Improved Electrocatalytic Activity and Durability of Pt Nanoparticles Supported on Boron-Doped Carbon Black
Abstract
:1. Introduction
2. Results and Discussion
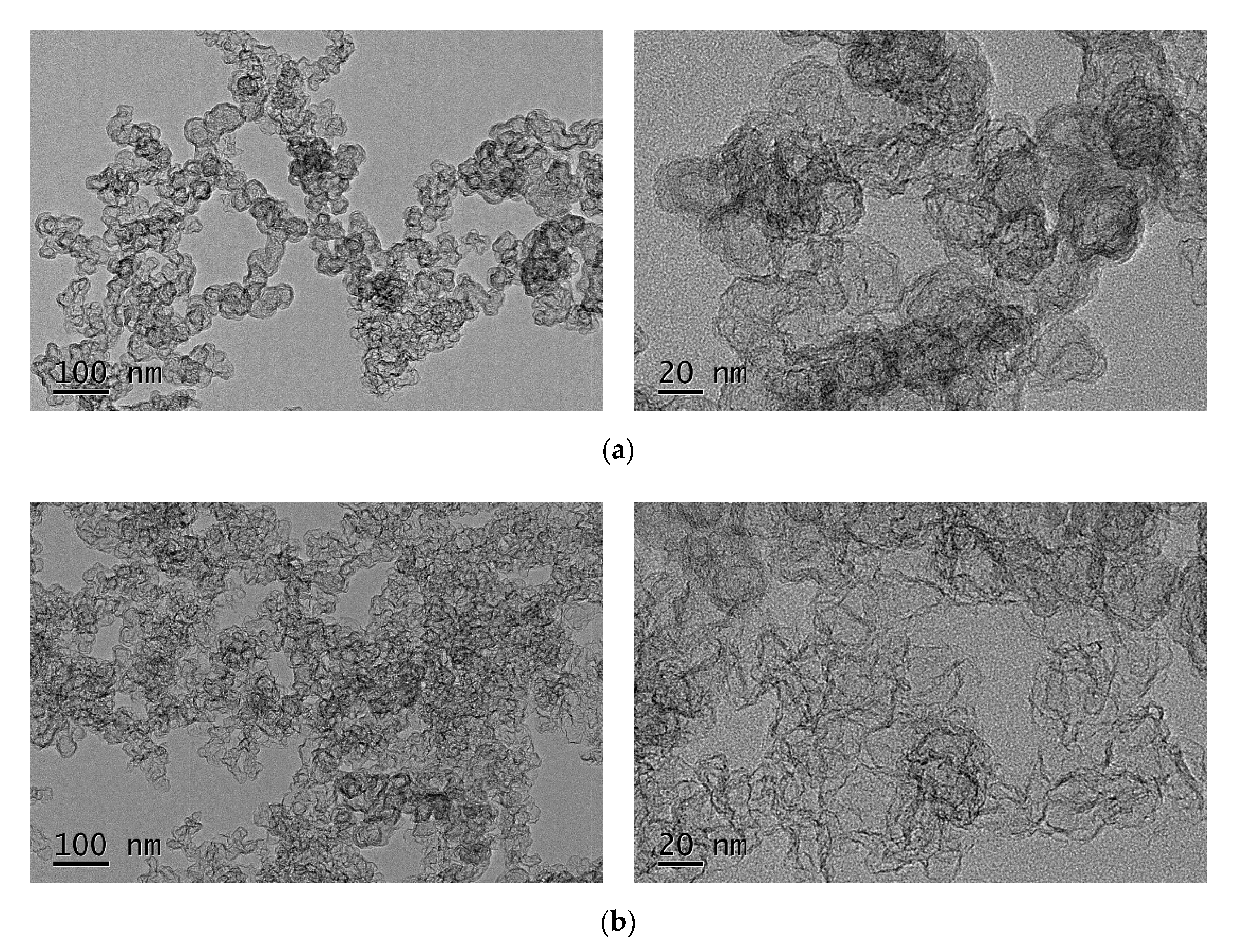
2.1. Characterization of B-ECP600
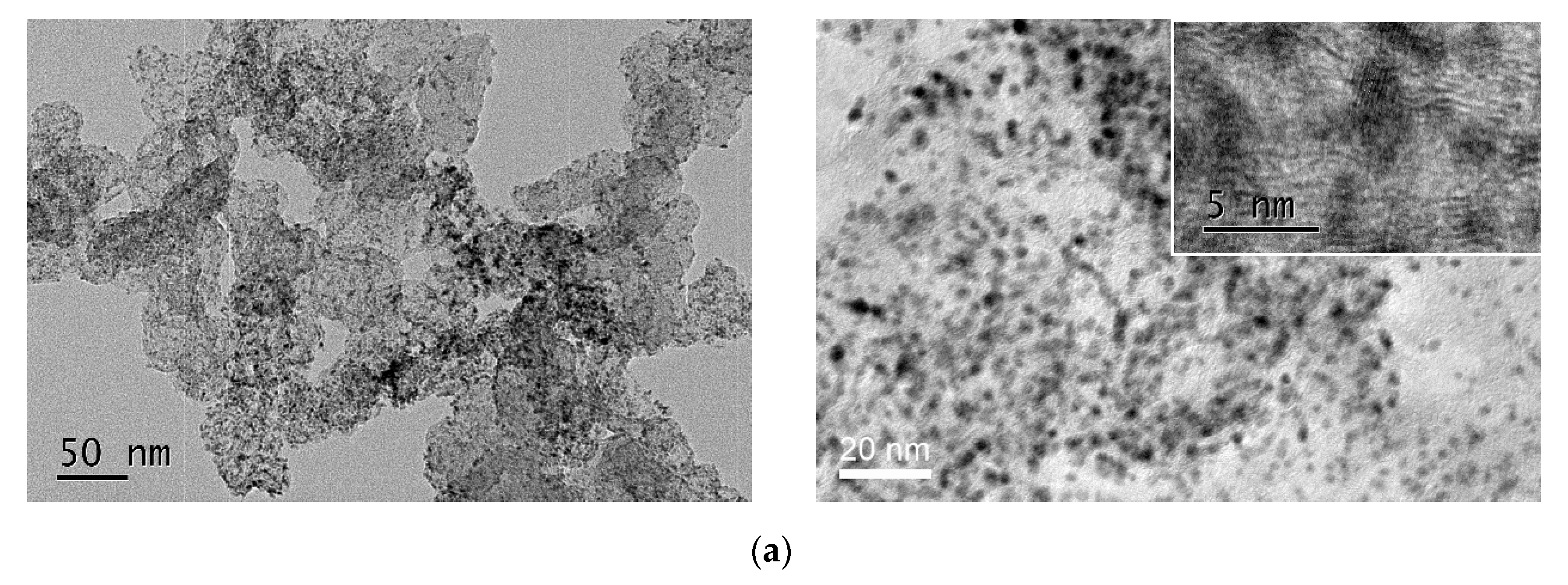
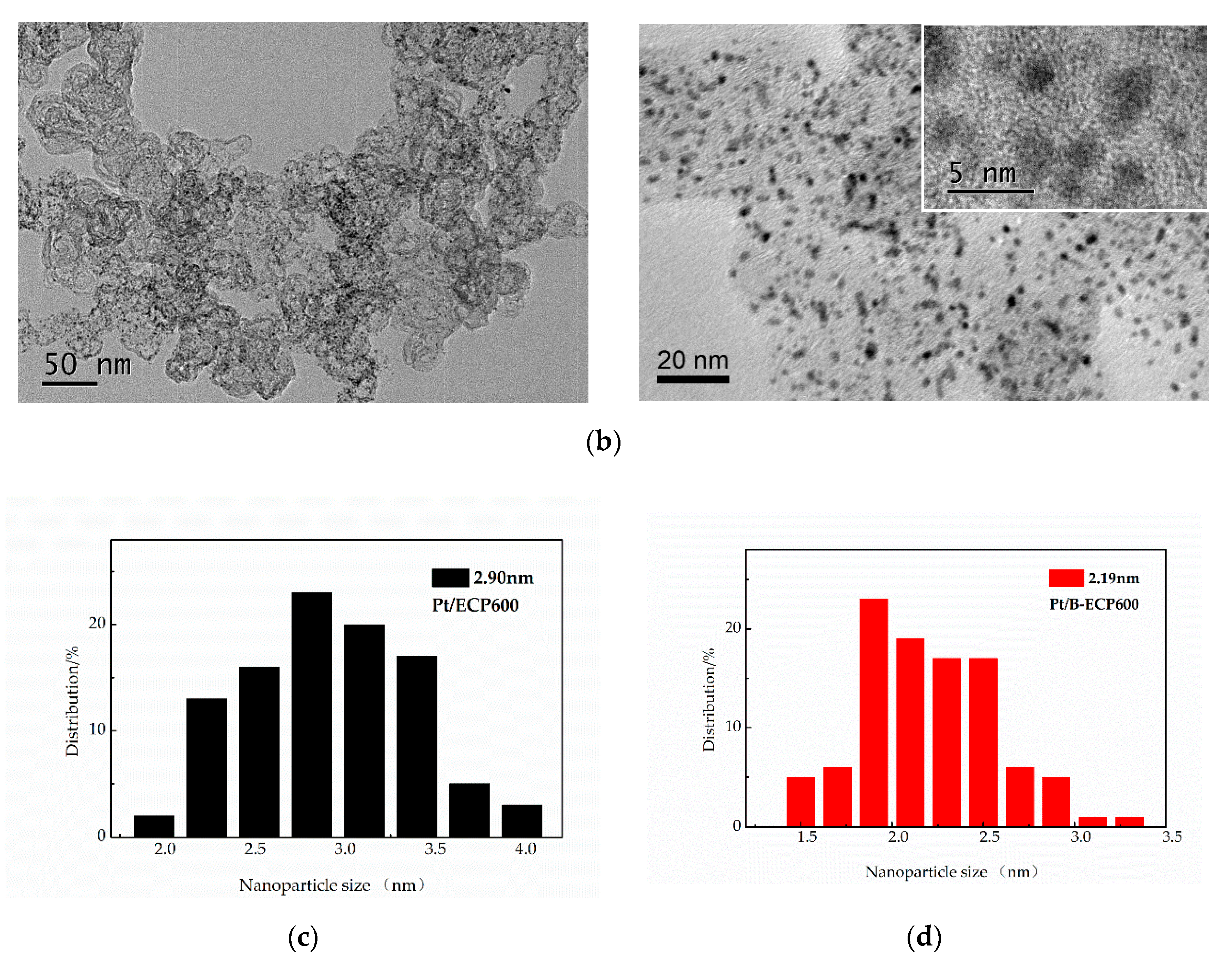
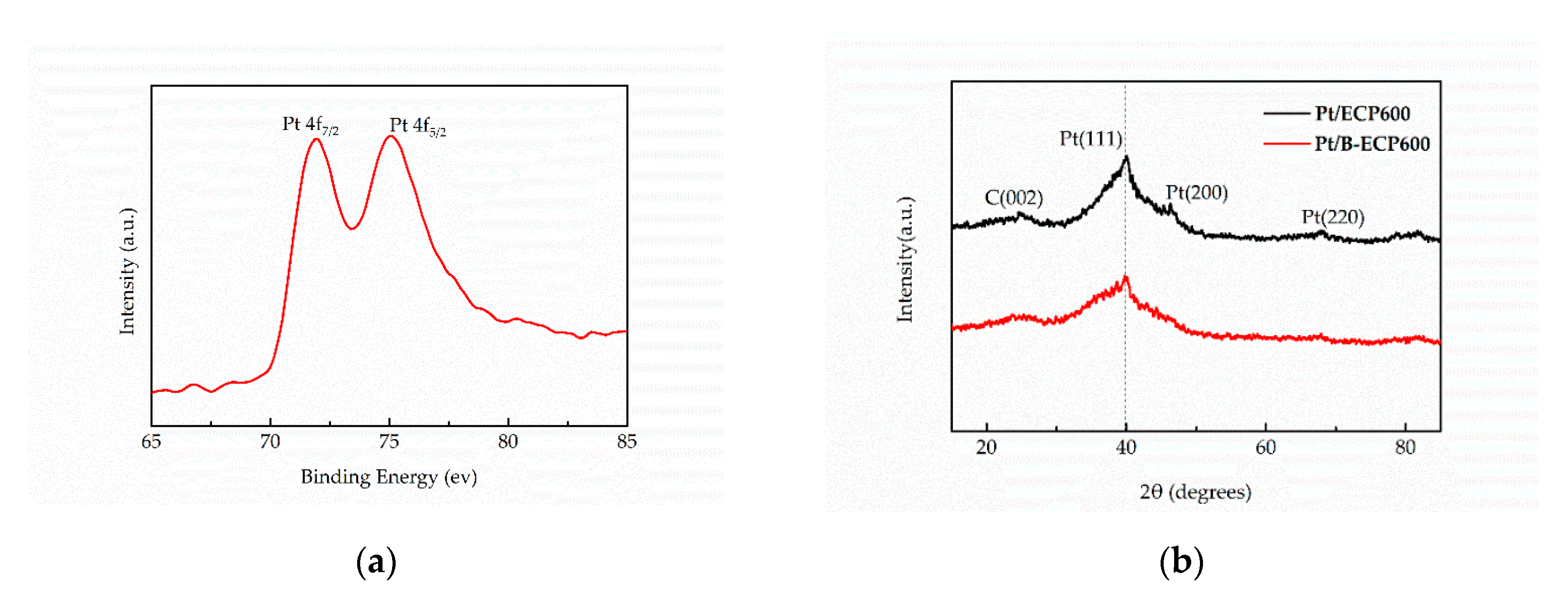
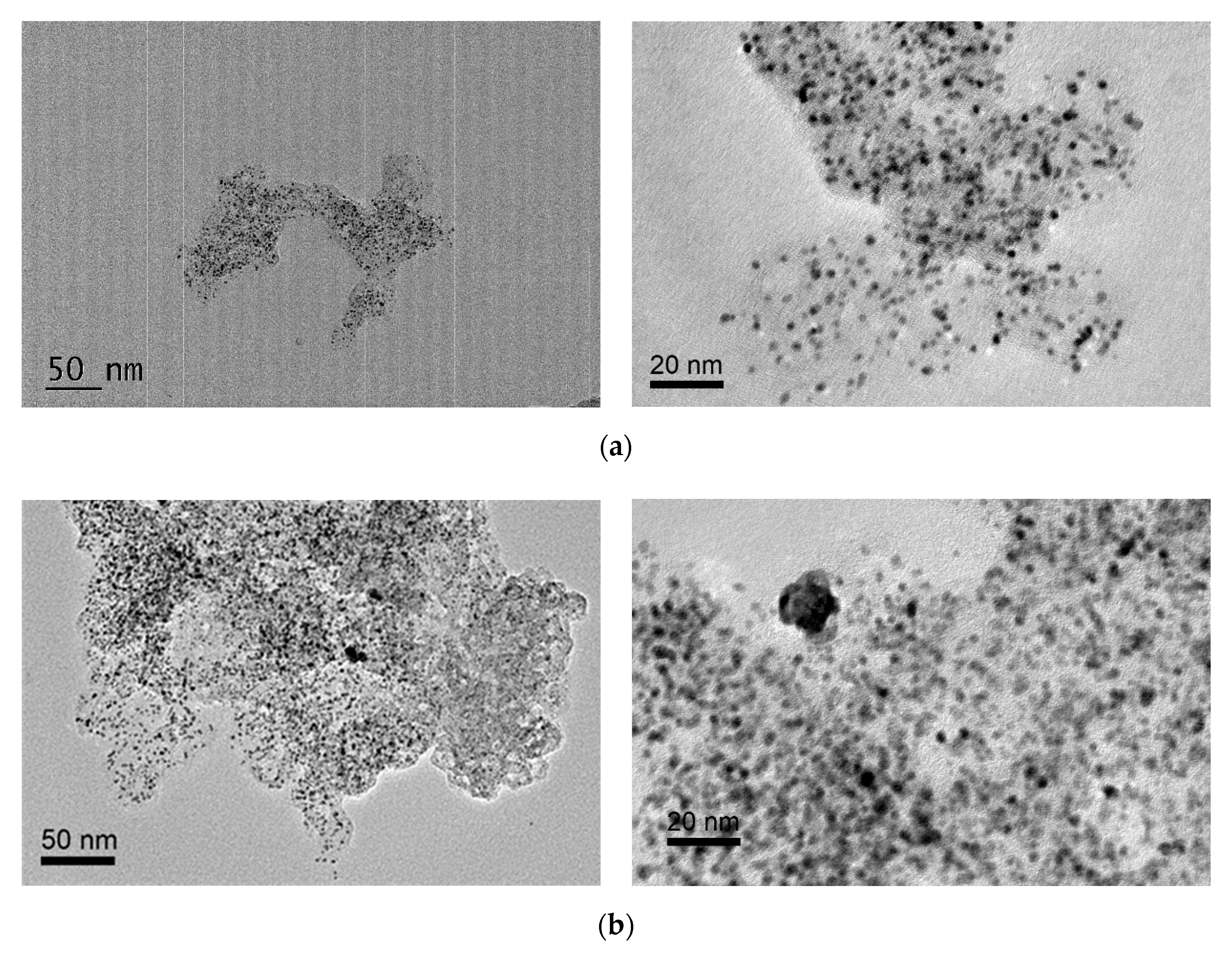
2.2. Characterization of Pt/B-ECP600
2.3. Electrochemical Properties of Pt/B-ECP600
3. Materials and Methods
3.1. Chemicals
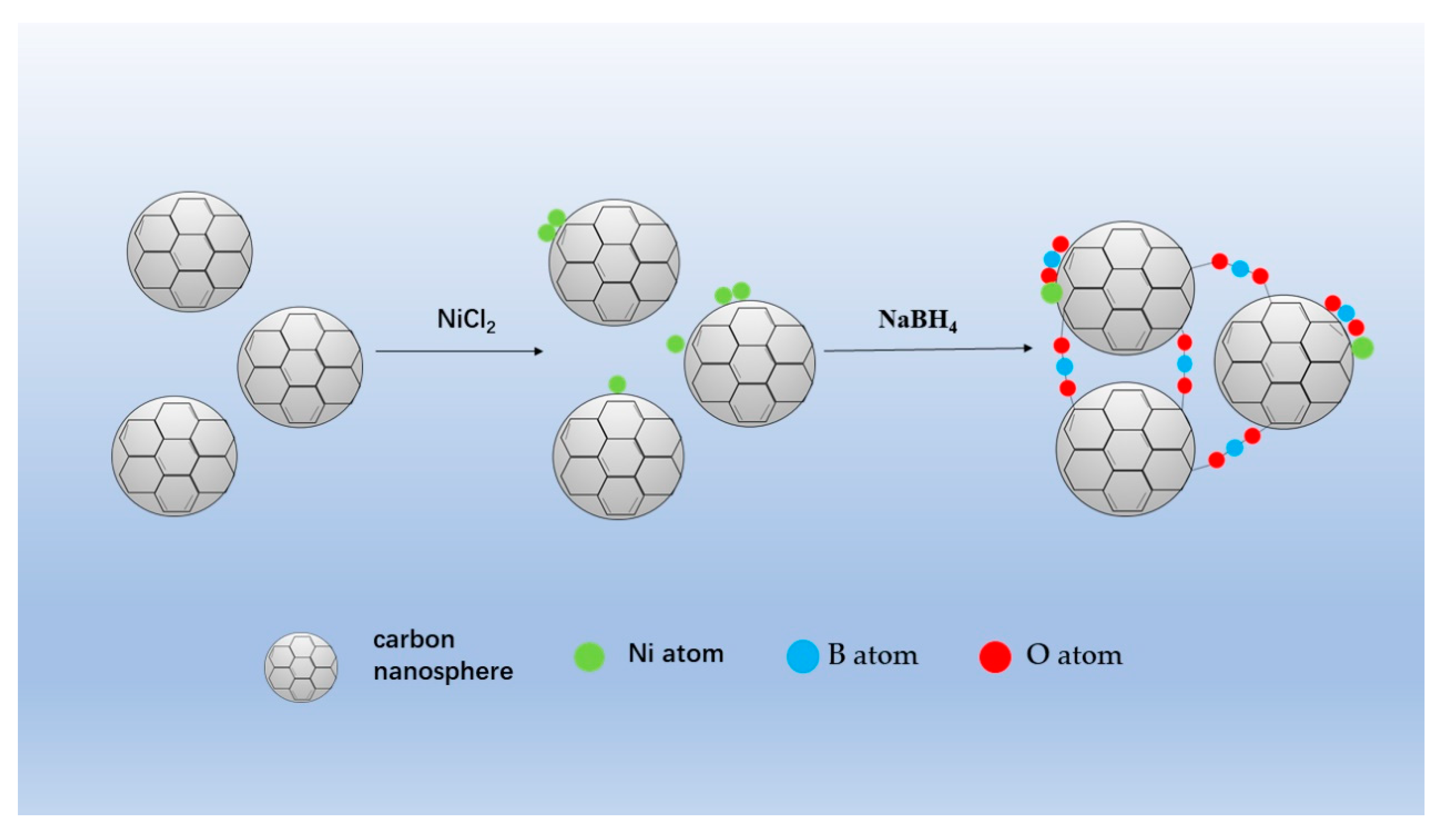
3.2. Preparation of Support and Catalysts
3.3. Physical Characterization
3.4. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. One-dimensional nanostructured electrocatalysts for polymer electrolyte membrane fuel cells—A review. Appl. Catal. B Environ. 2016, 199, 292–314. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Mondal, B.; Chatterjee, S.; Rana, A.; Amanullah, S.; Dey, A. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem. 2017, 1. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Kuttiyiel, K.; Sasaki, K.; Su, D.; Yang, T.; Park, G.; Zhang, C.; Chen, G.; Adzic, R. Pt Monolayer Shell on Nitrided Alloy Core—A Path to Highly Stable Oxygen Reduction Catalyst. Catalysts 2015, 5, 1321–1332. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Duan, W.; Wang, W.; Lin, Y.; Wang, Y.; Liu, J. Nanostructured Electrocatalysts for PEM Fuel Cells and Redox Flow Batteries: A Selected Review. ACS Catal. 2015, 5, 7288–7298. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, J.; Yang, L.; Zhang, J. A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1301523. [Google Scholar] [CrossRef]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.; Jia, Q.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 2017, 8, 957. [Google Scholar] [CrossRef]
- Saputro, A.G.; Fajrial, A.K.; Maulana, A.L.; Fathurrahman, F.; Agusta, M.K.; Akbar, F.T.; Dipojono, H.K. Dissociative Oxygen Reduction Reaction Mechanism on the Neighboring Active Sites of a Boron-Doped Pyrolyzed Fe–N–C Catalyst. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Yang, D.; Gu, J.; Liu, X.; He, H.; Wang, M.; Wang, P.; Zhu, Y.; Fan, Q.; Huang, R. Monodispersed Pt3Ni Nanoparticles as a Highly Efficient Electrocatalyst for PEMFCs. Catalysts 2019, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Lai, Q.; Zhao, Y.; Zhong, J.; Liang, Y. An effective FeCl3 template assisted synthesis of nitrogen, sulfur and iron-tridoped carbon nanosheets from a protic salt for oxygen reduction electrocatalysis. Chin. J. Catal. 2018, 39, 1453–1462. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Z.; Zhu, Y.; Ge, L.; Yuan, Z. P-doped mesoporous carbons for high-efficiency electrocatalytic oxygen reduction. Chin. J. Catal. 2019, 40, 1366–1374. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Park, S.; Jang, D.; Shin, Y.; Lim, D.; Park, S. Metal-free N-doped carbon blacks as excellent electrocatalysts for oxygen reduction reactions. Carbon 2019, 145, 481–487. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Siahrostami, S.; Higgins, D.; Nordlund, D.; Sokaras, D.; Kim, T.R.; Liu, Y.; Yan, X.; Nilsson, E.; et al. Designing Boron Nitride Islands in Carbon Materials for Efficient Electrochemical Synthesis of Hydrogen Peroxide. J. Am. Chem. Soc. 2018, 140, 7851–7859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, L. Heteroatom-Doped Graphitic Carbon Catalysts for Efficient Electrocatalysis of Oxygen Reduction Reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Nsabimana, A.; Bo, X.; Zhang, Y.; Li, M.; Han, C.; Guo, L. Electrochemical properties of boron-doped ordered mesoporous carbon as electrocatalyst and Pt catalyst support. J. Colloid Interface Sci. 2014, 428, 133–140. [Google Scholar] [CrossRef]
- Cao, C.; Wei, L.; Zhai, Q.; Wang, G.; Shen, J. Biomass-derived nitrogen and boron dual-doped hollow carbon tube as cost-effective and stable synergistic catalyst for oxygen electroreduction. Electrochim. Acta 2017, 249, 328–336. [Google Scholar] [CrossRef]
- Van Tam, T.; Kang, S.G.; Babu, K.F.; Oh, E.; Lee, S.G.; Choi, W.M. Synthesis of B-doped graphene quantum dots as a metal-free electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 10537–10543. [Google Scholar] [CrossRef]
- Du, N.; Wang, C.; Long, R.; Xiong, Y. N-doped carbon-stabilized PtCo nanoparticles derived from Pt@ZIF-67: Highly active and durable catalysts for oxygen reduction reaction. Nano Res. 2017, 10, 3228–3237. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, I.F.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Yu, D.; Nagelli, E.; Du, F.; Dai, L. Metal-Free Carbon Nanomaterials Become More Active than Metal Catalysts and Last Longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. [Google Scholar] [CrossRef]
- Charreteur, F.; Jaouen, F.; Ruggeri, S.; Dodelet, J. Fe/N/C non-precious catalysts for PEM fuel cells: Influence of the structural parameters of pristine commercial carbon blacks on their activity for oxygen reduction. Electrochim. Acta 2008, 53, 2925–2938. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Wu, X.; Fu, Y.; Wu, H.; Guo, S. Composites of boron-doped carbon nanosheets and iron oxide nanoneedles: Fabrication and lithium ion storage performance. J. Mater. Chem. A 2014, 2, 9111–9117. [Google Scholar] [CrossRef]
- Xu, C.; Su, Y.; Liu, D. Three-dimensional N,B-doped graphene aerogel as a synergistically enhanced metal-free catalyst for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 25440–25448. [Google Scholar] [CrossRef]
- Sun, D. Synthesis of Hierarchical Porous Carbons and Their Electrochemical Behavior. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2014. [Google Scholar]
- Fellinger, T.; White, R.J.; Titirici, M.; Antonietti, M. Borax-Mediated Formation of Carbon Aerogels from Glucose. Adv. Funct. Mater. 2012, 22, 3254–3260. [Google Scholar] [CrossRef]
- George, N.; Glavee, K.J.K.C.; Hadjapanayis, G.C. Borohydride Reductions of Metal Ions. A New Understanding of the Chemistry Leading to Nanoscale Particles of Metals, Borides, and Metal Borates. Langmuir 1992, 8, 771–773. [Google Scholar]
- He, D.; Zhang, L.; He, D.; Zhou, G.; Lin, Y.; Deng, Z.; Hong, X.; Wu, Y.; Chen, C.; Li, Y. Amorphous nickel boride membrane on a platinum–nickel alloy surface for enhanced oxygen reduction reaction. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Nesselberger, M.; Ashton, S.; Meier, J.C.; Katsounaros, I.; Mayrhofer, K.J.J.; Arenz, M. The Particle Size Effect on the Oxygen Reduction Reaction Activity of Pt Catalysts: Influence of Electrolyte and Relation to Single Crystal Models. J. Am. Chem. Soc. 2011, 133, 17428–17433. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, P.N.; Markovic, N.M. The Impact of Geometric and Surface Electronic Properties of Pt-Catalysts on the Particle Size Effect in Electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, Y.; Liu, Y.; Chen, S. A rotating disk electrode study of the particle size effects of Pt for the hydrogen oxidation reaction. Phys. Chem. Chem. Phys. (PCCP) 2012, 14, 2278–2285. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Universality in Oxygen Reduction Electrocatalysis on Metal Surfaces. ACS Catal. 2012, 2, 1654–1660. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S. Enhanced-electrocatalytic activity of Pt nanoparticles supported on nitrogen-doped carbon for the oxygen reduction reaction. J. Power Sources 2013, 240, 60–65. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Geng, D.; Chen, Y.; Li, R.; Cai, M.; Sun, X. A Highly Durable Platinum Nanocatalyst for Proton Exchange Membrane Fuel Cells: Multiarmed Starlike Nanowire Single Crystal. Angew. Chem. Int. Ed. 2011, 50, 422–426. [Google Scholar] [CrossRef]
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z. Stability of High-Performance Pt-Based Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2018, 30, 1705332. [Google Scholar] [CrossRef]



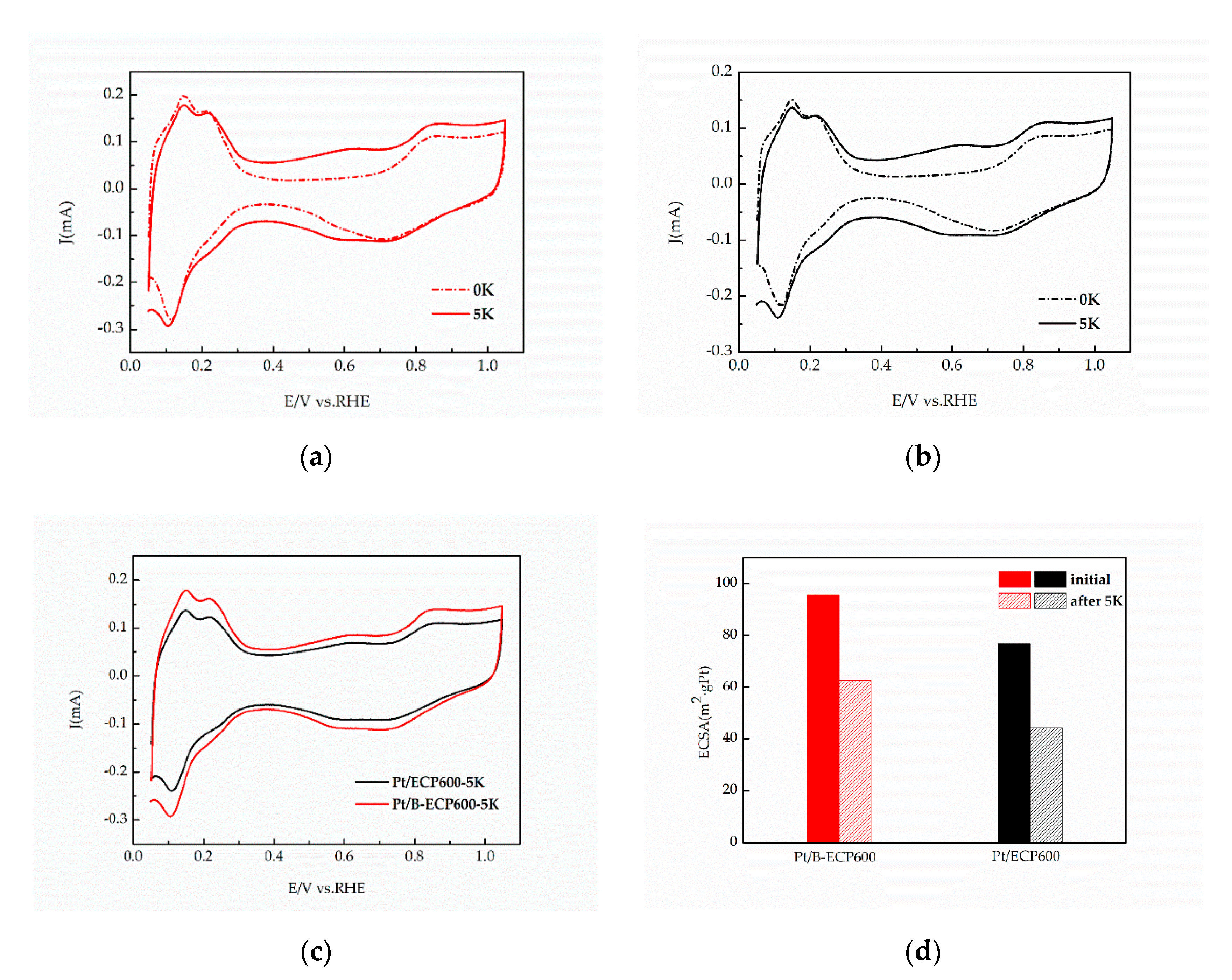
| RDE TESTING | ECSA (m2·gPt−1) | MA (A·mgPt−1) | SA (mA·cm−2) |
|---|---|---|---|
| Pt/ECP600 | 76.57 | 0.217 | 0.283 |
| Pt/B-ECP600 | 95.62 | 0.286 | 0.299 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, R.; Gu, J.; He, H.; Yu, T. Improved Electrocatalytic Activity and Durability of Pt Nanoparticles Supported on Boron-Doped Carbon Black. Catalysts 2020, 10, 862. https://doi.org/10.3390/catal10080862
Yao R, Gu J, He H, Yu T. Improved Electrocatalytic Activity and Durability of Pt Nanoparticles Supported on Boron-Doped Carbon Black. Catalysts. 2020; 10(8):862. https://doi.org/10.3390/catal10080862
Chicago/Turabian StyleYao, Rui, Jun Gu, Haitong He, and Tao Yu. 2020. "Improved Electrocatalytic Activity and Durability of Pt Nanoparticles Supported on Boron-Doped Carbon Black" Catalysts 10, no. 8: 862. https://doi.org/10.3390/catal10080862





