Nanoencapsulated Laccases Obtained by Double-Emulsion Technique. Effects on Enzyme Activity pH-Dependence and Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. Laccase Purification
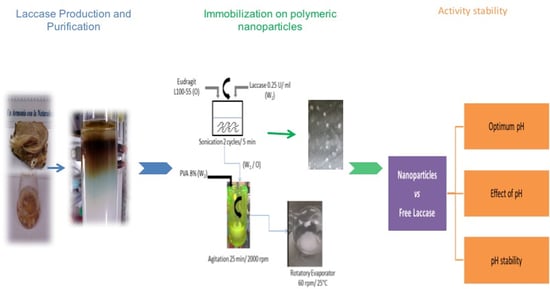
2.2. Nanoparticles Characterization
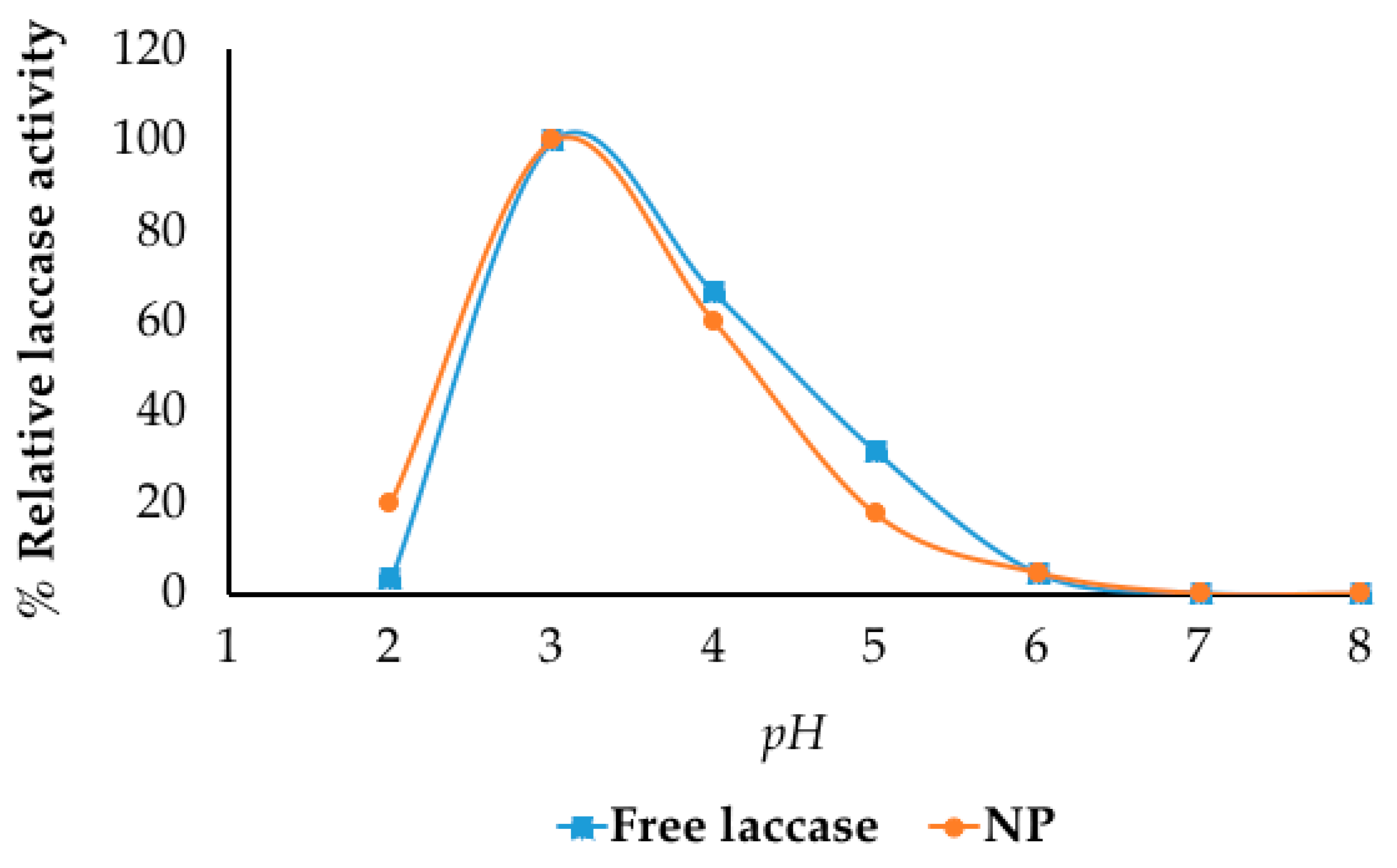
2.3. Optimum pH for Enzyme Activity
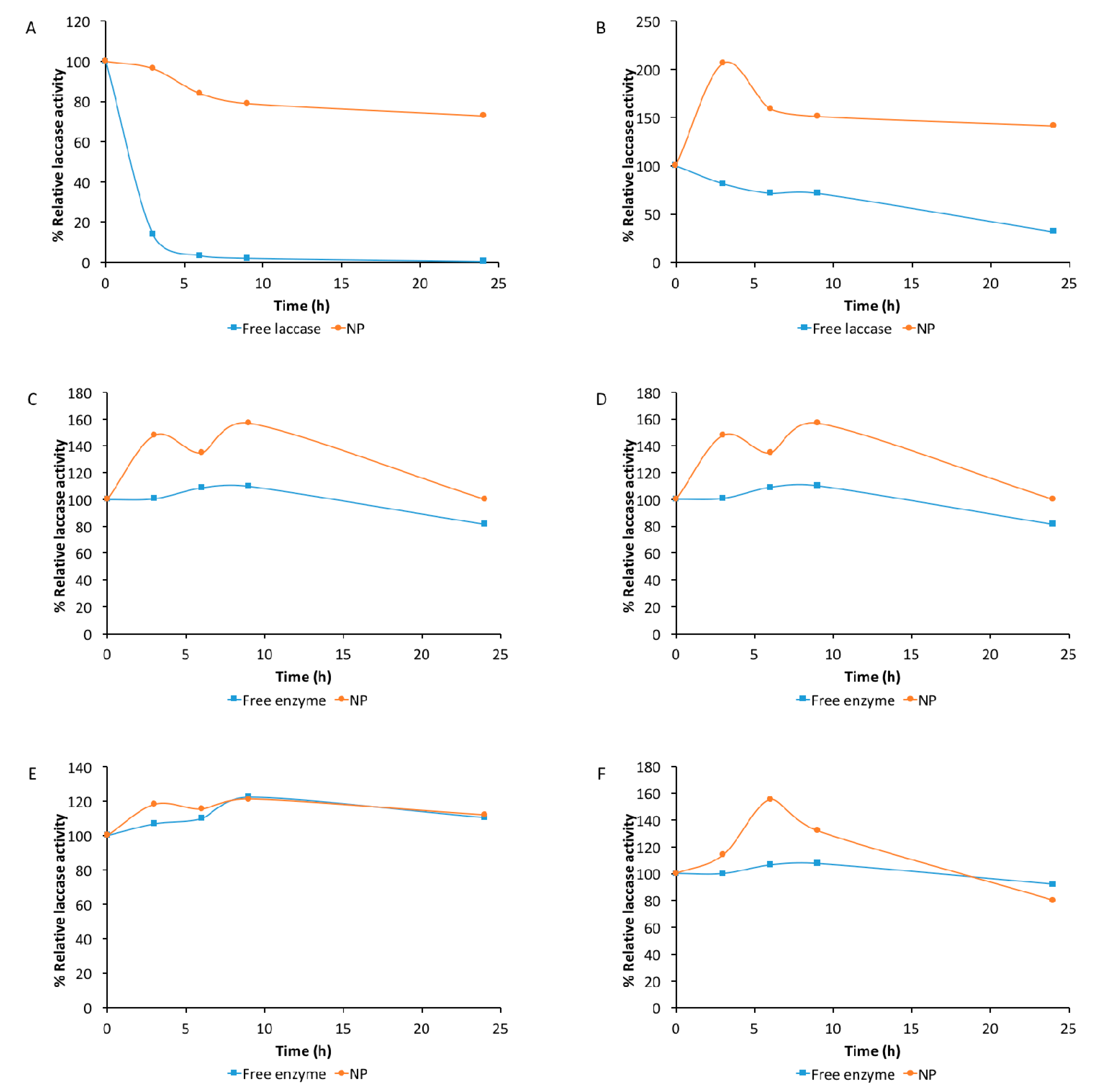
2.4. Effect of pH on Laccase Stability
2.5. pH Stability Determined in a Sequential Assay Involving Acidic pHs up to Alkaline Ones
3. Materials and Methods
3.1. Culture Media and Reagents
3.2. Laccase Purification
3.3. Laccase Immobilization on Polymeric Nanoparticles
3.4. Size and Morphology
3.5. Zeta Potential Analysis
3.6. Enzymatic Assays
3.7. Encapsulation Efficiency
3.8. Effects of pH on Laccase Activity and Stability
3.9. pH Stability Determined in a Sequential Assay Involving Acidic pHs up to Alkaline Ones
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, S.; Wu, X.L.; Xiong, J.; Zong, M.H.; Lou, W.Y. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review. Coord. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Daronch, N.A.; Kelbert, M.; Pereira, C.S.; de Araújo, P.H.H.; de Oliveira, D. Elucidating the choice for a precise matrix for laccase immobilization: A review. Int. J. Chem. Eng. 2020, 307, 125506. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zeng, G.; Liu, Y.; Yang, X.; Zhou, C.; Chen, M.; Liu, Y.; Jiang, J.; Yan, M. Immobilization of laccase on hollow mesoporous carbon nanospheres: Noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal. J. Hazard. Mater. 2019, 362, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar-Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Futur. J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Yuan, J.; Fan, X.; Wang, P. Co-immobilization of cellulase and laccase onto the reversibly soluble polymers for decolorization of denim fabrics. Fiber. Polym. 2017, 18, 993–999. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Zhu, L.; Wang, B.; Guo, T. Preparation of Eudragit L 100-55 enteric nanoparticles by a novel emulsion diffusion method. Colloids Surf. B 2013, 108, 127–133. [Google Scholar] [CrossRef]
- Sharma, K.K.; Shrivastava, B.; Sastry, V.R.B.; Sehgal, N.; Kuhad, R.C. Middle-redox potential laccase from Ganoderma sp.: Its application in improvement of feed for monogastric animals. Sci. Rep. 2013, 3, 1299. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, C.A.; Maldonado-Herrera, J.A.; Méndez-Zamora, G.; Hernández-Luna, C.E.; Gutierréz-Soto, G. Enzymatic extract of Trametes maxima CU1 on productive parameters and carcass yield of rabbits. Can. J. Anim. Sci. 2017, 688, 683–688. [Google Scholar] [CrossRef][Green Version]
- Zheng, F.; An, Q.; Meng, G.; Wu, X.J.; Dai, Y.C.; Si, J.; Cui, B.K. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int. J. Biol. Macromol. 2017, 102, 758–770. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Gutiérrez-Soto, G.; Urías-Orona, V.; Hernández-Luna, C.E. Effect of laccase from Trametes maxima CU1 on physicochemical quality of bread. Cogent Food Agric. 2017, 3, 1328762. [Google Scholar] [CrossRef]
- De la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, V.; Panda, A.K.; Majumdar, D.K. Enteric microsphere formulations of papain for oral delivery. Yakugaku Zasshi. 2011, 131, 697–709. [Google Scholar] [CrossRef]
- Pal, S.L.; Jana, U.; Manna, P.K.; Mohanta, G.P.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. Available online: https://www.japsonline.com/admin/php/uploads/159_pdf.pdf (accessed on 1 September 2020).
- Jelvehgari, M.; Zakeri-Milani, P.; Siahi-Shadbad, M.R.; Loveymi, B.D.; Nokhodchi, A.; Azari, Z.; Valizadeh, H. Development of pH-sensitive insulin nanoparticles using Eudragit L100-55 and chitosan with different molecular weights. Aaps Pharmscitech 2010, 11, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedcine 2006, 2, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Nareshkumar, S.A.; Raval, A.G. Formulation and evaluation of colon targeted pellets of bumadizone calcium. Int. J. Pharm. Biol. Sci. Arch. 2019, 7. [Google Scholar] [CrossRef]
- Ba, S.; Arsenault, A.; Hassani, T.; Jones, J.P.; Cabana, H. Laccase immobilization and insolubilization: From fundamentals to applications for the elimination of emerging contaminants in wastewater treatment. Crit. Rev. Biotechnol. 2013, 33, 404–418. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.K.; Kumar, P.; Ramchiary, N. Laccase isozymes from Ganoderma lucidum MDU-7: Isolation, characterization, catalytic properties and differential role during oxidative stress. J. Mol. Catal. B Enzym. 2015, 113, 68–75. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess. Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Sun, J.; Chen, S. Stimulation of laccase biocatalysis in ionic liquids: A review on recent progress. Curr. Protein Pept. Sci. 2018, 19, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Çetinus, Ş.A.; Öztop, H.N. Immobilization of catalase into chemically crosslinked chitosan beads. Enzym. Microb. Technol. 2003, 32, 889–894. [Google Scholar] [CrossRef]
- Thomas, S.; Chong, Y.N.; Chaw, C.S. Preparation and characterization of enteric microparticles by coacervation. Drug Dev. Ind. Pharm. 2013, 39, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Hernández-Luna, C.; Demarche, P.; Enaudd, E.; Garciía-Moralesa, R.; Agathos, S.N.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef]
- Yinghui, D.; Qiuling, W.; Shiyu, F. Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett. Appl. Microbiol. 2002, 35, 451–456. [Google Scholar] [CrossRef]
- Kumar, R.; Majumdar, D.K.; Panda, A.K.; Pathak, D.P. Eudragit coated microparticulate delivery. IJRPC 2014, 4, 698–712. [Google Scholar] [CrossRef]
- Fillat, Ú.; Ibarra, D.; Eugenio, E.; Moreno, A.D.; Tom, E.; Mart, R. Laccases as a potential tool for the efficient conversion of lignocellulosic biomass: A review. Fermentation 2017, 3, 17. [Google Scholar] [CrossRef]
- Chen, L.; Zou, M.; Hong, F.F. Evaluation of fungal laccase immobilized on natural nanostructured bacterial cellulose. Front. Microbiol. 2015, 6, 1245. [Google Scholar] [CrossRef]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. pH-sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur. J. Pharm. Biopharm. 2009, 72, 1–8. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Ma, Z.X.; Romero, J.J.; Arriola, K.G. Ruminant Nutrition Symposium: Improving cell wall digestion and animal performance with fibrolytic enzymes. J. Anim. Sci. 2014, 92, 1317–1330. [Google Scholar] [CrossRef]
- Pickard, M.A.; Vandertol, H.; Roman, R.; Vazquez-Duhalt, R. High production of ligninolytic enzymes from white rot fungi in cereal bran liquid medium. Can. J. Microbiol. 1999, 45, 627–631. [Google Scholar] [CrossRef]
- Abadulla, E.; Tzanov, T.; Costa, S.; Cavaco, A.; Gübitz, G. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000, 66, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Garfin, D.E. One dimensional gel electrophoresis. Methods Enzymol. 1990, 463, 497–513. [Google Scholar] [CrossRef]
- Catapane, M.; Nicolucci, C.; Menale, C.; Mita, L.; Rossi, S.; Mita, D.G.; Diano, N. Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from Trametes versicolor. J. Hazard. Mater. 2013, 248–249, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, Y.; Li, X.; Kong, X.Z. High yield preparation of uniform polyurea microspheres through precipitation polymerization and their application as laccase immobilization support. Chem. Eng. J. 2017, 328, 1043–1050. [Google Scholar] [CrossRef]
- Ranimol, G.; Venugopal, T.; Gopalakrishnan, S.; Sunkar, S. Production of laccase from Trichoderma harzianum and its application in dye decolourisation. Biocatal. Agric. Biotechnol. 2018, 16, 400–404. [Google Scholar] [CrossRef]


| Treatment | pH | Potential Z Values (mV) |
|---|---|---|
| NP + Lac | 2.0 | −0.638 |
| NP + Lac | 5.0 | −6.2 |
| NP + Lac | 6.1 | −17.00 |
| NP + Lac | 7.0 | −22.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong-Cerda, R.; Levin, L.; Castro-Ríos, R.; Hernández-Luna, C.E.; González-Horta, A.; Gutiérrez-Soto, G.; Chávez-Montes, A. Nanoencapsulated Laccases Obtained by Double-Emulsion Technique. Effects on Enzyme Activity pH-Dependence and Stability. Catalysts 2020, 10, 1085. https://doi.org/10.3390/catal10091085
Chong-Cerda R, Levin L, Castro-Ríos R, Hernández-Luna CE, González-Horta A, Gutiérrez-Soto G, Chávez-Montes A. Nanoencapsulated Laccases Obtained by Double-Emulsion Technique. Effects on Enzyme Activity pH-Dependence and Stability. Catalysts. 2020; 10(9):1085. https://doi.org/10.3390/catal10091085
Chicago/Turabian StyleChong-Cerda, Rocío, Laura Levin, Rocío Castro-Ríos, Carlos Eduardo Hernández-Luna, Azucena González-Horta, Guadalupe Gutiérrez-Soto, and Abelardo Chávez-Montes. 2020. "Nanoencapsulated Laccases Obtained by Double-Emulsion Technique. Effects on Enzyme Activity pH-Dependence and Stability" Catalysts 10, no. 9: 1085. https://doi.org/10.3390/catal10091085
APA StyleChong-Cerda, R., Levin, L., Castro-Ríos, R., Hernández-Luna, C. E., González-Horta, A., Gutiérrez-Soto, G., & Chávez-Montes, A. (2020). Nanoencapsulated Laccases Obtained by Double-Emulsion Technique. Effects on Enzyme Activity pH-Dependence and Stability. Catalysts, 10(9), 1085. https://doi.org/10.3390/catal10091085