Carbide-Modified Pd on ZrO2 as Active Phase for CO2-Reforming of Methane—A Model Phase Boundary Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Precatalyst Characterization
2.1.1. CVD Process toward the Pd(111) and Pd Foil-Based Subsurface Zr0–Pd Alloy Precatalysts
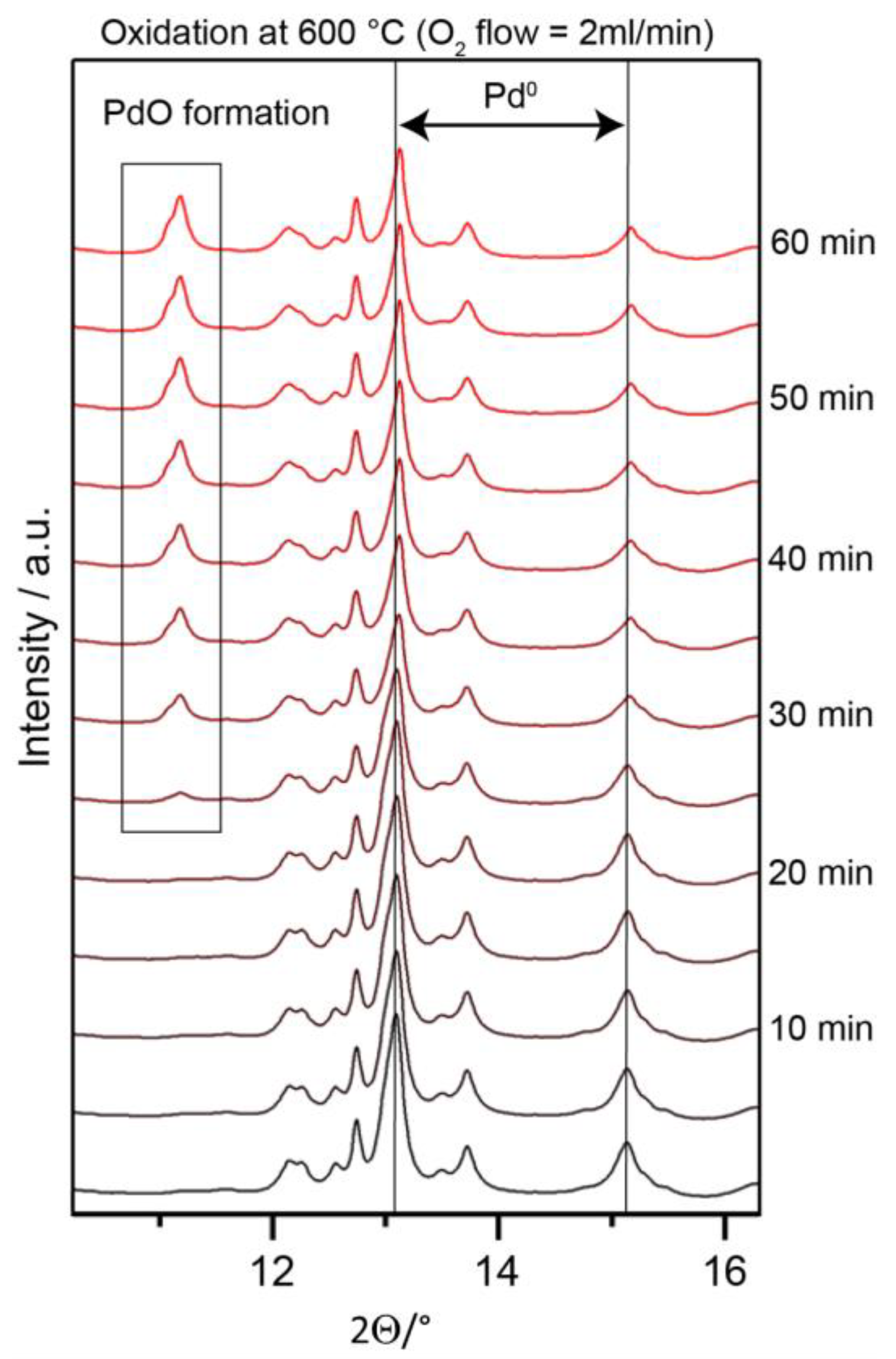
2.1.2. Characterization of the PdxZry Bulk-Intermetallic Precatalyst
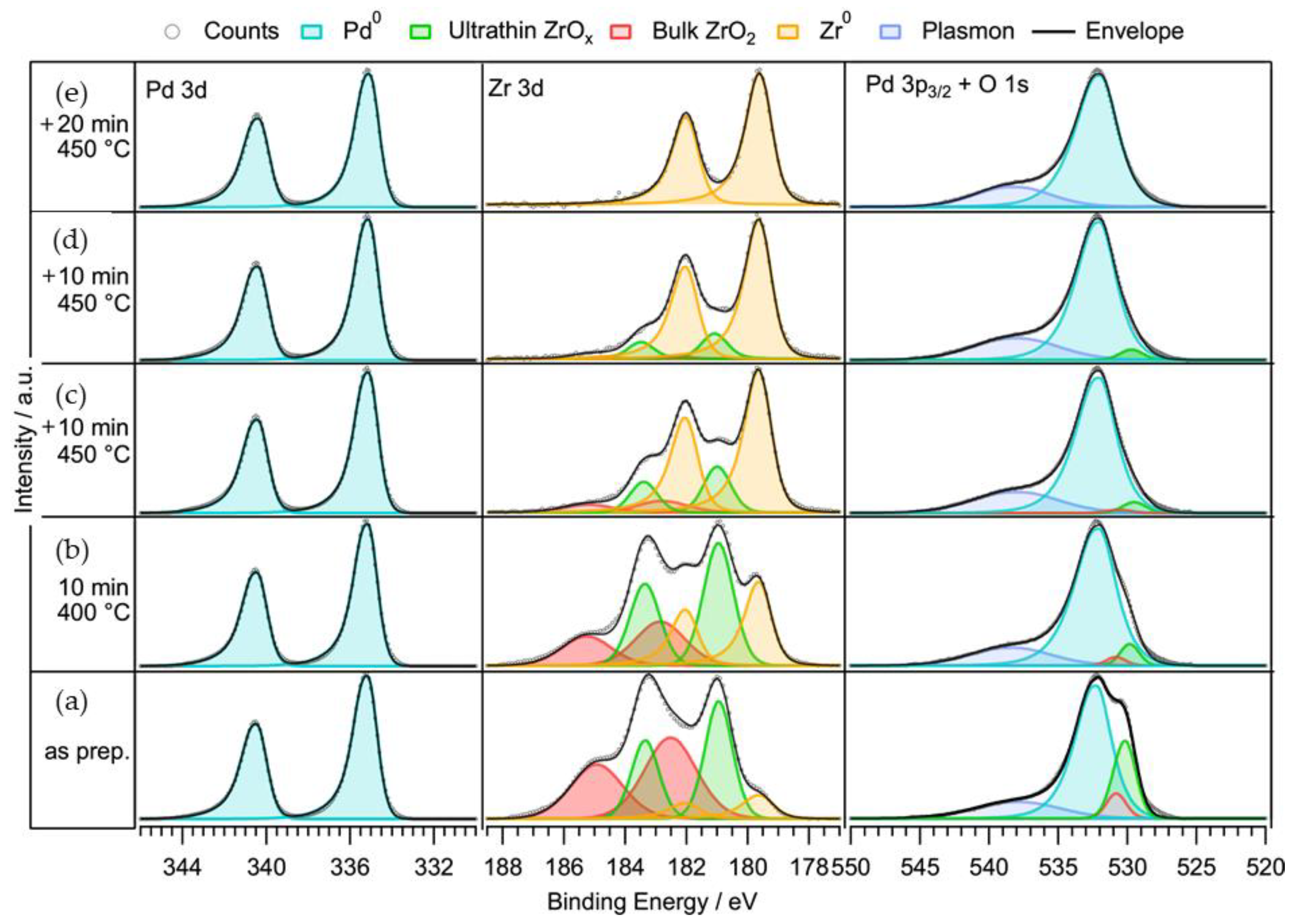
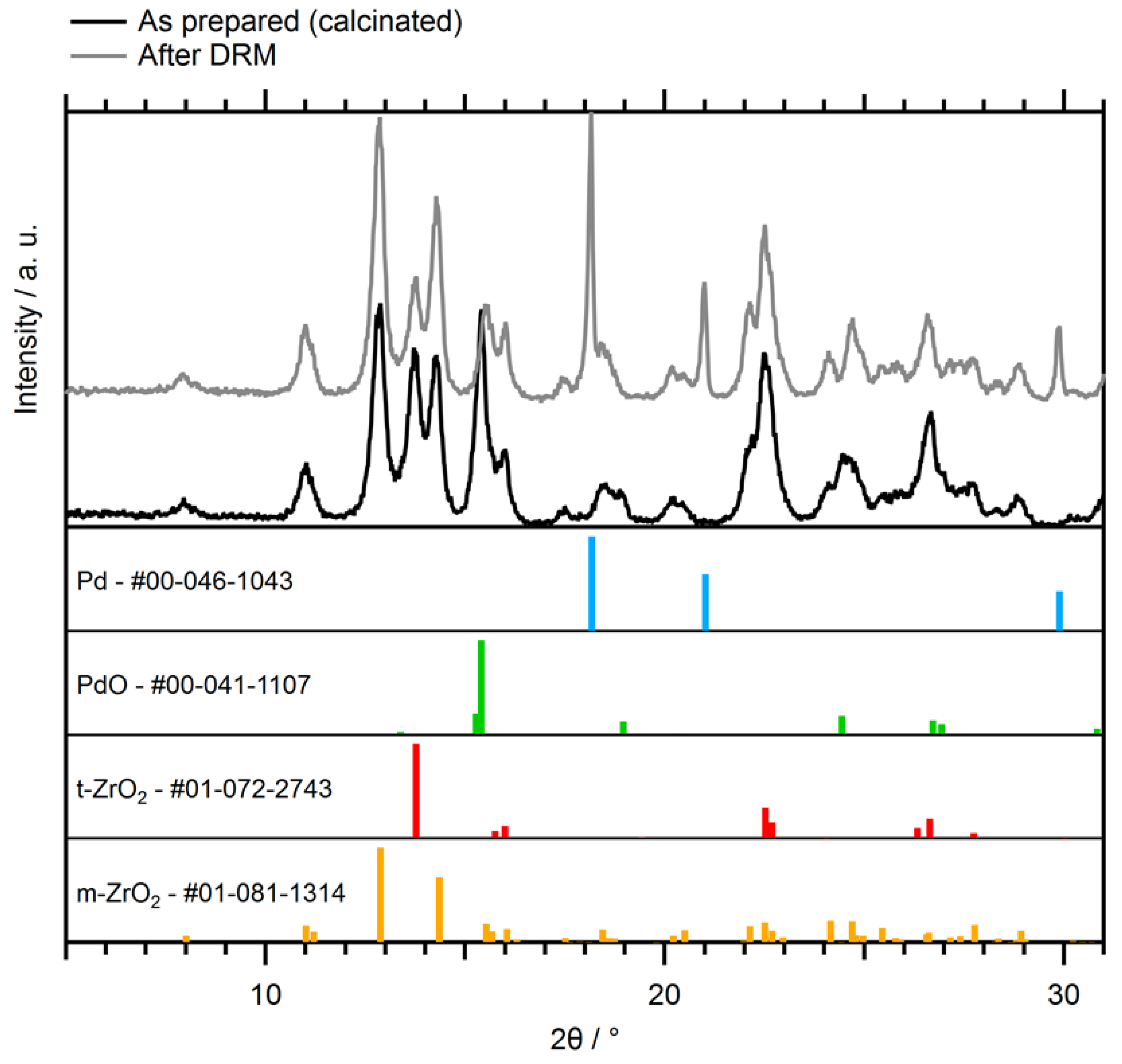
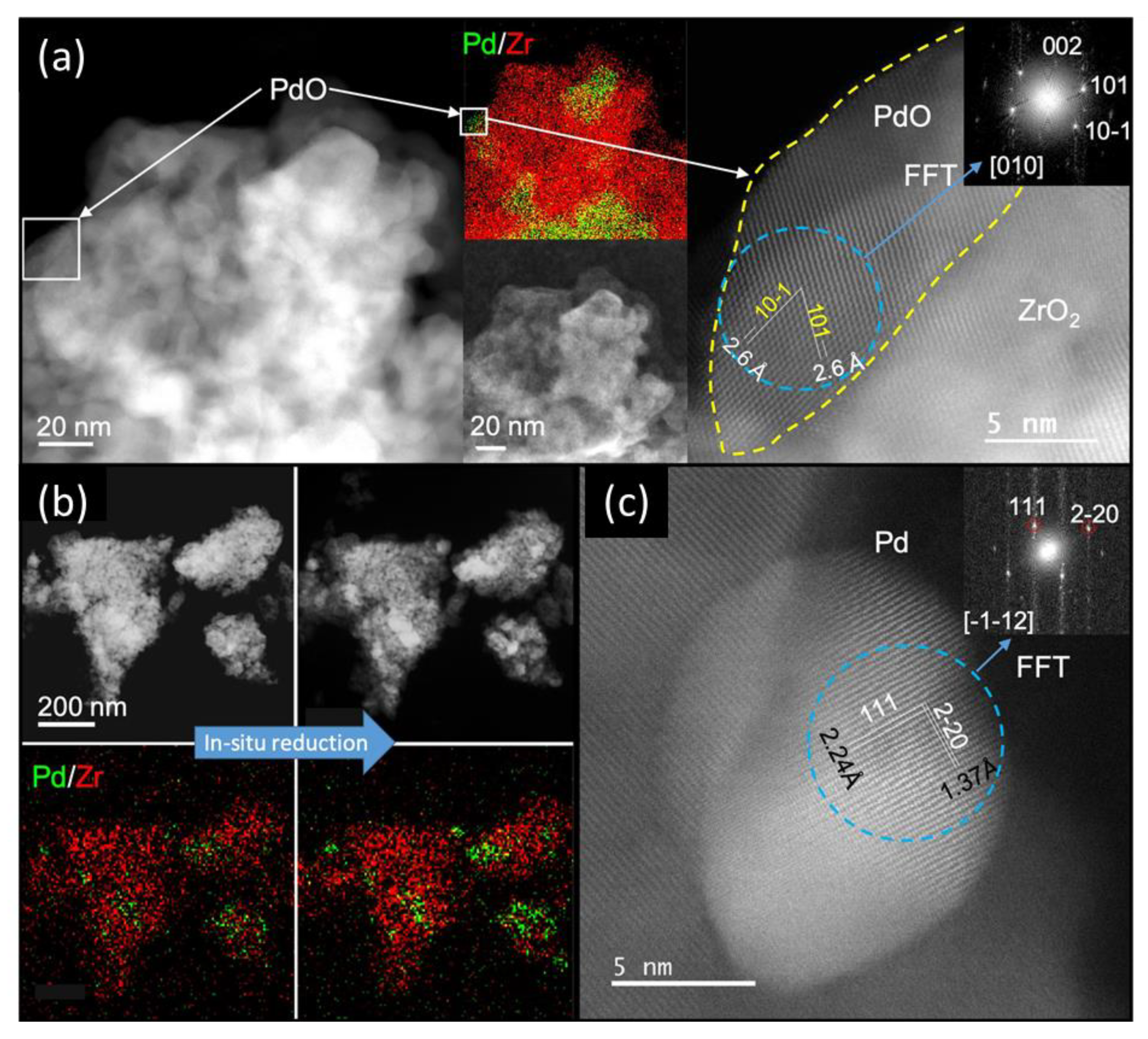
2.1.3. Characterization of the Supported Pd–ZrO2 Powder Catalyst
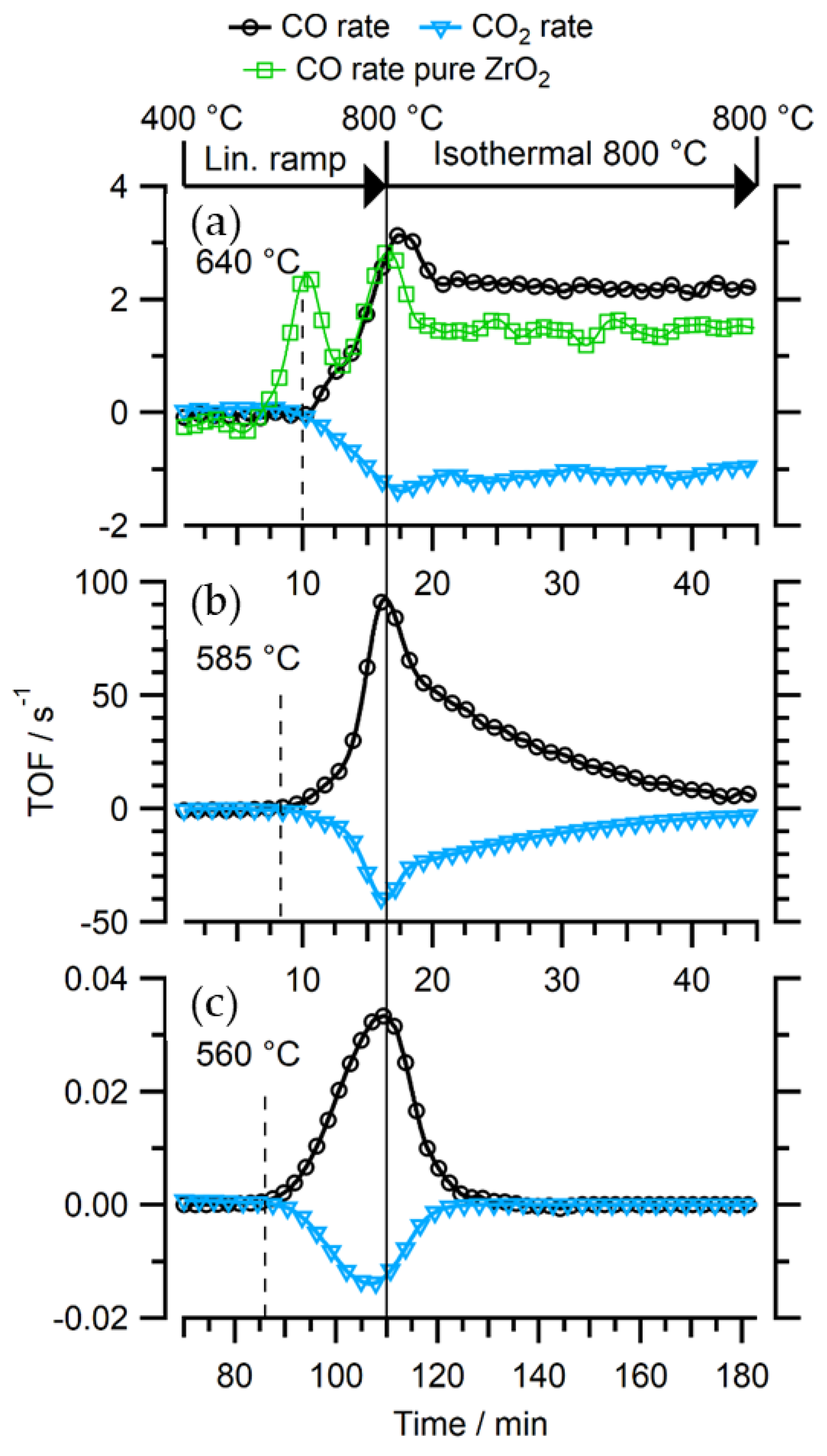
2.2. Catalytic Testing via Temperature-Programmed DRM Experiments
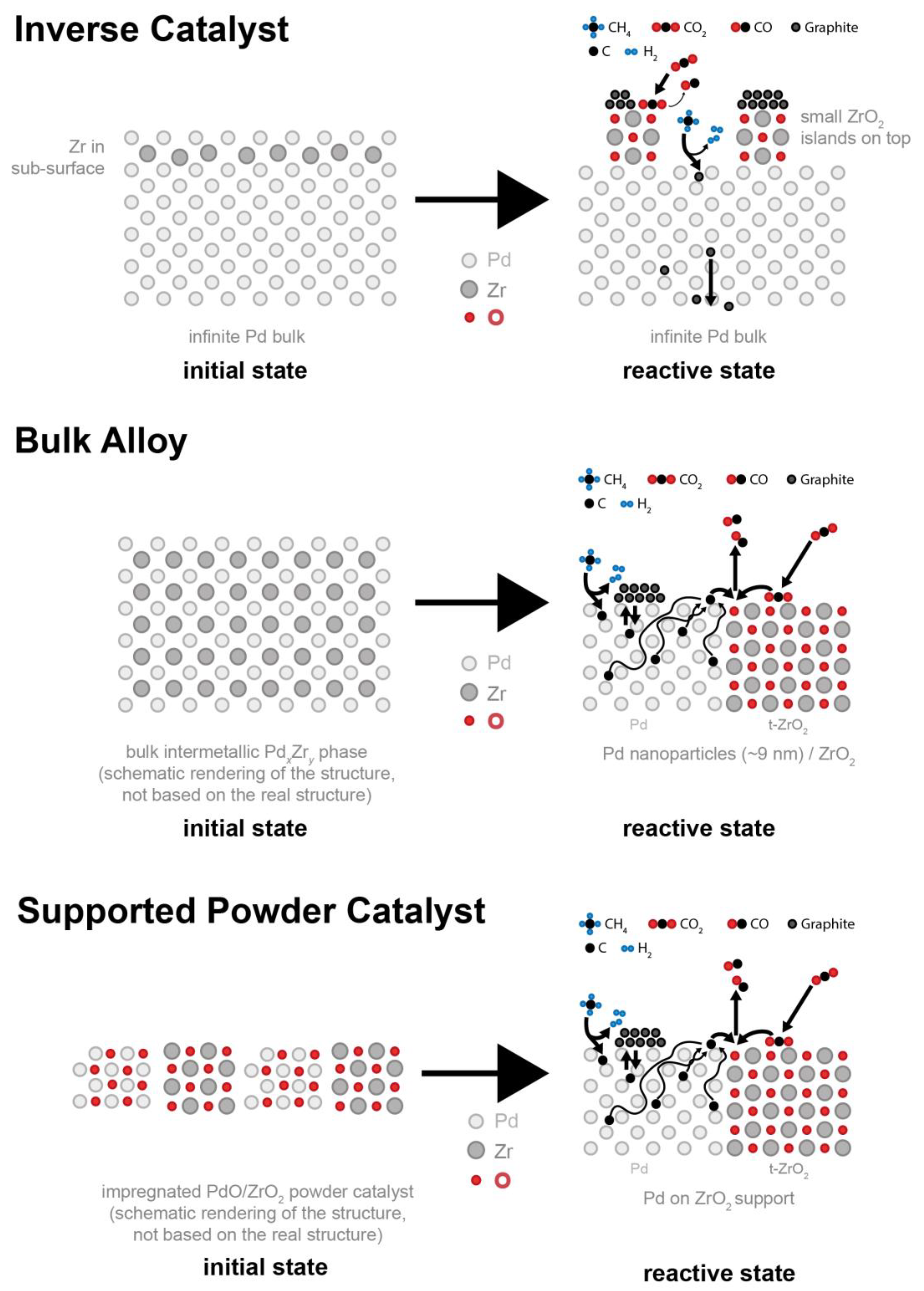
2.3. In-Situ Characterization under DRM Reaction Conditions
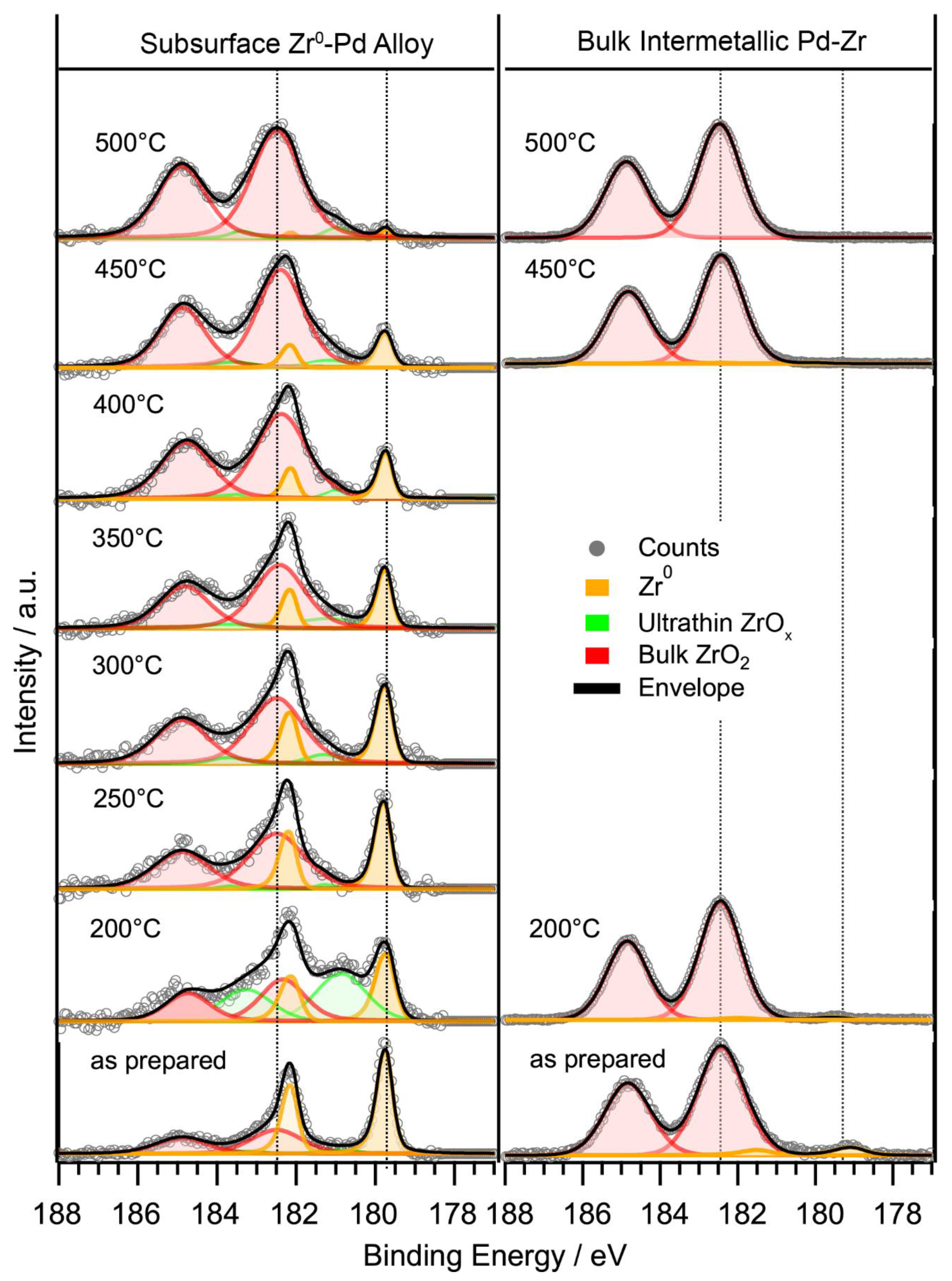
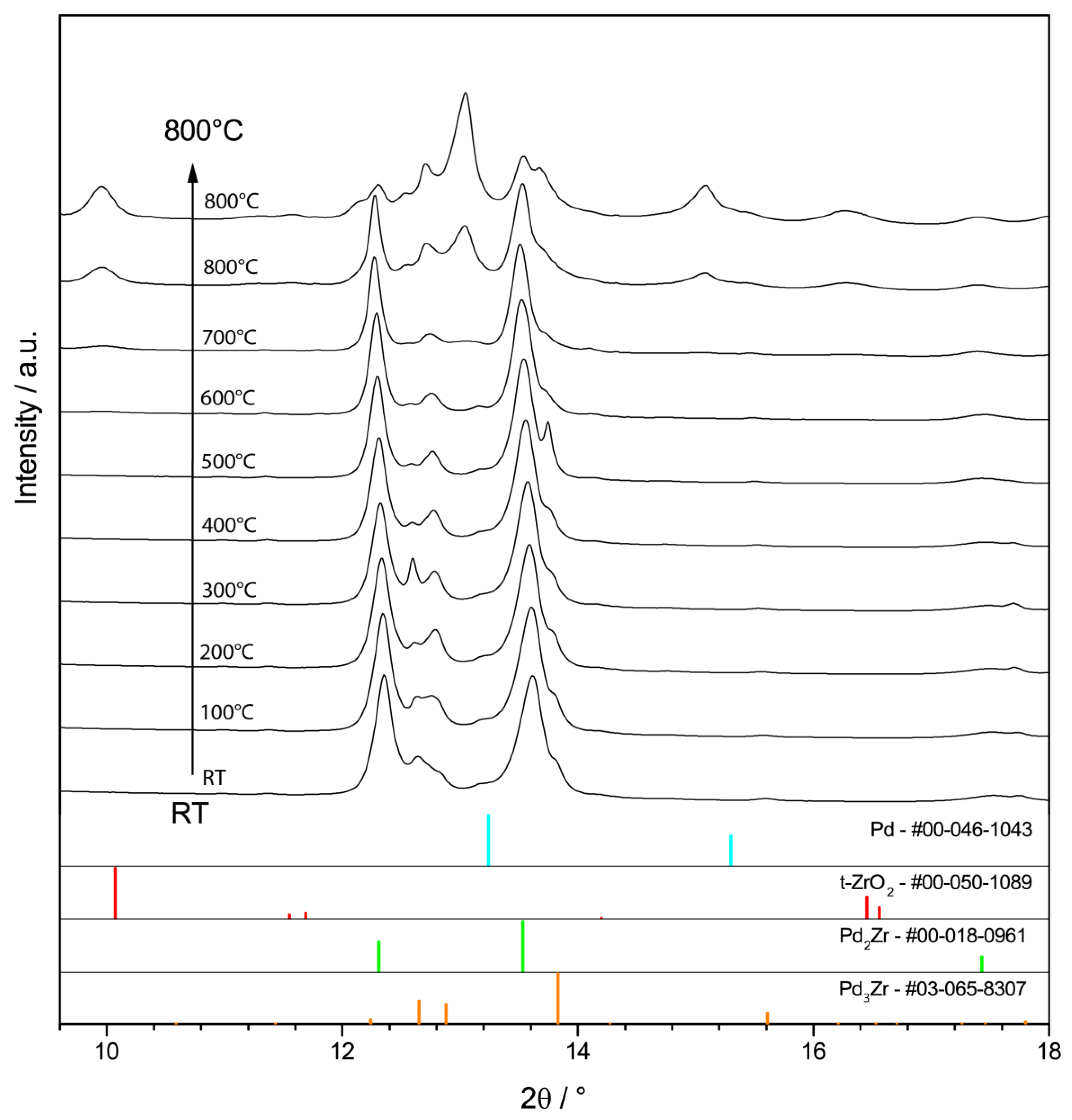
2.3.1. In-Situ Characterization of Precatalyst Activation
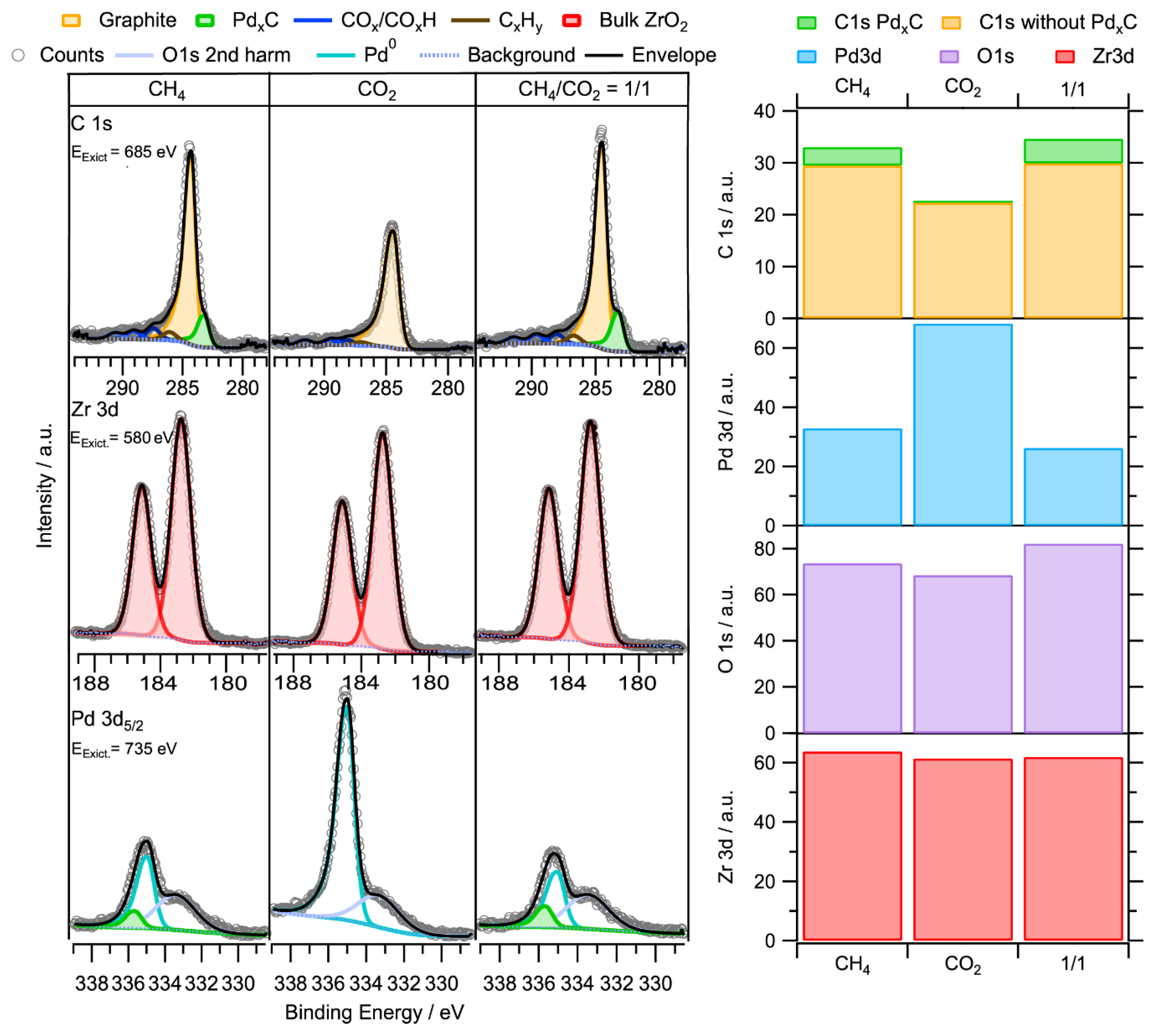
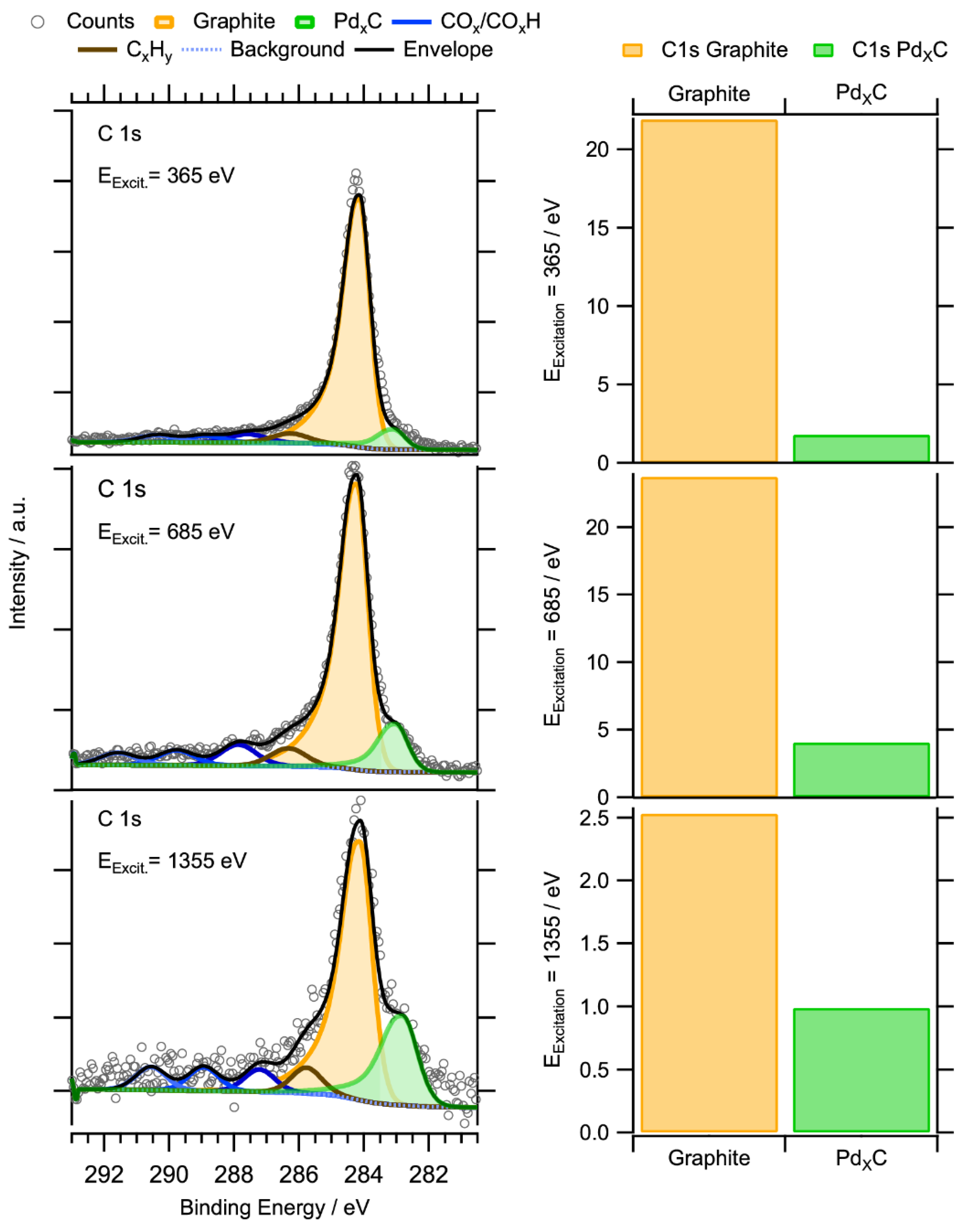
2.3.2. In-Situ NAP-XPS under Transient Reaction Conditions
Subsurface Zr0–Pd Foil Precatalyst
Bulk-Intermetallic PdxZry Precatalyst
3. Materials and Methods
3.1. Model Catalyst Preparation
3.2. Experimental Setups for in- and ex-Situ Characterization and Catalytic Testing of Model Catalysts
3.2.1. STM/XPS/LEIS/LEED Setup for Subsurface Alloy Characterization (TU Vienna)
3.2.2. Combined XPS/High Pressure Batch Reactor Setup (University of Innsbruck)
3.2.3. Recirculation Batch Reactor for Supported Powder Catalysts (University of Innsbruck)
3.2.4. In-Situ XRD at the Advanced Light Source (ALS) at LNBL
3.2.5. In-Situ NAP-XPS at ISISS End-Station at HZB/BESSYII
3.2.6. High-Resolution Transmission Electron Microscopy
3.3. Details of XPS Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alyea, E.C.; He, D.; Wang, J. Alcohol synthesis from syngas. Appl. Catal. A Gen. 1993, 104, 77–85. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.; Millar, G.J. Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: State of the art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Ghoneim, S.A.; El-Salamony, R.A.; El-Temtamy, S.A. Review on innovative catalytic reforming of natural gas to syngas. World J. Eng. Technol. 2016, 4, 116–139. [Google Scholar] [CrossRef]
- Minardi, E.; Chakraborty, S.; Curcio, S. Membrane reactors for dry reforming of methane. In Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier B.V., Radarweg 29, 1043 NX; Elsevier: Amsterdam, The Netherlands, 2015; pp. 99–144. [Google Scholar] [CrossRef]
- Bosko, M.; Múnera, J.; Lombardo, E.; Cornaglia, L. Dry reforming of methane in membrane reactors using Pd and Pd–Ag composite membranes on a NaA zeolite modified porous stainless steel support. J. Membr. Sci. 2010, 364, 17–26. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry reforming of methane has no future for hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrogen Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.; Hansen, J. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Bradford, M.C.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts I. Catalyst characterization and activity. Appl. Catal. A Gen. 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Sakai, Y.; Saito, H.; Sodesawa, T.; Nozaki, F. Catalytic reactions of hydrocarbon with carbon dioxide over metallic catalysts. React. Kinet. Catal. Lett. 1984, 24, 253–257. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Márquez-Álvarez, C.; Rodríguez-Ramos, I.; Schuurman, Y.; Ruiz, A.G.; Mirodatos, C. A transient kinetic study of the carbon dioxide reforming of methane over supported Ru catalysts. J. Catal. 1999, 184, 202–212. [Google Scholar] [CrossRef]
- Kehres, J.; Jakobsen, J.G.; Andreasen, J.W.; Wagner, J.B.; Liu, H.; Molenbroek, A.; Sehested, J.; Chorkendorff, I.; Vegge, T. Dynamical properties of a Ru/MgAl2O4 catalyst during reduction and dry methane reforming. J. Phys. Chem. C 2012, 116, 21407–21415. [Google Scholar] [CrossRef]
- Solymosi, F.; Kutsán, G.; Erdőhelyi, A. Catalytic reaction of CH4 with CO2 over alumina-supported Pt metals. Catal. Lett. 1991, 11, 149–156. [Google Scholar] [CrossRef]
- Bitter, J.; Seshan, K.; Lercher, J. Mono and Bifunctional Pathways of CO2/CH4 Reforming over Pt and Rh Based Catalysts. J. Catal. 1998, 176, 93–101. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, Y.-X.; Xie, Y.; Liu, C.-J. Carbon dioxide reforming of methane over glow discharge plasma-reduced Ir/Al2O3 catalyst. Catal. Commun. 2008, 9, 1558–1562. [Google Scholar] [CrossRef]
- Rameshan, C.; Li, H.; Anic, K.; Roiaz, M.; Pramhaas, V.; Blume, R.; Hävecker, M.; Knudsen, J.; Knop-Gericke, A.; Rupprechter, G.; et al. In situ NAP-XPS spectroscopy during methane dry reforming on ZrO2/Pt(111) inverse model catalyst. J. Phys. Condens. Matter 2018, 30, 264007. [Google Scholar] [CrossRef]
- Köpfle, N.; Götsch, T.; Grünbacher, M.; Carbonio, E.; Hävecker, M.; Knop-Gericke, A.; Schlicker, L.; Doran, A.; Kober, D.; Gurlo, A.; et al. Zirconium-assisted activation of palladium to boost syngas production by methane dry reforming. Angew. Chem. Int. Ed. 2018, 57, 14613–14618. [Google Scholar] [CrossRef]
- Steinhauer, B.; Reddy, K.M.; Radnik, J.; Martin, A. Development of Ni-Pd bimetallic catalysts for the utilization of carbon dioxide and methane by dry reforming. Appl. Catal. A Gen. 2009, 366, 333–341. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J.J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.L.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Liu, P.; Nørskov, J.K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001, 3, 3814–3818. [Google Scholar] [CrossRef]
- Gao, F.; Goodman, D.W. Pd–Au bimetallic catalysts: Understanding alloy effects from planar models and (supported) nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Foppa, L.; Silaghi, M.-C.; Larmier, K.; Comas-Vives, A. Intrinsic reactivity of Ni, Pd and Pt surfaces in dry reforming and competitive reactions: Insights from first principles calculations and microkinetic modeling simulations. J. Catal. 2016, 343, 196–207. [Google Scholar] [CrossRef]
- Bitter, J.; Seshan, K.; Lercher, J. The State of Zirconia Supported Platinum Catalysts for CO2/CH4 Reforming. J. Catal. 1997, 171, 279–286. [Google Scholar] [CrossRef]
- Foppa, L.; Margossian, T.; Kim, S.M.; Müller, C.R.; Coperet, C.; Larmier, K.; Comas-Vives, A. Contrasting the Role of Ni/Al2O3 Interfaces in Water–Gas Shift and Dry Reforming of Methane. J. Am. Chem. Soc. 2017, 139, 17128–17139. [Google Scholar] [CrossRef]
- Kühl, S.; Düdder, H.; Girgsdies, F.; Kähler, K.; Muhler, M.; Behrens, M. Perovskites as Precursors for Ni/La2O3Catalysts in the dry reforming of methane: Synthesis by constant pH co-precipitation, reduction mechanism and effect of Ru-doping. Z. Anorg. Allg. Chem. 2017, 643, 1088–1095. [Google Scholar] [CrossRef]
- Gallego, G.S.; Mondragón, F.; Tatibouët, J.-M.; Barrault, J.; Batiot-Dupeyrat, C. Carbon dioxide reforming of methane over La2NiO4 as catalyst precursor—Characterization of carbon deposition. Catal. Today 2008, 133, 200–209. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klötzer, B.; et al. In situ-determined catalytically active state of LaNiO3 in methane dry reforming. ACS Catal. 2019, 10, 1102–1112. [Google Scholar] [CrossRef]
- Armbruster, M. Intermetallic compounds in catalysis. Encycl. Catal. 2011. [Google Scholar] [CrossRef]
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A comparative discussion of the catalytic activity and CO2-Selectivity of Cu-Zr and Pd-Zr (Intermetallic) compounds in methanol steam reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef]
- Köpfle, N.; Mayr, L.; Lackner, P.; Schmid, M.; Schmidmair, D.; Götsch, T.; Penner, S.; Klötzer, B. Zirconium-palladium interactions during dry reforming of methane. ECS Trans. 2017, 78, 2419–2430. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic dry reforming of methane: Insights from model systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Li, H.; Choi, J.-I.J.; Mayr-Schmölzer, W.; Weilach, C.; Rameshan, C.; Mittendorfer, F.; Redinger, J.; Schmid, M.; Rupprechter, G. Growth of an ultrathin zirconia film on Pt3Zr examined by high-resolution X-ray photoelectron spectroscopy, temperature-programmed desorption, scanning tunneling microscopy, and density functional theory. J. Phys. Chem. C 2015, 119, 2462–2470. [Google Scholar] [CrossRef]
- Lackner, P.; Hulva, J.; Köck, E.-M.; Mayr-Schmölzer, W.; Choi, J.I.J.; Penner, S.; Diebold, U.; Mittendorfer, F.; Redinger, J.; Klötzer, B.; et al. Water adsorption at zirconia: From the ZrO2(111)/Pt3Zr(0001) model system to powder samples. J. Mater. Chem. A 2018, 6, 17587–17601. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef]
- Choi, J.I.J.; Mayr-Schmölzer, W.; Mittendorfer, F.; Redinger, J.; Diebold, U.; Schmid, M. The growth of ultra-thin zirconia films on Pd3Zr(0001). J. Phys. Condens. Matter 2014, 26, 225003. [Google Scholar] [CrossRef]
- Antlanger, M.; Mayr-Schmölzer, W.; Pavelec, J.; Mittendorfer, F.; Redinger, J.; Varga, P.; Diebold, U.; Schmid, M. Pt3Zr(0001): A substrate for growing well-ordered ultrathin zirconia films by oxidation. Phys. Rev. B 2012, 86, 035451. [Google Scholar] [CrossRef]
- Lackner, P.; Choi, J.I.J.; Diebold, U.; Schmid, M. Substoichiometric ultrathin zirconia films cause strong metal–support interaction. J. Mater. Chem. A 2019, 7, 24837–24846. [Google Scholar] [CrossRef]
- Konvicka, C.; Jeanvoine, Y.; Lundgren, E.; Kresse, G.; Schmid, M.; Hafner, J.; Varga, P. Surface and subsurface alloy formation of vanadium on Pd(111). Surf. Sci. 2000, 463, 199–210. [Google Scholar] [CrossRef]
- Niessen, A.K.; Miedema, A.R. The enthalpy effect on forming diluted solid solutions of two 4d and 5d transition metals. Ber. Bunsenges. Phys. Chem. 1983, 87, 717–725. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Klötzer, B.; Mayr, L.; Rameshan, R.; Zemlyanov, D.; Bernardi, J.; Föttinger, K.; Rupprechter, G. Surface modification processes during methane decomposition on Cu-promoted Ni–ZrO2 catalysts. Catal. Sci. Technol. 2014, 5, 967–978. [Google Scholar] [CrossRef]
- Belton, D.N. Electron spectroscopic identification of carbon species formed during diamond growth. J. Vac. Sci. Technol. A 1990, 8, 2353–2362. [Google Scholar] [CrossRef]
- Bastl, Z. X-Ray photoelectron spectroscopic studies of palladium dispersed on carbon surfaces modified by ion beams and plasmatic oxidation. Collect. Czechoslov. Chem. Commun. 1995, 60, 383–392. [Google Scholar] [CrossRef]
- Kogler, M.; Köck, E.-M.; Klötzer, B.; Perfler, L.; Penner, S. Surface reactivity of YSZ, Y2O3, and ZrO2 toward CO, CO2, and CH4: A comparative discussion. J. Phys. Chem. C 2016, 120, 3882–3898. [Google Scholar] [CrossRef]
- Balmes, O.; Resta, A.; Wermeille, D.; Felici, R.; Messing, M.E.; Deppert, K.; Liu, Z.; Grass, M.E.; Bluhm, H.; Van Rijn, R.; et al. Reversible formation of a PdCx phase in Pd nanoparticles upon CO and O2 exposure. Phys. Chem. Chem. Phys. 2012, 14, 4796–4801. [Google Scholar] [CrossRef]
- Teschner, D.; Borsodi, J.; Wootsch, A.; Révay, Z.; Hävecker, M.; Knop-Gericke, A.; Jackson, S.D.; Schlögl, R. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 2008, 320, 86–89. [Google Scholar] [CrossRef]
- Maciejewski, M.; Baiker, A. Incorporation of carbon into palladium during low-temperature disproportionation of carbon monoxide over palladium/zirconia prepared from glassy palladium-zirconium. J. Phys. Chem. 1994, 98, 285–290. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Rameshan, R.; Vonk, V.; Franz, D.; Drnec, J.; Penner, S.; Garhofer, A.; Mittendorfer, F.; Stierle, A.; Klötzer, B. Role of precursor carbides for graphene growth on Ni(111). Sci. Rep. 2018, 8, 2662. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.W.; Song, S.-H.; Chang, T.S.; Shin, C.-H.; Choi, M. Dry reforming of methane over cobalt catalysts: A literature review of catalyst development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Gadalla, A.M.; Bower, B. The role of catalyst support on the activity of nickel for reforming methane with CO2. Chem. Eng. Sci. 1988, 43, 3049–3062. [Google Scholar] [CrossRef]
- Dropsch, H.; Baerns, M. CO adsorption on supported Pd catalysts studied by adsorption microcalorimetry and temperature programmed desorption. Appl. Catal. A Gen. 1997, 158, 163–183. [Google Scholar] [CrossRef]
- Mayr, L.; Rameshan, R.; Klötzer, B.; Penner, S.; Rameshan, C. Combined UHV/high-pressure catalysis setup for depth-resolved near-surface spectroscopic characterization and catalytic testing of model catalysts. Rev. Sci. Instrum. 2014, 85, 055104. [Google Scholar] [CrossRef]
- Doran, A.; Schlicker, L.; Beavers, C.M.; Bhat, S.; Bekheet, M.F.; Gurlo, A. Compact low power infrared tube furnace for in situ X-ray powder diffraction. Rev. Sci. Instrum. 2017, 88, 013903. [Google Scholar] [CrossRef]
- Schlicker, L.; Doran, A.; Schneppmüller, P.; Gili, A.; Czasny, M.; Penner, S.; Gurlo, A. Transmission in situ and operando high temperature X-ray powder diffraction in variable gaseous environments. Rev. Sci. Instrum. 2018, 89, 033904. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Häusermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Bluhm, H.; Hävecker, M.; Knop-Gericke, A.; Kiskinova, M.; Schlogl, R.; Salmeron, M. In situ X-ray photoelectron spectroscopy studies of gas-solid interfaces at near-ambient conditions. MRS Bull. 2007, 32, 1022–1030. [Google Scholar] [CrossRef]
- CasaXPS Version 2.3.16 Pre-rel 1.4, Casa Software Ltd.: Teignmouth, UK, 2011.
- Scofield, J. Theoretical Photoionization Cross Sections from 1 to 1500 keV; Office of Scientific and Technical Information (OSTI), U.S. Department of Energy: Oak Ridge, TN, USA, 1973.
- Rodriguez, N.; Anderson, P.; Wootsch, A.; Wild, U.; Schlögl, R.; Paál, Z. XPS, EM, and catalytic studies of the accumulation of carbon on Pt black. J. Catal. 2001, 197, 365–377. [Google Scholar] [CrossRef]
- Ahn, B.S.; Jeon, S.G.; Lee, H.; Park, K.Y.; Shul, Y.-G. Hydrogenolysis of CFC-12 (CF2Cl2) over Pd/γ-Al2O3 pretreated with HCFC-22 (CHF2Cl). Appl. Catal. A Gen. 2000, 193, 87–93. [Google Scholar] [CrossRef]
- Gabasch, H.; Unterberger, W.; Hayek, K.; Klötzer, B.; Kleimenov, E.; Teschner, D.; Zafeiratos, S.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; et al. In situ XPS study of Pd(111) oxidation at elevated pressure, Part 2: Palladium oxidation in the 10−1 mbar range. Surf. Sci. 2006, 600, 2980–2989. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. NIST Electron Effective-Attenuation-Length Database SRD 82; Institute of Standards and Technology: Gaithersburg, MD, USA, 2011.
- Yeh, J.J. Atomic Calculation of Photoionization Cross-Sections and Asymmetry Parameters; Gordon and Breach Science Publishers: Langhorne, PE, USA, 1993. [Google Scholar]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Martinez-Huerta, M.; Fierro, J. The effect of CeO2 on the surface and catalytic properties of Pt/CeO2–ZrO2 catalysts for methane dry reforming. Appl. Catal. B Environ. 2009, 89, 149–159. [Google Scholar] [CrossRef]
- Makri, M.; Vasiliades, M.A.; Petallidou, K.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt% Ni/Ce1−xMxO2−δ (M=Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Simonov, M.N.; Rogov, V.A.; Smirnova, M.; Sadykov, V.A. Pulse microcalorimetry study of methane dry reforming reaction on Ni/Ceria-Zirconia catalyst. Catalysts 2017, 7, 268. [Google Scholar] [CrossRef]
- Grünbacher, M.; Götsch, T.; Opitz, A.K.; Klötzer, B.; Penner, S. CO2 reduction on the pre-reduced mixed ionic-electronic conducting Perovskites La0.6Sr0.4FeO3-δ and SrTi0.7Fe0.3O3-δ. ChemPhysChem 2017, 19, 93–107. [Google Scholar] [CrossRef]
- Opitz, A.K.; Nenning, A.; Rameshan, C.; Kubicek, M.; Götsch, T.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Rupprechter, G.; Klötzer, B.; et al. Surface chemistry of Perovskite-type electrodes during high temperature CO2 electrolysis investigated by operando photoelectron spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 35847–35860. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köpfle, N.; Ploner, K.; Lackner, P.; Götsch, T.; Thurner, C.; Carbonio, E.; Hävecker, M.; Knop-Gericke, A.; Schlicker, L.; Doran, A.; et al. Carbide-Modified Pd on ZrO2 as Active Phase for CO2-Reforming of Methane—A Model Phase Boundary Approach. Catalysts 2020, 10, 1000. https://doi.org/10.3390/catal10091000
Köpfle N, Ploner K, Lackner P, Götsch T, Thurner C, Carbonio E, Hävecker M, Knop-Gericke A, Schlicker L, Doran A, et al. Carbide-Modified Pd on ZrO2 as Active Phase for CO2-Reforming of Methane—A Model Phase Boundary Approach. Catalysts. 2020; 10(9):1000. https://doi.org/10.3390/catal10091000
Chicago/Turabian StyleKöpfle, Norbert, Kevin Ploner, Peter Lackner, Thomas Götsch, Christoph Thurner, Emilia Carbonio, Michael Hävecker, Axel Knop-Gericke, Lukas Schlicker, Andrew Doran, and et al. 2020. "Carbide-Modified Pd on ZrO2 as Active Phase for CO2-Reforming of Methane—A Model Phase Boundary Approach" Catalysts 10, no. 9: 1000. https://doi.org/10.3390/catal10091000
APA StyleKöpfle, N., Ploner, K., Lackner, P., Götsch, T., Thurner, C., Carbonio, E., Hävecker, M., Knop-Gericke, A., Schlicker, L., Doran, A., Kober, D., Gurlo, A., Willinger, M., Penner, S., Schmid, M., & Klötzer, B. (2020). Carbide-Modified Pd on ZrO2 as Active Phase for CO2-Reforming of Methane—A Model Phase Boundary Approach. Catalysts, 10(9), 1000. https://doi.org/10.3390/catal10091000





