Abstract
Plasma catalysis has recently gained traction as an alternative to ammonia synthesis. The current research is mostly fundamental and little attention has been given to the technical and economic feasibility of plasma-catalytic ammonia synthesis. In this study, the feasibility of plasma-catalytic ammonia is assessed for small-scale ammonia synthesis. A brief summary of the state of the art of plasma catalysis is provided as well as a targets and potential avenues for improvement in the conversion to ammonia, ammonia separation and a higher energy efficiency. A best-case scenario is provided for plasma-catalytic ammonia synthesis and this is compared to the Haber-Bosch ammonia process operated with a synthesis loop. An ammonia outlet concentration of at least 1.0 mol. % is required to limit the recycle size and to allow for efficient product separation. From the analysis, it follows that plasma-catalytic ammonia synthesis cannot compete with the conventional process even in the best-case scenario. Plasma catalysis potentially has a fast response to intermittent renewable electricity, although low pressure absorbent-enhanced Haber-Bosch processes are also expected to have fast responses to load variations. Low-temperature thermochemical ammonia synthesis is expected to be a more feasible alternative to intermittent decentralized ammonia synthesis than plasma-catalytic ammonia synthesis due to its superior energy efficiency.
1. Introduction
Renewable wind energy and solar power increasingly penetrate the electrical power grid, spurring the electrification of the energy landscape []. However, as these energy sources are intermittent, energy storage is required. A wide range of technology is available, including batteries and thermo-mechanical storage for short-term energy storage, typically up to a few days []. Chemical energy storage and pumped hydropower are the main alternatives for seasonal energy storage [,,]. Even though pumped hydropower is a potential solution for low-cost energy storage in naturally suited areas [], the energy density of such systems is low, and pumped hydropower heavily depends on the availability of large natural water formations.
Chemical energy storage in the form of hydrogen or hydrogen carriers has been proposed to solve the intermittency challenge. Renewable hydrogen is produced from water via electrolysis using renewable electricity, producing oxygen as a by-product. Hydrogen can be combusted to water in a fuel cell or in a gas turbine, producing electricity again. However, hydrogen is not easily stored on the long-term. Therefore, hydrogen carriers are required and ammonia (NH3) is one of the options [,]. Ammonia is currently mainly produced for fertilizer applications [,,]. However, ammonia may be a hydrogen carrier in the circular economy [,,]. In this case, intermittent renewables such as solar, tidal and wind power are coupled with chemical plants to produce ammonia.
Currently, ammonia is mainly produced with the Haber-Bosch process, continuously operated at high temperature and pressure, i.e., 400–500 °C and 100–300 bar [,,,]. The high temperature is required to activate the stable N≡N triple bond over the industrial catalyst, and a high pressure is required to shift the equilibrium to ammonia. Typically, Haber-Bosch plants have high production capacities of up to 3300 t-NH3 d−1, with a potential increase to 5000–6000 t-NH3 d−1 in the near future [,]. This corresponds to a few gigawatt (GW) in the case of an electricity-driven Haber-Bosch plant. Such electrolysis-based Haber-Bosch plants operate at an energy cost of about 26–40 GJ t-NH3−1, depending on the electrolysis technology used []. About 95% of this energy is required for the hydrogen production and the remaining part is required for nitrogen purification and ammonia synthesis. The theoretical minimum energy input for ammonia synthesis from air and water is 21.3 GJ t-NH3−1 [].
Upon scaling down the Haber-Bosch process, the energy losses increase and the energy consumption for ammonia synthesis increases (see Figure 1). Down to the 1–10 microwave (MW) scale (equivalent to 3–30 t-NH3 d−1), energy losses are limited and the energy consumption for ammonia synthesis is about 36–40 GJ t-NH3−1 []. However, upon further scaling down, energy losses become increasingly severe, and using alternative technologies becomes attractive. Non-conventional technologies typically cannot compete with Haber-Bosch at large-scale, due to the high energy efficiency of Haber-Bosch. However, alternative technologies operating under milder conditions can be beneficial for small-scale production. This is important for intermittent operation required in the case of rapid variation in the availability of solar and wind power. Small-scale ammonia synthesis may be relevant for energy storage, specifically for isolated small communities.
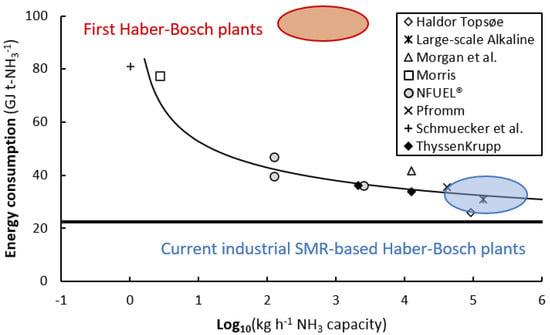
Figure 1.
Energy consumption of electrolysis-based Haber-Bosch processes as function of ammonia production capacity. Adapted and modified from []. Original references [,,,,,,,,,]. One microwave (MW) corresponds to approximately 100 kg h−1 ammonia.
1.1. State of the Art of Plasma-Catalytic Ammonia Synthesis
Plasma catalysis has recently gained attention for electrification of chemical processes and for energy storage applications [,,,,], and for ammonia synthesis in specific [,,]. Plasma is the fourth state of matter, in which electrons, ions, molecules, radicals, and excited species exist in a quasi-neutral state. In case of thermal plasmas, electrons and heavier species have the same temperature. On the other hand, non-thermal plasmas, the electron temperature is significantly higher than that of heavier ions and neutral species. Non-thermal plasmas can be coupled with a catalyst. Excited species generated in the plasma (for instance, vibrationally activated nitrogen, N2(v)) can have an enhanced adsorption rate as compared to ground-state molecules. As N2 dissociation is usually the bottleneck for ammonia synthesis, plasma activation of N2 may generate a synergistic effect with the catalyst.
Plasma catalysis potentially has a fast response to intermittent renewable electricity, although low pressure absorbent-enhanced Haber-Bosch processes are also expected to have fast responses to load variations. In the upcoming section, the state of the art of the electrolysis-based Haber-Bosch process and an alternative plasma-catalytic process are discussed. This serves as a starting point for process evaluation of plasma-catalytic ammonia synthesis.
An overview of reported energy consumptions for ammonia synthesis in various types of plasma reactors is shown in Figure 2. Among the plasma reactors, dielectric barrier discharge (DBD) reactors have been studied most extensively since the 2000s [,,]. Microwave (MW) and radiofrequency (RF) plasmas have been studied in the 1980s–1990s, although recently a few articles have been published on this subject as well []. Glow discharges, inspired by the commercial Birkeland-Eyde process for NOX production in the early 20th century, have been studied in the 1920s–1990s [,]. Other technologies, such as arc discharges and plasma jets have only been reported in a few studies, mostly with an exploratory character [,]. Various authors have discussed plasma reactors extensively [,,].
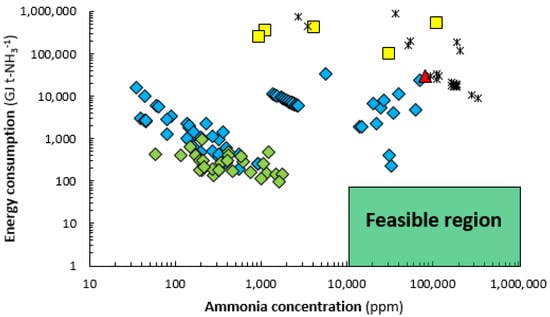
Figure 2.
Reported energy yield vs. ammonia concentration at various process conditions. Constructed and extended from []. Original references: dielectric barrier discharge (DBD) (alternating current, AC)  [,,,,,,,,,,,,,], DBD (pulse)
[,,,,,,,,,,,,,], DBD (pulse)  [,], glow discharge
[,], glow discharge  [], MW
[], MW  [,,,,] and radiofrequency (RF)
[,,,,] and radiofrequency (RF)  [,,,,,]. Note that the feed composition varies among the references, and therefore the ammonia yield. In case of a stoichiometric feed ratio, the ammonia outlet concentration is equal to the yield (at 0.0 mol. % NH3 inlet). The feasible region is discussed in Section 1.2.1.
[,,,,,]. Note that the feed composition varies among the references, and therefore the ammonia yield. In case of a stoichiometric feed ratio, the ammonia outlet concentration is equal to the yield (at 0.0 mol. % NH3 inlet). The feasible region is discussed in Section 1.2.1.
1.2. Comparison of the Small-Scale Haber-Bosch Processes and State-of-the-Art Plasma Catalysis
Figure 3 shows a comparison of reported state-of-the-art data on energy consumption of the electrolysis-based Haber-Bosch process, both at the 10 kW and 10 MW scale, as well as plasma-catalytic ammonia synthesis. Electrolysis-based hydrogen production is required for both the Haber-Bosch process and plasma catalysis at an energy consumption of about 31.4 GJ t-NH3−1 [,]. Nitrogen is purified for all alternatives via pressure swing adsorption at an energy consumption of about 1.0 GJ t-NH3 []. At the 10 MW scale, the high pressure ammonia synthesis loop of the Haber-Bosch process has a limited energy loss and consumes about 3.6 GJ t-NH−1 []. However, at 10 kW, the energy consumption of the high pressure ammonia synthesis loop is as high as 45–50 GJ t-NH3−1 (see Figure 3). This is mainly due to large heat losses from the synthesis reactor operating at high temperatures.
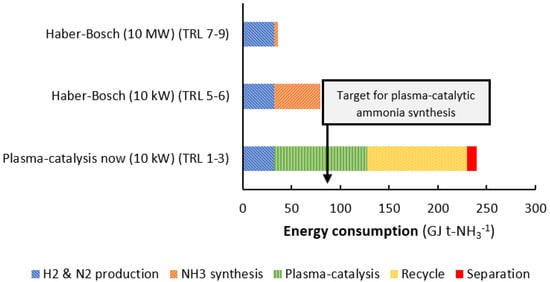
Figure 3.
Estimated energy consumption of state-of-the-art, small-scale, electrolysis-based Haber-Bosch, and plasma catalysis. For the Haber-Bosch process, estimates are based on reported energy consumption in Figure 1. For plasma catalysis, the energy consumption for gas recycling and ammonia separation is based on estimates in for low pressure, low conversion systems with solid sorbents as reported in [,]. The energy consumption for plasma catalysis is based on data from Kim et al. [] over a Ru-MgO/γ-Al2O3 catalyst at 0.2% conversion. The energy cost for recycling and separation with solid sorbents is discussed in Section 3.1. Technology readiness level (TRL). The TRL levels here apply for the complete system. TRL 1 is the basic idea, while TRL 9 is a commercial system. For more details, see ref. [].
As shown in Figure 3, the state-of-the-art system for a plasma-catalytic ammonia synthesis process has a considerably higher energy consumption than the electrolysis-based Haber-Bosch process, both at 10 kW and at 10 MW. Plasma catalysis allows for operation at milder conditions than conventional catalysis over the industrial iron catalyst. However, plasma catalysis is only beneficial when the energy consumption of a plasma-catalytic ammonia synthesis process is at least equal to the energy consumption of the electrolysis-based Haber-Bosch process at 10 kW (about 80 GJ t-NH3−1). The best reported value for plasma catalysis is 95 GJ t-NH3−1 at 0.2 mol. % NH3 [], while most reported values vary in the range between 103 and 106 GJ t-NH3−1 at various conversion levels (see Figure 2) []. Furthermore, the energy consumption of recycling of unconverted N2 and H2 for the best reported plasma-catalytic systems is high, due to the low conversion levels (see Figure 2). The recycling cost is usually not reported for plasma catalysis (like in Figure 2), but is significant in case of low conversions to ammonia, i.e., below 1.0 mol. % NH3 [].
1.2.1. Targets and Strategies for More Energy-Efficient Plasma-Catalytic Ammonia Synthesis
It is estimated that plasma catalysis may be competitive with the Haber-Bosch at a target total energy consumption of 80 GJ tNH3−1, based on the comparison with electrolysis-based Haber-Bosch process at 10 kW, equivalent to about ~10 kg t-NH3 d−1 production.
The energy costs of hydrogen production and nitrogen purification is the same for both processes and amount to about 32 GJ t-NH3−1. Ammonia separation in the Haber-Bosch process is achieved by condensation. However, under mild pressure (<70 bar), condensation is not feasible due to the high vapor pressure of ammonia under ambient conditions (about 7 bar) [,]. Therefore, sorbents are currently being developed for ammonia separation and storage, as discussed in Section 3.1. Typically, the energy consumption for ammonia separation with sorbents is about 10 GJ t-NH3−1, which changes little with ammonia concentration.
This implies that two functionalities primarily determine the feasibility of plasma-catalytic ammonia synthesis, namely (1) plasma catalysis, and (2) separation of NH3 and recycling of unconverted N2 and H2. In principle, the recycle cost is usually not an issue for industrial ammonia synthesis, as the energy cost of recycling is below 1 GJ t-NH3−1 at NH3 concentrations above 1.0 mol. % in the reactor outlet []. However, for the current best-reported data for plasma catalysis, the ammonia concentration is max 0.2% (see Figure 3), implying recycling costs as high as 100 GJ t-NH3−1. Clearly, increasing the NH3 concentration in the outlet of the plasma reactor to above 1.0%, at a reasonable energy cost, is desirable. Another benefit of increasing the conversion level and consequently the ammonia concentration is the reduction in capital investment for recycling, due to the smaller compressor. In the upcoming section, potential improvements for plasma catalysis are discussed.
2. Plasma Reactor and Catalyst Improvements to Plasma-Catalytic Ammonia Synthesis
The plasma reactor forms the core of the plasma-catalytic ammonia synthesis process. In this section, the plasma reactor types are evaluated, as well as the necessary coupling with a catalyst.
2.1. Plasma Reactor Type
The first choice to make is the plasma reactor type. While dielectric barrier discharge (DBD) reactors and glow discharge reactors operate at atmospheric pressure (or elevated pressures []), microwave (MW) reactors and radiofrequency (RF) reactors operate at sub-atmospheric pressures. In principle, operating at atmospheric pressures is beneficial, as vacuum conditions require expensive equipment and large volumes. Furthermore, separation of ammonia is difficult at low partial pressures.
Metrics to evaluate the plasma reactor type is the energy consumption for ammonia formation (requirement about 40 GJ t-NH3−1, see Figure 3), as well as the conversion (requirement ≥ 1.0 mol. % NH3). As shown in Figure 2, no data in the literature have attained this so far. It should be noted that the data shown in Figure 2 vary widely in temperature, pressure, H2:N2 ratio, and catalyst choice. However, the apparent general trend is that the energy consumption for ammonia formation is lowest in dielectric barrier discharge reactors. Other reactor types (glow discharges, microwave reactors, and radiofrequency reactors) have an energy consumption of one to four orders of magnitude higher. This may be attributed to the more effective plasma-catalyst coupling for a DBD reactor. Therefore, a dielectric barrier discharge reactor is the preferred plasma reactor type, with the current knowledge.
Plasma Optimization
Plasma optimization should be focused on maximizing the density of mildly activated molecular nitrogen species (N2(v) or N2(e)), rather than radical species, as the mildly activated molecular nitrogen species require less energy input than fully dissociated nitrogen radicals. Mild activation of N2 to N2(v) or N2(e) can primarily be achieved by a relatively low specific input energy (SIE). Upon increasing the SIE, the reduced electric field increases, thereby increasing the fraction of N2 dissociated in the plasma []. Furthermore, N2-rich feeds are beneficial for activating N2 rather than H2 [].
Various authors have reported an improved energy efficiency upon using pulsed plasmas in a dielectric barrier discharge reactor, rather than a continuous AC plasma [,]. For AC plasmas, excited N2 molecules are readily dissociated due to the continuous presence of activated species. This leads to dissociation and the formation of N radicals, which recombine with other species and form heat. For pulsed plasmas, less of the activated N2 is dissociated in the plasma due to climbing along the vibrational ladder []. Thus, a pulsed plasma dielectric barrier discharge reactor is preferred as the plasma reactor.
Further modifications can be made to the plasma properties, by changing the discharge frequency and the capacitive and discharge regimes. Plasma properties can be modified by the electrode material [,], as well as the dielectric constant in the reactor. Performance enhancement may be attained upon the physical mixture of the active catalyst with a dielectric material [], which can be attributed to a change in electron number density and average energy distribution.
2.2. Reaction Mechanisms and Catalyst Optimization
Several mechanisms towards ammonia synthesis in the presence of a plasma are being discussed, as previously reported in []. The various mechanisms for N2 activation are shown in Figure 4. The bond-dissociation energies of N2 and H2 are 945 kJ mol−1 and 436 kJ mol−1 respectively, which translates to 66.1 GJ t-NH3−1 at 100% efficient conversion to ammonia via complete dissociation. This implies that plasma-induced dissociation and subsequent radical reactions can never be sufficiently energy efficient to attain the required 40 GJ t-NH3−1 as explained in Section 1.2.1. Thus, a catalyst is required to aid in the dissociation of the molecules. As shown in Figure 4, the plasma can activate molecular N2 via vibrational or electronic excitation (denoted as N2(ex) in Figure 4), which lowers the barrier for N2 dissociation over the catalyst. Typically, catalysts which are able to dissociate N2 are also able to dissociate H2, while some mid-late transition metal catalysts such as Pd and Pt can dissociate H2, but not N2.
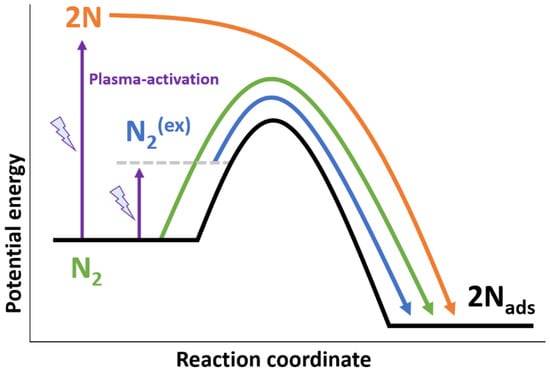
Figure 4.
Various mechanisms for N2 activation by the plasma and subsequent dissociative adsorption on a metal surface. Green: thermal N2 activation on a catalyst. Blue: plasma-activated molecular N2 dissociating over the metal with a barrier over the catalyst. Orange: complete dissociation of N2 by the plasma, followed by barrierless adsorption on the metal surface.
2.2.1. Plasma-Catalytic Ammonia Synthesis with Molecular Species
Thus, plasma activation of N2 without complete dissociation is the required pathway for plasma-catalytic ammonia synthesis with a low energy consumption. Mehta et al. [] proposed that plasma-activated molecular N2 can enhance the rate of ammonia formation, which was substantiated by Rouwenhorst et al. [] for Ru-based catalysts at a low plasma power input of 83–367 J L−1. Mehta et al. [] postulated that the plasma activates the N2, thereby lowering the barrier for N2 dissociation, while subsequent hydrogenation steps are not influenced by the plasma.
Furthermore, Mehta et al. [] postulated that thermally less active metals can become good catalysts for converting plasma-activated N2 to ammonia. However, the importance of plasma-activated molecular N2 (either vibrational or electronic) has been experimentally substantiated exclusively for Ru-based catalysts [,]. It remains an open question whether noble metals can become active for N2 dissociation after vibrational or electronic excitation. Over Ru-based catalysts, the apparent barrier for N2 dissociation over the catalyst decreases by about 40–70 kJ mol−1 upon plasma activation []. The discussion hereafter focuses on Ru-based catalysts, as a mechanism with excited N2 has only been proven to be possible for this metal. However, the role of the support and alkali promoters should also be valid for other metals [].
The support has a profound effect on the ammonia synthesis activity over Ru-catalysts []. The ammonia synthesis rate can vary by multiple orders of magnitude, depending on the catalyst formulation. For Ru catalysts it was found that oxide supports with a low electronegativity show a high ammonia synthesis activity for thermal catalysis []. A similar trend was confirmed for plasma catalysis (see Figure 5), suggesting that a pathway via excited molecular N2 is indeed dominant. The introduction of promoters (alkali metals and alkaline earth metals) can enhance the activity further [,]. The nitrogen dissociation barrier decreases upon the introduction of an alkali metal due to electrostatic interactions []. A similar activity improvement upon the introduction of an alkali metal was also reported for Co catalysts []. For this mechanism, the temperature should be such that N2 dissociation on the catalyst and NH3 desorption from the catalyst are possible. Thus, the temperature for plasma catalysis cannot be much lower than for thermal catalysis.
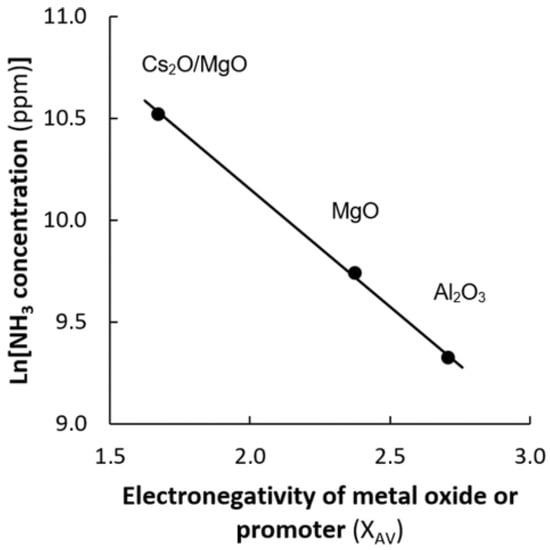
Figure 5.
Ammonia synthesis rate as a function of the electronegativity for plasma-catalytic ammonia synthesis. Reproduced from [].
The lowest reported energy requirement for plasma catalysis so far is for a promoted Ru/Al2O3 catalyst []. As listed in Table 1, trends in thermal catalysis indicate that changes in the catalyst may facilitate a lower energy cost for NH3 synthesis, given that the right plasma reactor is used. Promoted Ru/MgO catalysts are about 25 times more active than promoted Ru/Al2O3 catalysts for thermal catalysis []. As similar trends are valid for plasma catalysis at low conversions (see Figure 5), an increased productivity and a lower energy cost may be attained upon using promoted Ru/MgO catalysts. As listed in Table 1, the enhancement for plasma catalysis upon using promoted Ru/MgO is less profound at high conversions resulting in relatively high ammonia concentration (1.1–3.7 mol. % NH3).

Table 1.
Comparison of activity trends over Ru catalysts for thermal catalysis and for plasma catalysis. Thermal catalysis: data of Muhler et al. [], 385 °C, 1 atm, 40 mL min−1, H2:N2 = 3:1, 138 mg catalyst. Plasma catalysis: data of Ruan et al. [], 180 °C, 60 mL min−1, H2:N2 = 3:1, 1.1-3.7 mol. % NH3. Kim et al. [], 250–300 °C, 1 atm, 1–4 L min−1, H2:N2 = 1:4, 17.1 g catalyst, 0.01–0.16 mol. % NH3. Target value for plasma catalysis: 40 GJ t-NH3−1 at 1.0% NH3 at outlet. “Relative act.” refers to the relative ammonia synthesis rate on various catalysts, with Ru/Al2O3 as the base-case (1.0).
2.2.2. Best-Case Scenario for Plasma Catalysis
As shown in Figure 3, the energy costs of the state-of-the-art system for plasma catalysis and recycling are high (about 197 GJ t-NH3−1). Furthermore, the energy cost for recycling scales with the reciprocal of the single pass conversion. This implies that increasing the conversion by catalyst optimization and plasma optimization inherently decreases the energy cost of the recycle. An ammonia concentration of about 1.0 mol. % is required to effectively use the reactor volume for separation, as will be discussed in Section 3.1.
A target of 40 GJ t-NH3−1 at 1.0 mol. % ammonia outlet concentration was set for the plasma reactor (see Section 1.2.1). The lowest energy consumption reported so far is for a pulsed plasma DBD reactors with an energy consumption of 95 GJ t-NH3−1 at 0.16 mol. % over a Mg-Ru/Al2O3 catalyst []. Thus, an energy consumption enhancement by a factor 2.4 is required, whereas the conversion should increase by a factor 6.3 to attain feasible operation. Based on trends in thermal catalysis, a rate enhancement is expected by changing the catalyst from promoted Ru/Al2O3 to promoted Ru/MgO (see Table 1). It should be noted that the plasma activation of NH3 is an inherent limitation to the energy efficiency at high conversions, as NH3 is increasingly activated by the plasma with increasing NH3 concentration.
In order to get an estimate of the best-case scenario (BCS) for plasma-catalytic ammonia synthesis, we assume the barrier decrease for N2 dissociation equals the energy input for plasma-catalytic NH3 synthesis. The decrease in the N2 dissociation barrier over Ru catalysts is about 0.7 eV [], which translates to about 4.0 GJ t-NH3−1 or 1.1 kWh kg−1. From Figure 6, it follows that an ammonia outlet concentration of 0.35 mol. % is required to attain an energy cost of 40 GJ t-NH3−1. For 1.0 mol. % NH3 concentration at the reactor outlet, the total energy consumption for the plasma reactor (BCS) and the recycling is about 5.0 GJ t-NH3−1 (see Figure 6).
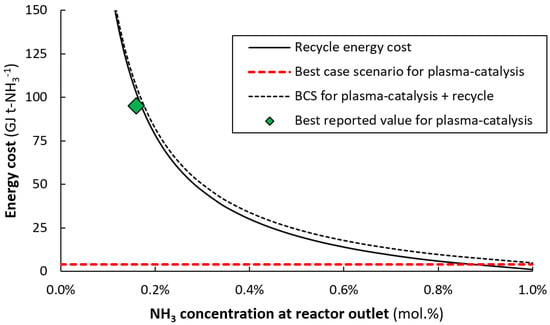
Figure 6.
Energy cost of the recycle as a function of the ammonia concentration at the reaction outlet, as well as the best reported value for plasma catalysis, and a best-case scenario for plasma catalysis. The energy cost of recycling is estimated based on values reported by Smith et al. []. The best reported value for plasma catalysis is found in the data of Kim et al. [].
3. Ammonia Separation and Conceptual Process Design
An evaluation of various process alternatives for ammonia separation in a plasma-catalytic ammonia synthesis process is presented. The cost of such a small-scale process is compared to a small-scale Haber-Bosch synthesis loop. As hydrogen and nitrogen production occur in a similar manner, this is not discussed here.
3.1. Separation and Storage
Ammonia is conventionally separated by condensation. However, this does not allow for efficient and complete separation at low operating pressures, as ammonia has a significant vapor pressure under ambient conditions (~7 bar). Therefore, an alternative method for ammonia separation is required for ammonia synthesis under mild pressures []. Solid sorbents can be used for this purpose and a wide range of materials has been tested [].
Among these sorbents, metal halides (e.g., metal chlorides and metal bromides) and zeolites are most promising (see Table 2). A benefit of metal halides over zeolites is the higher ammonia density of the storage material. Furthermore, ammonia can be separated at elevated temperatures using metal halides, minimizing the temperature difference between the plasma reactor and the separation step. This saves on the cost of heat integration between the plasma reactor (probably 200–300 °C) and the separation step, i.e., 150–250 °C for metal halides, as compared to 20–100 °C for zeolites. For low conversion systems such as plasma-catalytic ammonia synthesis, the investment cost of the recycle compressor and heat exchanger generally dominate the investment cost for the synthesis loop []. Therefore, metal halides are the preferred option in this case. However, it should be noted that zeolites are of interest if plasma-catalytic ammonia synthesis at room temperature is developed with plasma-activated N2 as the relevant species.

Table 2.
Comparison of ammonia separation technologies. Based on references [,,,,]. * The energy consumption increases to 20–25 GJ t-NH3−1 at 20 bar [].
Ammonia can be stored inside the metal halides. Metal halides absorb ammonia via diffusion of ammonia into the lattice, forming an ammine complex. For instance, ammonia can be absorbed into CaCl2 and MgCl2, forming Ca(NH3)XCl2 and Mg(NH3)XCl2. Ammonia leakage risks are reduced as compared to liquefied ammonia, as the ammonia vapor pressure in equilibrium with the ammonia loaded metal halides is significantly lower as compared to the vapor pressure of pure ammonia []. This decreases safety risks substantially. Thus, ammonia is stored inside metal halides, until ammonia is required for combustion in for instance a fuel cell or engine []. A simplified process scheme of a plasma-catalytic ammonia synthesis loop is shown in Figure 7. The adsorption-desorption cycle can be achieved by switching between multiple beds or by using moving bed reactors.
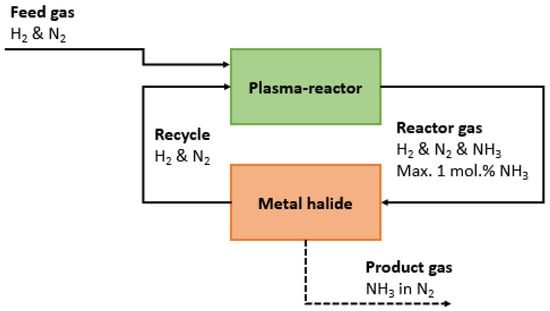
Figure 7.
Process scheme of a plasma-catalytic ammonia synthesis loop.
3.2. Synergy between Plasma Reactor and Ammonia Separation and Storage
As discussed above, synergy may be attained upon matching the operating temperature of the plasma reactor and the ammonia separator. The plasma reactor can operate at temperatures above the light-off temperature (typically > 150 °C), where a higher temperature implies a higher ammonia synthesis rate. On the other hand, upon increasing the temperature too much (i.e., above 300 °C), the benefits of plasma catalysis over heterogeneous catalysis without a plasma are negligible. Some catalysts show substantial thermal activity above 300 °C [,,,], which implies that the use of plasma is not necessary.
Among metal halides, MgCl2 shows suitable absorption-desorption cycles. At an ammonia partial pressure of 10 kPa and at 200 °C, two moles of ammonia can be absorbed in MgCl2, forming Mg(NH3)2Cl2. Upon increasing the temperature to 300 °C, all ammonia can be released again. As ammonia absorption is determined by the kinetic rate of the surface reaction [], maximizing the available MgCl2 surface area is beneficial. Supporting MgCl2 on an inert oxide can increase the available surface area of MgCl2. Furthermore, nano-pores are formed in the metal halide structure upon ammonia desorption. Possibly, the mechanical stability can be better maintained upon supporting MgCl2 on an inert oxide. Higher ammonia absorption capacities are obtained when loading metal halides on oxide supports []. MgCl2/SiO2 (40 wt. % MgCl2) is the best among the tested materials with an experimental sorbent capacity of about 5 wt. % ammonia at 200 °C, whereas the theoretical maximum is 11 wt. % ammonia.
The operating conditions and the energy consumption for the state-of-the-art plasma reactor and a best-case scenario (BCS) plasma reactor are listed in Table 3, as well as the operating conditions for ammonia separation with a MgCl2/SiO2 sorbent. Due to the similar reaction conditions in the plasma reactor and in the absorption step, heat integration can indeed be minimized. This simplifies the synthesis loop substantially in case of intermittent operation. From Table 3 it follows that the main improvement required is the energy consumption in the plasma reactor, as was also discussed in Section 2.2.2.

Table 3.
Synergy between plasma and ammonia separation and storage. * This includes the energy consumption for plasma-catalytic ammonia synthesis (PC) and the energy consumption for recycling (Rec). The energy consumption for recycling is based on the outlet NH3 concentration, based on interpolation of estimates in [,]. The state-of-the-art value for plasma catalysis is based on the data of Kim et al. [], while the best-case scenario is based on an energy input of 0.7 eV due to a decrease in the N2 dissociation barrier over Ru catalysts (see Section 2.2) []. The difference between the operating pressure of the plasma reactor and the separation step is to account for the pressure drop over the system.
3.3. Investment Cost Comparison
Most of the discussion so far has focused on the energy consumption in the ammonia synthesis loop, as the energy cost is usually the major cost contributor for electricity-driven ammonia synthesis []. Given that the energy consumption of small-scale systems is larger, the electricity cost is expected to be a major cost contributor in small-scale systems as well. However, investment costs can become substantial cost factors as well. In this section, an estimate is provided on the capital investment for plasma-catalytic ammonia synthesis as compared to a decentralized Haber-Bosch synthesis loop. As hydrogen and nitrogen are required for both a small-scale Haber-Bosch process and a plasma-catalytic ammonia synthesis process, the costs for hydrogen production and nitrogen purification are excluded in the analysis.
The capital investment for the ammonia synthesis loop scales with a cost-scaling factor of 0.6 [], while electrolyzers scale modularly. Thus, the contribution of the ammonia synthesis loop to the total cost increases upon scale-down. Figure 8 shows a comparison between the capital investment for the Haber-Bosch synthesis loop, the state-of-the-art plasma-catalytic ammonia synthesis loop, and a best-case scenario for the plasma-catalytic ammonia synthesis loop. For reference, the capital investment for a low-temperature, absorbent-enhanced Haber-Bosch synthesis loop is also shown. The equipment cost for the plasma generator is estimated to be 0.9 € W−1, based on estimates of van Rooij et al. []. The equipment cost of all other components are estimated, based on cost-scaling relations described in [].
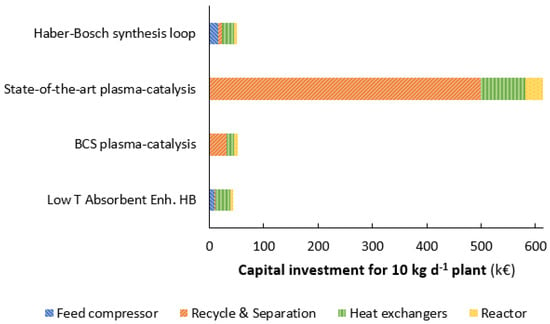
Figure 8.
Capital investment for the conventional Haber-Bosch synthesis loop, state-of-the-art plasma catalysis, a best-case scenario for plasma catalysis, and an absorbent-enhanced Haber-Bosch synthesis loop at 10 kg t-NH3 d−1 (~10 kW). Estimates for the conventional Haber-Bosch synthesis loop and the absorbent-enhanced Haber-Bosch synthesis loop are based on []. The cost of the plasma reactor is assumed to be the combination of a conventional reactor and a plasma generator source. A cost-scaling factor of 0.6 is used for scale-down of equipment. See also Table 3.
The investment cost of the state-of-the-art plasma-catalytic ammonia synthesis loop is about ten times as high as that of the small-scale Haber-Bosch synthesis loop. The main reason for this is the large recycle, implying a large compressor is required. Furthermore, separation of ammonia is less efficient at low ammonia concentrations, implying large equipment for ammonia absorption. Even for the best-case scenario with minimal heat exchange between the reactor and the sorbent, plasma-catalytic ammonia synthesis is equally expensive as the Haber-Bosch synthesis loop. The high-pressure Haber-Bosch synthesis loop requires a large feed compressor, as well as substantial heat integration between ammonia synthesis at 400–500 °C and ammonia separation at near-ambient temperature. On the other hand, the cost of ammonia separation and the recycle compressor are low due to high ammonia partial pressures and relatively high single pass conversions of about 15%. The best-case scenario plasma-catalytic synthesis loop operates at low pressures, thereby eliminating the requirement for a feed compressor. However, the recycle compressor is more expensive, due to low single pass conversions of about 1%. Furthermore, the low ammonia partial pressure also leads to more expensive ammonia separation equipment.
4. Plasma-Catalytic Ammonia Synthesis in Perspective
All in all, it can be concluded that plasma-catalytic ammonia synthesis does not provide significant investment cost advantages over the Haber-Bosch synthesis loop on a small scale (about 10 kW), even in the best base scenario (see Section 3.3). The energy consumption of the best-case scenario for plasma-catalytic ammonia synthesis is lower than that of the Haber-Bosch process at 10 kW (see Figure 9). At larger scale, the Haber-Bosch process is more energy efficient in any case (see Figure 3), implying plasma-catalytic ammonia synthesis is not a viable alternative.
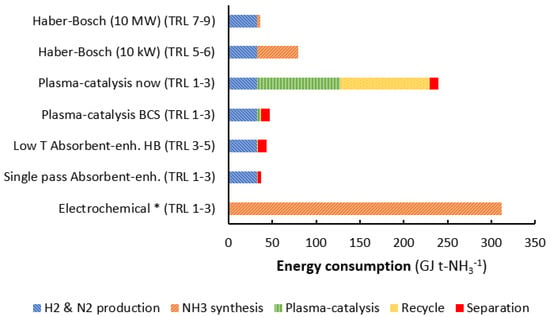
Figure 9.
Estimated energy consumption of state-of-the-art small-scale, electrolysis-based Haber-Bosch, plasma catalysis (also best-case scenario), absorbent-enhanced Haber-Bosch, single pass process absorbent-enhanced process, and electrochemical ammonia synthesis. Estimates based on [,,,]. For plasma catalysis, the energy consumption for gas recycling and ammonia separation is based on estimates in for low pressure (1.5 bar), low conversion (0.4 mol. % NH3) systems with solid sorbents (see also Section 3.1) [,]. * For electrochemical ammonia synthesis, only the energy consumption of ammonia production is included and the energy cost of ammonia separation and recycling is not included. TRL stands for technology readiness level. The TRL levels here apply for the complete system. TRL 1 is the basic idea, while TRL 9 is a commercial system. For more details, see ref. [].
In the previous sections, the focus was on improvements of plasma-catalytic ammonia synthesis. However, a wide range of technology is currently being researched as an alternative to the Haber-Bosch process on a small scale. Electrochemical synthesis, photochemical synthesis, homogeneous catalysis, and chemical looping approaches have been reported. The estimated state-of-the-art energy consumption for these technologies is shown in Figure 9. An extensive account on these technologies is given by Rouwenhorst et al. []. Electrochemical ammonia synthesis is often proposed as an alternative to ammonia synthesis under mild conditions. However, producing significant ammonia concentrations at a low energy cost has proven to be difficult [,,]. Even if ammonia is produced at a sufficiently low energy cost, the conversion levels and separation of ammonia from the electrolyte become key issues []. None of the other technologies can be implemented on a near-term, due to high energy cost and low ammonia yields obtained [].
Gradual improvements to the Haber-Bosch process have gained considerable research interest as well. The current industrial multiple promoted iron catalyst has been developed over the past century with minor changes in catalyst formulation and optimization of catalyst preparation [,]. On the other hand, activated carbon supported Ru catalysts (Ru/AC) have also been implemented in industry to a lesser extent []. The implementation of Ru/AC has been limited, due to a higher catalyst cost and a shorter catalyst lifetime than iron-based catalysts. For the industrial iron-based catalysts and the first generation of Ru-based catalysts (Ru/AC, Ru/Oxide), N2 dissociation is the rate limiting step, which can be enhanced by the introduction of alkali and alkaline earth promoters []. In various cases, ruthenium-based catalysts have been replaced by wüstite-based iron catalysts in ammonia converters, because these catalysts show similar activity []. A catalyst that is active at substantially lower temperatures is required to replace iron-based catalysts. Operation at lower temperatures can minimize heat loss to the surroundings during a small-scale operation and lower the required energy input.
Over the past years, Ru-based catalysts with substantially improved activity have been developed by using new support materials, such as electrides, among others [,,,,,,,,,,,]. The C12A7:e− structure used is an electride, stable at ambient temperature, consisting of a positively charged framework with the chemical formula [Ca24Al28O64]4+ and four extra-framework electrons, accommodated in the cages as counter ions []. It has been proposed that the N2 dissociation rate is enhanced on electride supported Ru catalyst, due to a small band gap between the valence band of the electride and the conduction band of the metal []. Furthermore, the hydrogen poisoning effect commonly encountered for Ru catalysts is thought to be suppressed []. For these catalysts, hydrogenation over the catalyst is the rate-limiting step, rather than the N2 dissociation step []. These catalysts show activity at 200–250 °C, similar to that of industrial Fe-based catalysts at 350–400 °C []. These highly active Ru-based catalysts are clearly a competitive alternative to a plasma reactor, as these catalysts are sufficiently active to decrease the reactor temperature similarly to plasma-catalytic ammonia synthesis. Furthermore, plasma catalysis can only be employed at a high energy efficiency (i.e., a low energy consumption), when ammonia conversions are low, as the plasma also activates the product and we expect NH3 concentrations should be limited to about 1.0 mol. % in order to limit plasma activation of the product. This problem is not present for the new generation of Ru-based catalysts, which no longer have the N2 dissociation as the rate-limiting step. Solid sorbents such as metal halides can also be combined with the new generation of Ru-based catalysts, thereby allowing to decrease the pressure of the ammonia synthesis loop to that of the hydrogen production and nitrogen purification pressure (about 7 bar) []. This is coined the absorbent-enhanced Haber-Bosch process.
Even for the best-case scenario (BCS) for plasma catalysis with a 5.8 times improvement in energy consumption for plasma catalysis (see Section 2.2.2), the energy consumption for a plasma-catalytic ammonia synthesis process is higher than for a low temperature synthesis, absorbent-enhanced Haber-Bosch process (see Figure 9). Furthermore, the capital investment for a plasma-catalytic process is expected to be higher than for the absorbent-enhanced Haber-Bosch process, because of the required larger heat exchanger and larger recycle compressor, due to a lower single pass conversion []. For reference, the electrolysis-based Haber-Bosch process at 10 MW has a power-to-ammonia efficiency of 52% (LHV), while the best-case scenario for plasma catalysis has a power-to-ammonia efficiency of 39% (LHV). The current energy consumption for plasma-catalytic ammonia synthesis is about 240 GJ t-NH3−1, leading to a power-to-ammonia efficiency as low as 8% (LHV).
5. Outlook
Plasma-catalytic ammonia synthesis does not appear to be a feasible alternative to long-term energy storage in NH3. In case of N2 activation for ammonia synthesis, alternative technologies under development for ammonia synthesis appear to be more feasible in the short term and in the long term (see Section 4). Ammonia synthesis from hydrogen and nitrogen is an exothermic reaction and an exergonic reaction and under most conditions [], implying plasma activation is, in principle, not desirable.
However, plasma catalysis is an interesting alternative to electrifying in the chemical industry, due to the ability of plasma to activate strong chemical bonds such as CH4, CO2 and N2 []. For instance, the bond dissociation energy of the N≡N is 9.79 eV, while electrons in DBD reactors typically have energies in the range 2–4 eV. The total barrier for CH4 activation can be decreased upon vibrational excitation [], thereby increasing the dissociative sticking probability []. Furthermore, plasma-assisted technologies have a fast response to electricity load variations. The plasma may provide very localized heating, thus limiting the heat requirement for the reactor. This can be beneficial for endergonic reactions. Lastly, plasma activation has the potential to provide process intensification. For instance, N2 activation with a plasma can be of interest for the direct synthesis of NOX [,], thereby eliminating the ammonia synthesis step altogether. As discussed in this article, the product outlet concentration should typically be above 1.0 mol. %, in order to limit the recycle size and to allow for efficient product separation.
Author Contributions
Conceptualization, K.H.R.R.; methodology, K.H.R.R., L.L.; formal analysis, K.H.R.R.; investigation, K.H.R.R.; writing—original draft preparation, K.H.R.R.; writing—review and editing, K.H.R.R., L.L.; visualization, K.H.R.R.; supervision, L.L.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Toeslag voor Topconsortia voor Kennis en Innovatie (TKI) from the Ministry of Economic Affairs and Climate Policy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Geem, K.M.; Galvita, V.V.; Marin, G.B. Making chemicals with electricity. Science 2019, 364, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Simon, E. Green ammonia. In Proceedings of the REFUEL Kickoff Meeting, Denver, CO, USA, 17–18 August 2017; Available online: https://arpa-e.energy.gov/sites/default/files/04cDenver-GreenAmmonia-Siemens-final.pdf (accessed on 1 September 2019).
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Hunt, J.D.; Byers, E.; Wada, Y.; Parkinson, S.; Gernaat, D.E.H.J.; Langan, S.J.; Van Vuuren, D.P.; Riahi, K. Global resource potential of seasonal pumped hydropower storage for energy and water storage. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, P. Catalyst: NH3 as an Energy Carrier. Chem 2017, 3, 709–712. [Google Scholar] [CrossRef]
- Nielsen, A. Ammonia: Catalysis and Manufacture, 1st ed.; Nielsen, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Liu, H. Ruthenium Based Ammonia Synthesis Catalysts; World Scientific Pub Co Pte Ltd.: Singapore, 2013; pp. 425–542. [Google Scholar]
- Jennings, J.M. Catalytic Ammonia Synthesis: Fundamentals and Practice, 1st ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Avery, W. A role for ammonia in the hydrogen economy. Int. J. Hydrogen Energy 1988, 13, 761–773. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Appl, M. Ammonia, 2. Production Processes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- European Fertilizer Manufacturers’ Association. Production of Ammonia. Best Available Techniques for Pollution Prevention and Control in the European Fertilizer Industry; European Fertilizer Manufacturers’ Association: Brussels, Belgium, 2000; Available online: https://www.ocinitrogen.com/MediaLibrary/Ammoniaprocess—BATProductionofammonia(2000)—Brochure.pdf (accessed on 1 September 2019).
- Brightling, J. Ammonia and the Fertiliser Industry: The Development of Ammonia at Billingham. Johns. Matthey Technol. Rev. 2018, 62, 32–47. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Ammonia, 4. Green Ammonia Production. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Proton Ventures, B.V. Sustainable ammonia for food and power. In Proceedings of the Nitrogen + Syngas, Gotheburg, Sweden, 6 February–1 March 2018; pp. 1–10. Available online: http://www.protonventures.com/wp-content/uploads/2018/09/NS-354-Small-scale-plant-design-PROTON-VENTURES-3-1.pdf (accessed on 1 September 2019).
- Rouwenhorst, K.H.R.; Van Der Ham, A.G.J.; Mul, G.; Kersten, S.R. Islanded ammonia power systems: Technology review & conceptual process design. Renew. Sustain. Energy Rev. 2019, 114. [Google Scholar] [CrossRef]
- Vrijenhoef, H. Dutch initiatives to store sustainable energy in the form of ammonia. In Proceedings of the NH3 Fuel Conference, Minneapolis, MN, USA, 1–2 November 2017; Available online: https://nh3fuelassociation.org/2017/09/25/dutch-initiatives-to-store-sustainable-energy-in-the-form-of-ammonia/ (accessed on 1 September 2019).
- Reese, M.; Marquart, C.; Malmali, M.; Wagner, K.; Buchanan, E.; McCormick, A.; Cussler, E.L. Performance of a Small-Scale Haber Process. Ind. Eng. Chem. Res. 2016, 55, 3742–3750. [Google Scholar] [CrossRef]
- Brown, T. Ammonia Technology Portfolio: Optimize for Energy Efficiency and Carbon Efficiency. Available online: https://ammoniaindustry.com/ammonia-technology-portfolio-optimize-for-energy-efficiency-and-carbon-efficiency/ (accessed on 23 March 2018).
- Morgan, E.R.; Manwell, J.F.; McGowan, J.G. Sustainable Ammonia Production from U.S. Offshore Wind Farms: A Techno-Economic Review. ACS Sustain. Chem. Eng. 2017, 5, 9554–9567. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards sustainable agriculture: Fossil-free ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef]
- Will, M. Realisation of Large-Scale Green Ammonia Plants. In Proceedings of the NH3 Fuel Conference, Pittsburgh, PA, USA, 31 October–1 November 2018. [Google Scholar]
- Will, M.; Lüke, L. Realisation of large-scale Green Ammonia plants. In Proceedings of the NH3 Event, Rotterdam, The Netherlands, 2–5 June 2018; Available online: https://nh3event.com/day-2/ (accessed on 15 July 2018).
- Vrijenhoef, J.P. Opportunities for Small scale Ammonia Production; International Fertiliser Society: London, UK, 2017; pp. 1–16. Available online: http://www.protonventures.com/wp-content/uploads/2017/07/Paper-Opportunities-for-small-scale-ammonia-production_ProtonVentures_HansVrijenhoef.pdf (accessed on 1 September 2019).
- Schmuecker, J.; Toyne, D. Making demonstration amounts of renewable ammonia and using it to fuel a farm tractor. In Proceedings of the NH3 Event, Rotterdam, The Netherlands, 6–7 June 2019. [Google Scholar]
- Hansen, J.B.; Han, P. The SOC4NH3 Project in Denmark. In Proceedings of the NH3 Event, Rotterdam, The Netherlands, 6–7 June 2019. [Google Scholar]
- Bogaerts, A.; Neyts, E.C. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Lett. 2018, 3, 1013–1027. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis Enabled by Plasma Activation of Strong Chemical Bonds: A Review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Brandenburg, R.; Bogaerts, A.; Bongers, W.; Fridman, A.; Fridman, G.; Locke, B.R.; Miller, V.; Reuter, S.; Schiorlin, M.; Verreycken, T.; et al. White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process. Polym. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 Plasma Catalysis Roadmap. J. Phys. D Appl. Phys. 2020, 53, 1–51. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Engelmann, Y.; van’t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-Driven catalysis: Green ammonia synthesis from intermittent electricity. Green Chem. 2020, in press. [Google Scholar]
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma Catalysis as an Alternative Route for Ammonia Production: Status, Mechanisms, and Prospects for Progress. ACS Sustain. Chem. Eng. 2017, 6, 15–31. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalytic ammonia synthesis: State of the art and future directions. J. Phys. D Appl. Phys. 2019, 52, 483001. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem. Plasma Process. 2015, 36, 45–72. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Schiappacasse, C.; Zhou, N.; Anderson, E.; Chen, D.; Liu, J.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; et al. A review on the non-thermal plasma-assisted ammonia synthesis technologies. J. Clean. Prod. 2018, 177, 597–609. [Google Scholar] [CrossRef]
- Peng, P.; Schiappacasse, C.; Zhou, N.; Addy, M.; Cheng, Y.; Zhang, Y.; Ding, K.; Wang, Y.; Chen, P.; Ruan, R. Sustainable Non-Thermal Plasma-Assisted Nitrogen Fixation—Synergistic Catalysis. ChemSusChem 2019, 12, 3702–3712. [Google Scholar] [CrossRef]
- Neyts, E.C. Plasma-Surface Interactions in Plasma Catalysis. Plasma Chem. Plasma Process. 2015, 36, 185–212. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Kim, H.-H.; Lefferts, L. Vibrationally Excited Activation of N2 in Plasma-Enhanced Catalytic Ammonia Synthesis: A Kinetic Analysis. ACS Sustain. Chem. Eng. 2019, 7, 17515–17522. [Google Scholar] [CrossRef]
- Barboun, P.M.; Mehta, P.; Herrera, F.A.; Go, D.B.; Schneider, W.F.; Hicks, J.C. Distinguishing Plasma Contributions to Catalyst Performance in Plasma-Assisted Ammonia Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 8621–8630. [Google Scholar] [CrossRef]
- Akay, G.; Zhang, K. Process Intensification in Ammonia Synthesis Using Novel Coassembled Supported Microporous Catalysts Promoted by Nonthermal Plasma. Ind. Eng. Chem. Res. 2017, 56, 457–468. [Google Scholar] [CrossRef]
- Peng, P.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; Zhou, N.; Schiappacasse, C.; Chen, D.; Zhang, Y.; Anderson, E.; Liu, Y.; et al. Ru-Based multifunctional mesoporous catalyst for low-pressure and non-thermal plasma synthesis of ammonia. Int. J. Hydrog. Energy 2017, 42, 19056–19066. [Google Scholar] [CrossRef]
- Li, S.; Van Raak, T.; Gallucci, F. Investigating the operation parameters for ammonia synthesis in dielectric barrier discharge reactors. J. Phys. D Appl. Phys. 2019, 53, 014008. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Atmospheric-Pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process. Polym. 2016, 14, 1600157. [Google Scholar] [CrossRef]
- Shah, J.R.; Gorky, F.; Lucero, J.; Carreon, M.A.; Carreon, M.L. Ammonia Synthesis via Atmospheric Plasma Catalysis: Zeolite 5A, a Case of Study. Ind. Eng. Chem. Res. 2020, 59, 5167–5176. [Google Scholar] [CrossRef]
- Iwamoto, M.; Akiyama, M.; Aihara, K.; Deguchi, T. Ammonia Synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu Electrode Catalysts in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. ACS Catal. 2017, 7, 6924–6929. [Google Scholar] [CrossRef]
- Hong, J.; Prawer, S.; Murphy, A.B. Production of Ammonia by Heterogeneous Catalysis in a Packed-Bed Dielectric-Barrier Discharge: Influence of Argon Addition and Voltage. IEEE Trans. Plasma Sci. 2014, 42, 2338–2339. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.M.; Cotrino, J.; Lambert, R.M.; González-Elipe, A.R. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 2015, 24, 65011. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.M.; Montoro-Damas, A.M.; Cotrino, J.; Lambert, R.M.; González-Elipe, A.R. About the enhancement of chemical yield during the atmospheric plasma synthesis of ammonia in a ferroelectric packed bed reactor. Plasma Process. Polym. 2016, 14, 1600081. [Google Scholar] [CrossRef]
- Mizushima, T.; Matsumoto, K.; Sugoh, J.-I.; Ohkita, H.; Kakuta, N. Tubular membrane-like catalyst for reactor with dielectric-barrier-discharge plasma and its performance in ammonia synthesis. Appl. Catal. A Gen. 2004, 265, 53–59. [Google Scholar] [CrossRef]
- Srinath, N.V. Plasma Catalytic Ammonia Synthesis at Atmospheric Pressure in a Dielectric Barrier Discharge Reactor; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2017; Available online: https://pure.tue.nl/ws/files/91518802/Afstudeerverslag_Nadadur_Veeraraghavan_Srinath_0980194_.pdf (accessed on 1 September 2019).
- Patil, B.S. Plasma (Catalyst)—Assisted Nitrogen Fixation: Reactor Development for Nitric Oxide and Ammonia Production; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2017; Available online: https://pure.tue.nl/ws/files/64000562/20170510_Patil.pdf (accessed on 1 September 2019).
- Peng, P.; Li, Y.; Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Atmospheric Pressure Ammonia Synthesis Using Non-Thermal Plasma Assisted Catalysis. Plasma Chem. Plasma Process. 2016, 36, 1201–1210. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Addy, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Schiappacasse, C.; Zhang, Y.; Chen, D.; Hatzenbeller, R.; et al. Atmospheric Plasma-Assisted Ammonia Synthesis Enhanced via Synergistic Catalytic Absorption. ACS Sustain. Chem. Eng. 2018, 7, 100–104. [Google Scholar] [CrossRef]
- Yin, K.S.; Venugopalan, M. Plasma chemical synthesis. I. Effect of electrode material on the synthesis of ammonia. Plasma Chem. Plasma Process. 1983, 3, 343–350. [Google Scholar] [CrossRef]
- Wildfire, C.; Abdelsayed, V.; Shekhawat, D.; Spencer, M.J. Ambient pressure synthesis of ammonia using a microwave reactor. Catal. Commun. 2018, 115, 64–67. [Google Scholar] [CrossRef]
- Uyama, H.; Nakamura, T.; Tanaka, S.; Matsumoto, O. Catalytic effect of iron wires on the syntheses of ammonia and hydrazine in a radio-frequency discharge. Plasma Chem. Plasma Process. 1993, 13, 117–131. [Google Scholar] [CrossRef]
- Nakajima, J.; Sekiguchi, H. Synthesis of ammonia using microwave discharge at atmospheric pressure. Thin Solid Films 2008, 516, 4446–4451. [Google Scholar] [CrossRef]
- Bai, X.; Tiwari, S.; Robinson, B.; Killmer, C.P.; Li, L.; Hu, J. Microwave catalytic synthesis of ammonia from methane and nitrogen. Catal. Sci. Technol. 2018, 8, 6302–6305. [Google Scholar] [CrossRef]
- Siemsen, L.G. The Synthesis of Ammonia from Hydrogen and Atomic Nitrogen on the Rh(110) Surface; Iowa State University: Ames, IA, USA, 1990; Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=12220&context=rtd (accessed on 1 September 2019).
- Shah, J.; Gorky, F.; Psarras, P.; Seong, B.; Gómez-Gualdrón, D.A.; Carreon, M.L. Ammonia yield enhancement by hydrogen sink effect during plasma catalysis. ChemCatChem 2019, 12, 1200–1211. [Google Scholar] [CrossRef]
- Shah, J.; Wu, T.; Lucero, J.; Carreon, M.A.; Carreon, M.L. Nonthermal Plasma Synthesis of Ammonia over Ni-MOF-74. ACS Sustain. Chem. Eng. 2018, 7, 377–383. [Google Scholar] [CrossRef]
- Shah, J.; Wang, W.; Bogaerts, A.; Carreon, M.L. Ammonia Synthesis by Radio Frequency Plasma Catalysis: Revealing the Underlying Mechanisms. ACS Appl. Energy Mater. 2018, 1, 4824–4839. [Google Scholar] [CrossRef]
- Uyama, H.; Uchikura, T.; Niijima, H.; Matsumoto, O. Synthesis of ammonia with RF discharge. Adsorption of products on zeolite. Chem. Lett. 1987, 16, 555–558. [Google Scholar] [CrossRef]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. Plasma Chem. Plasma Process. 1989, 9, 13–24. [Google Scholar] [CrossRef]
- Shah, J.; Harrison, J.M.; Carreon, M.L. Ammonia Plasma-Catalytic Synthesis Using Low Melting Point Alloys. Catalysts 2018, 8, 437. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Green Ammonia Production. In Techno-Economic Challenges of Green Ammonia as Energy Vector; Bañares-Alcántara, R., Valera-Medina, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Buchner, G.A.; Stepputat, K.J.; Zimmermann, A.W.; Schomäcker, R. Specifying Technology Readiness Levels for the Chemical Industry. Ind. Eng. Chem. Res. 2019, 58, 6957–6969. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U.; Xue, B.; Zhou, L.-M. Hydrogenation of Carbon Dioxide to Methanol with a Discharge-Activated Catalyst. Ind. Eng. Chem. Res. 1998, 37, 3350–3357. [Google Scholar] [CrossRef]
- Teramoto, Y.; Kim, H.H. Effect of vibrationally excited N2(v) on atomic nitrogen generation using two consecutive pulse corona discharges under atmospheric pressure N2. J. Phys. D Appl. Phys. 2019, 52, 494003. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Herrera, F.; Kim, J.; Rumbach, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 2018, 1, 269–275. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Burbach, H.G.B.; Núñez Paulí, J.; Vogel, D.W.; Geerdink, B.; Lefferts, L. Plasma-Catalytic Ammonia Synthesis beyond Thermal Equilibrium over Ru-based Catalysts. In preparation.
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J. Exploring the limits: A low-pressure, low-temperature Haber-Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Aika, K.-I. Role of alkali promoter in ammonia synthesis over ruthenium catalysts—Effect on reaction mechanism. Catal. Today 2017, 286, 14–20. [Google Scholar] [CrossRef]
- Ozaki, A. Development of alkali-promoted ruthenium as a novel catalyst for ammonia synthesis. Acc. Chem. Res. 1981, 14, 16–21. [Google Scholar] [CrossRef]
- Mortensen, J.J.; Hammer, B.; Nørskov, J.K. Alkali Promotion of N2 Dissociation over Ru(0001). Phys. Rev. Lett. 1998, 80, 4333–4336. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Tokunari, M.; Taniguchi, T.; Ooya, K.; Abe, H.; Niwa, Y.; Sasase, M.; Hara, M.; Hosono, H. Direct Activation of Cobalt Catalyst by 12CaO·7Al2O3 Electride for Ammonia Synthesis. ACS Catal. 2019, 9, 1670–1679. [Google Scholar] [CrossRef]
- Muhler, M.; Rosowski, F.; Hinrichsen, O.; Hornung, A.; Ertl, G. Ruthenium as catalyst for ammonia synthesis. Stud. Surf. Sci. Catal. 1996, 101, 317–326. [Google Scholar] [CrossRef]
- Ruan, R.; Deng, S.; Le, Z.; Chen, P. Non-Thermal Plasma Synthesis of Ammonia; United States, 2009. Available online: https://patentimages.storage.googleapis.com/b8/04/25/03baed26ca11d4/WO2009025835A1.pdf (accessed on 1 September 2019).
- Liu, C.Y.; Aika, K.-I. Effect of the Cl/Br Molar Ratio of a CaCl2−CaBr2 Mixture Used as an Ammonia Storage Material. Ind. Eng. Chem. Res. 2004, 43, 6994–7000. [Google Scholar] [CrossRef]
- Beach, J.D.; Kintner, J.D.; Welch, A.W. Removal of Gaseous NH3 from an NH3 Reactor Product Stream. United States, 2018. Available online: https://patentimages.storage.googleapis.com/62/ef/a4/83ada6d54f30fe/US20180339911A1.pdf (accessed on 1 September 2019).
- Malmali, M.; Le, G.; Hendrickson, J.; Prince, J.; McCormick, A.V.; Cussler, E.L. Better Absorbents for Ammonia Separation. ACS Sustain. Chem. Eng. 2018, 6, 6536–6546. [Google Scholar] [CrossRef]
- Liu, C.Y.; Aika, K.-I. Modification of active carbon and zeolite as ammonia separation materials for a new de-NOx process with ammonia on-site synthesis. Res. Chem. Intermed. 2002, 28, 409–417. [Google Scholar] [CrossRef]
- Zhang, T.; Miyaoka, H.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Review on Ammonia Absorption Materials: Metal Hydrides, Halides, and Borohydrides. ACS Appl. Energy Mater. 2018, 1, 232–242. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-Organized Ruthenium-Barium Core-Shell Nanoparticles on a Mesoporous Calcium Amide Matrix for Efficient Low-Temperature Ammonia Synthesis. Angew. Chem. 2018, 130, 2678–2682. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.-W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Zhao, Y.; Zhang, T. The Journey toward Low Temperature, Low Pressure Catalytic Nitrogen Fixation. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Smith, C.; Malmali, M.; Liu, C.-Y.; McCormick, A.V.; Cussler, E.L. Rates of Ammonia Absorption and Release in Calcium Chloride. ACS Sustain. Chem. Eng. 2018, 6, 11827–11835. [Google Scholar] [CrossRef]
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrog. Energy 2020, 45, 1541–1558. [Google Scholar] [CrossRef]
- Van Rooij, G.J.; Akse, H.N.; A Bongers, W.A.; Van De Sanden, M.C.M. Plasma for electrification of chemical industry: A case study on CO2 reduction. Plasma Phys. Control. Fusion 2017, 60, 014019. [Google Scholar] [CrossRef]
- Singh, A.R.; Rohr, B.A.; Statt, M.J.; Schwalbe, J.A.; Cargnello, M.; Nørskov, J.K. Strategies toward Selective Electrochemical Ammonia Synthesis. ACS Catal. 2019, 9, 8316–8324. [Google Scholar] [CrossRef]
- Andersen, S.Z.; Čolić, V.; Yang, S.; Schwalbe, J.A.; Nielander, A.C.; McEnaney, J.M.; Enemark-Rasmussen, K.; Baker, J.; Singh, A.R.; Rohr, B.A.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Nørskov, J.K.; Chorkendorff, I. The Difficulty of Proving Electrochemical Ammonia Synthesis. ACS Energy Lett. 2019, 4, 2986–2988. [Google Scholar] [CrossRef]
- Hollevoet, L.; De Ras, M.; Roeffaers, M.; Hofkens, J.; Martens, J.A. Energy-Efficient Ammonia Production from Air and Water Using Electrocatalysts with Limited Faradaic Efficiency. ACS Energy Lett. 2020, 5, 1124–1127. [Google Scholar] [CrossRef]
- Liu, H.; Han, W.; Huo, C.; Cen, Y. Development and application of wüstite-based ammonia synthesis catalysts. Catal. Today 2019, 1–18. [Google Scholar] [CrossRef]
- Mittasch, A.; Frankenburg, W. Early Studies of Multicomponent Catalysts. Adv. Catal. 1950, 2, 81–104. [Google Scholar] [CrossRef]
- Hattori, M.; Iijima, S.; Nakao, T.; Hosono, H.; Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kammert, J.; Moon, J.; Cheng, Y.; Daemen, L.L.; Irle, S.; Fung, V.; Liu, J.; Page, K.; Ma, X.; Phaneuf, V.; et al. Nature of Reactive Hydrogen for Ammonia Synthesis over a Ru/C12A7 Electride Catalyst. J. Am. Chem. Soc. 2020, 142, 7655–7667. [Google Scholar] [CrossRef]
- Hara, M.; Kitano, M.; Hosono, H. Ru-Loaded C12A7:e− Electride as a Catalyst for Ammonia Synthesis. ACS Catal. 2017, 7, 2313–2324. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kawamura, S.; Kitano, M.; Yokoyama, T.; Hosono, H. Kinetic evidence: The rate-determining step for ammonia synthesis over electride-supported Ru catalysts is no longer the nitrogen dissociation step. Catal. Sci. Technol. 2017, 7, 47–50. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Kitano, M.; Wang, J.; Ye, T.-N.; Li, J.; Kobayashi, Y.; Kishida, K.; Abe, H.; Niwa, Y.; et al. Ternary intermetallic LaCoSi as a catalyst for N2 activation. Nat. Catal. 2018, 1, 178–185. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Gong, Y.; Kitano, M.; Inoshita, T.; Hosono, H. Intermetallic Electride Catalyst as a Platform for Ammonia Synthesis. Angew. Chem. Int. Ed. 2019, 58, 825–829. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Kishida, K.; Abe, H.; Niwa, Y.; Sasase, M.; Fujita, Y.; Ishikawa, H.; Yokoyama, T.; Hara, M.; et al. Efficient and Stable Ammonia Synthesis by Self-Organized Flat Ru Nanoparticles on Calcium Amide. ACS Catal. 2016, 6, 7577–7584. [Google Scholar] [CrossRef]
- Gao, W.; Wang, P.; Guo, J.; Chang, F.; He, T.; Wang, Q.; Wu, G.; Chen, P. Barium Hydride-Mediated Nitrogen Transfer and Hydrogenation for Ammonia Synthesis: A Case Study of Cobalt. ACS Catal. 2017, 7, 3654–3661. [Google Scholar] [CrossRef]
- Matsuishi, S.; Toda, Y.; Miyakawa, M.; Hayashi, K.; Kamiya, T.; Hirano, M.; Tanaka, I.; Hosono, H. High-Density Electron Anions in a Nanoporous Single Crystal: [Ca24Al28O64]4+(4e-). Science 2003, 301, 626–629. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Jaramillo, T.F.; Nørskov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef]
- Jiao, F.; Xu, B. Electrochemical Ammonia Synthesis and Ammonia Fuel Cells. Adv. Mater. 2018, 31, 1–5. [Google Scholar] [CrossRef]
- Smith, R.R.; Killelea, D.R.; DelSesto, D.F.; Utz, A.L. Preference for Vibrational over Translational Energy in a Gas-Surface Reaction. Science 2004, 304, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Holmblad, P.M.; Wambach, J.; Chorkendorff, I. Molecular beam study of dissociative sticking of methane on Ni(100). J. Chem. Phys. 1995, 102, 8255–8263. [Google Scholar] [CrossRef][Green Version]
- Patil, B.S.; Hessel, V.; Seefeldt, L.C.; Dean, D.R.; Hoffman, B.M.; Cook, B.J.; Murray, L.J. Nitrogen Fixation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Patil, B.S.; Wang, Q.; Hessel, V.; Lang, J. Plasma N2-fixation: 1900–2014. Catal. Today 2015, 256, 49–66. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).