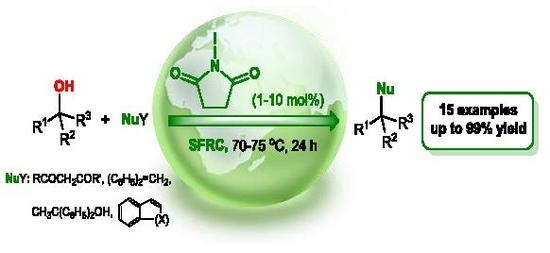
N-Iodosuccinimide as a Precatalyst for Direct Cross-Coupling of Alcohols with C-Nucleophiles under Solvent-Free Reaction Conditions
Abstract
:1. Introduction
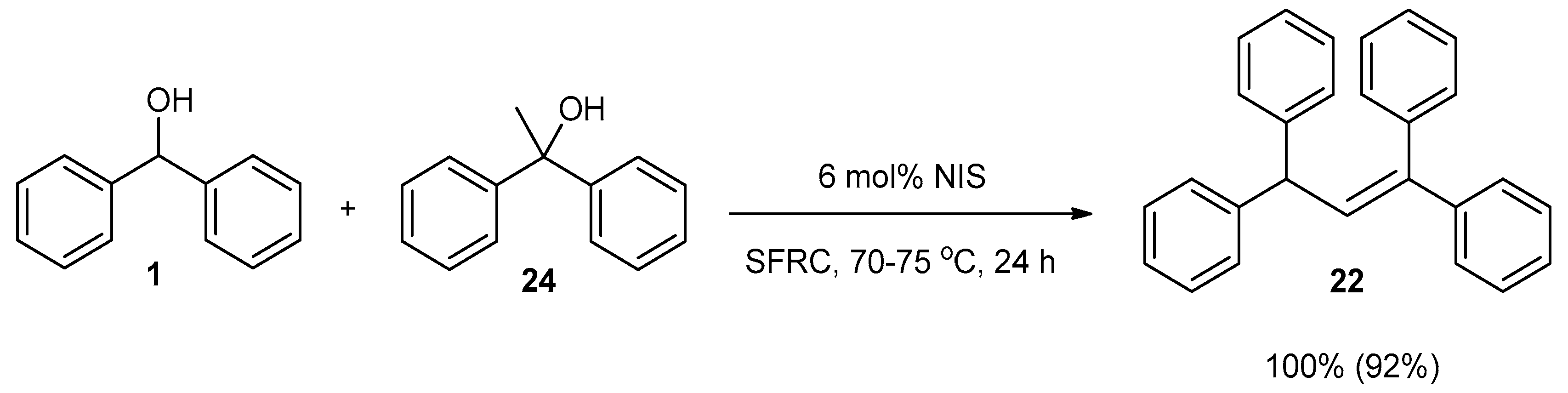
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Van Der Eycken, E.V. Recent approaches for C–C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 2013, 42, 1121–1146. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Saito, T.; Ueba, M.; Baba, A. Direct substitution of the hydroxy group in alcohols with silyl nucleophiles catalyzed by indium trichloride. Angew. Chem. Int. Ed. 2004, 43, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Emer, E.; Sinisi, R.; Capdevila, M.G.; Petruzziello, D.; De Vincentiis, F.; Cozzi, P.G. ChemInform Abstract: Direct nucleophilic SN1-type reactions of alcohols. Eur. J. Org. Chem. 2011, 2011, 647–666. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.-P.; Wang, C.-H.; Zhou, J. Catalytic functionalization of tertiary alcohols to fully substituted carbon centres. Org. Biomol. Chem. 2014, 12, 6033–6048. [Google Scholar] [CrossRef] [PubMed]
- Uchuskin, M.G.; Makarov, A.S.; Butin, A.V. Catalytic Alkylation of furans by π-activated alcohols (Review). Chem. Heterocycl. Compd. 2014, 50, 791–806. [Google Scholar] [CrossRef]
- Chaskar, A.; Murugan, K. Direct allylation of alcohols using allyltrimethylsilane: A move towards an economical and ecological protocol for C-C bond formation. Catal. Sci. Tech. 2014, 4, 1852–1868. [Google Scholar] [CrossRef]
- Shang, X.-J.; Liu, Z.-Q. Iron-catalyzed alkylation of alkenes and alkynes using alcohols as the alkylating reagent. Synthesis 2015, 47, 1706–1708. [Google Scholar] [CrossRef]
- Moran, J.; Dryzhakov, M.; Richmond, E. Recent advances in direct catalytic dehydrative substitution of alcohols. Synthesis 2016, 48, 935–959. [Google Scholar] [CrossRef] [Green Version]
- Ajvazi, N.; Stavber, S. Alcohols in direct carbon-carbon and carbon-heteroatom bond-forming reactions: Recent advances. Arkivoc 2018, 2018, 288–329. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.-P.; Jiang, N.; Huang, X.-Q.; Yu, B.; Hu, C.-W. Non-corrosive heteropolyacid-based recyclable ionic liquid catalyzed direct dehydrative coupling of alcohols with alcohols or alkenes. Mol. Catal. 2019, 468, 80–85. [Google Scholar] [CrossRef]
- Chevella, D.; Macharla, A.K.; Kodumuri, S.; Banothu, R.; Gajula, K.S.; Amrutham, V.; Gennadievna, G.N.; Nama, N.; Durgaiah, C.; Kumar, M.A.; et al. Synthesis of internal olefins by direct coupling of alcohols and olefins over Moβ zeolite. Catal. Commun. 2019, 123, 114–118. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bora, U. Molecular iodine-catalyzed selective C-3 benzylation of indoles with benzylic alcohols: A greener approach toward benzylated indoles. ACS Omega 2019, 4, 11770–11776. [Google Scholar] [CrossRef] [PubMed]
- Böldl, M.; Fleischer, I. Dehydrative coupling of benzylic alcohols catalyzed by Brønsted Acid/Lewis Base. Eur. J. Org. Chem. 2019, 2019, 5856–5861. [Google Scholar] [CrossRef] [Green Version]
- Čebular, K.; Stavber, S. Molecular iodine as a mild catalyst for cross-coupling of alkenes and alcohols. Pure Appl. Chem. 2018, 90, 377–386. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Ajvazi, N.; Stavber, S. N-halosuccinimides as precatalysts for C-, N-, O-, and X-nucleophilic substitution reactions of alcohols under mild reaction conditions. Catalysts 2020, 10, 460. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. aerobic oxidative iodination of organic compounds with iodide catalyzed by sodium nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite “on water” or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262. [Google Scholar] [CrossRef]
- Stavber, G.; Stavber, S. Towards greener fluorine organic chemistry: Direct electrophilic fluorination of carbonyl compounds in water and under solvent-free reaction conditions. Adv. Synth. Catal. 2010, 352, 2838–2846. [Google Scholar] [CrossRef]
- Eames, J. Acid–Base Properties of Enols and Enolates. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chun, S.; Chung, Y.K. Silver/NBS-catalyzed synthesis of α-alkylated aryl ketones from internal alkynes and benzyl alcohols via ether intermediates. Org. Lett. 2018, 20, 5583–5586. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. A Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef]
- Breugst, M.; Von Der Heiden, D. Mechanisms in Iodine Catalysis. Chem. A Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2006, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Čebular, K.; Božić, B.; Stavber, S. 1,3-dibromo-5,5-dimethylhydantoin as a precatalyst for activation of carbonyl functionality. Molecules 2019, 24, 2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Entry | 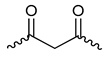 | R1, R2, R3 | NIS (Mol%) | Yield b (%) | |||
|---|---|---|---|---|---|---|---|
| 1 | 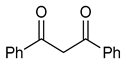 | 2 | R1=R2=H, R3=Ph | 4 | 1 | - | 100 (99) |
| 2 | 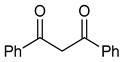 | 2 | R1=Me, R2=H, R3=Ph | 11 | 3 | 12 | 100 (99) |
| 3 | 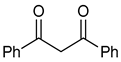 | 2 | R1=Cl, R2=H, R3=Ph | 13 | 10 | 14 | 100 (98) |
| 4 | 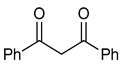 | 2 | R1=OMe, R2=R3=H | 15 | 6 | 16 | 100 (98) |
| 5 | 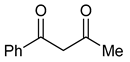 | 7 | R1=R2=H, R3=Ph | 1 | 2 | 8 | 100 (98) |
| 6 | 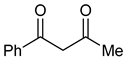 | 7 | R1=Me, R2=H, R3=Ph | 11 | 5 | 17 | 100 (99) |
| 7 |  | 9 | R1=R2=H, R3=Ph | 1 | 8 | 10 | 100 (98) |
| 8 |  | 9 | R1=Me, R2= H, R3=Ph | 11 | 7 | 18 | 100 (99) |
| 9 |  | 19 | R1=R2=H, R3=Ph | 1 | 4 | 20 | 100 (99) |
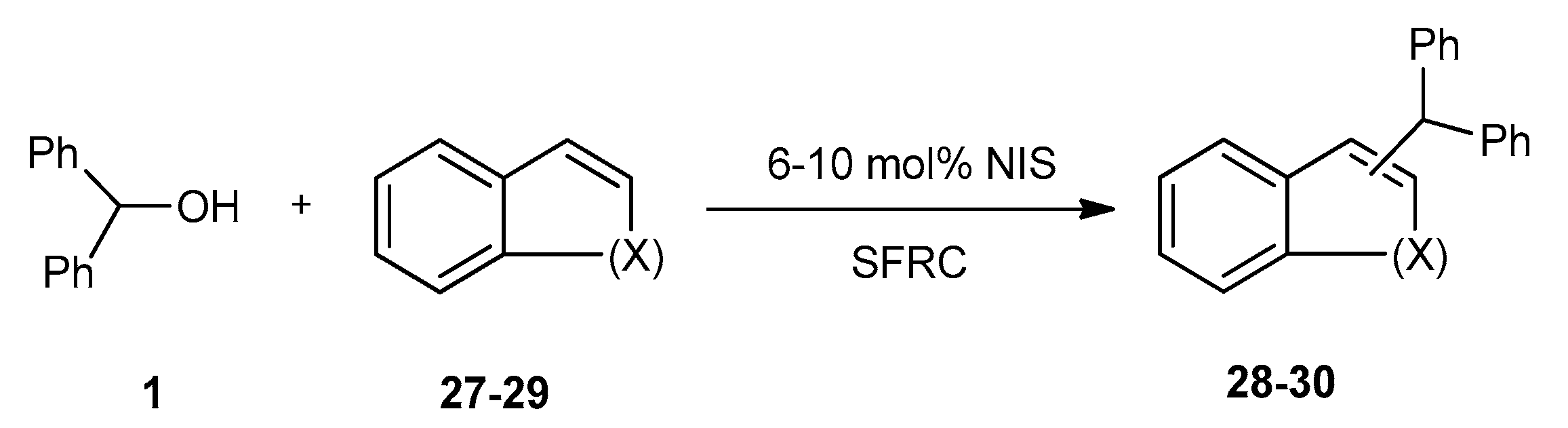
| Entry | X | Product 28–30 | Yield (%) b | ||
|---|---|---|---|---|---|
| 1 | NAc | 27 | 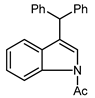 | 28 | 91 |
| 2 | O | 29 | 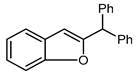 | 30 | 61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajvazi, N.; Stavber, S. N-Iodosuccinimide as a Precatalyst for Direct Cross-Coupling of Alcohols with C-Nucleophiles under Solvent-Free Reaction Conditions. Catalysts 2020, 10, 850. https://doi.org/10.3390/catal10080850
Ajvazi N, Stavber S. N-Iodosuccinimide as a Precatalyst for Direct Cross-Coupling of Alcohols with C-Nucleophiles under Solvent-Free Reaction Conditions. Catalysts. 2020; 10(8):850. https://doi.org/10.3390/catal10080850
Chicago/Turabian StyleAjvazi, Njomza, and Stojan Stavber. 2020. "N-Iodosuccinimide as a Precatalyst for Direct Cross-Coupling of Alcohols with C-Nucleophiles under Solvent-Free Reaction Conditions" Catalysts 10, no. 8: 850. https://doi.org/10.3390/catal10080850