Syntheses of 3,3-Disubstituted Dihydrobenzofurans, Indolines, Indolinones and Isochromanes by Palladium-Catalyzed Tandem Reaction Using Pd(PPh3)2Cl2/(±)-BINAP as a Catalytic System
Abstract
:1. Introduction
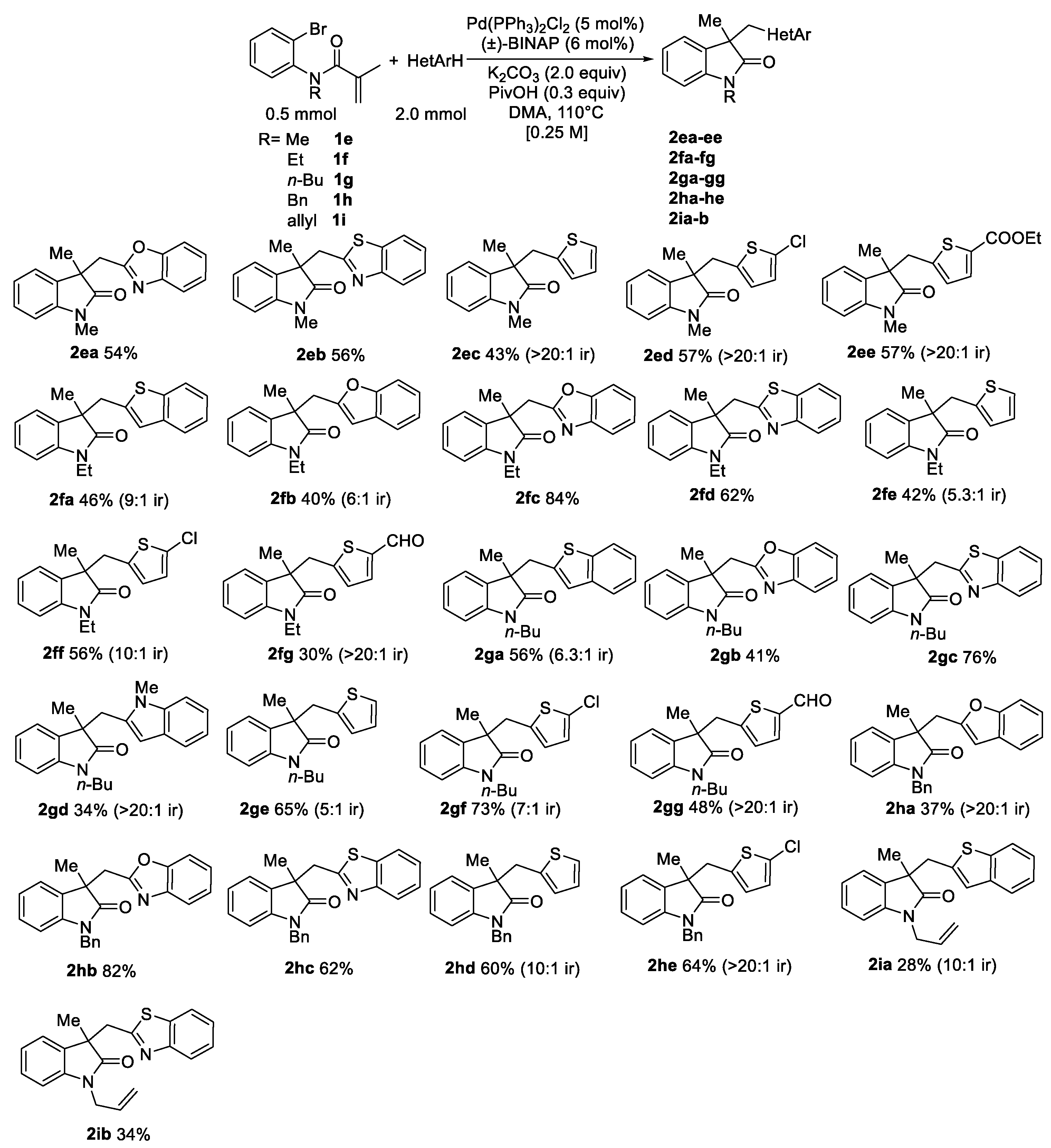
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Procedure A for the Preparation of O or N-methylallyl Arylbromides





3.3. General Procedure B for the Preparation of Starting Materials






3.4. General Procedure for Condition Optimization of the Tandem Reaction
3.5. General Procedure for the Typical Procedure for Tandem Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pang, J.; Xu, Z. Advances in the biological activities and synthesis of 2-arylbenzofurans. Chin. J. Org. Chem. 2005, 25, 25–33. (In Chinese) [Google Scholar]
- Galliford, C.V.; Scheidt, K. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar]
- Liu, S.; Zhou, J. Research progress for biological activity of benzothiazolone compounds. Agrochemicals 2012, 51, 863–865. (In Chinese) [Google Scholar]
- Chen, H.; Li, K. Recent progress on biological activity and synthesis of 2-substituted benzofuran derivatives. J. Pharm. Pract. 2013, 31, 5–11. (In Chinese) [Google Scholar]
- Zhang, H.; Ouyang, G.; Chen, H.; Zhang, L.; Wang, Y. Review of oxindole compounds studies. Fine Chem. Intermed. 2016, 46, 9–16. (In Chinese) [Google Scholar]
- Gupta, A.K.; Bharadwaj, M.; Kumar, A.; Mehrotra, R. Spiro-oxindoles as a promising class of small molecule inhibitors of p53-MDM2 interaction useful in targeted cancer therapy. Top. Curr. Chem. 2017, 375, 1–25. [Google Scholar]
- Jarvis, B.B.; Comezoglu, S.N.; Rao, M.M.; Pena, N.B.; Boettner, F.E.; Forsyth, G.; Epling, B. Isolation of macrocyclic trichothecenes from a large-scale extract of baccharis megapotamica. J. Org. Chem. 1987, 52, 45–56. [Google Scholar]
- Bernard, C.B.; Krishnamurty, H.G.; Chauret, D.; Durst, T.; Philogene, B.J.R.; Sanchez-Vindas, P.; Hasbun, C.; Poveda, L.; San Roman, L.; Arnason, J.T. Insecticidal defenses of piperaceae from the neotropics. J. Chem. Ecol. 1995, 21, 801–814. [Google Scholar]
- Chauret, D.C.; Bernard, C.B.; Arnason, J.T.; Durst, T. Insecticidal neolignans from piper decurrens. J. Nat. Prod. 1996, 59, 152–155. [Google Scholar]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Wang, G.; Qiu, S.; Shangary, S.; Gao, W.; Qin, D.; Stuckey, J.; Krajewski, K.; et al. Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2−p53 interaction. J. Med. Chem. 2006, 49, 3432–3435. [Google Scholar]
- Yu, S.; Qin, D.; Shangary, S.; Chen, J.; Wang, G.; Ding, K.; McEachern, D.; Qiu, S.; Nikolovska-Coleska, Z.; Miller, R.; et al. Potent and orally active small-molecule inhibitors of the MDM2—p53 interaction. J. Med. Chem. 2009, 52, 7970–7973. [Google Scholar]
- Luk, K.-C.; So, S.-S.; Zhang, J.; Zhang, Z. Oxindole Derivatives. W.O. Patent 2006136606, 28 December 2006. [Google Scholar]
- Volk, B.; Barkoczy, J.; Hegedus, E.; Udvari, S.; Gacsalyi, I.; Mezei, T.; Pallagi, K.; Kompagne, H.; Levay, G.; Egyed, A.; et al. (Phenylpiperazinyl-butyl)oxindoles as selective 5-HT7 receptor antagonists. J. Med. Chem. 2008, 51, 2522–2532. [Google Scholar]
- Cheenpracha, S.; Ritthiwigrom, T.; Laphookhieo, S. Alstoniaphyllines A–C, unusual nitrogenous derivatives from the bark of Alstonia macrophylla. J. Nat. Prod. 2013, 76, 723–726. [Google Scholar]
- Grigg, R.; Sridharan, V. Heterocycles via Pd catalysed molecular queuing processes. Relay switches and the maximisation of molecular complexity. Pure Appl. Chem. 1998, 70, 1047–1057. [Google Scholar]
- Grigg, R.; Sridharan, V. Palladium catalysed cascade cyclisation-anion capture, relay switches and molecular queues. J. Organometal. Chem. 1999, 576, 65–87. [Google Scholar]
- Shen, J.; Cheng, G.; Cui, X. Domino reactions based on Pd-catalyzed C-H bond activation. Prog. Chem. 2012, 24, 1324–1336. (In Chinese) [Google Scholar]
- Mai, W.; Wang, J.; Yang, L.; Yuan, J.; Mao, P.; Xiao, Y.; Qu, L. Progress in synthesis of oxindoles by radical addition-cyclization. Chin. J. Org. Chem. 2014, 34, 1958–1965. (In Chinese) [Google Scholar]
- Xiao, W.-J.; Chen, J.-R.; Yu, X.-Y. Tandem radical cyclization of N-arylacrylamides: An emerging platform for the construction of 3,3-disubstituted oxindoles. Synthesis 2014, 47, 604–629. [Google Scholar]
- Ni, C.; Zhu, L.; Hu, J. Advances in transition-metal-mediated di-and monofluoroalkylations. Acta Chim. Sin. 2015, 73, 90–115. (In Chinese) [Google Scholar]
- Li, J.; Song, R.; Liu, Y.; Xie, Y. Difunctionalization of acrylamides through C–H oxidative radical coupling: New approaches to oxindoles. Synthesis 2015, 47, 1195–1209. [Google Scholar]
- Ding, G.; Wang, Z.; Yin, Z.; Yue, G. Progress in palladium-catalyzed tandem reaction of constructing benzo-five-membered heterocycles. Chin. J. Org. Chem. 2016, 36, 43–59. (In Chinese) [Google Scholar]
- Abdukader, A.; Zhang, Y.; Zhang, Z.; Chenjiang, L. Recent advances of the access to indolinones via C-H bond functionalization/cyclization cascade strategy. Chin. J. Org. Chem. 2016, 36, 875–888. (In Chinese) [Google Scholar]
- Phillips, D.; France, D.J. Palladium-catalyzed heterocyclization: A carbon-centered Approach. Asian J. Org. Chem. 2017, 6, 27–40. [Google Scholar]
- Blouin, S.; Blond, G.; Donnard, M.; Gulea, M.; Suffert, J. Cyclocarbopalladation as a key step in cascade reactions: Recent developments. Synthesis 2017, 49, 1767–1784. [Google Scholar]
- Zhao, J.; Duan, X.; Guo, L. Recent advances in persulfates-promoted radical reaction. Chin. J. Org. Chem. 2017, 37, 2498–2511. (In Chinese) [Google Scholar]
- Ping, Y.; Li, Y.; Zhu, J.; Kong, W. Construction of quaternary stereocenters by palladium-catalyzed carbopalladation-initiated cascade reactions. Angew. Chem. Int. Ed. 2019, 58, 1562–1573. [Google Scholar]
- Ju, B.; Kong, W. Recent progress in the consecutive double Heck reaction. Asian J. Org. Chem. 2020, 9, 1154–1161. [Google Scholar]
- Yoon, H.; Marchese, A.D. Carboiodination catalyzed by nickel. J. Am. Chem. Soc. 2018, 140, 10950–10954. [Google Scholar]
- Liu, J.-G.; Chen, W.-W.; Gu, C.-X.; Xu, B.; Xu, M.-H. Access to spiroindolines and spirodihydrobenzofurans via Pd-catalyzed domino Heck spiroyclization through C–H activation and carbene insertion. Org. Lett. 2018, 20, 2728–2732. [Google Scholar]
- Wang, K.; Ding, Z.; Zhou, Z.; Kong, W. Ni-catalyzed enantioselective reductive diarylation of activated alkenes by domino cyclization/cross-coupling. J. Am. Chem. Soc. 2018, 140, 12364–12368. [Google Scholar]
- Wu, X.-X.; Tian, H.; Wang, Y.; Liu, A.; Chen, H.; Fan, Z.; Li, X.; Chen, S. A facile approach to synthesize azaindoline functionalized spirocarbocyclic scaffolds via a Pd-catalyzed cascade cyclization/dearomatization process. Org. Chem. Front. 2018, 5, 3310–3314. [Google Scholar]
- Wu, X.X.; Liu, A.; Mou, M.; Chen, H.; Chen, S. Palladium-catalyzed cascade carbopalladation/phenol dearomatization reaction: Construction of diversely functionalized spirocarbocyclic scaffolds. J. Org. Chem. 2018, 83, 14184–14194. [Google Scholar]
- Tian, Z.X.; Qiao, J.B.; Xu, G.L.; Pang, X.; Qi, L.; Ma, W.Y.; Zhao, Z.Z.; Duan, J.; Du, Y.F.; Su, P.; et al. Highly enantioselective cross-electrophile aryl-alkenylation of unactivated alkenes. J. Am. Chem. Soc. 2019, 141, 7637–7643. [Google Scholar]
- Koy, M.; Bellotti, P.; Katzenburg, F.; Daniliuc, C.G.; Glorius, F. Synthesis of all-carbon quaternary centers by palladium-catalyzed olefin dicarbofunctionalization. Angew. Chem. Int. Ed. 2019, 59, 2375–2379. [Google Scholar]
- Hu, H.; Teng, F.; Liu, J.; Hu, W.; Luo, S.; Zhu, Q. Enantioselective synthesis of 2-oxindole spiro-fused lactones and lactams by Heck/carbonylative cyclization: Method development and applications. Angew. Chem. Int. Ed. 2019, 58, 9225–9229. [Google Scholar]
- Zhang, Z.-M.; Xu, B.; Wu, L.; Wu, Y.; Qian, Y.; Zhou, L.; Liu, Y.; Zhang, J. Enantioselective dicarbofunctionalization of unactivated alkenes by palladium-catalyzed tandem Heck/Suzuki coupling reaction. Angew. Chem. Int. Ed. 2019, 58, 14653–14659. [Google Scholar]
- Yang, F.; Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Intramolecular Reductive Heck Reaction of Unactivated Alkenes. Org. Lett. 2019, 21, 6989–6994. [Google Scholar]
- Kong, W.; Wang, K. Enantioselective reductive diarylation of alkenes by Ni-catalyzed domino Heck cyclization/cross coupling. Synlett 2019, 30, 1008–1014. [Google Scholar]
- Ju, B.; Chen, S.; Kong, W. Pd-catalyzed enantioselective double Heck reaction. Org. Lett. 2019, 21, 9343–9347. [Google Scholar]
- Liang, R.-X.; Chen, R.-Y.; Zhong, C.; Zhu, J.-W.; Cao, Z.-Y.; Jia, Y.-X. 3,3′-Disubstituted oxindoles formation via copper-catalyzed arylboration and arylsilylation of alkenes. Org. Lett. 2020, 22, 3215–3218. [Google Scholar]
- Wu, X.; Tang, Z.; Zhang, C.; Wang, C.; Wu, L.; Qu, J.; Chen, Y. Pd-Catalyzed regiodivergent synthesis of diverse oxindoles enabled by the versatile Heck reaction of carbamoyl chlorides. Org. Lett. 2020, 22, 3915–3921. [Google Scholar]
- Chen, M.; Wang, X.; Yang, P.; Kou, X.; Ren, Z.H.; Guan, Z.H. Palladium-catalyzed enantioselective Heck carbonylation with a monodentate phosphoramidite ligand: Asymmetric synthesis of (+)-physostigmine, (+)-physovenine, and (+)-folicanthine. Angew. Chem. Int. Ed. 2020, 59, 12199–12205. [Google Scholar]
- Bai, X.; Wu, C.; Ge, S.; Lu, Y. Pd/Cu-Catalyzed enantioselective sequential Heck/Sonogashira coupling: Asymmetric synthesis of oxindoles containing trifluoromethylated quaternary stereogenic centers. Angew. Chem. Int. Ed. 2020, 59, 2764–2768. [Google Scholar]
- Yu, H.; Xuan, P.; Lin, J.; Jiao, M. Copper (I)-catalyzed synthesis of 3, 3-disubstituted isoindolin-1-ones from enamides via cascade radical addition and cyclization. Tetrahedron Lett. 2018, 59, 3636–3641. [Google Scholar]
- Yu, H.; Hu, B.; Huang, H. Nickel-catalyzed alkylarylation of activated alkenes with benzyl-amines via C–N bond activation. Chem. A Eur. J. 2018, 24, 7114–7117. [Google Scholar]
- Lu, K.; Han, X.-W.; Yao, W.-W.; Luan, Y.-X.; Wang, Y.-X.; Chen, H.; Xu, X.-T.; Zhang, K.; Ye, M. DMF-Promoted redox-neutral Ni-catalyzed intramolecular hydroarylation of alkene with simple arene. ACS Catal. 2018, 8, 3913–3917. [Google Scholar]
- Kanyiva, K.S.; Makino, S.; Shibata, T. Silver-catalyzed efficient synthesis of oxindoles and pyrroloindolines via α-aminoalkylation of N-arylacrylamides with amino acid derivatives. Chem. Asian J. 2018, 13, 496–499. [Google Scholar]
- Chen, F.-X.; Karmaker, P.; Qiu, J.; Wu, D.; Yin, H. Free radical cyclization of N-arylacrylamides: Mild and facile synthesis of 3-thiocyanato oxindoles. Synlett 2018, 29, 954–958. [Google Scholar]
- Shi, Y.; Xiao, H.; Xu, X.H.; Huang, Y. Transition metal free decarboxylative fluoroalkylation of N-acrylamides with 3, 3, 3-trifluoro-2, 2-dimethylpropanoic acid (TFDMPA). Org. Biomol. Chem. 2018, 16, 8472–8476. [Google Scholar]
- Yu, Y.; Zheng, P.; Wu, Y.; Ye, X. Electrochemical cobalt-catalyzed C–H or N–H oxidation: A facile route to synthesis of substituted oxindoles. Org. Biomol. Chem. 2018, 16, 8917–8921. [Google Scholar]
- Zhang, Z.; Zhang, L.; Cao, Y.; Li, F.; Bai, G.; Liu, G.; Yang, Y.; Mo, F. Mn-Mediated electrochemical trifluoromethylation/C(sp2)-H functionalization cascade for the synthesis of azaheterocycles. Org. Lett. 2019, 21, 762–766. [Google Scholar]
- Yang, Z.; Tang, A. Synthesis of perfluoroalkyl-substituted oxindoles through organophotoredox-catalyzed perfluoroalkylation of N-arylacrylamides with perfluoroalkyl iodides. Synlett 2019, 30, 1061–1066. [Google Scholar]
- Wang, J.; Sun, K.; Chen, X.; Chen, T.; Liu, Y.; Qu, L.; Zhao, Y.; Yu, B. An external-catalyst-free trifluoromethylation/cyclization strategy to access trifluoromethylated-dihydroisoquinolinones/indolines with togni reagent II. Org. Lett. 2019, 21, 1863–1867. [Google Scholar]
- Biswas, P.; Mandal, S.; Guin, J. Aerobic acylarylation of α,β-unsaturated amides with aldehydes. Org. Lett. 2020, 22, 4294–4299. [Google Scholar]
- Ye, J.; Shi, Z.; Sperger, T.; Yasukawa, Y.; Kingston, C.; Schoenebeck, F.; Lautens, M. Remote C−H alkylation and C−C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem. 2016, 9, 361–368. [Google Scholar]
- Sun, H.; Jiang, Y.; Yang, Y.-S.; Li, Y.-Y.; Li, L.; Wang, W.; Feng, T.; Li, Z.-H.; Liu, J. Synthesis of difluoromethylated 2-oxindoles and quinoline-2,4-diones via visible light-induced tandem radical cyclization of N-arylacrylamides. Org. Biomol. Chem. 2019, 17, 6629–6638. [Google Scholar]
- Ruan, Z.; Huang, Z.; Xu, Z.; Mo, G.; Tian, X.; Yu, X.Y.; Ackermann, L. Catalyst-free, direct electrochemical tri- and difluoroalkylation/cyclization: Access to functionalized oxindoles and quinolinones. Org. Lett. 2019, 21, 1237–1240. [Google Scholar]
- Luo, X.; Zhou, L.; Lu, H.; Deng, G.; Liang, Y.; Yang, C.; Yang, Y. Palladium-catalyzed domino Heck/C–H activation/decarboxylation: A rapid entry to fused isoquinolinediones and isoquinolinones. Org. Lett. 2019, 21, 9960–9964. [Google Scholar]
- Guo, J.; Xu, C.; Wang, L.; Huang, W.; Wang, M. Catalyst-free and selective trifluoromethylative cyclization of acryloanilides using PhICF3Cl. Org. Biomol. Chem. 2019, 17, 4593–4599. [Google Scholar]
- Guan, Z.; Chen, S.; Huang, Y.; Yao, H. Rhodium(III)-catalyzed intramolecular olefin hydroarylation of aromatic aldehydes using a transient directing group. Org. Lett. 2019, 21, 3959–3962. [Google Scholar]
- René, O.; Lapointe, D.; Fagnou, K. Domino palladium-catalyzed Heck-intermolecular direct arylation reactions. Org. Lett. 2009, 11, 4560–4563. [Google Scholar]
- Kong, W.; Wang, Q.; Zhu, J. Palladium-catalyzed enantioselective domino heck/intermolecular C–H bond functionalization: Development and application to the synthesis of (+)-esermethole. J. Am. Chem. Soc. 2015, 137, 16028–16031. [Google Scholar]
- Wu, X.-X.; Chen, W.-L.; Shen, Y.; Chen, S.; Xu, P.-F.; Liang, Y.-M. Palladium-catalyzed domino Heck/intermolecular C–H bond functionalization: Efficient synthesis of alkylated polyfluoroarene derivatives. Org. Lett. 2016, 18, 1784–1787. [Google Scholar]
- Yue, G.; Lei, K.; Hirao, H.; Zhou, J. Palladium-catalyzed asymmetric reductive heck reaction of aryl halides. Angew. Chem. Int. Ed. 2015, 54, 6531–6535. [Google Scholar]
- Yue, G.; Wu, Y.; Wu, C.; Yin, Z.; Chen, H.; Wang, X.; Zhang, Z. Synthesis of 2-arylindoles by Suzuki coupling reaction of 3-bromoindoles with hindered benzoboronic acids. Tetrahedron Lett. 2017, 58, 666–669. [Google Scholar]
- Wu, Y.; Dou, Z.; Wu, C.; Zhang, Z.; Wang, X.; Yin, Z.; Song, X.; He, C.; Yue, G. Application of mestiylboronic acid and its esters in coupling reacitons. Chin. J. Org. Chem. 2018, 38, 2896–2926. (In Chinese) [Google Scholar]
- Yue, G.; Wu, Y.; Dou, Z.; Chen, H.; Yin, Z.; Song, X.; He, C.; Wang, X.; Feng, J.; Zhang, Z.; et al. Synthesis of spiropyrrolidine oxindoles via Ag-catalyzed stereo- and regioselective 1,3-diploar cycloaddition of indole-based azomethine ylides with chalcones. New J. Chem. 2018, 42, 20024–20031. [Google Scholar]
- Yue, G.; Dou, Z.; Zhou, Z.; Zhang, L.; Feng, J.; Chen, H.; Yin, Z.; Song, X.; Liang, X.; Wang, X.; et al. Rapid abnormal [3+2]-cycloaddition of isatin N,N′-cyclic azomethine imine 1,3-dipoles with chalcones. New J. Chem. 2020, 44, 8813–8817. [Google Scholar]
- Lu, Z.; Hu, C.; Guo, J.; Li, J.; Cui, Y.; Jia, Y. Water-controlled regioselectivity of Pd-catalyzed domino reaction involving a C−H activation process: Rapid synthesis of diverse carbo- and heterocyclic skeletons. Org. Lett. 2010, 12, 480–483. [Google Scholar]
- Yang, T.; Zhou, J.-L.; Li, J.; Shen, Y.; Gao, C.; Li, Y.-M. Radical addition/cyclization cascade: An efficient approach to nitro-containing quinoline-2,4(1H, 3H)-diones. Synthesis 2018, 50, 3460–3466. [Google Scholar]
- Huang, S.; Niu, P.; Su, Y.; Hu, D.; Huo, C. Tandem radical cyclization of N-methacryloyl benzamides with CBr4 to construct brominated isoquinolinediones. Org. Biomol. Chem. 2018, 16, 7748–7752. [Google Scholar]
- Hédouin, J.; Schneider, C.; Gillaizeau, I.; Hoarau, C. Palladium-catalyzed domino allenamide carbopalladation/direct C–H allylation of heteroarenes: Synthesis of primprinine and papaverine analogues. Org. Lett. 2018, 20, 6027–6032. [Google Scholar]
- Marchese, A.D.; Kersting, L.; Lautens, M. Diastereoselective nickel-catalyzed carboiodination generating six-membered nitrogen-based heterocycles. Org. Lett. 2019, 21, 7163–7168. [Google Scholar]
- Palladium(II)-catalyzed intramolecular C-H alkenylation for the synthesis of chromanes. J. Org. Chem. 2019, 84, 2048–2060.
- Wollenburg, M.; Bajohr, J.; Marchese, A.D.; Whyte, A.; Glorius, F.; Lautens, M. Palladium-catalyzed disilylation and digermanylation of alkene tethered aryl halides: Direct access to versatile silylated and germanylated heterocycles. Org. Lett. 2020, 22, 3679–3683. [Google Scholar]
- Ilies, M.A.; Vullo, D.; Pastorek, J.; Scozzafava, A.; Ilies, M.; Caproiu, M.T.; Pastorekova, S.; Supuran, C.T. Carbonic anhydrase inhibitors.Inhibition of tumor-associated isozyme IX by halogenosulfanilamide and halogenophenylaminobenzolamide derivatives. J. Med. Chem. 2003, 46, 2187–2196. [Google Scholar]
- Song, H.; Liu, Y.; Liu, Y.; Wang, Q. Self-induced stereoselective in situ trifluoromethylation: Preparation of spiro [indoline-3, 3′-quinoline] via palladium-catalyzed cascade reaction. Org. Lett. 2014, 16, 3240–3243. [Google Scholar]
- Ortgies, S.; Breder, A. Selenium-catalyzed oxidative C(sp2)–H amination of alkenes exemplified in the expedient synthesis of (aza-)indoles. Org. Lett. 2015, 17, 2748–2751. [Google Scholar]
- Pinto, A.; Jia, Y.; Neuville, L.; Zhu, J. Palladium-Catalyzed Enantioselective Domino Heck–Cyanation Sequence: Development and Application to the Total Synthesis of Esermethole and Physostigmine. Chem. A Eur. J. 2007, 13, 961–967. [Google Scholar]






| Entry | Pd Salt | Additive | Base | Yield b |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | PivOH | K2CO3 | 61 |
| 2 | Pd2(dba)3 | PivOH | K2CO3 | 16 |
| 3 | Pd(dppf)2Cl2 | PivOH | K2CO3 | 39 |
| 4 | Pd(PPh3)4 | PivOH | K2CO3 | 61 |
| 5 | PdCl2 | PivOH | K2CO3 | 54 |
| 6 | Pd/C | PivOH | K2CO3 | - c |
| 7 | Pd(PPh3)2Cl2 | PivOH | K2CO3 | 80 |
| 8 | Pd(PPh3)2Cl2 | PivOH | KOAc | 57 |
| 9 | Pd(PPh3)2Cl2 | PivOH | K3PO4 | 15 |
| 10 | Pd(PPh3)2Cl2 | PivOH | KOtBu | 55 |
| 11 | Pd(PPh3)2Cl2 | PivOH | Na2CO3 | 22 |
| 12 | Pd(PPh3)2Cl2 | PivOH | Cs2CO3 | 65 |
| 13 | Pd(PPh3)2Cl2 | PivOH | LiOH·H2O | 31 |
| 14 | Pd(PPh3)2Cl2 | PivOH | NaOH | 25 |
| 15 | Pd(PPh3)2Cl2 | PivOH | KOH | 42 |
| 16 | Pd(PPh3)2Cl2 | PivOH | TEA | - c |
| 17 | Pd(PPh3)2Cl2 (1 mol%) | PivOH | K2CO3 | 59 |
| 18 | Pd(PPh3)2Cl2 (3 mol%) | PivOH | K2CO3 | 75 |
| 19 | Pd(PPh3)2Cl2 | - d | K2CO3 | 66 |
| 20 | Pd(PPh3)2Cl2 | CsF | K2CO3 | 61 |
| 21 | Pd(PPh3)2Cl2 | Ag2CO3 | K2CO3 | <20% |
| 22 | Pd(PPh3)2Cl2 | AgNO3 | K2CO3 | 20% |

| Entry | Ligand | Solvent | Yield b |
|---|---|---|---|
| 1 | Ph3P | DMA | 36 |
| 2 | Cy3P | DMA | 56 |
| 3 | Cy3P·HBF4 | DMA | 61 |
| 4 | tBu3P·HBF4 | DMA | 50 |
| 5 | dppe | DMA | 60 |
| 6 | dppb | DMA | 62 |
| 7 | dppp | DMA | 65 |
| 8 | (±)-BINAP | DMA | 80 |
| 9 | (±)-BINAP | DMF | 76 |
| 10 | (±)-BINAP | PhMe | 59 |
| 11 | (±)-BINAP | ACN | 44 |
| 12 | (±)-BINAP | PhCN | <10 |
| 13 | (±)-BINAP | DMSO | -c |
| 14 | (±)-BINAP | 1,4-dioxane | <10 |
| 15 | (±)-BINAP | DMA (90 °C) | 75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, G.; Li, S.; Jiang, D.; Ding, G.; Feng, J.; Chen, H.; Yang, C.; Yin, Z.; Song, X.; Liang, X.; et al. Syntheses of 3,3-Disubstituted Dihydrobenzofurans, Indolines, Indolinones and Isochromanes by Palladium-Catalyzed Tandem Reaction Using Pd(PPh3)2Cl2/(±)-BINAP as a Catalytic System. Catalysts 2020, 10, 1084. https://doi.org/10.3390/catal10091084
Yue G, Li S, Jiang D, Ding G, Feng J, Chen H, Yang C, Yin Z, Song X, Liang X, et al. Syntheses of 3,3-Disubstituted Dihydrobenzofurans, Indolines, Indolinones and Isochromanes by Palladium-Catalyzed Tandem Reaction Using Pd(PPh3)2Cl2/(±)-BINAP as a Catalytic System. Catalysts. 2020; 10(9):1084. https://doi.org/10.3390/catal10091084
Chicago/Turabian StyleYue, Guizhou, Sicheng Li, Dan Jiang, Gang Ding, Juhua Feng, Huabao Chen, Chunping Yang, Zhongqiong Yin, Xu Song, Xiaoxia Liang, and et al. 2020. "Syntheses of 3,3-Disubstituted Dihydrobenzofurans, Indolines, Indolinones and Isochromanes by Palladium-Catalyzed Tandem Reaction Using Pd(PPh3)2Cl2/(±)-BINAP as a Catalytic System" Catalysts 10, no. 9: 1084. https://doi.org/10.3390/catal10091084
APA StyleYue, G., Li, S., Jiang, D., Ding, G., Feng, J., Chen, H., Yang, C., Yin, Z., Song, X., Liang, X., Zhang, L., Wang, X., & Lu, C. (2020). Syntheses of 3,3-Disubstituted Dihydrobenzofurans, Indolines, Indolinones and Isochromanes by Palladium-Catalyzed Tandem Reaction Using Pd(PPh3)2Cl2/(±)-BINAP as a Catalytic System. Catalysts, 10(9), 1084. https://doi.org/10.3390/catal10091084





