Effect of La2O3 as a Promoter on the Pt,Pd,Ni/MgO Catalyst in Dry Reforming of Methane Reaction
Abstract
1. Introduction
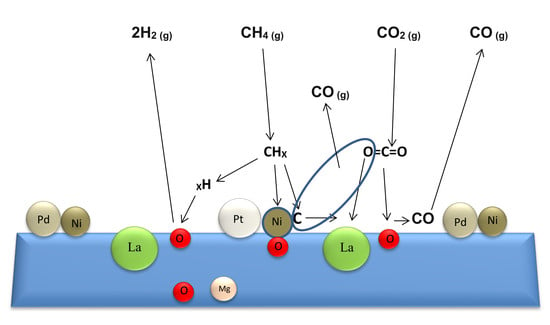
2. Results and Discussion
2.1. Characterization of the Catalysts
2.1.1. XRD Patterns
2.1.2. FT-IR Spectra
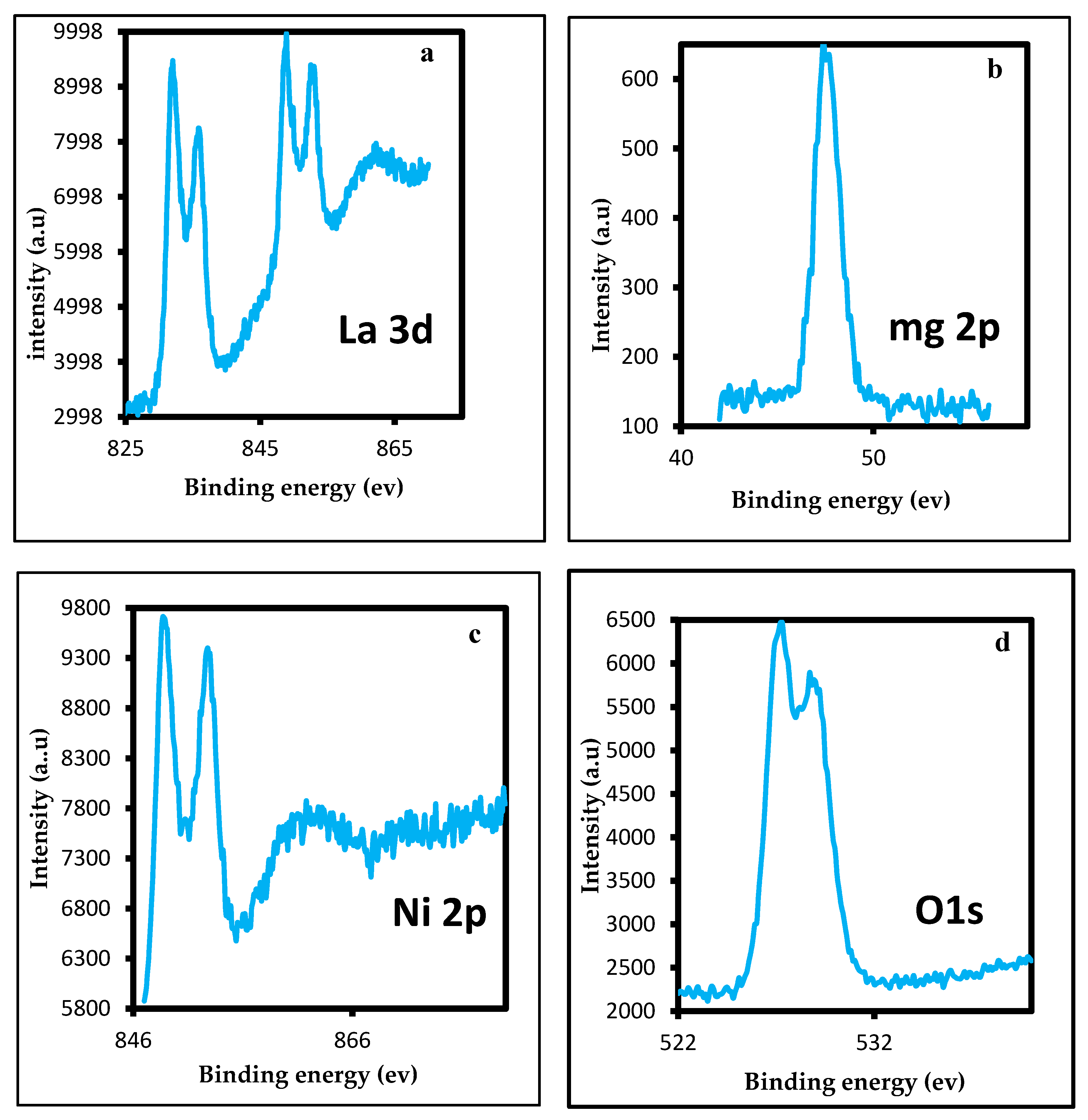
2.1.3. XPS Analysis
2.1.4. Temperature Programmed Reduction (H2-TPR)
2.1.5. Brunauer–Emmett–Teller (BET) Surface Area
2.1.6. TEM Characterization
2.1.7. Thermal Analysis
2.2. Catalytic Performance in Biogas Reforming
2.2.1. Effects of Reactant Concentration on Conversion
2.2.2. Effects of Concentration of the Catalyst on Conversion
2.2.3. Effects of Temperature on Conversion
2.2.4. Stability Tests
2.2.5. Post-Reaction Characterization
3. Experimental Section
3.1. Materials
3.2. Preparation of Catalysts
3.3. Characterization of the Catalysts
3.4. Catalytic Evaluations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning carbon dioxide into fuel. Philos. Trans. R. Soc. A 2010, 368, 3346–3364. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulose agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M.; Balat, H. Biowastes to biofuels. Energy Convers. Manag. 2011, 52, 1815–1828. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry reforming of methane has no future for hydrogen Hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Djinovic, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh-CeO2 catalyst. Int. J. Hydrogen Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- Chen, J.; Yao, C.; Zhao, Y.; Jia, P. Synthesis gas production from dry reforming of methane over Ce0.75Zr0.25O2-supported Ru catalysts. Int. J. Hydrogen Energy 2010, 35, 1630–1642. [Google Scholar] [CrossRef]
- Gaur, S.; Haynes, D.J.; Spivey, J.J. Rh, Ni, and Ca substituted pyrochlore catalysts for dry reforming of methane. Appl. Catal. A 2011, 403, 142–151. [Google Scholar] [CrossRef]
- Gamba, O.; Moreno, S.; Molina, R. Catalytic performance of Ni-Pr supported on delaminated clay in the dry reforming of methane. Int. J. Hydrog. Energy 2011, 36, 1540–1550. [Google Scholar] [CrossRef]
- Gonzalez-Delacruz, V.M.; Perenguez, R.; Ternero, F.; Holgado, J.P.; Caballero, A. In situ XAS study of synergic effects on Ni-Co/ ZrO2 methane reforming catalysts. J. Phys. Chem. C 2012, 116, 2919–2926. [Google Scholar] [CrossRef]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.S. Manganese promoting effects on the Co-Ce-Zr-Ox nano catalysts for methane dry reforming with carbon dioxide to hydrogen and carbon monoxide. Chem. Eng. J. 2011, 170, 457–463. [Google Scholar] [CrossRef]
- Djokovic, P.; Crniveca, I.O.; Erjaveca, B.; Pintara, A. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni-Co bimetallic catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar]
- Al-Doghachi, F.A. Effects of Platinum and Palladium Metals on Ni/Mg1−xZrxO Catalysts in the CO2 Reforming of Methane. Bull. Chem. React. Eng. Catal. 2018, 13, 295–310. [Google Scholar] [CrossRef]
- Grange, P. Catalytic Hydrodesulfurization. Catal. Rev. Sci. Eng. 1980, 21, 135–181. [Google Scholar] [CrossRef]
- Devaraja, P.B.; Avadhani, D.N.; Prashant, S.C.; Nagabhushana, H.; Sharma, S.C.; Nagabhushana, B.M.; Nagaswarupa, H.P. Synthesis, Structural and Luminescence Studies of Magnesium Oxide Nanopowder. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 847–851. [Google Scholar]
- Hidalgo, C.; Jalila, S.; Alberto, M.; Said, S. XPS Evidence for Structure—Performance Relationship in Selective Hydrogenation of Croton aldehyde to Crotyl Alcohol on Platinum Systems Supported on Natural Phosphates. J. Colloid Interface Sci. 2012, 382, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, E.G.; Pusel, J.; Stagg, W.S.; Faraji, S. The Effects of Pt Addition to Supported Ni Catalysts on Dry (CO2) Reforming of Methane to Syngas. J. CO2 Util. 2014, 6, 40–44. [Google Scholar] [CrossRef]
- Bao, Z.; Lu, Y.; Han, J.; Li, Y.; Yu, F. Highly Active and Stable Ni-based Bimodal Pore Catalyst for Dry Reforming of Methane. Appl. Catal. A 2015, 491, 116–126. [Google Scholar] [CrossRef]
- Roberto, B.S.; Juniora, R.C.; Rabelo, N.S.; Gomes, F.N. Steam reforming of acetic acid over Ni-based catalysts derived from La1−xCaxNiO3 perovskite type oxides. Fuel 2019, 82, 20081–20312. [Google Scholar]
- Al-Doghachi, F.A.; Taufiq-Yap, Y.H. CO2 Reforming of Methane over Ni/MgO Catalysts Promoted with Zr and La Oxides. Chem. Sel. 2018, 3, 816–827. [Google Scholar] [CrossRef]
- Djadja, A.; Libs, S.; Kiennemann, A.; Barama, A. Characterization and Activity in Dry Reforming of Methane on Ni,Mg/Al and Ni/MgO Catalysts. Catal. Today 2006, 113, 194–200. [Google Scholar] [CrossRef]
- Ahmed, W.; Awadallah, A.E.; Aboul, E.A. Ni/CeO2-Al2O3 Catalysts for Methane Thermo-catalytic Decomposition to COx-free H2 Production. Int. J. Hydrogen Energy 2016, 41, 18484–18493. [Google Scholar] [CrossRef]
- Mojović, Z.; Mentus, S.; Tesic, Z. Introduction of Pt and Pd Nanoclusters in Zeolite Cavities by Thermal Degradation of Acetylacetonates. Mater. Sci. Forum 2004, 453, 257–262. [Google Scholar] [CrossRef]
- Al-Doghachi, F.A.; Islam, A.; Zainal, Z.; Saiman, M.I.; Embong, Z.; Taufiq-Yap, Y.H. High Coke-Resistance Pt/Mg1−xNixO Catalyst for Dry Reforming of Methane. PLoS ONE 2016, 11, 145–862. [Google Scholar] [CrossRef]
- Aramouni, N.A.; Zeaiter, J.; Kwapinski, W.; Leahy, J.J.; Ahmad, M.N. Eclectic trimetallic Ni–Co–Ru catalyst for the dry reforming of methane. Int. J. Hydrog. Energy 2020. In Press. [Google Scholar] [CrossRef]
- Kehres, J.; Jakobsen, J.G.; Andreasen, J.W.; Wagner, J.B.; Liu, H.; Molenbroek, A.; Vegge, T. Dynamical Properties of a Ru/MgAl2O4 Catalyst During Reduction and Dry Methane Reforming. J. Phys. Chem. 2012, 116, 21407–21415. [Google Scholar] [CrossRef]
- Al-Doghachi, F.A.; Rashid, U.; Taufiq-Yap, Y.H. Investigation of Ce (III) Promoter Effects on the Tri-metallic Pt,Pd,Ni/MgO Catalyst in Dry-reforming of Methane. RSC Adv. 2016, 6, 10372–10384. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; Leitenburg, C.; Giona, M.A. Model for the Temperature—Programmed Reduction of Low and High Surface Area Ceria. Catalysis 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Steinhauer, B.; Kasireddy, M.; Radnik, J.; Martin, A. Development of Ni-Pd Bimetallic Catalysts for the Utilization of Carbon Dioxide and Methane by Dry Reforming. Appl. Catal. A 2009, 366, 333–341. [Google Scholar] [CrossRef]
- Istadi, I.; Anggoro, D.D.; Amin, N.S.; Ling, D.W. Catalyst deactivation simulation through carbon deposition in carbon dioxide reforming over Ni/CaO-Al2O3 catalyst. Bull. Chem. React. Eng. Catal. 2011, 6, 129–136. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Xiao, P.; Liu, D.; Zou, C.J. Structure and Catalytic Performance of Mg-SBA-15-Supported Nickel Catalysts for CO2 Reforming of Methane to Syngas. Chem. Eng. Technol. 2013, 36, 1701–1707. [Google Scholar]
- Al-Doghachi, F.A.; Rashid, U.; Zainal, Z.; Saiman, M.I.; Taufiq-Yap, Y.H. Influence of Ce2O3 and CeO2 Promoterson Pd/MgO Catalysts in the Dry-reforming of Methane. RSC Adv. 2015, 5, 81739–81752. [Google Scholar] [CrossRef]
- Al-Doghachi, F.A.; Islam, A.; Zainal, Z.; Saiman, M.I.; Embong, Z.; Taufiq-Yap, Y.H. Hydrogen Production from Dry-Reforming of Biogas over Pt/Mg1−xNixO Catalysts. Energy Procedia 2015, 79, 18–25. [Google Scholar] [CrossRef]












| Catalysts | TEM (nm) | a Crystal Size (D) | XRF | |||
|---|---|---|---|---|---|---|
| Debye–Sherrer Eq. (nm) | Ni% | Pd% | Pt% | Mg and La% | ||
| Pt,Pd,Ni/MgO | 42.1 | 49 | 1.03 | 0.96 | 1.14 | 96.31 |
| Pt,Pd,Ni/Mg0.97La3+0.03O3 | 44.6 | 66 | 0.95 | 1.05 | 0.93 | 95.93 |
| Pt,Pd,Ni/Mg0.93La3+0.07O3 | 40.4 | 52 | 1.11 | 1.21 | 0.99 | 96.15 |
| Pt,Pd,Ni/Mg0.85La3+0.15O3 | 38.6 | 50 | 1.14 | 0.98 | 1.09 | 96.56 |
| Catalysts | Temp. °C | Temp. °C | Temp. °C | Temp. °C | Temp. °C | Amount of Adsorbed H2 Gas (μmol/g) |
|---|---|---|---|---|---|---|
| Pt,Pd,Ni/MgO | 130 | 184 | 621 | - | - | 464.8 |
| Pt,Pd,Ni/Mg0.97La3+0.03O | 115 | 175 | 573 | 532 | 635 | 503.7 |
| Pt,Pd,Ni/Mg0.93 La 3+0.07O | 123 | 170 | 578 | 545 | 652 | 515 |
| Pt,Pd,Ni/Mg0.85 La 3+0.15O | 126 | 163 | 572 | 559 | 677 | 572 |
| Sample Name | Specific Surface | Pore Volume | Pore Radius |
|---|---|---|---|
| Area (m2/g) | (cm3/g) | (Å) | |
| MgO | 11.1 | 0.21 | 9.9 |
| Pt,Pd,Ni/MgO | 12.97 | 0.12 | 9.7 |
| Pt,Pd,Ni/Mg0.97La3+0.03O3 | 13.79 | 0.054 | 44.53 |
| Pt,Pd,Ni/Mg0.93La3+0.07O3 | 14.19 | 0.082 | 30.87 |
| Pt,Pd,Ni/Mg0.85La3+0.15O3 | 17.17 | 0.095 | 18.27 |
| Catalyst | CH4 | CO2 | H2/CO |
|---|---|---|---|
| Conversion % | Conversion % | Conversion % | |
| Mg1−xLaxO | 36 | 50 | 0.3 |
| Pt,Pd,Ni/MgO | 72.83 | 81.14 | 0.7 |
| Pt,Pd,Ni/Mg0.97La3+0.03O | 80.86 | 94.17 | 1.07 |
| Pt,Pd,Ni/Mg0.93La3+0.07O | 84.92 | 98.96 | 1.15 |
| Pt,Pd,Ni/Mg0.85La3+0.15O | 85.01 | 98.97 | 1.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Najar, A.M.A.; Al-Doghachi, F.A.J.; Al-Riyahee, A.A.A.; Taufiq-Yap, Y.H. Effect of La2O3 as a Promoter on the Pt,Pd,Ni/MgO Catalyst in Dry Reforming of Methane Reaction. Catalysts 2020, 10, 750. https://doi.org/10.3390/catal10070750
Al-Najar AMA, Al-Doghachi FAJ, Al-Riyahee AAA, Taufiq-Yap YH. Effect of La2O3 as a Promoter on the Pt,Pd,Ni/MgO Catalyst in Dry Reforming of Methane Reaction. Catalysts. 2020; 10(7):750. https://doi.org/10.3390/catal10070750
Chicago/Turabian StyleAl-Najar, Ali M. A., Faris A. J. Al-Doghachi, Ali A. A. Al-Riyahee, and Yun Hin Taufiq-Yap. 2020. "Effect of La2O3 as a Promoter on the Pt,Pd,Ni/MgO Catalyst in Dry Reforming of Methane Reaction" Catalysts 10, no. 7: 750. https://doi.org/10.3390/catal10070750
APA StyleAl-Najar, A. M. A., Al-Doghachi, F. A. J., Al-Riyahee, A. A. A., & Taufiq-Yap, Y. H. (2020). Effect of La2O3 as a Promoter on the Pt,Pd,Ni/MgO Catalyst in Dry Reforming of Methane Reaction. Catalysts, 10(7), 750. https://doi.org/10.3390/catal10070750