Iron-Catalyzed Carbonyl–Alkyne and Carbonyl–Olefin Metathesis Reactions
Abstract
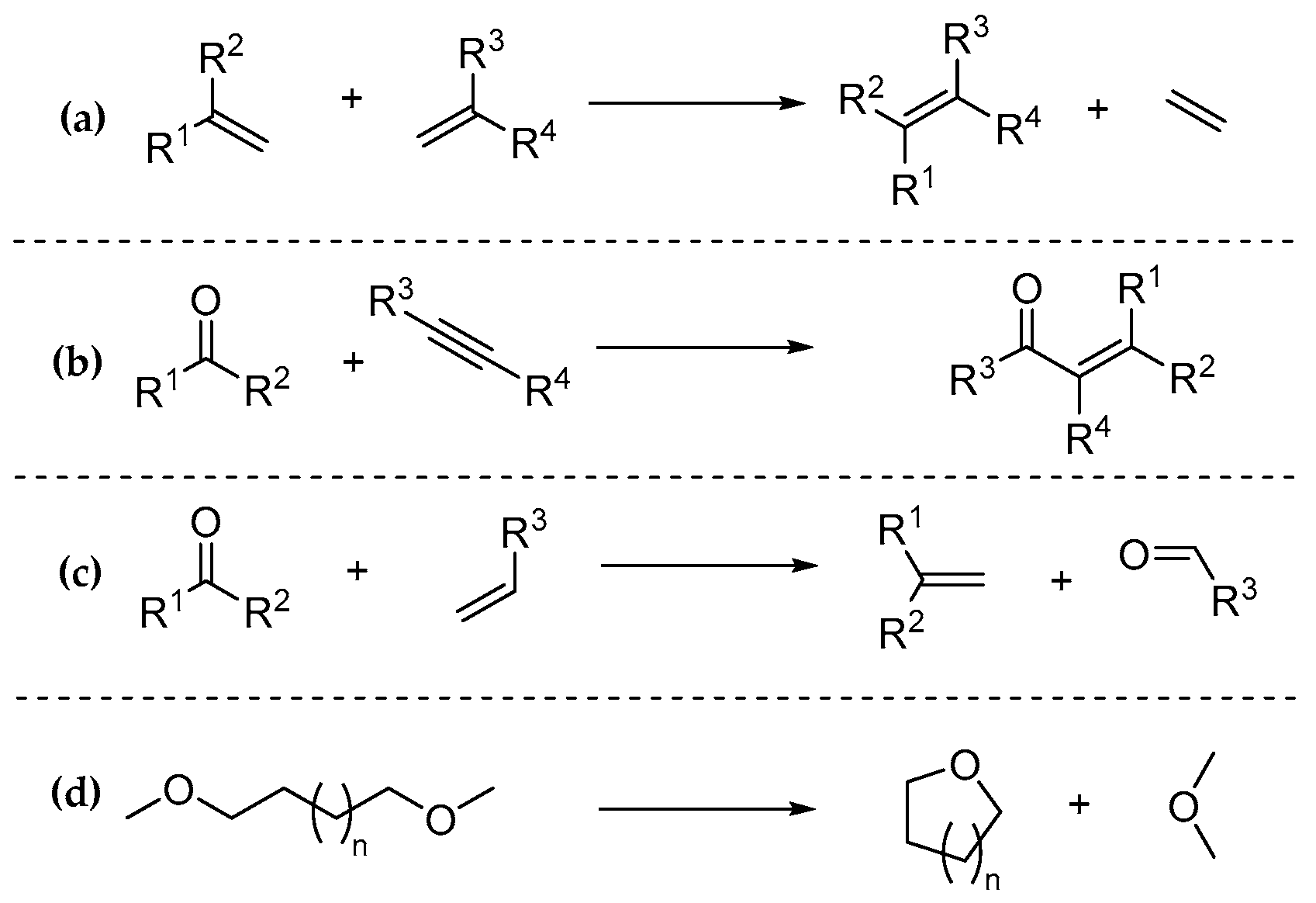
1. Introduction
2. Results
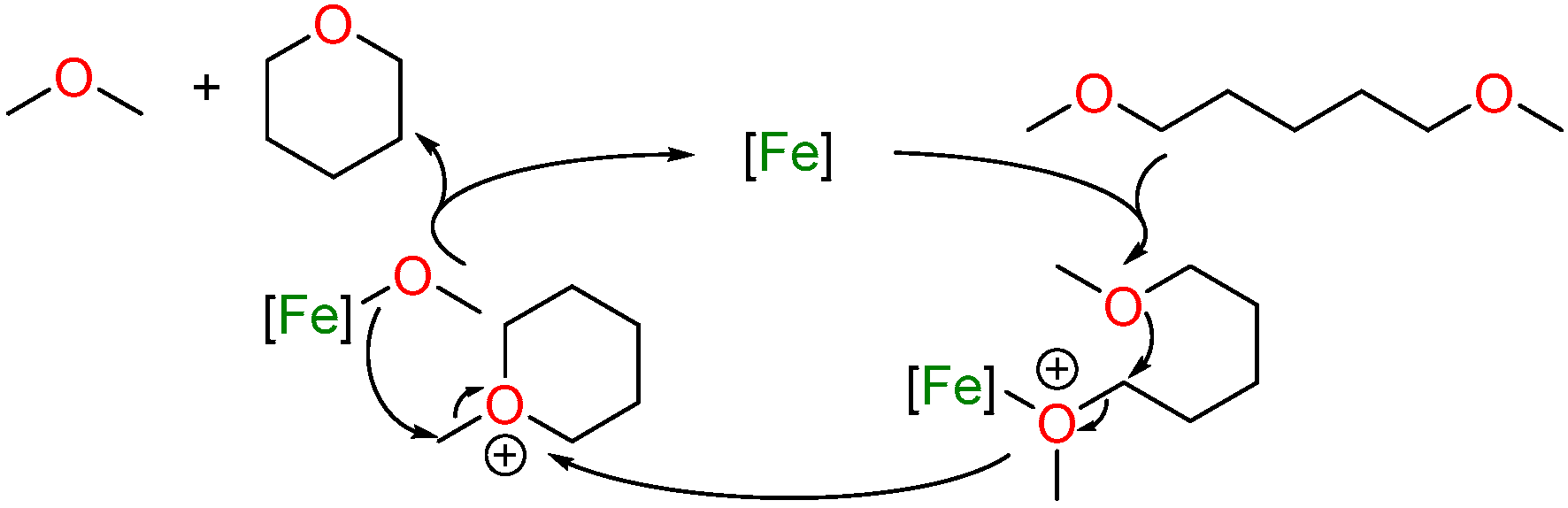
2.1. Iron-Catalyzed C–O/C–O Ring-Closing Metathesis
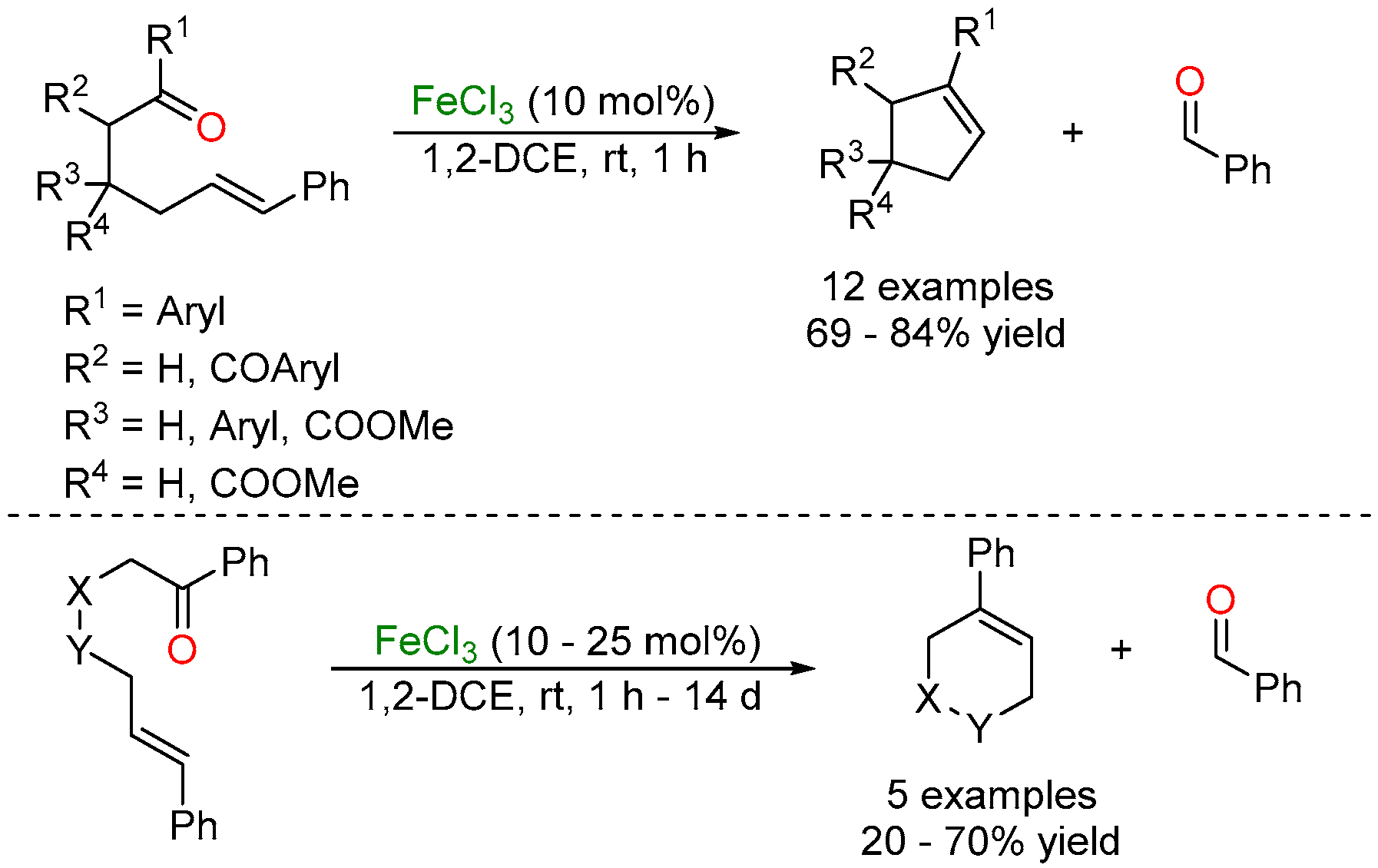
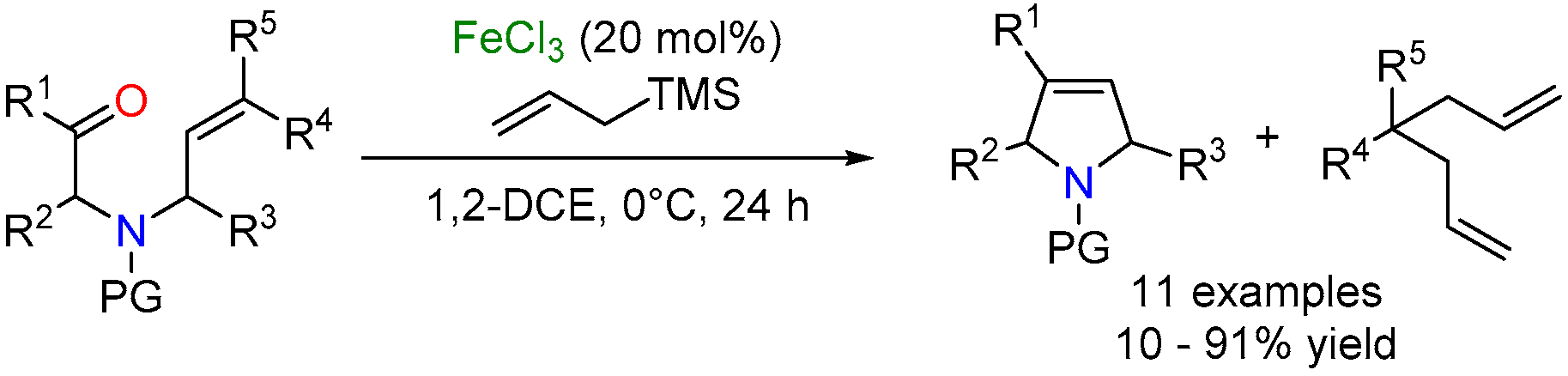
2.2. Iron-Catalyzed Carbonyl-Alkyne Metathesis
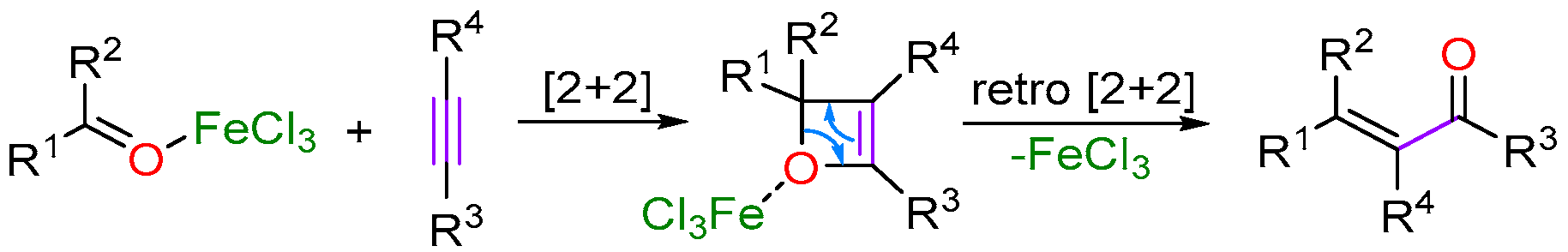
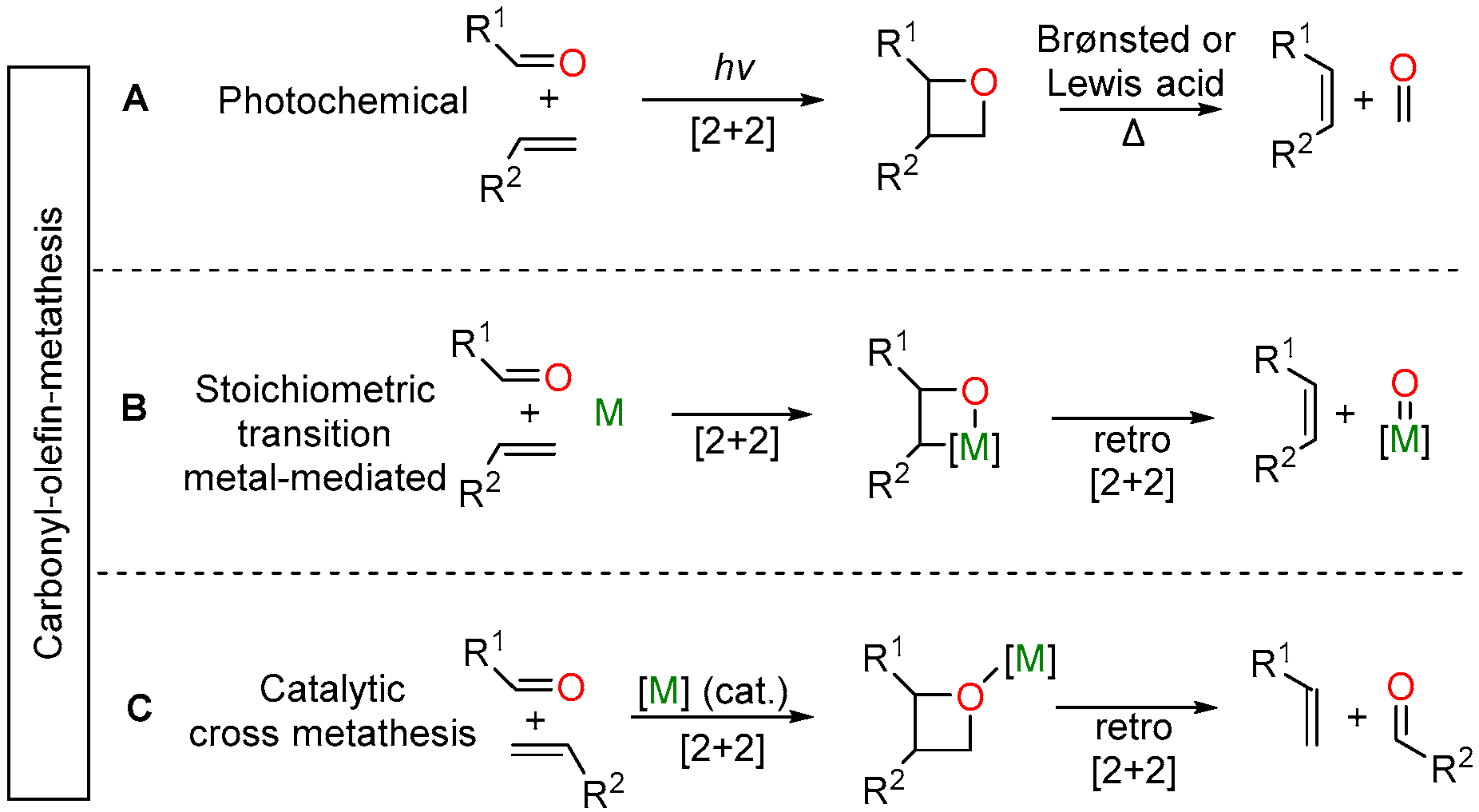
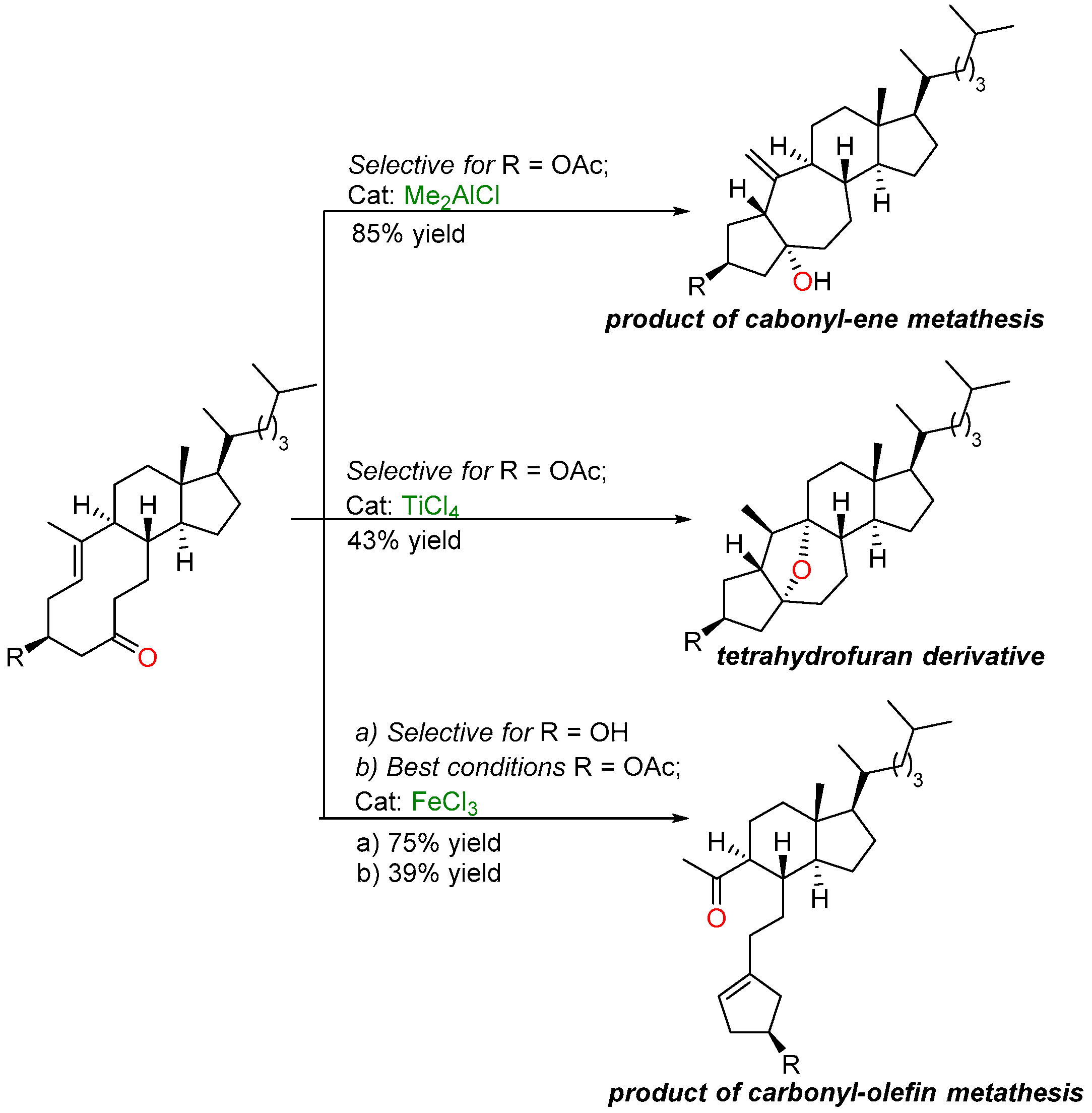
2.3. Iron-Catalyzed Carbonyl-Olefin Metathesis
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Melchor, M.; Solans-Monfort, X.; Ujaque, G. C−C BondFormation. In Comprehensive Inorganic Chemistry II; Poeppelmeier, J.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 9, pp. 767–805. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Khusnutdinov, R.I.; Tolstikov, G.A. Synthesis of cyclobutane and cyclopentane compounds using homogeneous metal complex catalysts. J. Organomet. Chem. 1991, 409, 15–65. [Google Scholar] [CrossRef]
- Schuster, M.; Blechert, S. Olefin Metathesis in Organic Chemistry. Angew. Chem. Int. Ed. Engl. 1997, 36, 2036–2056. [Google Scholar] [CrossRef]
- Bolm, C.; Legros, J.; Le Paih, J.; Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef] [PubMed]
- Amador, A.G.; Scholz, S.O.; Skubi, K.L.; Yoon, T.P. Photocatalysis in Organic Synthesis, 2019 ed.; König, B., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2019; pp. 467–516. [Google Scholar]
- Horibe, T.; Ishihara, K. Initiators for Radical Cation-induced [2 + 2]- and [4 + 2]-Cycloadditions of Electron-rich Alkenes. Chem. Lett. 2020, 49, 107–113. [Google Scholar] [CrossRef]
- Ojima, I.; Tzamarioudaki, M.; Li, Z.; Donovan, R.J. Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis. Chem. Rev. 1996, 96, 635–662. [Google Scholar] [CrossRef]
- Iwasawa, N. 5.08 Thermal and Metal-Induced [3 + 2] Cycloadditions. In Comprehensive Organic Synthesis II, Vol. 5; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–350. [Google Scholar] [CrossRef]
- Schrock, R.R. Multiple metal-carbon bonds for catalytic metathesis reactions (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2006, 45, 3748–3759. [Google Scholar] [CrossRef]
- Grubbs, R.H. Olefin-metathesis catalysts for the preparation of molecules and materials (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2006, 45, 3760–3765. [Google Scholar] [CrossRef]
- Chauvin, Y. Olefin metathesis: The early days (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2006, 45, 3740–3747. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Zhugralin, A.R. The remarkable metal-catalysed olefin metathesis reaction. Nature 2007, 450, 243–251. [Google Scholar] [CrossRef]
- Aitken, S.G.; Abell, A.D. Olefin Metathesis: Catalyst Development, Microwave Catalysis, and Domino Applications. Aust. J. Chem. 2005, 58, 3–13. [Google Scholar] [CrossRef][Green Version]
- Ravindar, L.; Lekkala, R.; Rakesh, K.P.; Asiri, A.M.; Marwani, H.M.; Qin, H.-L. Carbonyl–olefin metathesis: A key review. Org. Chem. Front. 2018, 5, 1381–1391. [Google Scholar] [CrossRef]
- Saito, A.; Tateishi, K. Syntheses of Heterocycles Via Alkyne-Carbonyl Metathesis of Unactivated Alkynes. Heterocycles 2016, 92, 607–630. [Google Scholar] [CrossRef]
- Schindler, C.; Ludwig, J. Lewis Acid Catalyzed Carbonyl–Olefin Metathesis. Synlett 2017, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Sarkar, S.; Biswas, S.; Maiti, S.; Jana, U. Iron-catalyzed synthesis of functionalized 2H-chromenes via intramolecular alkyne-carbonyl metathesis. J. Org. Chem. 2011, 76, 3539–3544. [Google Scholar] [CrossRef]
- Biberger, T.; Makai, S.; Lian, Z.; Morandi, B. Iron-Catalyzed Ring-Closing C-O/C-O Metathesis of Aliphatic Ethers. Angew. Chem. Int. Ed. Engl. 2018, 57, 6940–6944. [Google Scholar] [CrossRef]
- Khripach, V.A.; Zhabinskii, V.N.; Kuchto, A.I.; Zhiburtovich, Y.Y.; Gromak, V.V.; Groen, M.B.; van der Louw, J.; de Groot, A. Intramolecular cycloaddition/cycloreversion of (E)-3β,17β-diacetoxy-5,10-secoandrost-1(10)-en-5-one. Tetrahedron Lett. 2006, 47, 6715–6718. [Google Scholar] [CrossRef]
- Hayashi, A.; Yamaguchi, M.; Hirama, M. SbF5-Promoted Addition of Aldehydes to Alkynes. Synlett 1995, 1995, 195–196. [Google Scholar] [CrossRef]
- Poater, A.; Chaitanya Vummaleti, S.V.; Pump, E.; Cavallo, L. Comparing Ru and Fe-catalyzed olefin metathesis. Dalton Trans. 2014, 43, 11216–11220. [Google Scholar] [CrossRef]
- Poater, A. Environmental friendly Fe substitutive of Ru in water oxidation catalysis. Catal. Commun. 2014, 44, 2–5. [Google Scholar] [CrossRef]
- Liu, L.; Xu, B.; Hammond, G.B. Construction of cyclic enones via gold-catalyzed oxygen transfer reactions. Beilstein J. Org. Chem. 2011, 7, 606–614. [Google Scholar] [CrossRef]
- Rhee, J.U.; Krische, M.J. Alkynes as synthetic equivalents to stabilized Wittig reagents: Intra- and intermolecular carbonyl olefinations catalyzed by Ag(I), BF3, and HBF4. Org. Lett. 2005, 7, 2493–2495. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Bera, K.; Jalal, S.; Sarkar, S.; Jana, U. FeCl3-catalyzed synthesis of functionally diverse dibenzo[b,f]oxepines and benzo[b]oxepines via alkyne-aldehyde metathesis. Org. Biomol. Chem. 2014, 12, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Sarkar, S.; Jalal, S.; Jana, U. Synthesis of substituted phenanthrene by iron(III)-catalyzed intramolecular alkyne-carbonyl metathesis. J. Org. Chem. 2012, 77, 8780–8786. [Google Scholar] [CrossRef]
- Chang, S.; Grubbs, R.H. A Highly Efficient and Practical Synthesis of Chromene Derivatives Using Ring-Closing Olefin Metathesis. J. Org. Chem. 1998, 63, 864–866. [Google Scholar] [CrossRef]
- Menon, R.S.; Findlay, A.D.; Bissember, A.C.; Banwell, M.G. The Au(I)-catalyzed intramolecular hydroarylation of terminal alkynes under mild conditions: Application to the synthesis of 2H-chromenes, coumarins, benzofurans, and dihydroquinolines. J. Org. Chem. 2009, 74, 8901–8903. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, X.H.; He, Q.; Chen, Y.; Xie, Y.Y.; Ding, J.; Miao, Z.H.; Yang, C.H. Design, synthesis and biological evaluation of substituted 11H-benzo[a]carbazole-5-carboxamides as novel antitumor agents. Eur. J. Med. Chem. 2011, 46, 5878–5884. [Google Scholar] [CrossRef]
- von Angerer, E.; Prekajac, J. Benzo[a]carbazole derivatives. Synthesis, estrogen receptor binding affinities, and mammary tumor inhibiting activity. J. Med. Chem. 1986, 29, 380–386. [Google Scholar] [CrossRef]
- Paterno, E.; Chieffii, G. Synthesis in organic chemistry using light. Note II. Compounds of unsaturated hydrocarbons with aldehydes and ketones. Gazz. Chim. Ital. 1909, 39, 341–361. [Google Scholar]
- Büchi, G.; Inman, C.G.; Lipinsky, E.S. Light-catalyzed Organic Reactions. I. The Reaction of Carbonyl Compounds with 2-Methyl-2-butene in the Presence of Ultraviolet Light. J. Am. Chem. Soc. 1954, 76, 4327–4331. [Google Scholar] [CrossRef]
- Jones, G.; Acquadro, M.A.; Carmody, M.A. Long-chain enals via carbonyl–olefin metathesis. An application in pheromone synthesis. J. Chem. Soc. Chem. Commun. 1975, 206–207. [Google Scholar] [CrossRef]
- Stille, J.R.; Santarsiero, B.D.; Grubbs, R.H. Rearrangement of bicyclo[2.2.1]heptane ring systems by titanocene alkylidene complexes to bicyclo[3.2.0]heptane enol ethers. Total synthesis of (.+-.)-.DELTA.9(12)-capnellene. J. Org. Chem. 1990, 55, 843–862. [Google Scholar] [CrossRef]
- Schopov, I.; Jossifov, C. A Carbonyl-Olefin Exchange Reaction – New Route to Polyconjugated Polymers, 1; A New Synthesis of Polyphenylacetylene. Makromol. Chem. 1983, 4, 659–662. [Google Scholar] [CrossRef]
- Demole, E.; Enggist, P.; Borer, M.C. Applications synthétiques de la cyclisation d’alcools tertiaires γ-éthyléniques en α-bromotétrahydrofurannes sous l’action du N-bromosuccinimide. II. Cyclisation du (±)-nérolidol en diméthyl-2,5-(méthyl-4-pentène-3-yl)-2-cycloheptène-4-one, tétraméthyl-3, 3, 7, 10-oxa-2-tricyclo[5.5.0.01,4]-dodécène-9, β-acoratriène, cédradiène-2,8, épi-2-α-cédrène et α-cédrène. Helv. Chim. Acta 1971, 54, 1845–1864. [Google Scholar] [CrossRef]
- Jackson, A.C.; Goldman, B.E.; Snider, B.B. Intramolecular and intermolecular Lewis acid catalyzed ene reactions using ketones as enophiles. J. Org. Chem. 1984, 49, 3988–3994. [Google Scholar] [CrossRef]
- van Schaik, H.-P.; Vijn, R.-J.; Bickelhaupt, F. Acid-Catalyzed Olefination of Benzaldehyde. Angew. Chem. Int. Ed. Engl. 1994, 33, 1611–1612. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Zimmerman, P.M.; Gianino, J.B.; Schindler, C.S. Iron(III)-catalysed carbonyl-olefin metathesis. Nature 2016, 533, 374–379. [Google Scholar] [CrossRef]
- Saa, C. Iron(III)-Catalyzed Ring-Closing Carbonyl-Olefin Metathesis. Angew. Chem. Int. Ed. Engl. 2016, 55, 10960–10961. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Xi, H.; Bai, X.; Ma, E.; Yan, X.; Li, Z. FeCl3 -Catalyzed Ring-Closing Carbonyl-Olefin Metathesis. Angew. Chem. Int. Ed. Engl. 2016, 55, 10410–10413. [Google Scholar] [CrossRef]
- McAtee, C.C.; Riehl, P.S.; Schindler, C.S. Polycyclic Aromatic Hydrocarbons via Iron(III)-Catalyzed Carbonyl-Olefin Metathesis. J. Am. Chem. Soc. 2017, 139, 2960–2963. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Phan, S.; McAtee, C.C.; Zimmerman, P.M.; Devery, J.J., III; Schindler, C.S. Mechanistic Investigations of the Iron(III)-Catalyzed Carbonyl-Olefin Metathesis Reaction. J. Am. Chem. Soc. 2017, 139, 10832–10842. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, P.M. Automated discovery of chemically reasonable elementary reaction steps. J. Comput. Chem. 2013, 34, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Riehl, P.S.; Nasrallah, D.J.; Schindler, C.S. Catalytic, transannular carbonyl-olefin metathesis reactions. Chem. Sci. 2019, 10, 10267–10274. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhuang, D.; Zhao, Y.; Xie, Q.; Zhu, J. Reaction mechanisms of iron(iii) catalyzed carbonyl–olefin metatheses in 2,5- and 3,5-hexadienals: Significant substituent and aromaticity effects. Org. Chem. Front. 2019, 6, 3917–3924. [Google Scholar] [CrossRef]
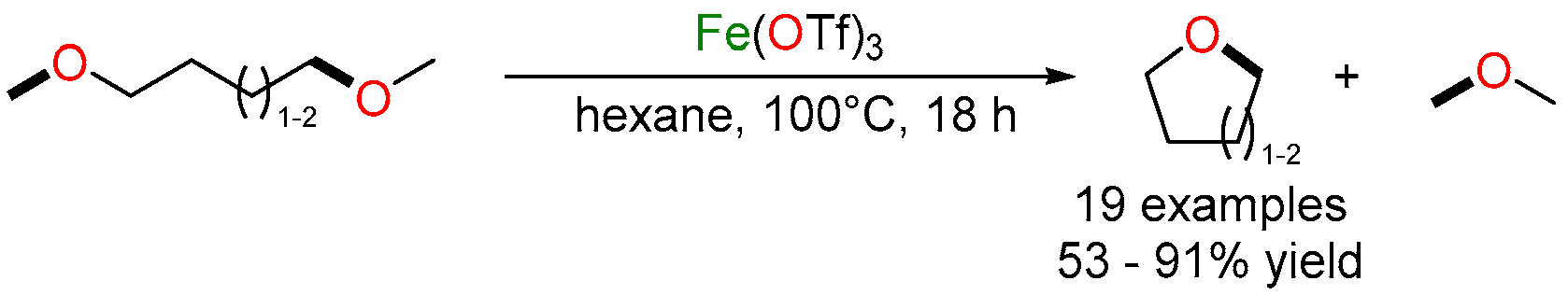
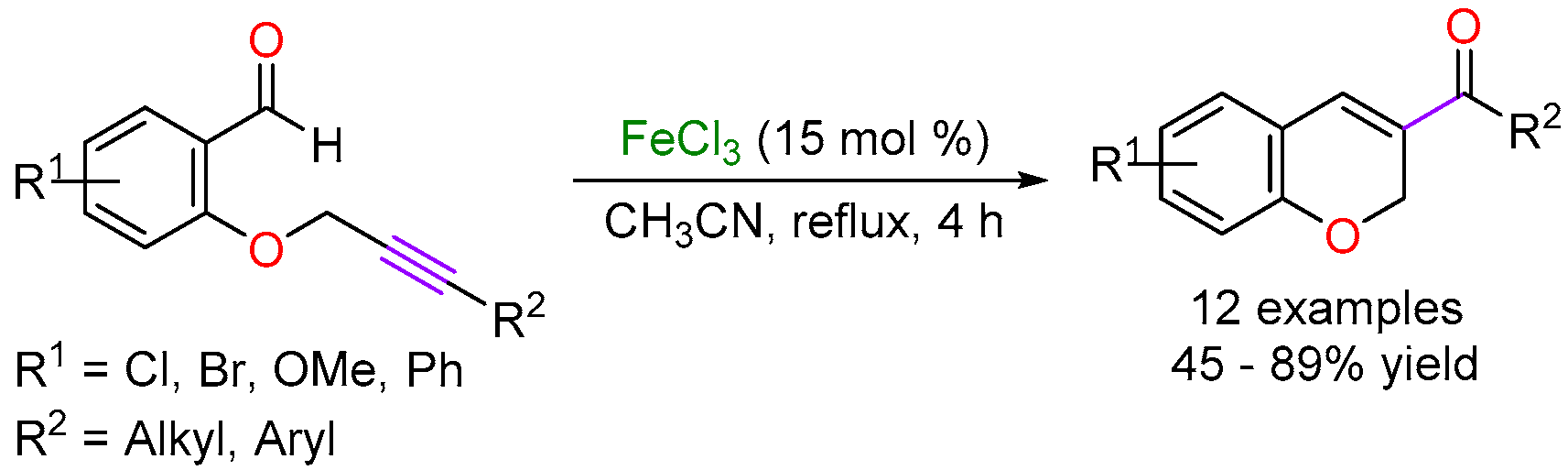
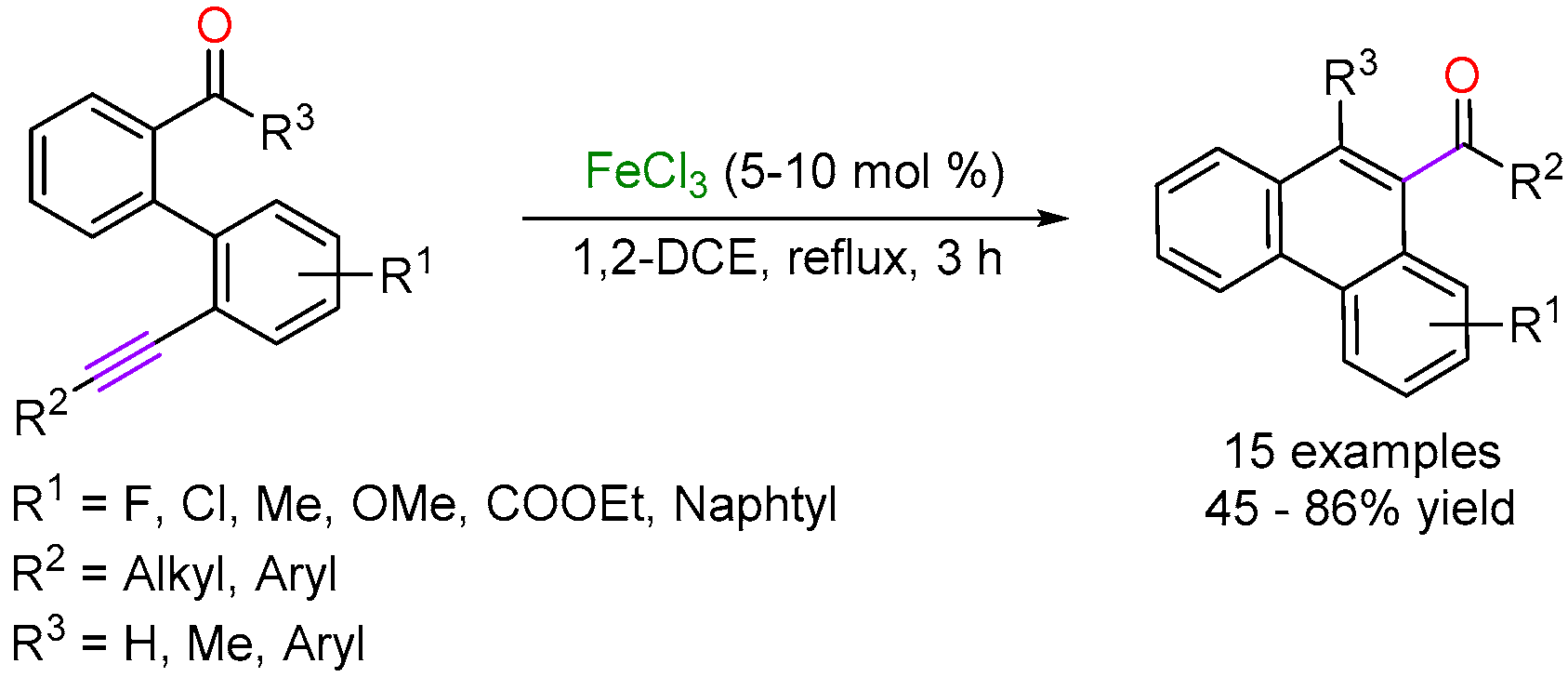

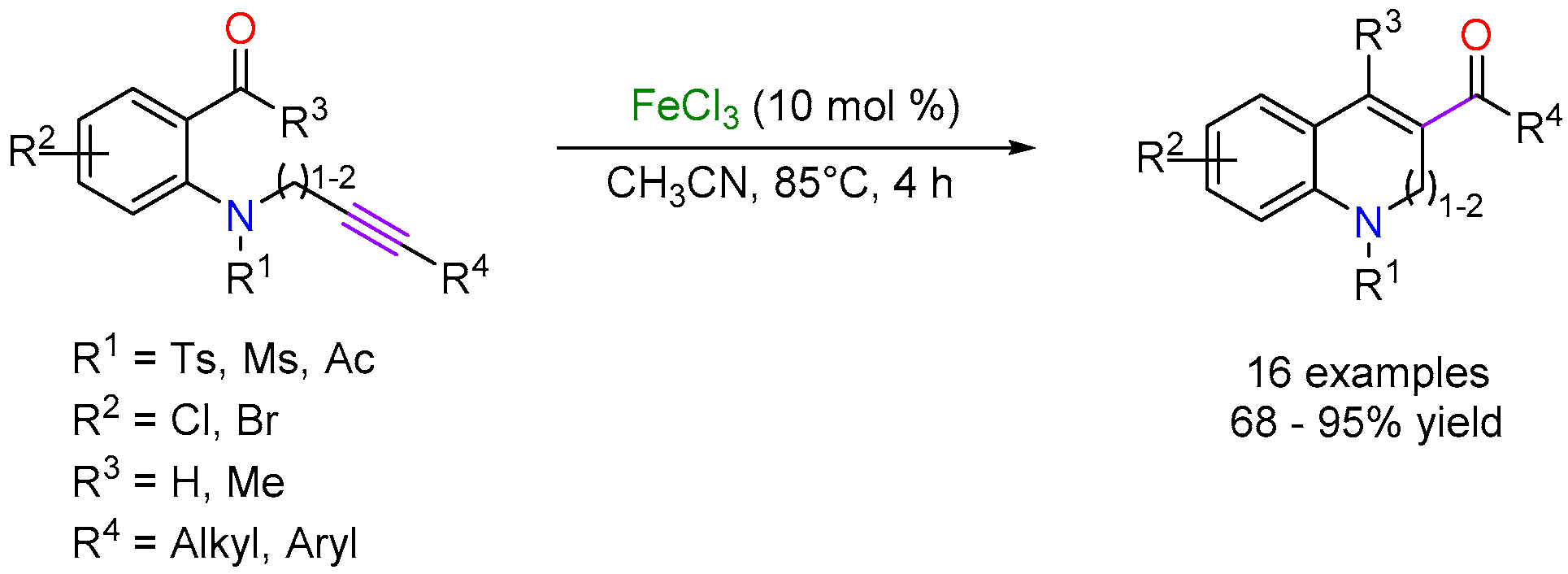


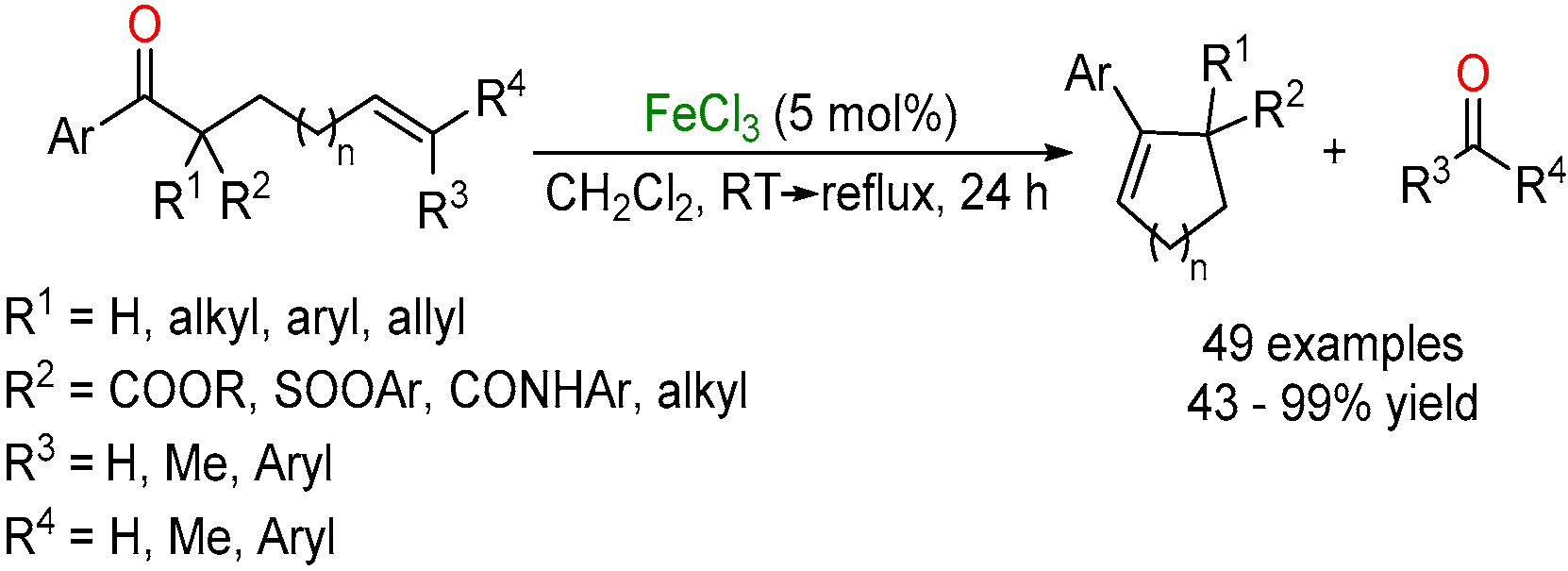


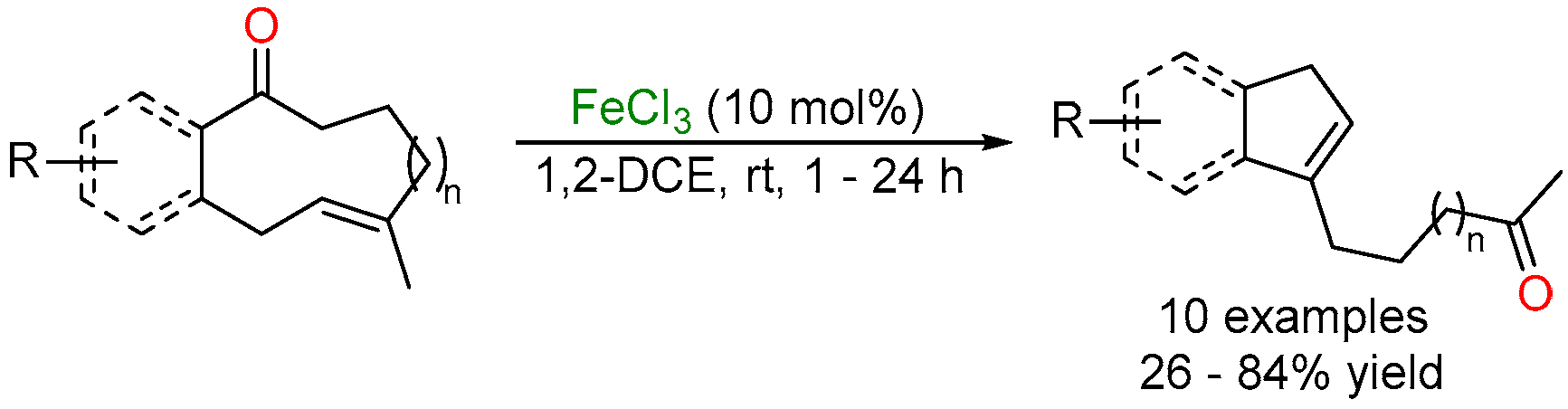
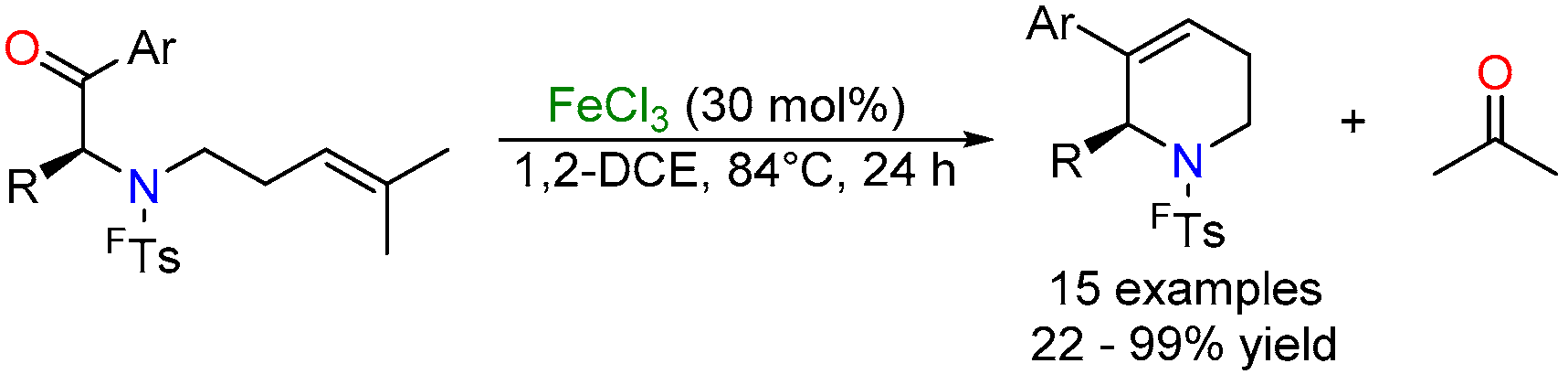

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, B.W.; Tsogoeva, S.B. Iron-Catalyzed Carbonyl–Alkyne and Carbonyl–Olefin Metathesis Reactions. Catalysts 2020, 10, 1092. https://doi.org/10.3390/catal10091092
Grau BW, Tsogoeva SB. Iron-Catalyzed Carbonyl–Alkyne and Carbonyl–Olefin Metathesis Reactions. Catalysts. 2020; 10(9):1092. https://doi.org/10.3390/catal10091092
Chicago/Turabian StyleGrau, Benedikt W., and Svetlana B. Tsogoeva. 2020. "Iron-Catalyzed Carbonyl–Alkyne and Carbonyl–Olefin Metathesis Reactions" Catalysts 10, no. 9: 1092. https://doi.org/10.3390/catal10091092
APA StyleGrau, B. W., & Tsogoeva, S. B. (2020). Iron-Catalyzed Carbonyl–Alkyne and Carbonyl–Olefin Metathesis Reactions. Catalysts, 10(9), 1092. https://doi.org/10.3390/catal10091092





