Electrospun Active Media Based on Polyvinylidene Fluoride (PVDF)-Graphene-TiO2 Nanocomposite Materials for Methanol and Acetaldehyde Gas-Phase Abatement
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials and Mats Characterization
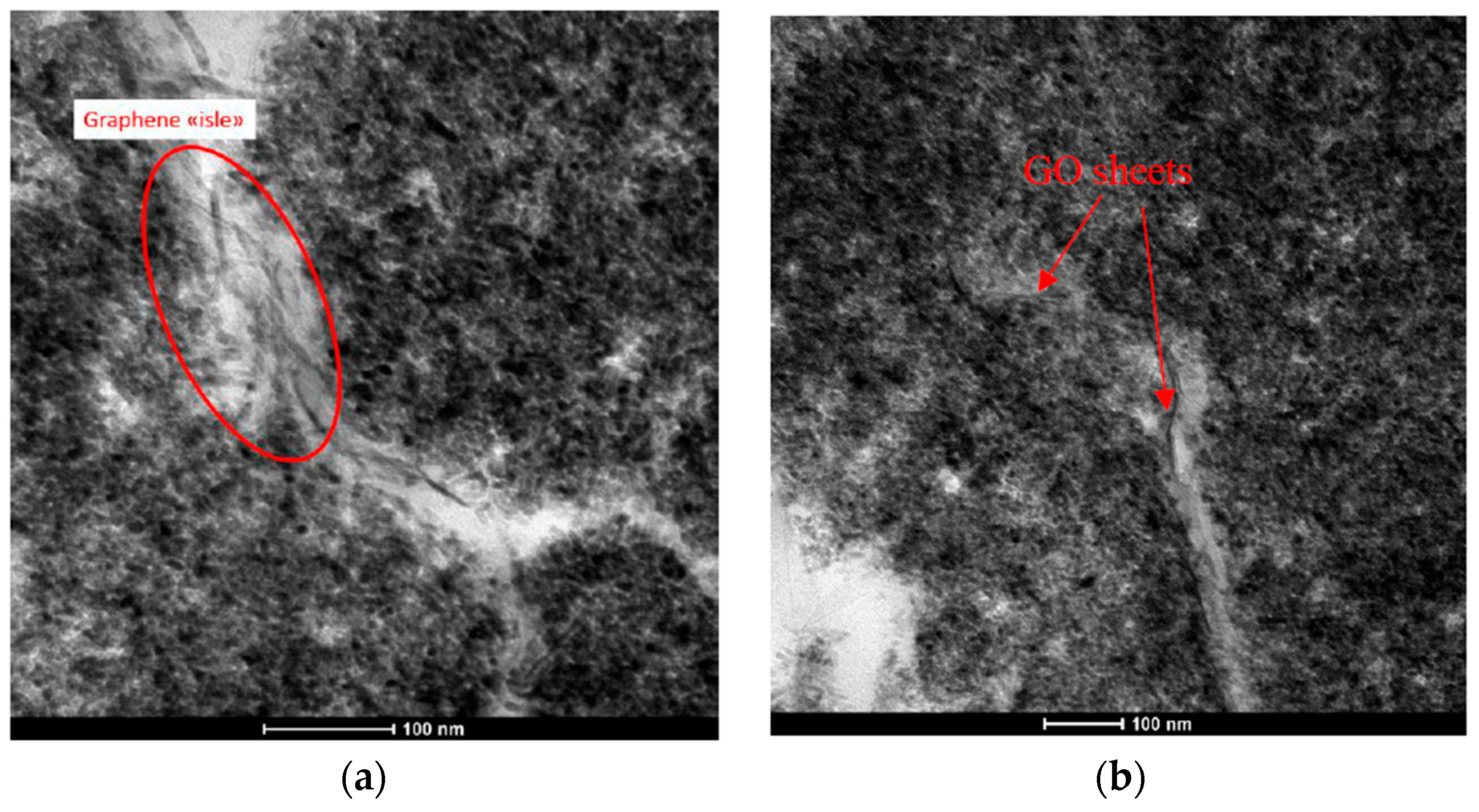
2.1.1. Photo-Catalytic Systems
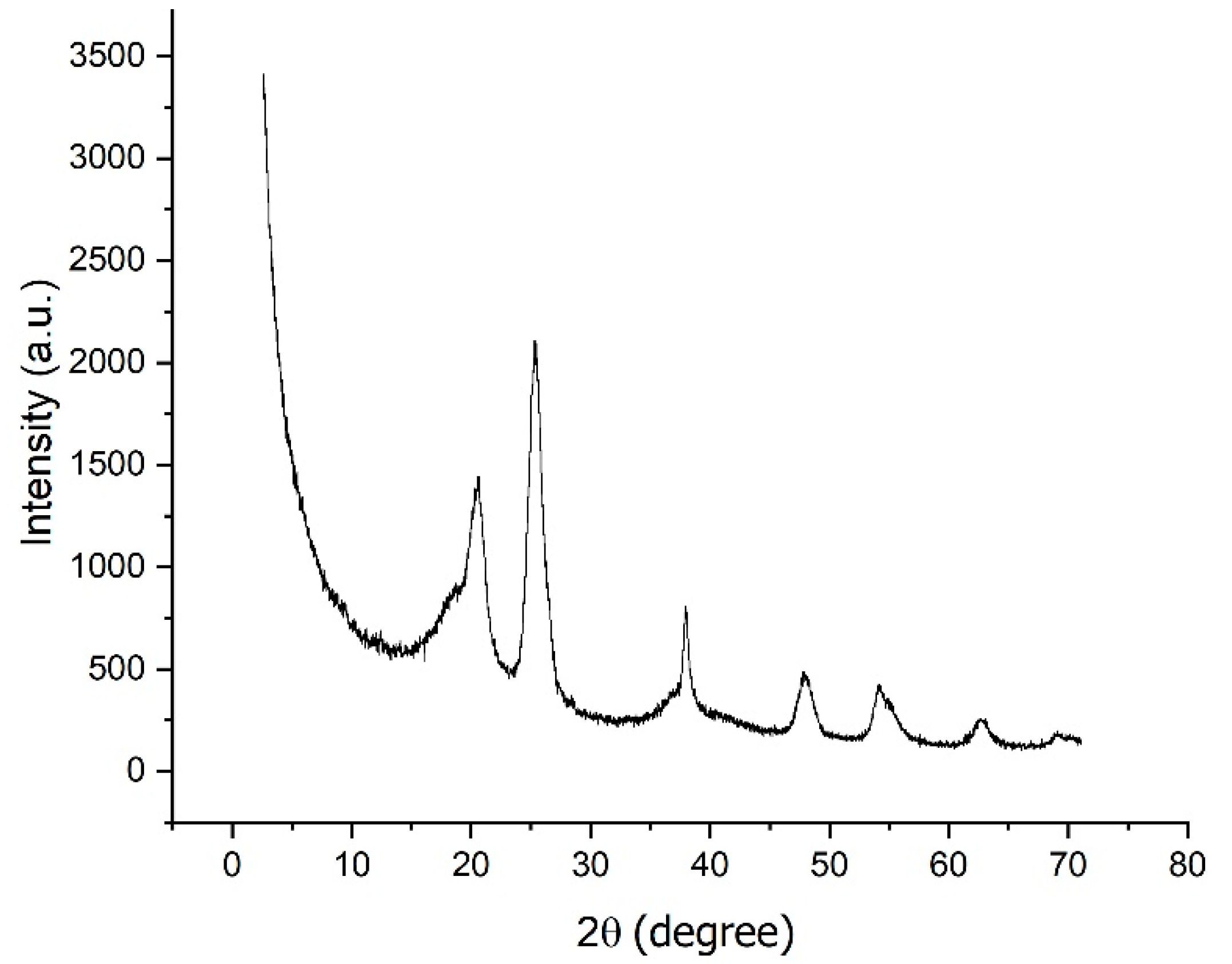
2.1.2. Nonwoven Mats Characterization
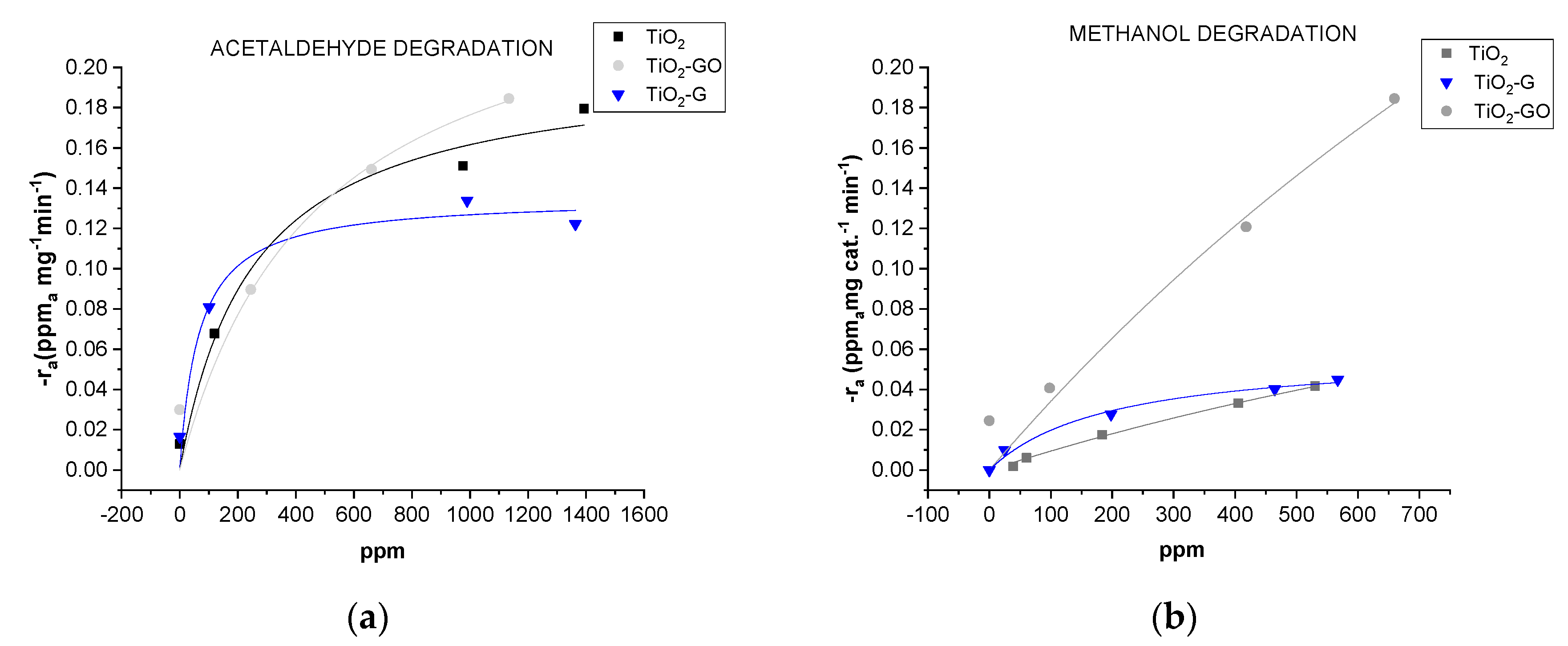
2.2. Photo-Catalytic Performance
3. Experimental
3.1. Materials
3.2. Catalytic Systems and Nonwowen Mats Production
- Titanium dioxide (TiO2)
- TiO2-Graphene (TiO2-G)
- TiO2-Graphene oxide (TiO2-GO)
3.3. Methods
3.4. Photo-Catalytic Testing Station
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parmar, G.R.; Rao, N.N. Emerging control technologies for volatile organic compounds. Crit. Rev. Environ. Sci. Technol. 2009, 39, 41–78. [Google Scholar] [CrossRef]
- Mozaffara, A.; Zhang, Y.L.; Fan, M.; Cao, F.; Lin, Y.C. Characteristics of summertime ambient VOCs and their contributions to O3 and SOA formation in a suburban area of Nanjing, China. Atmos. Res. 2020, 240, 104923. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.D. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Weon, S.; He, F.; Choi, W. Status and challenges in photocatalytic nanotechnology for cleaning air polluted with volatile organic compounds: Visible light utilization and catalyst deactivation. Environ. Sci. Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Ho, W.; Cao, J.; Shen, Z.; Lee, S.C. Fabrication of Bi2O2CO3/g-C3N4 heterojunctions for efficiently photocatalytic NO in air removal: In-situ self-sacrificial synthesis, characterizations and mechanistic study. Appl. Catal. B Environ. 2016, 199, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, H.; Liu, Y.; Li, J. Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy Environ. Sci. 2013, 6, 1362–1387. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.C.; Li, Y. Oxygen-deficient metal oxide nanostructures for photoelectrochemical water oxidation and other applications. Nanoscale 2012, 4, 6682–6691. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.N.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The Effect of Gold Loading and Particle Size on Photocatalytic Hydrogen Production from Ethanol over Au/TiO2 Nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef]
- Weber, A.S.; Grady, A.M.; Koodali, R.T. Lanthanide modified semiconductor photo-catalysts. Catal. Sci. Technol. 2012, 2, 683–693. [Google Scholar] [CrossRef]
- Gurunathan, K. Photocatalytic hydrogen production using transition metal ions-doped γ-Bi2O3 semiconductor particles. Int. J. Hydrogen Energ. 2004, 29, 933–940. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Luciani, G.; Andreozzi, R.; Maddalena, P.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J Hydrogen Energ. 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R.; et al. Near UV-Irradiation of CuOx-Impregnated TiO2 providing active species for H2 production through methanol photoreforming. ChemCatChem 2019, 11, 4314–4326. [Google Scholar] [CrossRef]
- Hou, Y.D.; Wang, X.C.; Wu, L.; Chen, X.F.; Ding, Z.X.; Wang, X.X.; Fu, X.Z. N-Doped SiO2/TiO2 mesoporous nanoparticles with enhanced photocatalytic activity under visible-light irradiation. Chemosphere 2008, 72, 414–421. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.H.; Smith, S.C.; Chen, Z.G.; Lu, G.Q.; Cheng, H.M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, H.; Hu, S.; Li, J.H. Preparation and Enhanced Photoelectrochemical Performance of Coupled Bicomponent ZnO−TiO2 Nanocomposites. J. Phys. Chem. C 2008, 112, 117–122. [Google Scholar] [CrossRef]
- Chen, X.F.; Wang, X.C.; Fu, X.Z. Hierarchical macro/mesoporous TiO2/SiO2 and TiO2/ZrO2 nanocomposites for environmental photocatalysis. Energy Environ. Sci. 2009, 2, 872–877. [Google Scholar] [CrossRef]
- Dal Lago, E.; Boaretti, C.; Piovesan, F.; Roso, M.; Lorenzetti, A.; Modesti, M. The effect of different compatibilizers on the properties of a post-industrial PC/PET blend. Materials 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.L.; Zhang, H.C.; Huang, H.; Liu, Y.; Kang, Z.H. Ag3PO4/SnO2 semiconductor nanocomposites with enhanced photocatalytic activity and stability. New J. Chem. 2012, 36, 1541–1544. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sokhi, S.; Balomajumder, C.; Satapathi, S. Reusable graphene oxide nanofibers for enhanced photocatalytic activity: A detailed mechanistic study. J. Mater Sci. 2017, 52, 5390–5403. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, L.; Xie, R.; Xu, H.; Zhong, Y.; Sui, X.; Mao, Z. Fabrication of novel rGO/Bi20TiO32 heterojunction for enhanced visible-light photocatalytic activity. J. Photochem. Photobiol. A 2016, 329, 18–25. [Google Scholar] [CrossRef]
- Maira, A.J.; Coronado, J.M.; Augugliaro, V.; Yeung, K.L.; Conesa, J.C.; Soria, J. Fourier Transform infrared study of the performance of nanostructured TiO2 particles for the photocatalytic oxidation of gaseous toluene. J. Catal. 2001, 202, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Xie, X.; Wang, X.; Wang, Y.; Segets, D.; Sun, J. Efficient adsorption and sustainable degradation of gaseous acetaldehyde and o-xylene using rGO-TiO2 photo-catalyst. Chem. Eng. J. 2018, 349, 708–718. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Ballari, M.M.; Cassano, A.E.; Alfano, O.M.; Kubacka, A.; Fernández-García, M. Ceria promotion of acetaldehyde photo-oxidation in a TiO2-based catalyst: A spectroscopic and kinetic study. Catal. Sci. Technol. 2015, 5, 1521. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Masschaele, K.; Moortgat, E. Factors driving the activity of commercial titanium dioxide powder towards gas phase photocatalytic oxidation of acetaldehyde. Catal. Sci. Technol. 2012, 2, 2311–2318. [Google Scholar] [CrossRef]
- Danon, A.; Bhattacharyya, K.; Vijayan, B.K. The effect of reactor materials on the properties of titanium oxide nanotubes. ACS Catal. 2012, 2, 45–49. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Wahab, R.; Tripathy, S.K.; Shin, H.-S.; Mohapatra, M.; Musarrat, J.; Al-Khedhairy, A.A.; Kumar Kaushik, N. Photocatalytic oxidation of acetaldehyde with ZnO-quantum dots. Chem. Eng. J. 2013, 226, 154–160. [Google Scholar] [CrossRef]
- Vijayan, B.K.; Dimitrijevic, N.M.; Finkelstein-Shapiro, D. Coupling titania nanotubes and carbon nanotubes to create photocatalytic nanocomposites. ACS Catal. 2012, 2, 223–229. [Google Scholar] [CrossRef]
- Hamal, D.B.; Klabunde, K.J. Valence state and catalytic role of cobalt ions in cobalt TiO2 nanoparticle photo-catalysts for acetaldehyde degradation under visible light. J. Phys. Chem. C 2011, 115, 17359–17367. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, J.; Shi, J.W. Ozone-assisted photocatalytic oxidation of gaseous acetaldehyde on TiO2/H-ZSM-5 catalysts. J. Hazard Mater. 2009, 171, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Modesti, M.; Roso, M.; Boaretti, C.; Besco, S.; Hrelja, D.; Sgarbossa, P.; Lorenzetti, A. Preparation of smart nano-engineered electrospun membranes formethanol gas-phase photoxidation. Appl. Catal. B Environ. 2014, 144, 216–222. [Google Scholar] [CrossRef]
- Roso, M.; Lorenzetti, A.; Boaretti, C.; Hrelja, D.; Modesti, M. Graphene/TiO2 based photo-catalysts on nanostructured membranes as a potential active filter media for methanol gas-phase degradation. Appl. Catal. B Environ. 2015, 176, 225–232. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Pelizzo, M.G.; Lauria, A.; Modesti, M.; Lorenzetti, A. Nanostructured photo-catalysts based on different oxidized graphenes for VOCs removal. Ind. Eng. Chem. Res. 2017, 56, 9980–9992. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Bonora, R.; Modesti, M.; Lorenzetti, A. Nanostructured active media for VOCs abatement: The synergy of graphene oxide and semiconductors coupling. Ind. Eng. Chem. Res. 2018, 57, 16635–16644. [Google Scholar] [CrossRef]
- Pant, H.R.; Park, C.H.; Pokharel, P.; Tijing, L.D.; Lee, D.S.; Kim, C.S. ZnO micro-flowers assembled on reduced graphene sheets with high photocatalytic activity for removal of pollutants. Powder Technol. 2013, 235, 853–858. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and Characterization of Phosphated Mesoporous Titanium Dioxide with High Photocatalytic Activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.; Pham, V.H.; Shin, E.W.; Pham, H.S.; Chung, K.J.S.; Kim, E.J.; Hur, S.H. The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chem. Eng. J. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Calcagno, V.; Silvestri, B.; Raiola, L.; D’Errico, G.; Costantini, A.; Branda, F.; Luciani, G. 5,6-Dihydroxyindole-2-carboxylic Acid–TiO2 Charge Transfer Complexes in the Radical Polymerization of Melanogenic Precursor(s). J. Phys. Chem. C 2016, 120, 6262–6268. [Google Scholar] [CrossRef]
- Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.K. Reduced graphene oxide (RGO) on TiO2 for an improved photoelectrochemical (PEC) and photocatalytic activity. Sol. Energy 2019, 190, 185–194. [Google Scholar] [CrossRef]
- Jiang, B.; Yin, H.; Jiang, T.; Jiang, Y.; Feng, H.; Chen, K.; Zhou, W.; Wada, Y. Hydrothermal synthesis of rutile TiO2 nanoparticles using hydroxyl and carboxyl group-containing organics as modifiers. Mater. Chem. Phys. 2006, 98, 231–235. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Zanfardino, A.; Varcamonti, M.; Silvestri, B.; Costantini, A.; Branda, F.; Luciani, G. Titania as a driving agent for DHICA polymerization: A novel strategy for the design of bioinspired antimicrobial nanomaterials. J. Mater. Chem. B 2015, 3, 2808–2815. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Zanfardino, A.; Silvestri, B.; Giudicianni, P.; Costantini, A.; Varcamonti, M.; Branda, F.; Luciani, G. Antimicrobial activity of eumelanin-based hybrids: The role of TiO2 in modulating the structure and biological performance. Mater. Sci. Engin. 2017, 75, 454–462. [Google Scholar] [CrossRef]
- Oskam, G.; Nellore, A.; Penn, R.L.; Searson, P.C. The Growth Kinetics of TiO2 Nanoparticles from Titanium (IV) Alkoxide at High Water/Titanium Ratio. J. Phys. Chem. B 2003, 107, 1734–1738. [Google Scholar] [CrossRef]
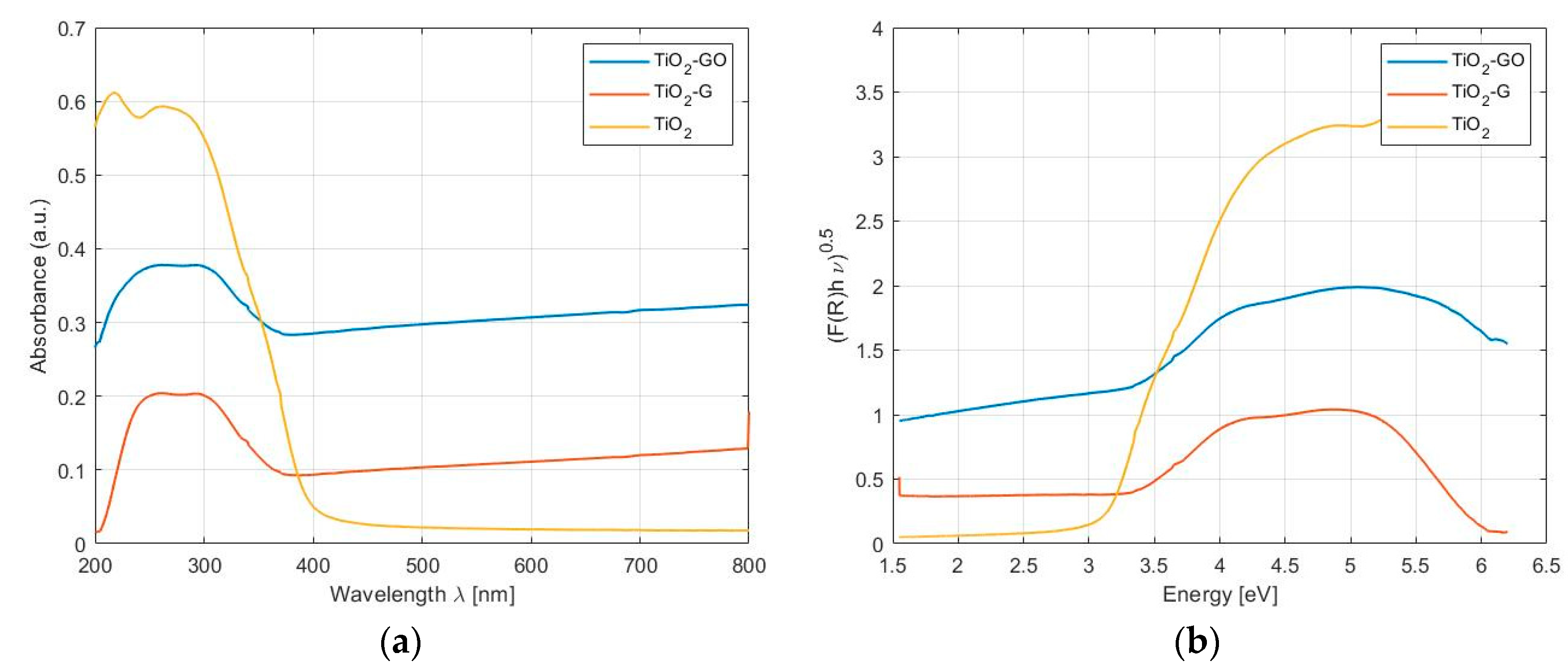


| Photocatalytic System | Absorption Limit [nm] | Band Gap [eV] |
|---|---|---|
| TiO2 | 388 | 3.21 |
| TiO2-G | 420 | 2.9 |
| TiO2-GO | 608 | 2.25 |
| Mat Type | Specific Amount of Photo-Catalyst∙(103) [mg/cm2] |
|---|---|
| PVDF TiO2 | 0.41 ± 0.01 |
| PVDF TiO2-G | 0.85 ± 0.01 |
| PVDF TiO2-GO | 0.82 ± 0.01 |
| - | Acetaldheyde Degradation | Methanol Degradation | ||||
|---|---|---|---|---|---|---|
| Mat Type | kT [mg cat−1 min−1] | K | Adj-R2 | kT [mg cat−1 min−1] | K | Adj-R2 |
| PVDF/TiO2 | 0.20 ± 0.02 | 3.90 × 10−3 | 0.9713 | 0.198 ± 0.08 | 5.00 × 10−4 | 0.9983 |
| PVDF/TiO2-GO | 0.26 ± 0.02 | 2.10 × 10−3 | 0.9140 | 0.813 ± 0.08 | 4.38 × 10−4 | 0.9380 |
| PVDF/TiO2-G | 0.14 ± 0.01 | 1.49 × 10−2 | 0.9337 | 0.059 ± 0.006 | 5.11 × 10−3 | 0.9848 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boaretti, C.; Vitiello, G.; Luciani, G.; Lorenzetti, A.; Modesti, M.; Roso, M. Electrospun Active Media Based on Polyvinylidene Fluoride (PVDF)-Graphene-TiO2 Nanocomposite Materials for Methanol and Acetaldehyde Gas-Phase Abatement. Catalysts 2020, 10, 1017. https://doi.org/10.3390/catal10091017
Boaretti C, Vitiello G, Luciani G, Lorenzetti A, Modesti M, Roso M. Electrospun Active Media Based on Polyvinylidene Fluoride (PVDF)-Graphene-TiO2 Nanocomposite Materials for Methanol and Acetaldehyde Gas-Phase Abatement. Catalysts. 2020; 10(9):1017. https://doi.org/10.3390/catal10091017
Chicago/Turabian StyleBoaretti, Carlo, Giuseppe Vitiello, Giuseppina Luciani, Alessandra Lorenzetti, Michele Modesti, and Martina Roso. 2020. "Electrospun Active Media Based on Polyvinylidene Fluoride (PVDF)-Graphene-TiO2 Nanocomposite Materials for Methanol and Acetaldehyde Gas-Phase Abatement" Catalysts 10, no. 9: 1017. https://doi.org/10.3390/catal10091017






