.; Zhai, J.J.; Cai, W.B. Carbon-supported Pd-Pt nanoalloy with low Pt content and superior catalysis for formic acid electro-oxidation. J. Phys. Chem. C 2010, 114, 6446–6451. [Google Scholar] [CrossRef]
- Xu, H.; Yan, B.; Li, S.M.; Wang, J.; Wang, C.Q.; Guo, J.; Du, Y.K. One-pot fabrication of N-doped graphene supported dandelion-like PtRu nanocrystals as efficient and robust electrocatalysts towards formic acid oxidation. J. Colloid Interface Sci. 2018, 512, 96–104. [Google Scholar] [CrossRef]
- Salomé, S.; Ferraria, A.M.; Rego, A.M.B.; Alcaide, F.; Savadogo, O.; Rego, R. Enhanced activity and durability of novel activated carbon-supported PdSn heat-treated cathode catalyst for polymer electrolyte fuel cells. Electrochim. Acta 2016, 192, 268–282. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yao, Q.S.; Wu, X.D.; Fu, Y.S.; Deng, K.M.; Wang, X. Intimately coupled hybrid of graphitic carbon nitride nanoflakelets with reduced graphene oxide for supporting Pd nanoparticles: A stable nanocatalyst with high catalytic activity towards formic acid and methanol electrooxidation. Electrochim. Acta 2016, 200, 131–141. [Google Scholar] [CrossRef]
- Liu, X.; Bu, Y.F.; Cheng, T.; Gao, W.; Jiang, Q. Flower-like carbon supported Pd-Ni bimetal nanoparticles catalyst for formic acid electrooxidation. Electrochim. Acta 2019, 324, 134816. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, Z.L.; Li, C.M. Formic acid-reduced ultrasmall Pd nanocrystals on graphene to provide superior electrocatalytic activity and stability toward formic acid oxidation. Nano Energy 2015, 11, 71–77. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, S.H.; Jiang, K.; André, T.; Cai, W.B. In situ spectroscopic investigation of CO accumulation and poisoning on Pd black surfaces in concentrated HCOOH. J. Power Sources 2012, 199, 165–169. [Google Scholar] [CrossRef]
- Hu, S.Z.; Munoz, F.; Noborikawa, J.; Haan, J.; Scudiero, L.; Ha, S. Carbon supported Pd-based bimetallic and trimetallic catalyst for formic acid electrochemical oxidation. Appl. Catal. B Environ. 2016, 180, 758–765. [Google Scholar] [CrossRef]
- Zhang, J.W.; Chen, M.S.; Li, H.Q.; Li, Y.J.; Cao, Z.M.; Fang, M.L.; Kuang, Q.; Zheng, J.; Xie, Z.X. Stable palladium hydride as a superior anode electrocatalyst for direct formic acid fuel cells. Nano Energy 2018, 44, 127–134. [Google Scholar] [CrossRef]
- Jin, Y.X.; Han, D.M.; Jia, W.P.; Huang, G.B.; Li, F.; Chen, X.Y.; Li, R.R.; Zheng, M.M.; Gao, W.Y. B-N codoped graphene as a novel support for Pd catalyst with enhanced catalysis for ethanol electrooxidation in alkaline medium. J. Electrochem. Soc. 2017, 164, F638–F644. [Google Scholar] [CrossRef]
- Wu, Y.R.; Wang, C.M.; Zou, L.L.; Huang, Q.H.; Yang, H. Incorporation of cobalt into Pd2Sn intermetallic nanoparticles as durable oxygen reduction electrocatalyst. J. Electroanal. Chem. 2017, 789, 167–173. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Perazzolo, V.; Agnoli, S.; Schneider, O.; Granozzi, G.; Gennaro, A. Metal-support interaction in platinum and palladium nanoparticles loaded on nitrogen-doped mesoporous carbon for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, L.; Zhao, K.; Liu, Z.; Zhang, X.S.; Hu, S.Z. Effect of pyridinic- and pyrrolic-nitrogen on electrochemical performance of Pd for formic acid electrooxidation. Electrochim. Acta 2020, 337, 135758. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Sun, G.Q.; Xin, Q. Effect of alloying degree in PtSn catalyst on the catalytic behavior for ethanol electro-oxidation. Electrochim. Acta 2009, 54, 1511–1518. [Google Scholar] [CrossRef]
- Feng, Y.; Bin, D.; Zhang, K.; Ren, F.F.; Wang, J.; Du, Y.K. One-step synthesis of nitrogen-doped graphene supported PdSn bimetallic catalysts for ethanol oxidation in alkaline media. RSC Adv. 2016, 6, 19314–19321. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]





| Catalyst | Crystallite Size/nm | Lattice Parameter/Å | Alloying Degree χ/% | Atomic Ratio Pd:Sn (Nominal) | Atomic Ratio Pd:Sn (ICP) |
|---|---|---|---|---|---|
| 4Pd1Sn/BN-G | 4.8 | 3.920 | 19.33 | 4:1 | 4.2:1 |
| 3Pd1Sn/BN-G | 4.5 | 3.928 | 26.36 | 3:1 | 3.1:1 |
| 2Pd1Sn/BN-G | 5.0 | 3.933 | 30.75 | 2:1 | 2.1:1 |
| 1Pd1Sn/BN-G | 5.9 | 3.947 | 43.05 | 1:1 | 1.2:1 |
| Pd/BN-G | 7.6 | 3.918 | - | - | - |
| 3Pd1Sn/G | 8.8 | 3.922 | - | 3:1 | 3.2:1 |
| Pd/G | 10.2 | 3.898 | - | - | - |
| Samples | B.E. of Pd3d5/2/eV | B.E. of Pd3d3/2/eV | Species | at% | B.E. of Sn3d5/2 /eV | B.E. of Sn3d5/2/eV | Species | at% |
|---|---|---|---|---|---|---|---|---|
| 3Pd1Sn/G | 335.71 | 340.96 | Pd0 | 57 | 485.06 | 493.46 | Sn0 | 10 |
| 337.61 | 342.86 | Pd(II) | 25 | 486.86 | 495.31 | Sn(II) | 90 | |
| 338.46 | 343.96 | Pd(IV) | 18 | - | - | - | - | |
| 3Pd1Sn/BN-G | 335.66 | 340.91 | Pd0 | 61 | 485.21 | 493.66 | Sn0 | 5 |
| 336.81 | 342.16 | Pd(II) | 26 | 486.91 | 495.36 | Sn(II) | 95 | |
| 338.26 | 343.61 | Pd(IV) | 13 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Pei, S.; He, Z.; Shao, H.; Wang, J.; Wang, K.; Wang, Y.; Jin, Y. High Active PdSn Binary Alloyed Catalysts Supported on B and N Codoped Graphene for Formic Acid Electro-Oxidation. Catalysts 2020, 10, 751. https://doi.org/10.3390/catal10070751
Chen D, Pei S, He Z, Shao H, Wang J, Wang K, Wang Y, Jin Y. High Active PdSn Binary Alloyed Catalysts Supported on B and N Codoped Graphene for Formic Acid Electro-Oxidation. Catalysts. 2020; 10(7):751. https://doi.org/10.3390/catal10070751
Chicago/Turabian StyleChen, Dan, Shien Pei, Zhishun He, Haibo Shao, Jianming Wang, Kai Wang, Yong Wang, and Yanxian Jin. 2020. "High Active PdSn Binary Alloyed Catalysts Supported on B and N Codoped Graphene for Formic Acid Electro-Oxidation" Catalysts 10, no. 7: 751. https://doi.org/10.3390/catal10070751




