Effect of Ag2S Nanocrystals/Reduced Graphene Oxide Interface on Hydrogen Evolution Reaction
Abstract
:1. Introduction
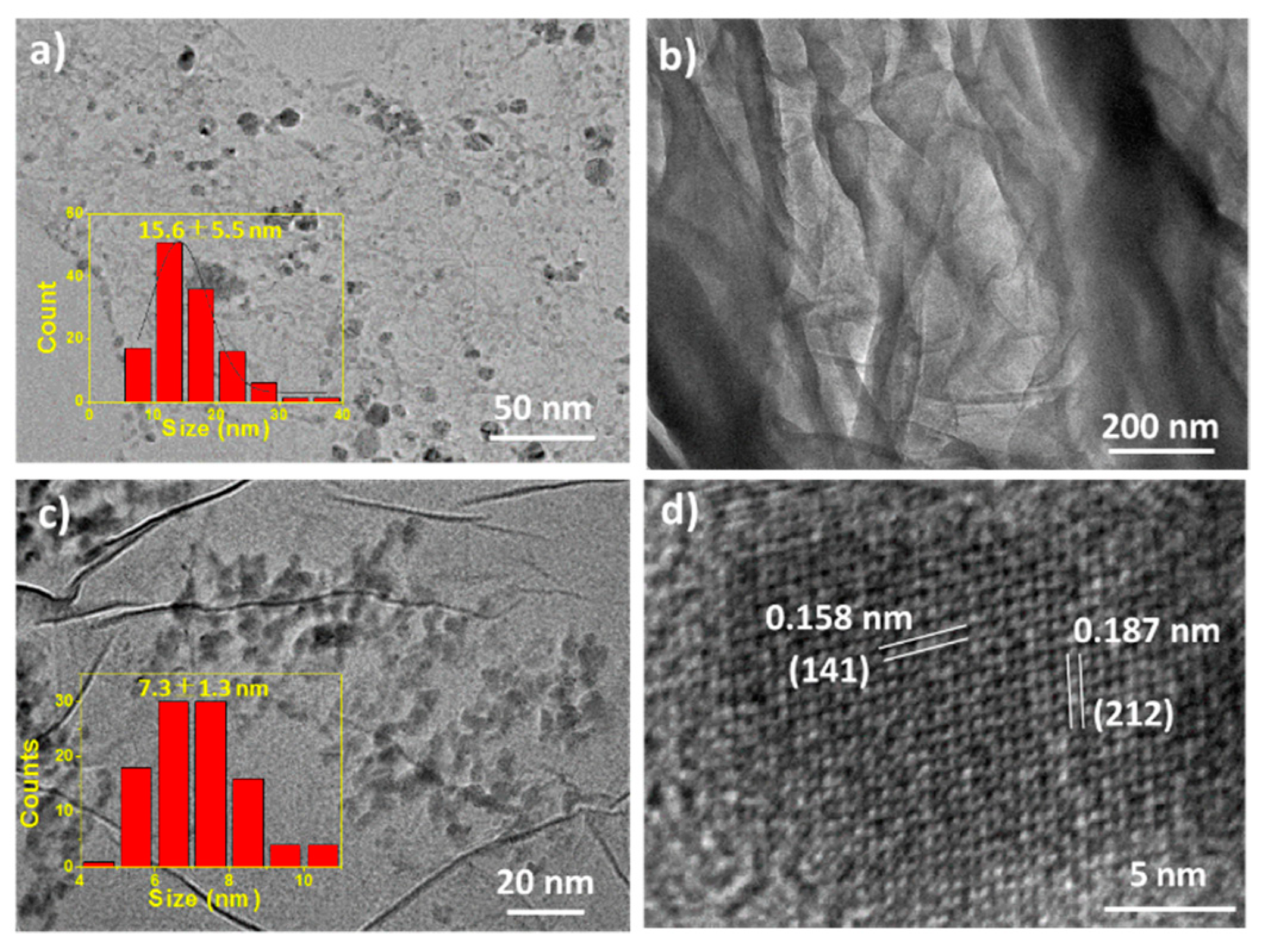
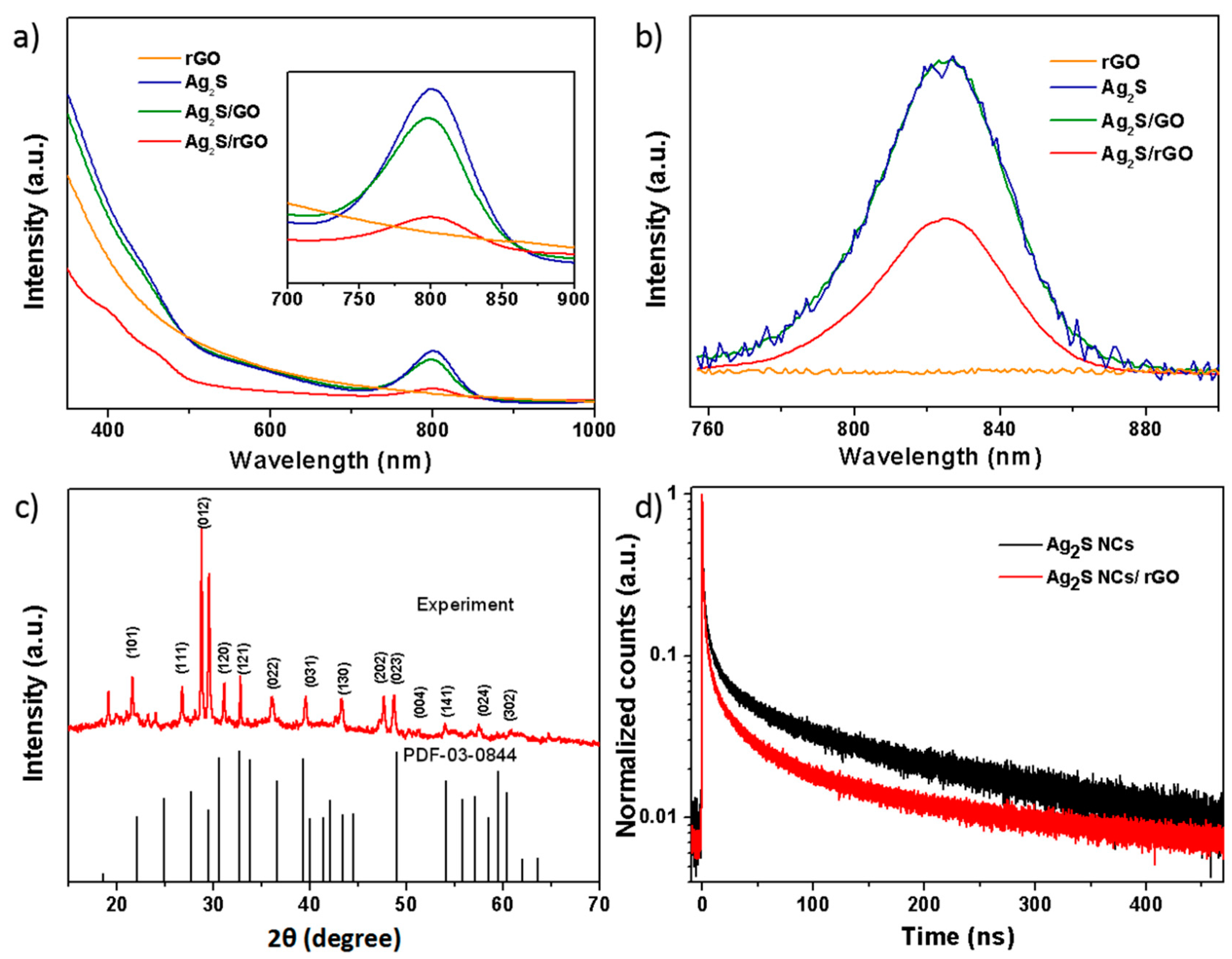
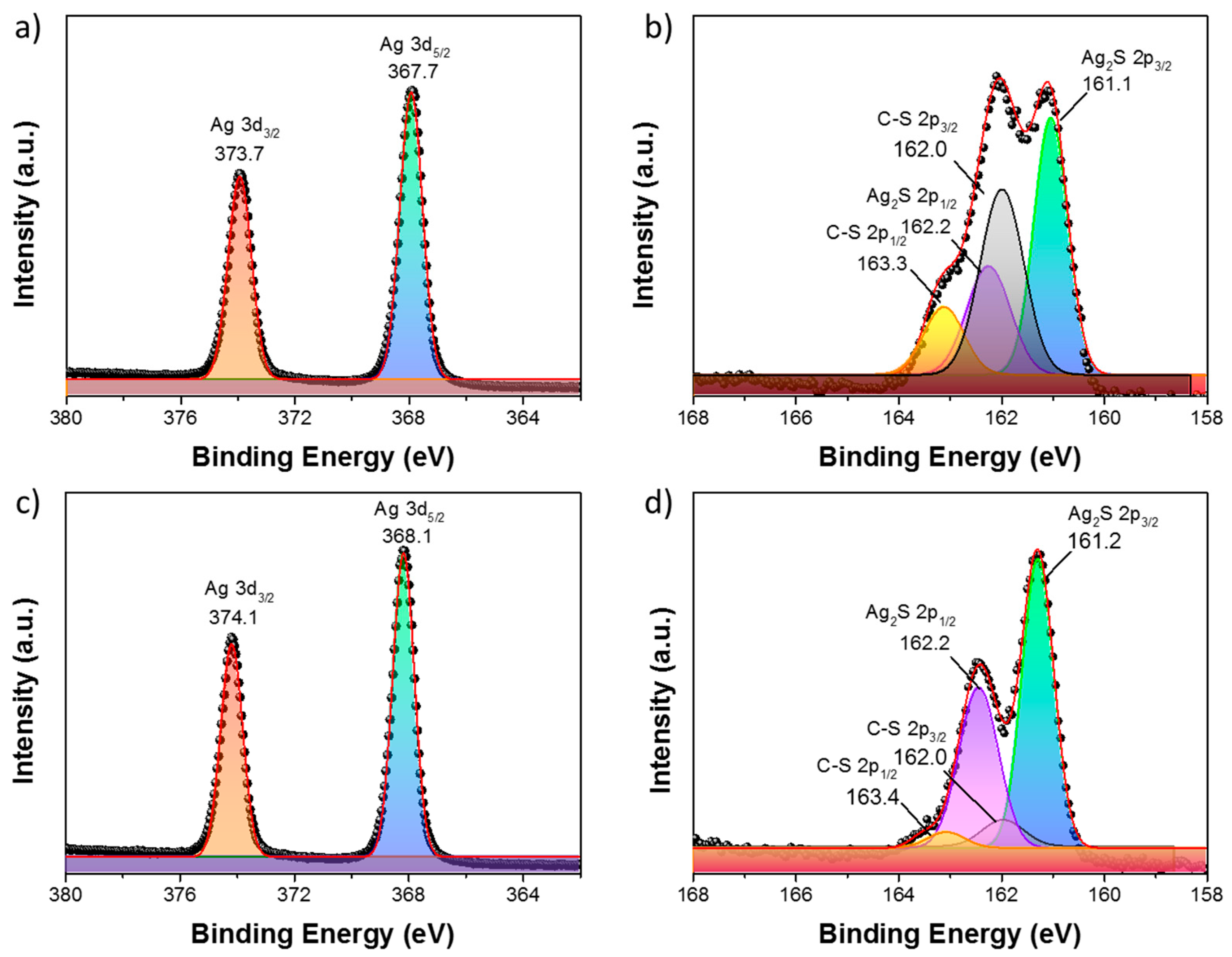
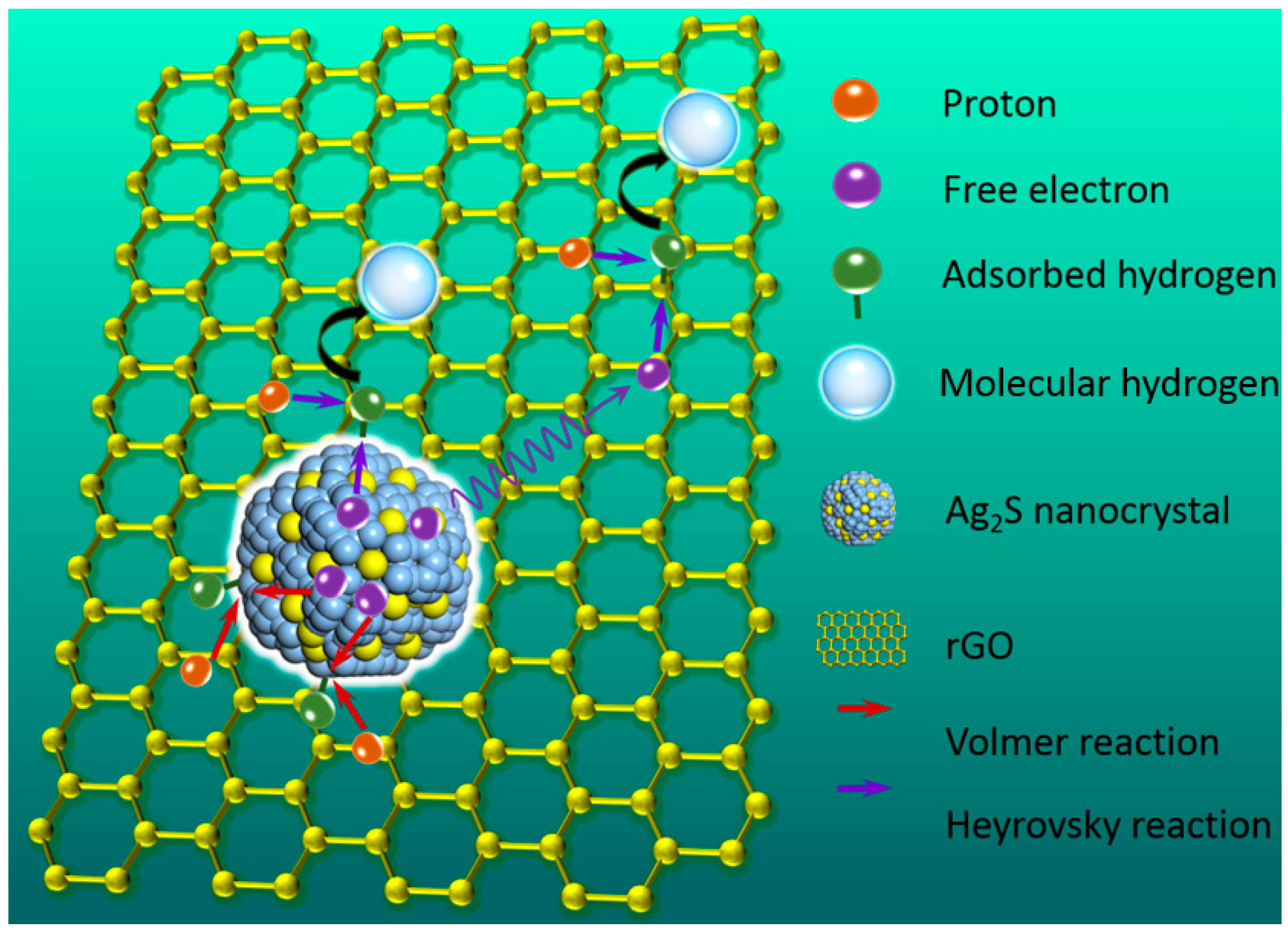
2. Result and Discussion
3. Experimental
3.1. Chemicals and Materials
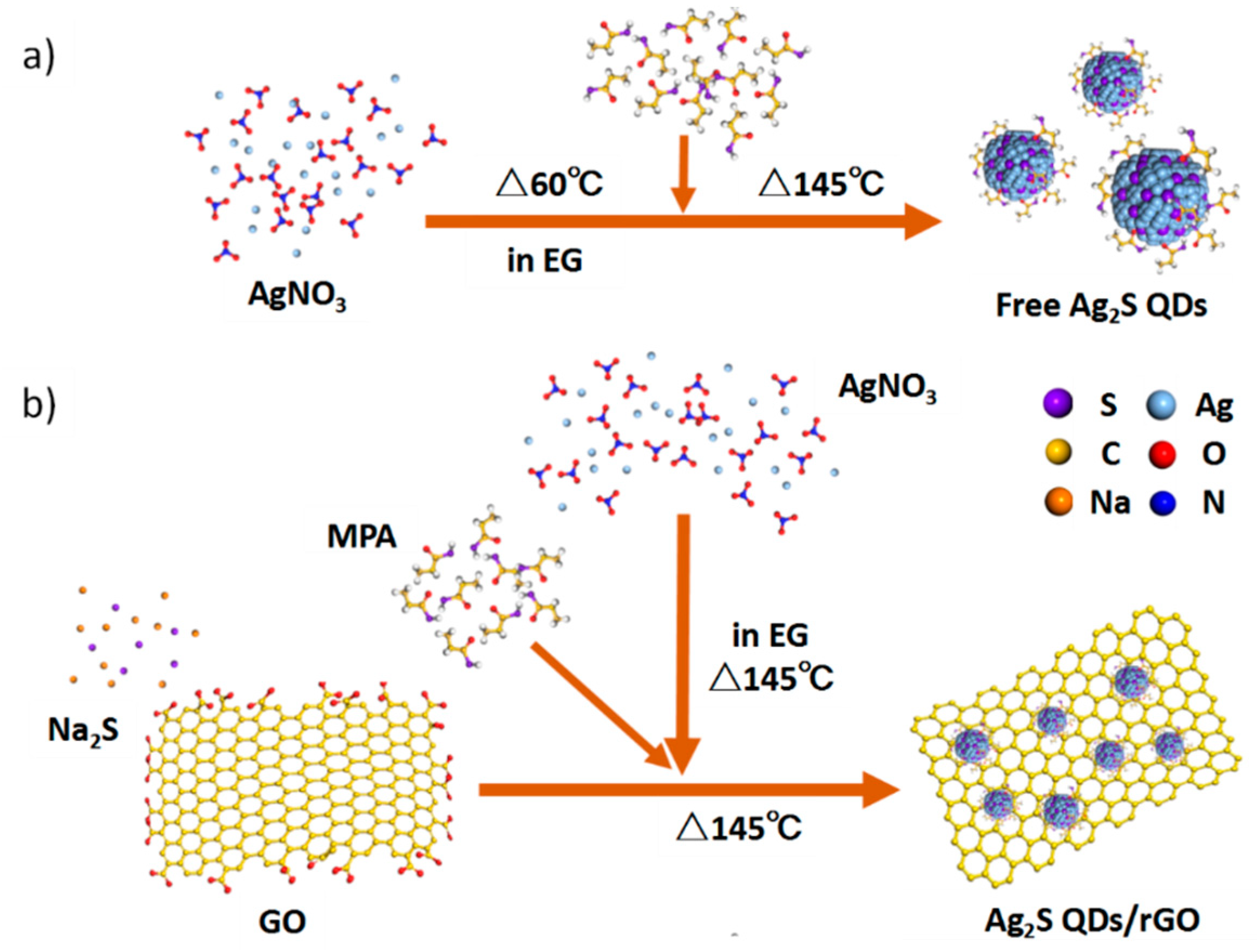
3.2. Synthesis of Ag2S Nanocrystals
3.3. Synthesis of Ag2S/rGO Composite
3.4. Characterizations
3.5. Electrochemical HER Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huxter, V.M.; Mirkovic, T.; Nair, P.S.; Scholes, G.D. Demonstration of bulk semiconductor optical properties in processable Ag2S and EuS nanocrystalline systems. Adv. Mater. 2008, 20, 2439–2443. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Wang, D.; Chen, Y.X.; Tian, Y.F.; Yan, S.S.; Mei, L.M.; Jiao, J. Ag2S/ZnO core-shell nanoheterojunction for a self-powered solid-state photodetector with wide spectral response. J. Alloys Compd. 2018, 735, 2491–2496. [Google Scholar] [CrossRef]
- Tang, S.L.; He, C.S.; Li, D.; Cai, W.H.; Fan, L.Z.; Li, Y.C. Precursor reactivity differentiation for single-step preparation of Ag2Se@Ag2S core-shell nanocrystals with distinct absorption and emission properties enabling sensitive near-infrared photodetection. J. Mater. Sci. 2018, 53, 11355–11366. [Google Scholar] [CrossRef]
- Kang, M.H.; Kim, S.H.; Jang, S.; Lim, J.E.; Chang, H.; Kong, K.J.; Myung, S.; Park, J.K. Synthesis of silver sulfide nanoparticles and their photodetector applications. RSC Adv. 2018, 8, 28447–28452. [Google Scholar] [CrossRef] [Green Version]
- Ghafoor, S.; Ata, S.; Mahmood, N.; Arshad, S.N. Photosensitization of TiO2 nanofibers by Ag2S with the synergistic effect of excess surface Ti3+ states for enhanced photocatalytic activity under simulated sunlight. Sci. Rep. 2017, 7, 255. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liu, D.; Yang, Y.; Xu, R.; Zhang, T.; Sheng, K.; Song, H. Photoelectrochemical detection of alpha-fetoprotein based on ZnO inverse opals structure electrodes modified by Ag2S nanoparticles. Sci. Rep. 2016, 6, 38400. [Google Scholar] [CrossRef]
- Jiang, P.; Zhu, C.N.; Zhang, Z.L.; Tian, Z.Q.; Pang, D.W. Water-soluble Ag2S quantum dots for near-infrared fluorescence imaging in vivo. Biomaterials 2012, 33, 5130–5135. [Google Scholar] [CrossRef]
- Hong, G.; Robinson, J.T.; Zhang, Y.; Diao, S.; Antaris, A.L.; Wang, Q.; Dai, H. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem. 2012, 51, 9818–9821. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Yang, W.; Liu, Y.; Zhai, X. Synthesis of SERS active Ag2S nanocrystals using oleylamine as solvent, reducing agent and stabilizer. Mater. Res. Bull. 2012, 47, 2579–2583. [Google Scholar] [CrossRef]
- Fu, X.; Jiang, T.; Zhao, Q.; Yin, H. Charge-transfer contributions in surface-enhanced Raman scattering from Ag, Ag2S and Ag2Se substrates. J. Raman Spectrosc. 2012, 43, 1191–1195. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.X.; Mao, S.; Wang, Y.; Shen, W.F.; Wang, W.; Huang, L.J.; Li, H.L.; Tang, J.G. Recent progress in synthetic methods and applications in solar cells of Ag2S quantum dots. Mater. Res. Bull. 2018, 106, 113–123. [Google Scholar] [CrossRef]
- Karimipour, M.; Bagheri, M.; Johansson, E.M.J.; Molaei, M. Excellent growth of ZnS shell on Ag2S QDs using a photochemical-microwave irradiation approach and fabrication of their indoor QD thin film solar cells. Mater. Technol. 2018, 33, 784–792. [Google Scholar] [CrossRef]
- Öberg, V.; Zhang, X.; Johansson, M.; Johansson, E. Solution-Processed Environmentally Friendly Ag2S Colloidal Quantum Dot Solar Cells with Broad Spectral Absorption. Appl. Sci. 2017, 7, 1020. [Google Scholar] [CrossRef]
- Suarez, J.A.; Plata, J.J.; Marquez, A.M.; Sanz, J.F. Ag2S Quantum Dot-Sensitized Solar Cells by First Principles: The Effect of Capping Ligands and Linkers. J. Phys. Chem. A 2017, 121, 7290–7296. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Ding, H.M.; Chen, C.H.; Wang, N.N. Ag2S/Bi2S3 co-sensitized TiO2 nanorod arrays prepared on conductive glass as a photoanode for solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 3234–3239. [Google Scholar] [CrossRef]
- Zilio, P.; Dipalo, M.; Tantussi, F.; Messina, G.C.; de Angelis, F. Hot electrons in water: Injection and ponderomotive acceleration by means of plasmonic nanoelectrodes. Light Sci. Appl. 2017, 6, e17002. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Basu, M.; Nazir, R.; Mahala, C.; Fageria, P.; Chaudhary, S.; Gangopadhyay, S.; Pande, S. Ag2S/Ag Heterostructure: A Promising Electrocatalyst for the Hydrogen Evolution Reaction. ACS J. Surf. Colloid. 2017, 33, 3178–3186. [Google Scholar] [CrossRef]
- Yu, W.; Yin, J.; Li, Y.; Lai, B.; Jiang, T.; Li, Y.; Liu, H.; Liu, J.; Zhao, C.; Singh, S.C.; et al. Ag2S Quantum Dots as an Infrared Excited Photocatalyst for Hydrogen Production. ACS Appl. Energy Mater. 2019, 2, 2751–2759. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, K.; Jiang, B.; Li, J.; Zeng, M.; Fu, L. Emerging two-dimensional nanomaterials for electrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 8187–8208. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yang, J.; Dong, Q.C.; Geng, H.B.; Zheng, Y.; Liu, Y.L.; Wang, W.J.; Li, C.C.; Dong, X.C. Highly Dispersive MoP Nanoparticles Anchored on Reduced Graphene Oxide Nanosheets for an Efficient Hydrogen Evolution Reaction Electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 26258–26263. [Google Scholar] [CrossRef] [PubMed]
- Ravula, S.; Zhang, C.; Essner, J.; Robertson, J.; Lin, J.; Baker, G. Ionic liquid-assisted synthesis of nanoscale (MoS2)x(SnO2)1−x on reduced graphene oxide for the electrocatalytic hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 8065–8074. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.V.; Zana, A.; Arenz, M.; Thiele, S. [Mo3S13]2− Cluster Decorated Sulfur-doped Reduced Graphene Oxide as Noble Metal-Free Catalyst for Hydrogen Evolution Reaction in Polymer Electrolyte Membrane Electrolyzers. Chemelectrochem 2018, 5, 2672–2680. [Google Scholar] [CrossRef]
- Fu, H.Y.; Chen, Y.J.; Ren, Z.Y.; Xiao, Y.T.; Liu, Y.Y.; Zhang, X.; Tian, G.H. Highly dispersed of Ni0.85Se nanoparticles on nitrogen-doped graphene oxide as efficient and durable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 262, 107–114. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, S.K.; Jeon, K.M.; Piao, Y.; Kang, Y.C. Mesoporous reduced graphene oxide/WSe2 composite particles for efficient sodium-ion batteries and hydrogen evolution reactions. Appl. Surf. Sci. 2018, 459, 309–317. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Li, F.; Li, W.Z.; Gao, W.B.; Wen, H.; Li, J.; Hu, Y.P.; Luo, Y.T.; Li, R. Porous CoS2 nanostructures based on ZIF-9 supported on reduced graphene oxide: Favourable electrocatalysis for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 6665–6673. [Google Scholar] [CrossRef]
- Tan, S.M.; Pumera, M. Electrosynthesis of Bifunctional WS3−x/Reduced Graphene Oxide Hybrid for Hydrogen Evolution Reaction and Oxygen Reduction Reaction Electrocatalysis. Chem. Eur. J. 2017, 23, 8510–8519. [Google Scholar] [CrossRef]
- Sun, W.Y.; Li, P.; Liu, X.; Shi, J.J.; Sun, H.M.; Tao, Z.L.; Li, F.J.; Chen, J. Size-controlled MoS2 nanodots supported on reduced graphene oxide for hydrogen evolution reaction and sodium-ion batteries. Nano Res. 2017, 10, 2210–2222. [Google Scholar] [CrossRef]
- Liu, Y.R.; Shang, X.; Gao, W.K.; Dong, B.; Li, X.; Li, X.H.; Zhao, J.C.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. In situ sulfurized CoMoS/CoMoO4 shell-core nanorods supported on N-doped reduced graphene oxide (NRGO) as efficient electrocatalyst for hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 2885–2896. [Google Scholar] [CrossRef]
- Li, C.X.; Grantham, J.; Zhao, W. One-pot growth of 3D reduced graphene oxide foams embedded with MoS2 and Ni-doped MoS2 nanocatalysts for hydrogen evolution reaction. Abstr. Pap. Am. Chem. Soc. 2017, 253, 3158–3163. [Google Scholar]
- Lee, J.E.; Jung, J.; Ko, T.Y.; Kim, S.; Kim, S.I.; Nah, J.; Ryu, S.; Nam, K.T.; Lee, M.H. Catalytic synergy effect of MoS2/reduced graphene oxide hybrids for a highly efficient hydrogen evolution reaction. RSC Adv. 2017, 7, 5480–5487. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.H.; Zhao, X.J.; Ye, W.C.; Wang, C.M. Highly porous Ag-Ag2S/MoS2 with additional active sites synthesized by chemical etching method for enhanced electrocatalytic hydrogen evolution. Electrochim. Acta 2014, 142, 173–181. [Google Scholar] [CrossRef]
- Kosmala, T.; Mosconi, D.; Giallongo, G.; Rizzi, G.A.; Granozzi, G. Highly Efficient MoS2/Ag2S/Ag Photoelectrocatalyst Obtained from a Recycled DVD Surface. ACS Sustain. Chem. Eng. 2018, 6, 7818–7825. [Google Scholar] [CrossRef]
- Liu, X.X.; Zang, J.B.; Chen, L.; Chen, L.B.; Chen, X.; Wu, P.; Zhou, S.Y.; Wang, Y.H. A microwave-assisted synthesis of CoO@Co core-shell structures coupled with N-doped reduced graphene oxide used as a superior multi-functional electrocatalyst for hydrogen evolution, oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2017, 5, 5865–5872. [Google Scholar] [CrossRef]
- Qin, Y.C.; Dai, X.P.; Zhang, X.; Huang, X.L.; Sun, H.; Gao, D.W.; Yu, Y.B.; Zhang, P.F.; Jiang, Y.; Zhuo, H.Y.; et al. Microwave-assisted synthesis of multiply-twinned Au-Ag nanocrystals on reduced graphene oxide for high catalytic performance towards hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 3865–3871. [Google Scholar] [CrossRef]
- Konkena, B.; Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. MoSSe@reduced graphene oxide nanocomposite heterostructures as efficient and stable electrocatalysts for the hydrogen evolution reaction. Nano Energy 2016, 29, 46–53. [Google Scholar] [CrossRef]
- Akyuz, D.; Keskin, B.; Sahinturk, U.; Koca, A. Electrocatalytic hydrogen evolution reaction on reduced graphene oxide electrode decorated with cobaltphthalocyanine. Appl. Catal. B Environ. 2016, 188, 217–226. [Google Scholar] [CrossRef]
- Yan, H.J.; Tian, C.G.; Wang, L.; Wu, A.P.; Meng, M.C.; Zhao, L.; Fu, H.G. Phosphorus-Modified Tungsten Nitride/Reduced Graphene Oxide as a High-Performance, Non-Noble-Metal Electrocatalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 6325–6329. [Google Scholar] [CrossRef]
- Ma, L.B.; Shen, X.P.; Zhou, H.; Zhu, G.X.; Ji, Z.Y.; Chen, K.M. CoP nanoparticles deposited on reduced graphene oxide sheets as an active electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 5337–5343. [Google Scholar] [CrossRef]
- Lang, B.; Yu, H.-K. Novel Ag2S nanoparticles on reduced graphene oxide sheets as a super-efficient catalyst for the reduction of 4-nitrophenol. Chin. Chem. Lett. 2017, 28, 417–421. [Google Scholar] [CrossRef]
- Solomon, G.; Mazzaro, R.; You, S.; Natile, M.M.; Morandi, V.; Concina, I.; Vomiero, A. Ag2S/MoS2 Nanocomposites Anchored on Reduced Graphene Oxide: Fast Interfacial Charge Transfer for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 22380–22389. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.T.; Xu, W.C.; Zhu, S.L.; Cui, Z.D.; Yang, X.J.; Inoue, A. Synthesis and properties of nanoporous Ag2S/CuS catalyst for hydrogen evolution reaction. Electrochim. Acta 2016, 190, 221–228. [Google Scholar] [CrossRef]
- Aazam, E.S. Photocatalytic oxidation of methylene blue dye under visible light by Ni doped Ag2S nanoparticles. J. Ind. Eng. Chem. 2014, 20, 4033–4038. [Google Scholar] [CrossRef]
- Romand, M.; Roubin, M.; Deloume, J.P. ESCA studies of some copper and silver selenides. J. Electron Spectrosc. Relat. Phenom. 1978, 13, 229–242. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron Spectrosc. Relat. Phenom. 1991, 57, 273–277. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Cook, J.M. Interactions of NO and SO2 with iron deposited on silica. J. Colloid Interface Sci. 1985, 104, 250–257. [Google Scholar] [CrossRef]
- Chen, D.J.; Zou, L.L.; Li, S.X.; Zheng, F.Y. Nanospherical like reduced graphene oxide decorated TiO2 nanoparticles: An advanced catalyst for the hydrogen evolution reaction. Sci. Rep. 2016, 6, 20335. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef]

| Samples | Overpotential (mV) | Tafel Slope (mV/dec) |
|---|---|---|
| Ag2S/rGO | 120 | 49.1 |
| Ag2S/GO | 131 | 62.3 |
| Ag2S | 207 | 99.2 |
| rGO | 240 | 120.6 |
| Peak | Binding Energy (BE) Position | Full Width Half Maximum (FWHM) | ΔE | Peak Area | % Lorentzian–Gaussian |
|---|---|---|---|---|---|
| (eV) | (eV) | (eV) | |||
| 1 | 161.1 2p3/2 | 0.77 | 1.1 | 10,802 | 22% |
| 2 | 162.0 2p3/2 | 0.92 | 1.3 | 8041 | |
| 3 | 162.2 2p1/2 | 0.90 | 5522 | ||
| 4 | 163.3 2p1/2 | 1.10 | 4135 |
| Peak | BE Position | FWHM | ΔE | Peak Area | % Lorentzian–Gaussian |
|---|---|---|---|---|---|
| (eV) | (eV) | (eV) | |||
| 1 | 161.2 2p3/2 | 0.73 | 1.0 | 9566 | 21% |
| 2 | 162.0 2p3/2 | 0.91 | 1.4 | 1207 | |
| 3 | 162.2 2p1/2 | 0.90 | 5034 | ||
| 4 | 163.4 2p1/2 | 0.99 | 589 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Yu, Z.; Xing, J.; Zou, Y.; Liu, H.; Zhang, H.; Yu, W.; Idriss, H.; Guo, C. Effect of Ag2S Nanocrystals/Reduced Graphene Oxide Interface on Hydrogen Evolution Reaction. Catalysts 2020, 10, 948. https://doi.org/10.3390/catal10090948
Zhao C, Yu Z, Xing J, Zou Y, Liu H, Zhang H, Yu W, Idriss H, Guo C. Effect of Ag2S Nanocrystals/Reduced Graphene Oxide Interface on Hydrogen Evolution Reaction. Catalysts. 2020; 10(9):948. https://doi.org/10.3390/catal10090948
Chicago/Turabian StyleZhao, Chen, Zhi Yu, Jun Xing, Yuting Zou, Huiwen Liu, Hao Zhang, Weili Yu, Hicham Idriss, and Chunlei Guo. 2020. "Effect of Ag2S Nanocrystals/Reduced Graphene Oxide Interface on Hydrogen Evolution Reaction" Catalysts 10, no. 9: 948. https://doi.org/10.3390/catal10090948
APA StyleZhao, C., Yu, Z., Xing, J., Zou, Y., Liu, H., Zhang, H., Yu, W., Idriss, H., & Guo, C. (2020). Effect of Ag2S Nanocrystals/Reduced Graphene Oxide Interface on Hydrogen Evolution Reaction. Catalysts, 10(9), 948. https://doi.org/10.3390/catal10090948







