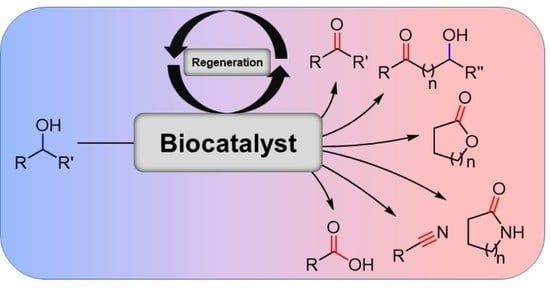
Biocatalytic Oxidation of Alcohols
Abstract
:1. Introduction
1.1. Why Use Biocatalysis for Alcohol Oxidations
1.2. Biocatalysis for Alcohol Oxidation: Perceived and Real Limitations
1.2.1. Availability of Oxidative Enzymes
1.2.2. Substrate Scope
1.2.3. Stability
1.2.4. Biocatalyst Costs
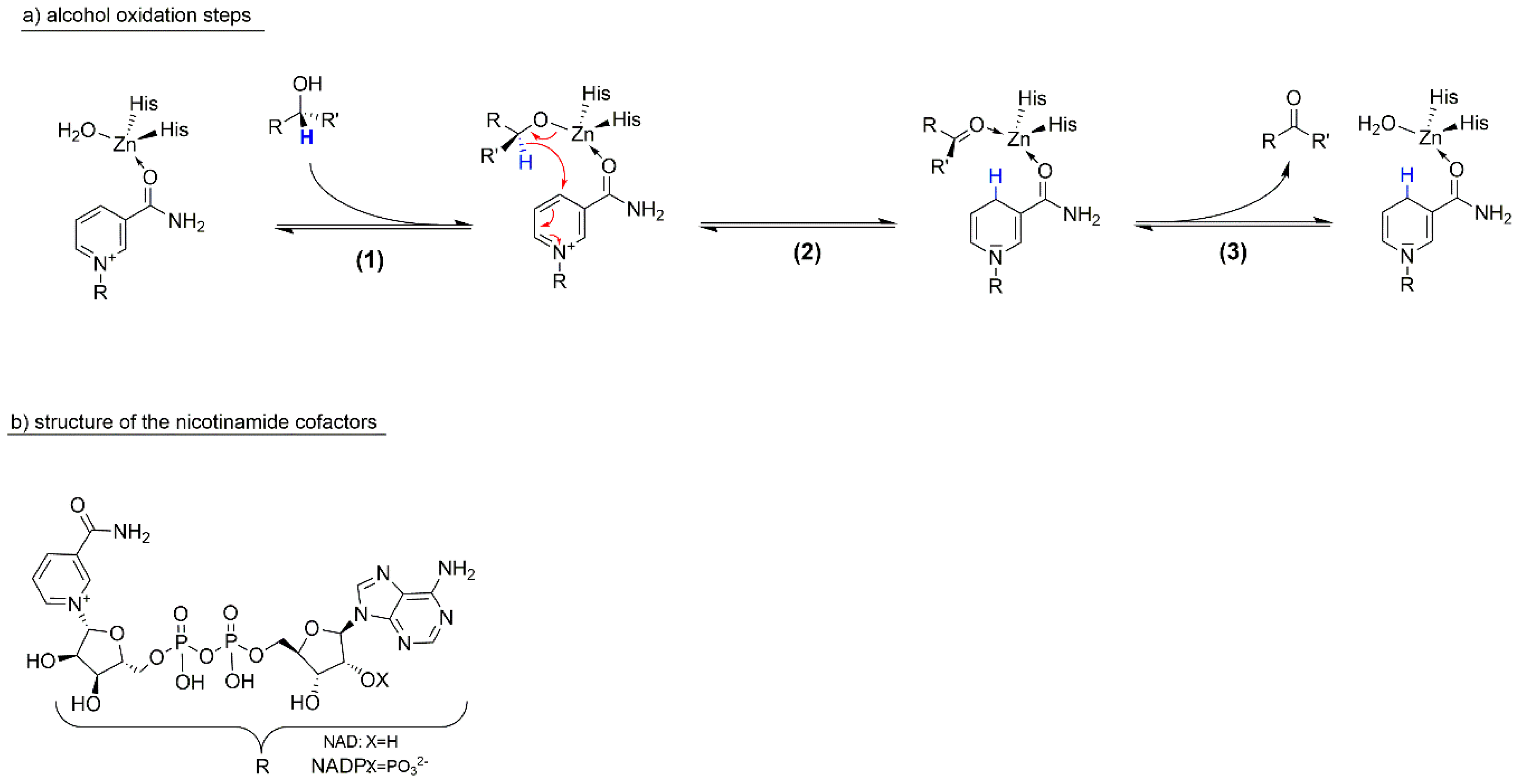
1.3. The Catalysts Used
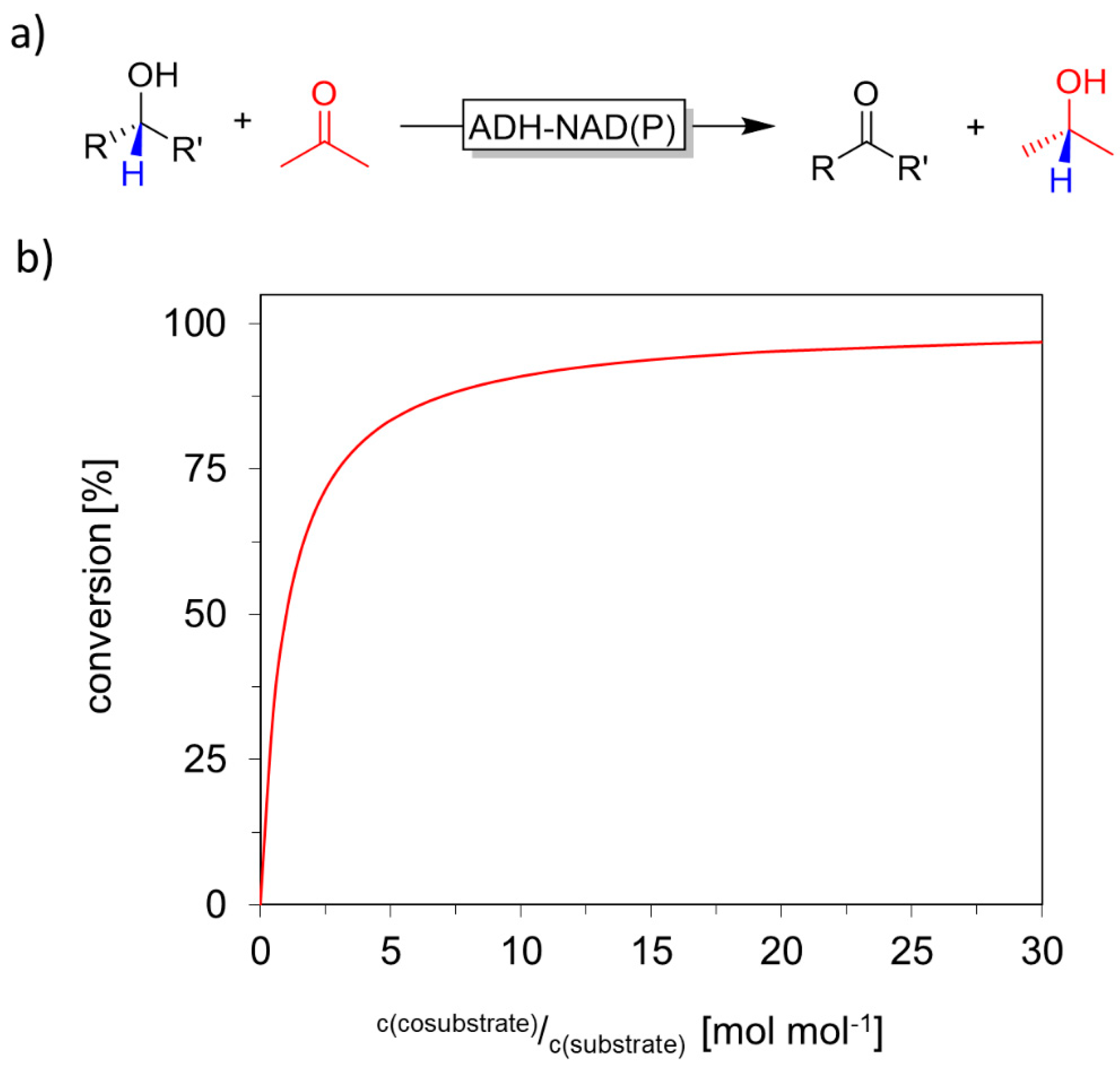
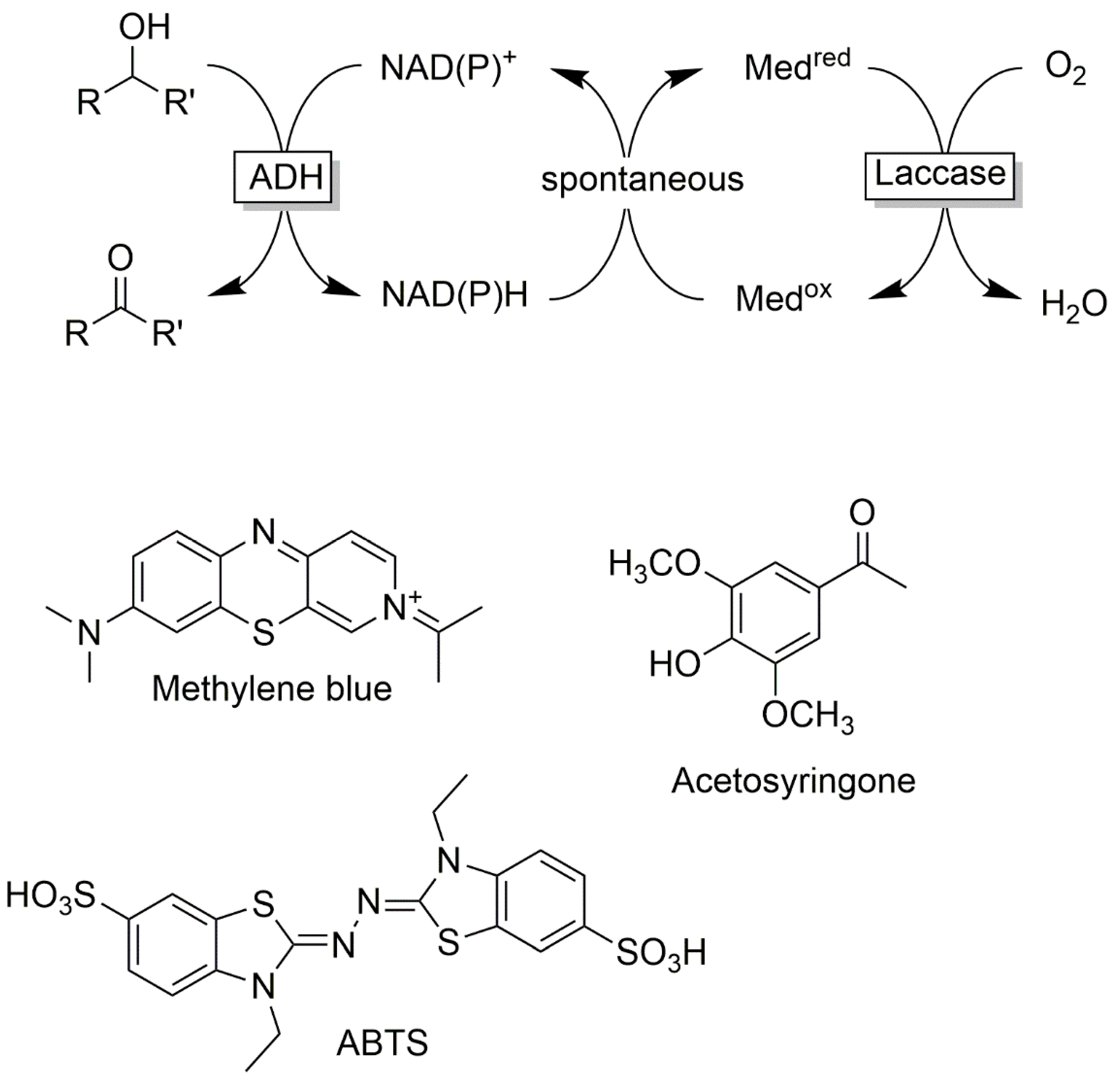
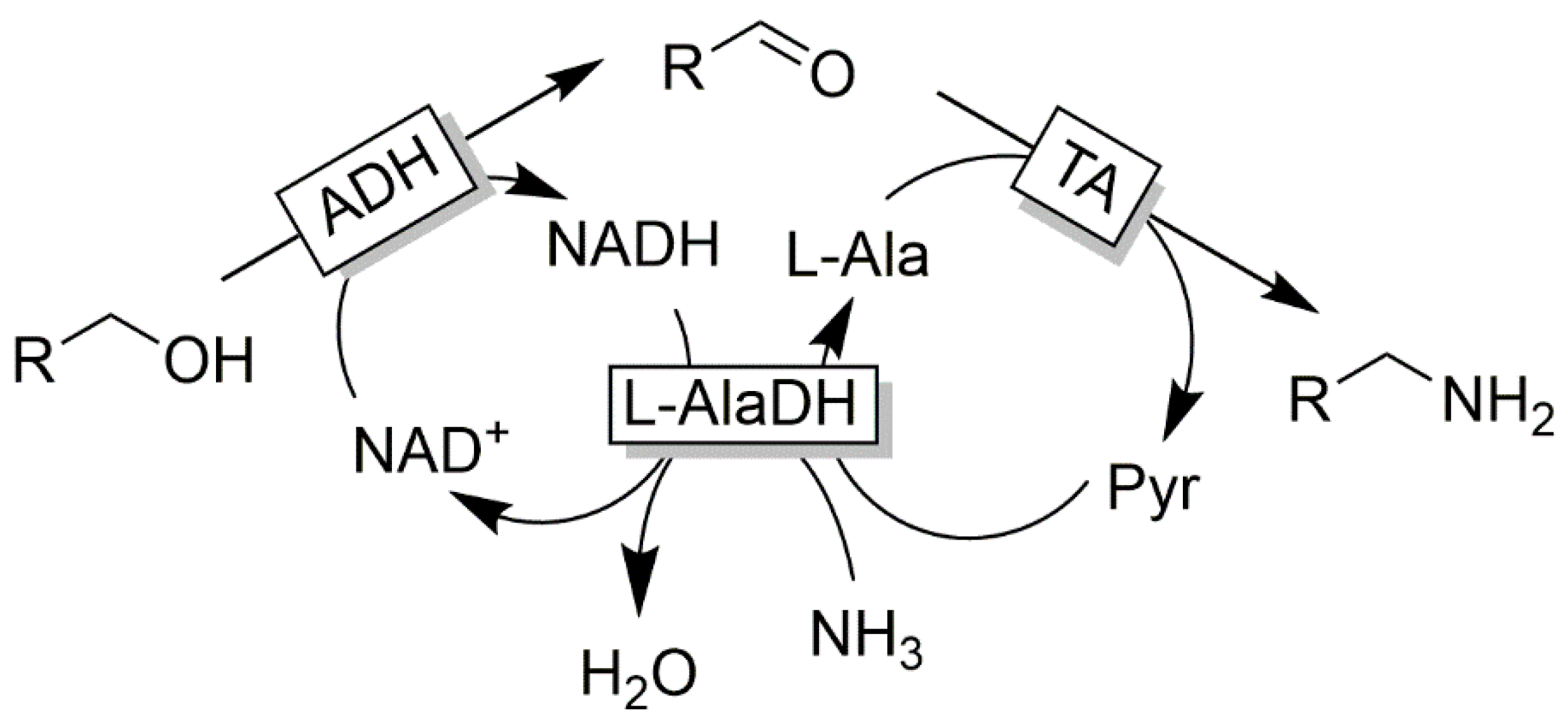
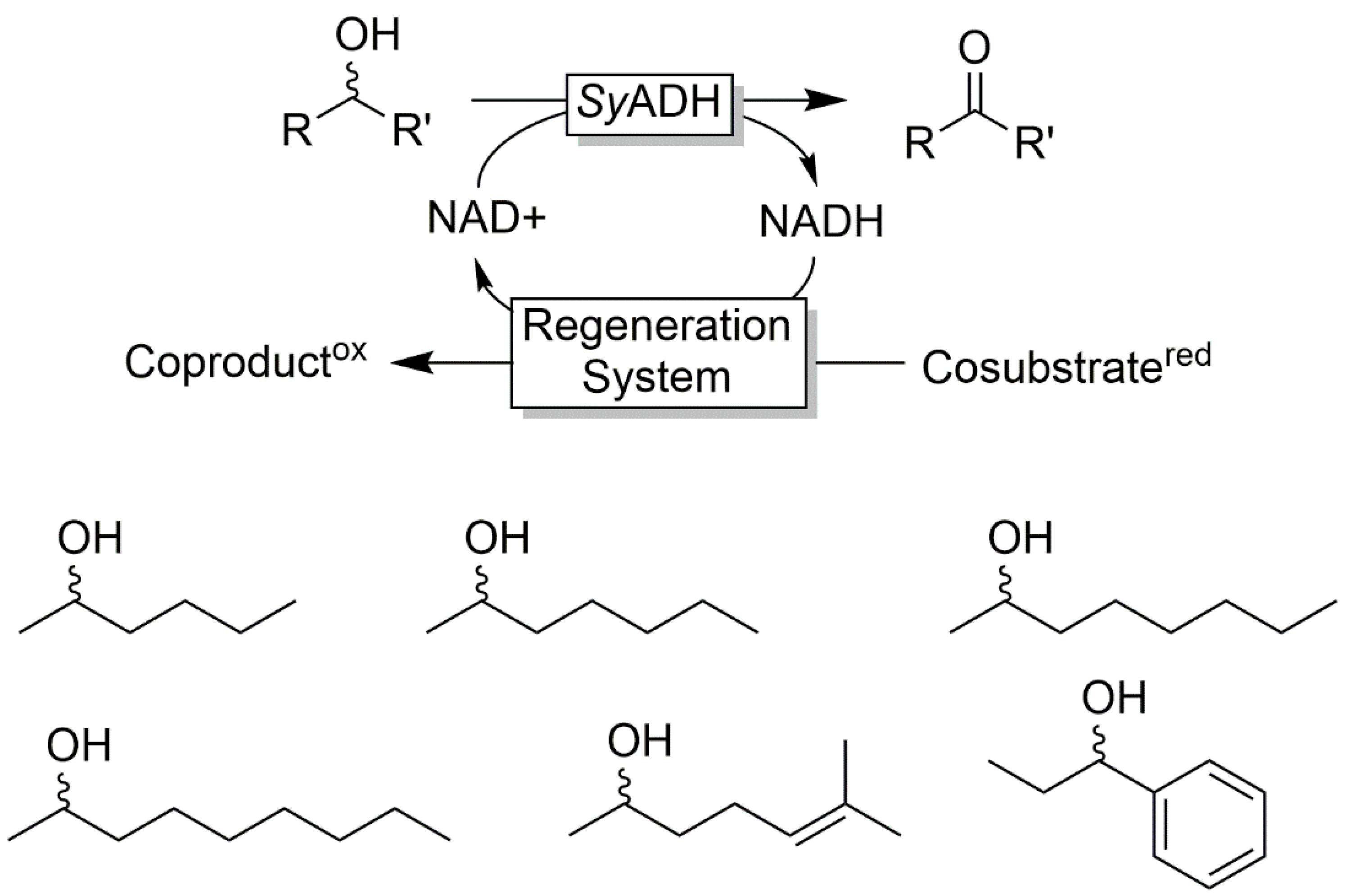
Cofactor Regeneration Strategies
2. Oxidation of Primary Alcohols
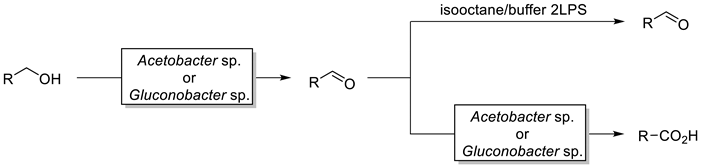
2.1. Oxidation of Primary Alcohols to Aldehydes
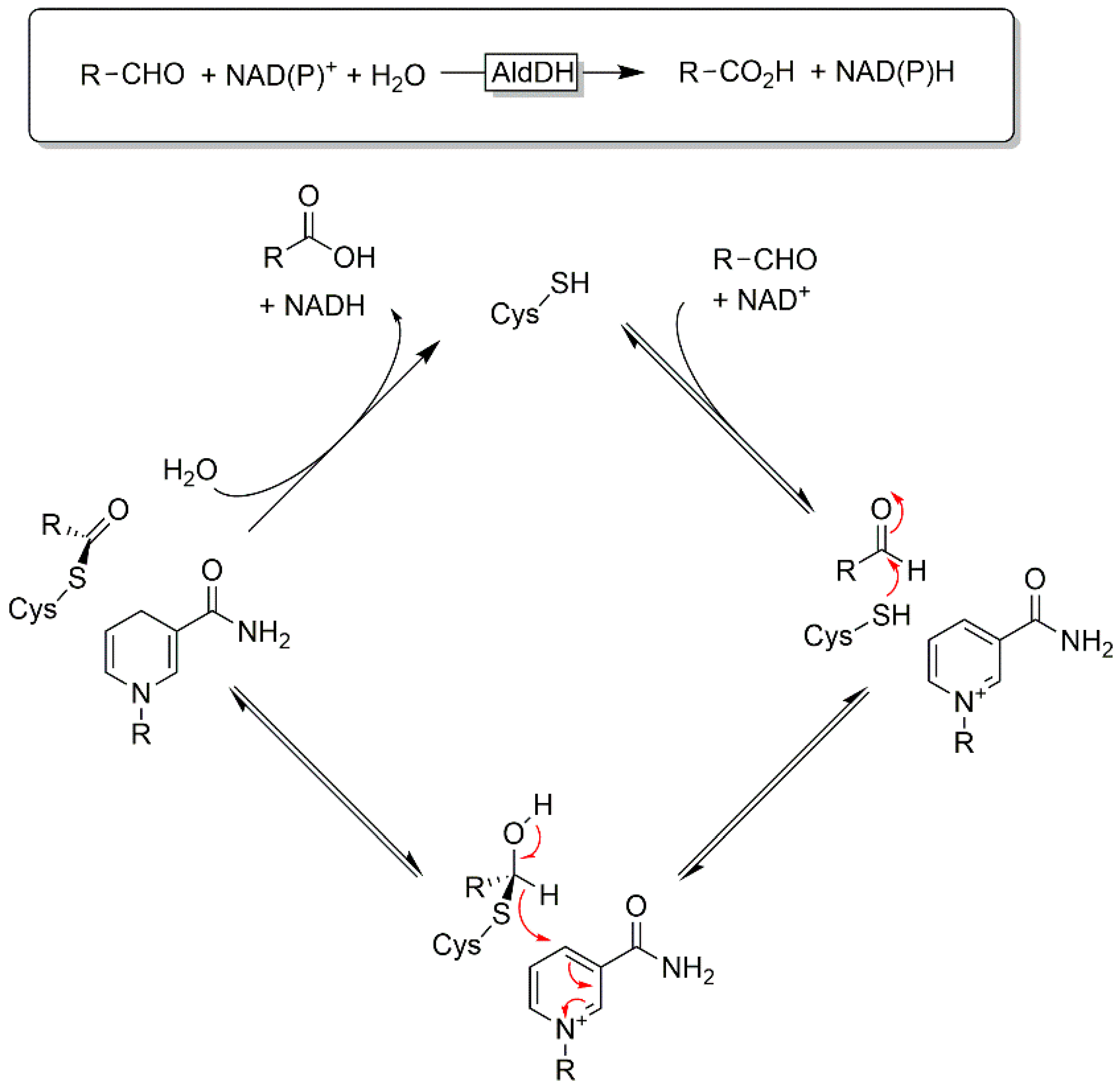
2.2. Oxidation of Primary Alcohols to Acids
3. Oxidation of Secondary Alcohols
3.1. Complete Oxidation of Racemic Secondary Alcohols
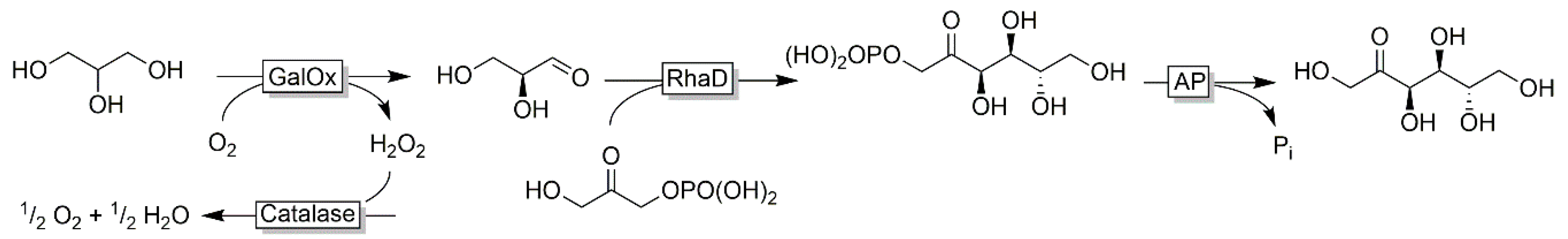
3.2. Regioselective Oxidation of Polyols
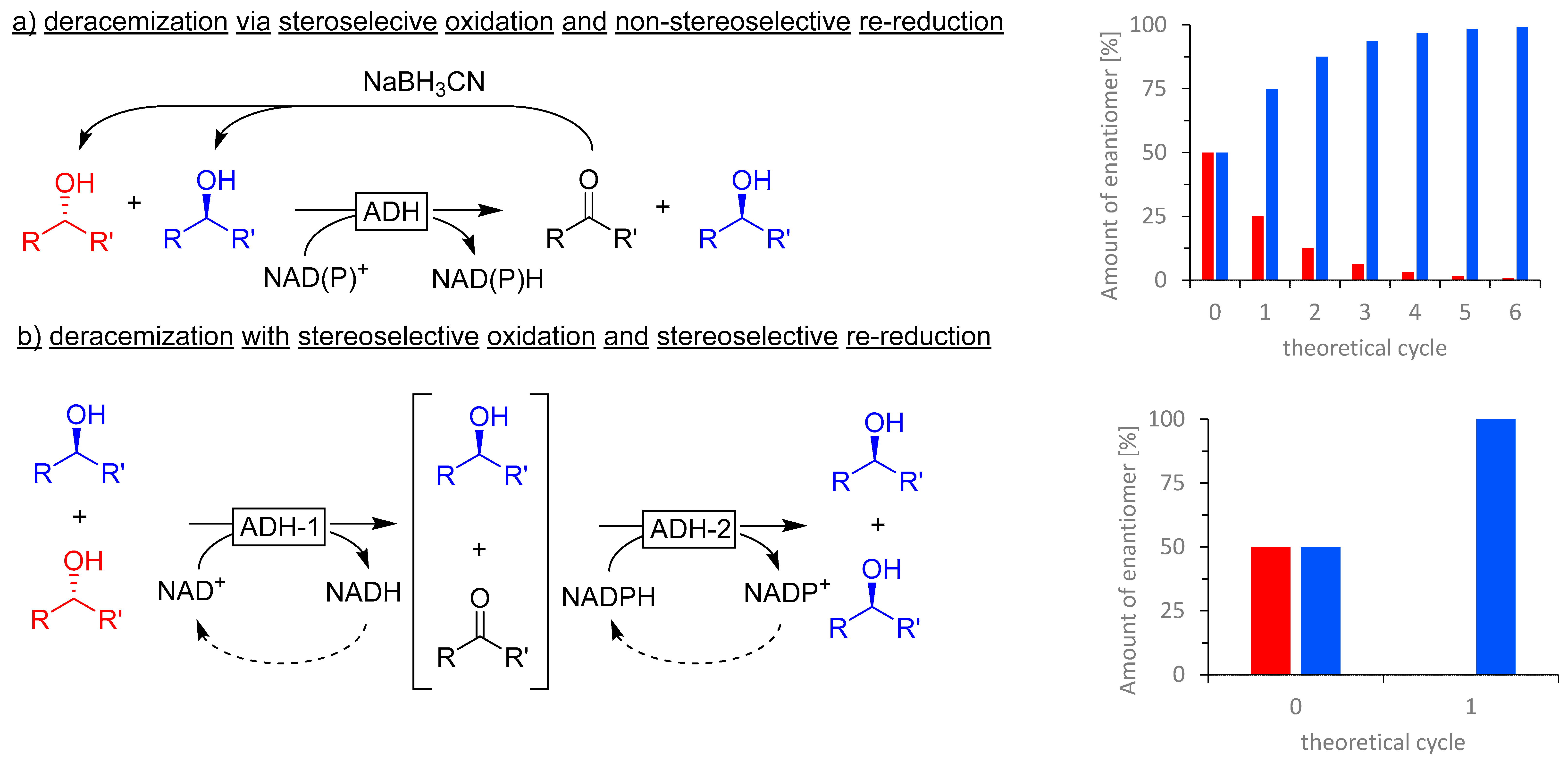
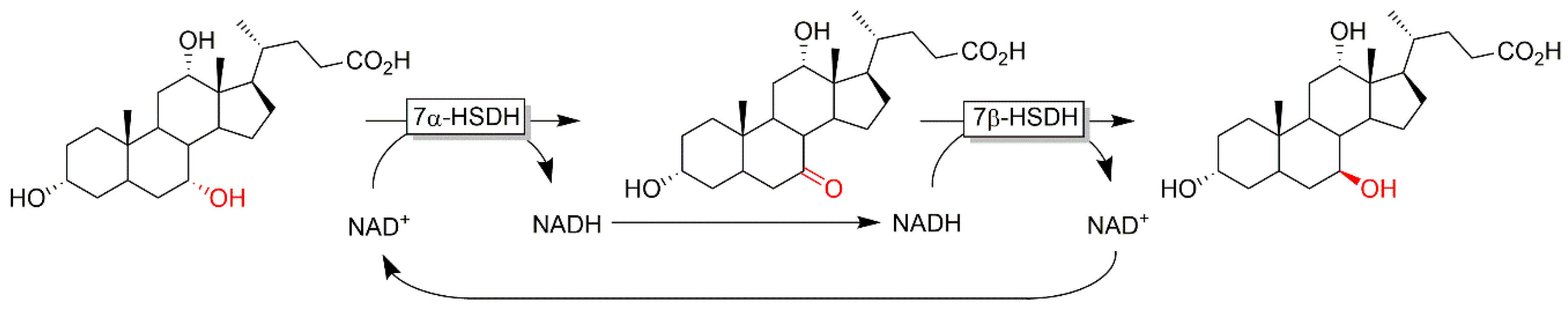
3.3. Kinetic Oxidative Resolution and Deracemization of Racemic Secondary Alcohols
4. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 16, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Schoemaker, H.E.; Mink, D.; Wubbolts, M.G. Dispelling the myths—Biocatalysis in industrial synthesis. Science 2003, 299, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.F.; Williams, G.R. The mechanism of liver alcohol dehydrogenase. Arch. Biochem. Biophys. 1968, 124, 344–348. [Google Scholar] [CrossRef]
- Tsai, C.S. Relative reactivities of primary alcohols as substrates of liver alcohol dehydrogenase. Can. J. Biochem. 1968, 46, 381–385. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [Green Version]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast expression systems: Overview and recent advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Quaglia, D.; Irwin, J.A.; Paradisi, F. Horse liver alcohol dehydrogenase: New perspectives for an old enzyme. Mol. Biotechnol. 2012, 52, 244–250. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Wang, H.L.; Zhang, Y.P.; Chen, L.F.; Wu, K.; Wei, D.Z. Cloning and characterization of three ketoreductases from soil metagenome for preparing optically active alcohols. Biotechnol. Lett. 2016, 38, 1799–1808. [Google Scholar] [CrossRef]
- Berini, F.; Casciello, C.; Marcone, G.L.; Marinelli, F. Metagenomics: Novel enzymes from non-culturable microbes. FEMS Microbiol. Lett. 2017, 364, 19. [Google Scholar] [CrossRef]
- Ausec, L.; Berini, F.; Casciello, C.; Cretoiu, M.S.; van Elsas, J.D.; Marinelli, F.; Mandic-Mulec, I. The first acidobacterial laccase-like multicopper oxidase revealed by metagenomics shows high salt and thermo-tolerance. Appl. Microbiol. Biotechnol. 2017, 101, 6261–6276. [Google Scholar] [CrossRef] [PubMed]
- Ufarté, L.; Potocki-Veronese, G.; Cecchini, D.; Tauzin, A.S.; Rizzo, A.; Morgavi, D.P.; Cathala, B.; Moreau, C.; Cleret, M.; Robe, P.; et al. Highly promiscuous oxidases discovered in the bovine rumen microbiome. Front. Microbiol. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radianingtyas, H.; Wright, P.C. Alcohol dehydrogenases from thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiol. Rev. 2003, 27, 593–616. [Google Scholar] [CrossRef]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 2013, 512840. [Google Scholar] [CrossRef] [PubMed]
- Petratos, K.; Gessmann, R.; Daskalakis, V.; Papadovasilaki, M.; Papanikolau, Y.; Tsigos, I.; Bouriotis, V. Structure and dynamics of a thermostable alcohol dehydrogenase from the antarctic psychrophile Moraxella sp. Tae123. ACS Omega 2020, 5, 14523–14534. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Wei, P.; Zhu, J.; Huang, L.; Cai, J.; Xu, Z. Efficient expression and purification of recombinant alcohol oxidase in Pichia pastoris. Biotechnol. Bioproc. Eng. 2012, 17, 693–702. [Google Scholar] [CrossRef]
- Hamnevik, E.; Maurer, D.; Enugala, T.R.; Chu, T.; Löfgren, R.; Dobritzsch, D.; Widersten, M. Directed evolution of alcohol dehydrogenase for improved stereoselective redox transformations of 1-phenylethane-1,2-diol and its corresponding acyloin. Biochemistry 2018, 57, 1059–1062. [Google Scholar]
- Qu, G.; Liu, B.; Jiang, Y.; Nie, Y.; Yu, H.; Sun, Z. Laboratory evolution of an alcohol dehydrogenase towards enantioselective reduction of difficult-to-reduce ketones. Biores. Bioproc. 2019, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Reetz, M.T. Learning lessons from directed evolution of stereoselective enzymes. Org. Chem. Front. 2016, 3, 1350–1358. [Google Scholar] [CrossRef]
- Viña-Gonzalez, J.; Jimenez-Lalana, D.; Sancho, F.; Serrano, A.; Martinez, A.T.; Guallar, V.; Alcalde, M. Structure-guided evolution of aryl alcohol oxidase from Pleurotus eryngii for the selective oxidation of secondary benzyl alcohols. Adv. Synth. Catal. 2019, 361, 2514–2525. [Google Scholar] [CrossRef] [Green Version]
- Viña-Gonzalez, J.; Gonzalez-Perez, D.; Ferreira, P.; Martinez, A.T.; Alcalde, M. Focused directed evolution of aryl-alcohol oxidase in Saccharomyces cerevisiae by using chimeric signal peptides. Appl. Environ. Microbiol. 2015, 81, 6451–6462. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; Garcia-Ruiz, E.; Gonzalez-Perez, D.; Ullrich, R.; Hofrichter, M.; Alcalde, M. Directed evolution of unspecific peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014, 80, 3496–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ruiz, E.; Gonzalez-Perez, D.; Ruiz-Duenas, F.J.; Martinez, A.T.; Alcalde, M. Directed evolution of a temperature-, peroxide- and alkaline pH-tolerant versatile peroxidase. Biochem. J. 2012, 441, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, G.; Li, A.; Acevedo-Rocha, C.G.; Sun, Z.; Reetz, M.T. The crucial role of methodology development in directed evolution of selective enzymes. Angew. Chem. Int. Ed. 2020, 59, 13204–13231. [Google Scholar] [CrossRef]
- Reetz, M.T. Directed evolution as a means to engineer enantioselective enzymes. In Asymmetric Organic Synthesis with Enzymes; Gotor, V., Alfonso, I., García-Urdiales, E., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2008; pp. 21–63. [Google Scholar]
- Arnold, F.H. Directed evolution: Bringing new chemistry to life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Roiban, G.D.; Reetz, M.T. Enzyme promiscuity: Using a P450 enzyme as a carbene transfer catalyst. Angew. Chem. Int. Ed. 2013, 52, 5439–5440. [Google Scholar] [CrossRef]
- Wang, M.; Si, T.; Zhao, H.M. Biocatalyst development by directed evolution. Biores. Technol. 2012, 115, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.Y.; Wang, Y.; Xu, G.C.; Wu, L.; Han, R.Z.; Schwaneberg, U.; Rao, Y.J.; Zhao, Y.L.; Zhou, J.H.; Ni, Y. Structural insight into enantioselective inversion of an alcohol dehydrogenase reveals a “polar gate” in stereorecognition of diaryl ketones. J. Am. Chem. Soc. 2018, 140, 12645–12654. [Google Scholar] [CrossRef]
- Maria-Solano, M.A.; Romero-Rivera, A.; Osuna, S. Exploring the reversal of enantioselectivity. Org. Biomol. Chem. 2017, 15, 4122–4129. [Google Scholar] [CrossRef] [Green Version]
- Aalbers, F.S.; Fürst, M.J.L.J.; Rovida, S.; Trajkovic, M.; Gómez Castellanos, J.R.; Bartsch, S.; Vogel, A.; Mattevi, A.; Fraaije, M.W. Approaching boiling point stability of an alcohol dehydrogenase through computationally-guided enzyme engineering. eLife 2020, 9, e54639. [Google Scholar] [CrossRef]
- Pickl, M.; Winkler, C.K.; Glueck, S.M.; Fraaije, M.W.; Faber, K. Rational engineering of a flavoprotein oxidase for improved direct oxidation of alcohols to carboxylic acids. Molecules 2017, 22, 2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Heuvel, R.H.H.; Fraaije, M.W.; Mattevi, A.; Laane, C.; van Berkel, W.J.H. Vanillyl-alcohol oxidase, a tasteful biocatalyst. J. Mol. Catal. B Enzym. 2001, 11, 185–188. [Google Scholar] [CrossRef]
- Van den Heuvel, R.H.H.; Fraaije, M.W.; van Berkel, W.J.H. Direction of the reactivity of vanillyl-alcohol oxidase with 4-alkylphenols. FEBS Lett. 2000, 481, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Van den Heuvel, R.H.H.; Fraaije, M.W.; Ferrer, M.; Mattevi, A.; van Berkel, W.J.H. Inversion of stereospecificity of vanillyl-alcohol oxidase. Proc. Natl. Acad. Sci. USA 2000, 97, 9455–9460. [Google Scholar] [CrossRef] [Green Version]
- Drijfhout, F.P.; Fraaije, M.W.; Jongejan, H.; van Berkel, W.J.H.; Franssen, M.C.R. Enantioselective hydroxylation of 4-alkylphenols by vanillyl alcohol oxidase. Biotechnol. Bioeng. 1998, 59, 171–177. [Google Scholar] [CrossRef]
- Fraaije, M.W.; van Berkel, W.J.H. Catalytic mechanism of the oxidative demethylation of 4-(methoxymethyl)phenol by vanillyl-alcohol oxidase - evidence for formation of a p-quinone methide intermediate. J. Biol. Chem. 1997, 272, 18111–18116. [Google Scholar] [CrossRef] [Green Version]
- Vina-Gonzalez, J.; Alcalde, M. Directed evolution of the aryl-alcohol oxidase: Beyond the lab bench. Comp. Struct. Biotechnol. J. 2020, 18, 1800–1810. [Google Scholar] [CrossRef]
- Vina-Gonzalez, J.; Elbl, K.; Ponte, X.; Valero, F.; Alcalde, M. Functional expression of aryl-alcohol oxidase in Saccharomyces cerevisiae and Pichia pastoris by directed evolution. Biotechnol. Bioeng. 2018, 115, 1666–1674. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.; Sancho, F.; Vina-Gonzalez, J.; Carro, J.; Alcalde, M.; Guallar, V.; Martinez, A.T. Switching the substrate preference of fungal aryl-alcohol oxidase: Towards stereoselective oxidation of secondary benzyl alcohols. Catal. Sci. Technol. 2019, 9, 833–841. [Google Scholar] [CrossRef] [Green Version]
- Machielsen, R.; Leferink, N.G.H.; Hendriks, A.; Brouns, S.J.J.; Hennemann, H.-G.; Dauβmann, T.; van der Oost, J. Laboratory evolution of Pyrococcus furiosus alcohol dehydrogenase to improve the production of (2S,5S)-hexanediol at moderate temperatures. Extremophiles 2008, 12, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Pennacchio, A.; Pucci, B.; Secundo, F.; La Cara, F.; Rossi, M.; Raia, C.A. Purification and characterization of a novel recombinant highly enantioselective short-chain NAD(H)-dependent alcohol dehydrogenase from Thermus thermophilus. Appl. Environ. Microbiol. 2008, 74, 3949–3958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höllrigl, V.; Hollmann, F.; Kleeb, A.; Buehler, K.; Schmid, A. TADH, the thermostable alcohol dehydrogenase from Thermus sp. ATN1: A versatile new biocatalyst for organic synthesis. Appl. Microbiol. Biotechnol. 2008, 81, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Raia, C.A.; Giordano, A.; Rossi, M.; Adams, M.W.W.; Kelly, R.M. Alcohol dehydrogenase from Sulfolobus solfataricus. In Methods Enzymol.; Academic Press: Cambridge, MA, USA, 2001; Volume 331, pp. 176–195. [Google Scholar]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Proc. Res. Dev. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Moa, S.; Himo, F. Quantum chemical study of mechanism and stereoselectivity of secondary alcohol dehydrogenase. J. Inorg. Biochem. 2017, 175, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Opperman, D.J.; Paul, C.E. Enzymatic reductions - a chemist’s perspective. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Musa, M.M.; Ziegelmann-Fjeld, K.I.; Vieille, C.; Phillips, R.S. Activity and selectivity of w110a secondary alcohol dehydrogenase from Thermoanaerobacter ethanolicus in organic solvents and ionic liquids: Mono-and biphasic media. Org. Biomol. Chem. 2008, 6, 887–892. [Google Scholar] [CrossRef]
- Fossati, E.; Polentini, F.; Carrea, G.; Riva, S. Exploitation of the alcohol dehydrogenase-acetone nadp-regeneration system for the enzymatic preparative-scale production of 12-ketochenodeoxycholic acid. Biotechnol. Bioeng. 2006, 93, 1216–1220. [Google Scholar] [CrossRef]
- Kosjek, B.; Stampfer, W.; Pogorevc, M.; Goessler, W.; Faber, K.; Kroutil, W. Purification and characterization of a chemotolerant alcohol dehydrogenase applicable to coupled redox reactions. Biotechnol. Bioeng. 2004, 86, 55–62. [Google Scholar] [CrossRef]
- Orbegozo, T.; Vries, J.G.d.; Kroutil, W. Biooxidation of primary alcohols to aldehydes through hydrogen transfer employing Janibacter terrae. Eur. J. Org. Chem. 2010, 2010, 3445–3448. [Google Scholar] [CrossRef] [Green Version]
- Chenault, H.; Whitesides, G. Regeneration of nicotinamide cofactors for use in organic synthesis. App. Biochem. Biotechnol. 1987, 14, 147–197. [Google Scholar] [CrossRef]
- Ni, Y.; Holtmann, D.; Hollmann, F. How green is biocatalysis? To calculate is to know. ChemCatChem 2014, 6, 930–943. [Google Scholar] [CrossRef]
- Lavandera, I.; Kern, A.; Resch, V.; Ferreira-Silva, B.; Glieder, A.; Fabian, W.M.F.; de Wildeman, S.; Kroutil, W. One-way biohydrogen transfer for oxidation of sec-alcohols. Org. Lett. 2008, 10, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Riebel, B.R.; Gibbs, P.R.; Wellborn, W.B.; Bommarius, A.S. Cofactor regeneration of NAD+ from NADH: Novel water-forming NADH oxidases. Adv. Synth. Catal. 2002, 344, 1156–1168. [Google Scholar] [CrossRef]
- Riebel, B.; Gibbs, P.; Wellborn, W.; Bommarius, A. Cofactor regeneration of both NAD+ from NADH and NADP+ from NADPH:NADH oxidase from Lactobacillus sanfranciscensis. Adv. Synth. Catal. 2003, 345, 707–712. [Google Scholar] [CrossRef]
- Hummel, W.; Riebel, B. Isolation and biochemical characterization of a new NADH oxidase from lactobacillus brevis. Biotechnol. Lett. 2003, 25, 51–54. [Google Scholar] [CrossRef]
- Hummel, W.; Kuzu, M.; Geueke, B. An efficient and selective enzymatic oxidation system for the synthesis of enantiomerically pure d-tert-leucine. Org. Lett. 2003, 5, 3649–3650. [Google Scholar] [CrossRef]
- Geueke, B.; Riebel, B.; Hummel, W. NADH oxidase from Lactobacillus brevis: A new catalyst for the regeneration of NAD. Enzym. Microb. Technol. 2003, 32, 205–211. [Google Scholar] [CrossRef]
- Jiang, R.; Bommarius, A.S. Hydrogen peroxide-producing NADH oxidase (nox-1) from Lactococcus lactis. Tetrahedron Asymm. 2004, 15, 2939–2944. [Google Scholar] [CrossRef]
- Hirano, J.; Miyamoto, K.; Ohta, H. Purification and characterization of thermostable H2O2-forming NADH oxidase from 2-phenylethanol-assimilating Brevibacterium sp. KU1309. Appl. Microbiol. Biotechnol. 2008, 80, 71–78. [Google Scholar] [CrossRef]
- Huang, L.; Sayoga, G.V.; Hollmann, F.; Kara, S. Horse liver alcohol dehydrogenase-catalyzed oxidative lactamization of amino alcohols. ACS Catal. 2018, 8, 8680–8684. [Google Scholar] [CrossRef] [Green Version]
- Nowak, C.; Beer, B.; Pick, A.; Roth, T.; Lommes, P.; Sieber, V. A water-forming NADH oxidase from Lactobacillus pentosus suitable for the regeneration of synthetic biomimetic cofactors. Front. Microbiol. 2015, 6, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, N.H.; Hollmann, F.; Kracher, D.; Preims, M.; Haltrich, D.; Ludwig, R. Engineering an enzymatic regeneration system for NAD(P)H oxidation. J. Mol. Catal. B Enzym. 2015, 120, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Kochius, S.; Ni, Y.; Kara, S.; Gargiulo, S.; Schrader, J.; Holtmann, D.; Hollmann, F. Light-accelerated biocatalytic oxidation reactions. ChemPlusChem 2014, 79, 1554–1557. [Google Scholar] [CrossRef]
- Könst, P.; Kara, S.; Kochius, S.; Holtmann, D.; Arends, I.W.C.E.; Ludwig, R.; Hollmann, F. Expanding the scope of laccase-mediator systems. ChemCatChem 2013, 5, 3027–3032. [Google Scholar] [CrossRef]
- Kara, S.; Spickermann, D.; Schrittwieser, J.H.; Weckbecker, A.; Leggewie, C.; Arends, I.W.C.E.; Hollmann, F. Access to lactone building blocks via horse liver alcohol dehydrogenase-catalyzed oxidative lactonization. ACS Catal. 2013, 3, 2436–2439. [Google Scholar] [CrossRef]
- Aksu, S.; Arends, I.W.C.E.; Hollmann, F. A new regeneration system for oxidized nicotinamide cofactors. Adv. Synth. Catal. 2009, 351, 1211–1216. [Google Scholar] [CrossRef]
- Ferrandi, E.E.; Monti, D.; Patel, I.; Kittl, R.; Haltrich, D.; Riva, S.; Ludwig, R. Exploitation of a laccase/meldola’s blue system for nad+ regeneration in preparative scale hydroxysteroid dehydrogenase-catalyzed oxidations. Adv. Synth. Catal. 2012, 354, 2821–2828. [Google Scholar] [CrossRef]
- Tonin, F.; Martì, E.; Arends, I.W.C.E.; Hanefeld, U. Laccase did it again: A scalable and clean regeneration system for NAD+ and its application in the synthesis of 12-oxo-hydroxysteroids. Catalysts 2020, 10, 677. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Q.; Pu, L.; Tan, Z.; Guo, K.; Ying, H.; Ouyang, P. Nonenzymatic and metal-free organocatalysis for in situ regeneration of oxidized cofactors by activation and reduction of molecular oxygen. ACS Catal. 2016, 6, 4989–4994. [Google Scholar] [CrossRef]
- Rauch, M.; Schmidt, S.; Arends, I.W.C.E.; Oppelt, K.; Kara, S.; Hollmann, F. Photobiocatalytic alcohol oxidation using led light sources. Green Chem. 2017, 19, 376–379. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, S.; Arends, I.W.C.E.; Hollmann, F. A photoenzymatic system for alcohol oxidation. ChemCatChem 2011, 3, 338–342. [Google Scholar] [CrossRef]
- Boratyński, F.; Dancewicz, K.; Paprocka, M.; Gabryś, B.; Wawrzeńcz, C. Chemo-enzymatic synthesis of optically active γ- and δ-decalactones and their effect on aphid probing, feeding and settling behavior. PLoS ONE 2016, 11, e0146160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boratynski, F.; Kielbowicz, G.; Wawrzenczyk, C. Lactones 34. Application of alcohol dehydrogenase from horse liver (hladh) in enantioselective synthesis of δ- and ε-lactones. J. Mol. Catal. B Enzym. 2010, 65, 30–36. [Google Scholar] [CrossRef]
- Irwin, A.J.; Jones, J.B. Asymmetric syntheses via enantiotopically selective horse liver alcohol dehydrogenase catalyzed oxidations of diols containing a prochiral center. J. Am. Chem. Soc. 1977, 99, 556–561. [Google Scholar] [CrossRef]
- Dias Gomes, M.; Bommarius, B.R.; Anderson, S.R.; Feske, B.D.; Woodley, J.M.; Bommarius, A.S. Bubble column enables higher reaction rate for deracemization of (R,S)-1-phenylethanol with coupled alcohol dehydrogenase/NADH oxidase system. Adv. Synth. Catal. 2019, 361, 2574–2581. [Google Scholar] [CrossRef] [Green Version]
- Presecki, A.V.; Vasic-Racki, Ð. Mathematical modelling of the dehydrogenase catalyzed hexanol oxidation with coenzyme regeneration by NADH oxidase. Proc. Biochem. 2009, 44, 54–61. [Google Scholar] [CrossRef]
- Könst, P.; Merkens, H.; Kara, S.; Kochius, S.; Vogel, A.; Zuhse, R.; Holtmann, D.; Arends, I.W.C.E.; Hollmann, F. Oxidation von aldehyden mit alkoholdehydrogenasen. Angew. Chem. Int. Ed. 2012, 51, 9914–9917. [Google Scholar] [CrossRef]
- Zhang, J.D.; Cui, Z.M.; Fan, X.J.; Wu, H.L.; Chang, H.H. Cloning and characterization of two distinct water-forming NADH oxidases from Lactobacillus pentosus for the regeneration of nad. Bioproc. Biosys. Eng. 2016, 39, 603–611. [Google Scholar] [CrossRef]
- Holec, C.; Neufeld, K.; Pietruszka, J. P450 bm3 monooxygenase as an efficient NAD(P)H-oxidase for regeneration of nicotinamide cofactors in ADH-catalysed preparative scale biotransformations. Adv. Synth. Catal. 2016, 358, 1810–1819. [Google Scholar] [CrossRef]
- Rehn, G.; Pedersen, A.T.; Woodley, J.M. Application of nad(p)h oxidase for cofactor regeneration in dehydrogenase catalyzed oxidations. J. Mol. Catal. B Enzym. 2016, 134, 331–339. [Google Scholar] [CrossRef]
- Ni, Y.; Fernández-Fueyo, E.; Baraibar, A.G.; Ullrich, R.; Hofrichter, M.; Yanase, H.; Alcalde, M.; van Berkel, W.J.H.; Hollmann, F. Peroxygenase-catalyzed oxyfunctionalization reactions promoted by the complete oxidation of methanol. Angew. Chem. Int. Ed. 2016, 55, 798–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.-Y.; Zong, M.-H.; Zheng, G.-W.; Li, N. Myoglobin-catalyzed efficient in situ regeneration of nad(p)+ and their synthetic biomimetic for dehydrogenase-mediated oxidations. ACS Catal. 2019, 9, 2196–2202. [Google Scholar] [CrossRef]
- Jia, H.-Y.; Zong, M.-H.; Zheng, G.-W.; Li, N. One-pot enzyme cascade for controlled synthesis of furancarboxylic acids from 5-hydroxymethylfurfural by H2O2 internal recycling. ChemSusChem 2019, 12, 4764–4768. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, H.; Mayr, T.; Hobisch, M.; Borisov, S.; Klimant, I.; Krühne, U.; Woodley, J.M. Measurement of oxygen transfer from air into organic solvents. J. Chem. Technol. Biotechnol. 2016, 91, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A.T.; Birmingham, W.R.; Rehn, G.; Charnock, S.J.; Turner, N.J.; Woodley, J.M. Process requirements of galactose oxidase catalyzed oxidation of alcohols. Org. Proc. Res. Dev. 2015, 19, 1580–1589. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Karau, A. Deactivation of formate dehydrogenase (FDH) in solution and at gas-liquid interfaces. Biotechnol. Prog. 2005, 21, 1663–1672. [Google Scholar] [CrossRef]
- Thomas, C.R.; Geer, D. Effects of shear on proteins in solution. Biotechnol. Lett. 2011, 33, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, W.; Ludwig, R.; Dewulf, J.; Auly, M.; Messiaen, T.; Haltrich, D.; Van Langenhove, H. Bubble-free oxygenation of a bi-enzymatic system: Effect on biocatalyst stability. Biotechnol. Bioeng. 2009, 102, 122–131. [Google Scholar] [CrossRef]
- Massey, V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 2000, 28, 283–296. [Google Scholar] [CrossRef]
- Massey, V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994, 269, 22459–22462. [Google Scholar]
- Whittaker, J.W. The radical chemistry of galactose oxidase. Arch. Biochem. Biophys. 2005, 433, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.W. Free radical catalysis by galactose oxidase. Chem. Rev. 2003, 103, 2347–2363. [Google Scholar] [CrossRef] [PubMed]
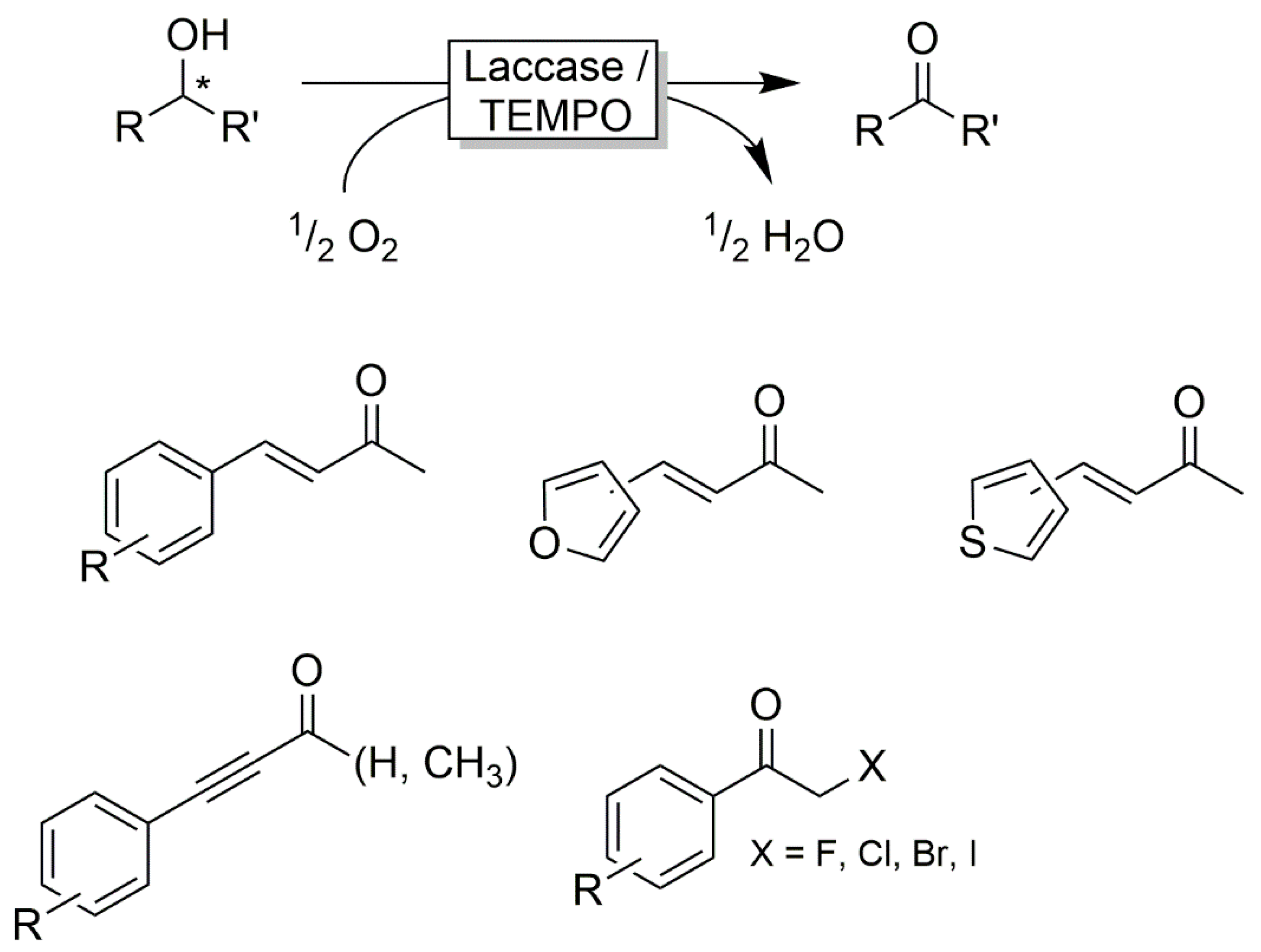
- Díaz-Rodríguez, A.; Lavandera, I.; Kanbak-Aksu, S.; Sheldon, R.A.; Gotor, V.; Gotor-Fernández, V. From diols to lactones under aerobic conditions using a laccase/TEMPO catalytic system in aqueous medium. Adv. Synth. Catal. 2012, 18, 3405–3408. [Google Scholar] [CrossRef] [Green Version]
- Tromp, S.; Matijošytė, I.; Sheldon, R.; Arends, I.; Mul, G.; Kreutzer, M.; Moulijn, J.; de Vries, S. Mechanism of laccase–TEMPO-catalyzed oxidation of benzyl alcohol. ChemCatChem 2010, 2, 827–833. [Google Scholar] [CrossRef]
- Arends, I.; Li, Y.X.; Ausan, R.; Sheldon, R.A. Comparison of tempo and its derivatives as mediators in laccase catalysed oxidation of alcohols. Tetrahedron 2006, 62, 6659–6665. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E. Organocatalytic oxidations mediated by nitroxyl radicals. Adv. Synth. Catal. 2004, 346, 1051–1071. [Google Scholar] [CrossRef]
- Bühler, B.; Bollhalder, I.; Hauer, B.; Witholt, B.; Schmid, A. Chemical biotechnology for the specific oxyfunctionalization of hydrocarbons on a technical scale. Biotechnol. Bioeng. 2003, 82, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Bühler, B.; Bollhalder, I.; Hauer, B.; Witholt, B.; Schmid, A. Use of the two-liquid phase concept to exploit kinetically controlled multistep biocatalysis. Biotechnol. Bioeng. 2003, 81, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Bühler, B.; Witholt, B.; Hauer, B.; Schmid, A. Characterization and application of xylene monooxygenase for multistep biocatalysis. Appl. Environ. Microbiol. 2002, 68, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bühler, B.; Schmid, A.; Hauer, B.; Witholt, B. Xylene monooxygenase catalyzes the multistep oxygenation of toluene and pseudocumene to corresponding alcohols, aldehydes, and acids in Escherichia coli jm101. J. Biol. Chem. 2000, 275, 10085–10092. [Google Scholar] [CrossRef] [Green Version]
- Molinari, F.; Gandolfi, R.; Aragozzini, F.; Leon, R.; Prazeres, D.M.F. Biotransformations in two-liquid-phase systems: Production of phenylacetaldehyde by oxidation of 2-phenylethanol with acetic acid bacteria. Enzym. Microb. Technol. 1999, 25, 729–735. [Google Scholar] [CrossRef]
- Gandolfi, R.; Cavenago, K.; Gualandris, R.; Sinisterra Gago, J.V.; Molinari, F. Production of 2-phenylacetic acid and phenylacetaldehyde by oxidation of 2-phenylethanol with free immobilized cells of Acetobacter aceti. Proc. Biochem. 2004, 39, 749–753. [Google Scholar] [CrossRef]
- Villa, R.; Romano, A.; Gandolfi, R.; Sinisterra Gago, J.V.; Molinari, F. Chemoselective oxidation of primary alcohols to aldehydes with Gluconobacter oxydans. Tetrahedron Lett. 2002, 43, 6059–6061. [Google Scholar] [CrossRef]
- Gandolfi, R.; Ferrara, N.; Molinari, F. An easy and efficient method for the production of carboxylic acids and aldehydes by microbial oxidation of primary alcohols. Tetrahedron Lett. 2001, 42, 513–514. [Google Scholar] [CrossRef]
- Ferreira, P.; Medina, M.; Guillén, F.; Martínez, M.J.; Van Berkel, W.J.H.; Martínez, Á.T. Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem. J. 2005, 389, 731–738. [Google Scholar] [CrossRef]
- Francisco, G.; Angel, T.M.; Maria Jesús, M. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur. J. Biochem. 1992, 209, 603–611. [Google Scholar]
- De Almeida, T.P.; van Schie, M.; Tieves, F.; Ma, A.; Younes, S.; Fernandez Fueyo, E.; Arends, I.; Riul, A.; Hollmann, F. Efficient aerobic oxidation of trans-2-hexen-1-ol using the aryl alcohol oxidase from Pleurotus eryngii. Adv. Synth. Catal. 2019, 361, 2668–2672. [Google Scholar] [CrossRef] [Green Version]
- Van Schie, M.M.C.H.; Pedroso de Almeida, T.; Laudadio, G.; Tieves, F.; Fernández-Fueyo, E.; Noël, T.; Arends, I.W.C.E.; Hollmann, F. Biocatalytic synthesis of the green note trans-2-hexenal in a continuous-flow microreactor. Beilstein J. Org. Chem. 2018, 14, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Ukeda, H.; Ishii, T.; Sawamura, M.; Isobe, K. Glycolaldehyde production from ethylene glycol with immobilized alcohol oxidase and catalase. Biosci. Biotech. Biochem. 1998, 62, 1589–1591. [Google Scholar] [CrossRef] [Green Version]
- Isobe, K.; Nishise, H. A new enzymatic method for glycolaldehyde production from ethylene glycol. J. Mol. Catal. B Enzym. 1995, 1, 37–43. [Google Scholar] [CrossRef]
- Matijosyte, I.; Arends, I.W.C.E.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (cleas) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Baratto, L.; Candido, A.; Marzorati, M.; Sagui, F.; Riva, S.; Danieli, B. Laccase-mediated oxidation of natural glycosides. J. Mol. Catal. B Enzym. 2006, 39, 3–8. [Google Scholar] [CrossRef]
- Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P.; Ljunggren, S. Laccase/mediated oxidation of a lignin model for improved delignification procedures. J. Mol. Catal. B Enzym. 2003, 26, 105–110. [Google Scholar] [CrossRef]
- Baiocco, P.; Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P. Promoting laccase activity towards non-phenolic substrates: A mechanistic investigation with some laccase-mediator systems. Org. Biomol. Chem. 2003, 1, 191–197. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P.; Macchitella, D. An oxidation of alcohols by oxygen with the enzyme laccase and mediation by tempo. Tetrahedron Lett. 2001, 42, 7551–7553. [Google Scholar] [CrossRef]
- Gavagan, J.E.; Fager, S.K.; Seip, J.E.; Clark, D.S.; Payne, M.S.; Anton, D.L.; DiCosimo, R. Chemoenzymic synthesis of n-(phosphonomethyl)glycine. J. Org. Chem. 1997, 62, 5419–5427. [Google Scholar] [CrossRef]
- Siebum, A.; van Wijk, A.; Schoevaart, R.; Kieboom, T. Galactose oxidase and alcohol oxidase: Scope and limitations for the enzymatic synthesis of aldehydes. J. Mol. Catal. B Enzym. 2006, 41, 141–145. [Google Scholar] [CrossRef]
- Franke, D.; Machajewski, T.; Hsu, C.-C.; Wong, C.-H. One-pot synthesis of l-fructose using coupled multienzyme systems based on rhamnulose-1-phosphate aldolase. J. Org. Chem. 2003, 68, 6828–6831. [Google Scholar] [CrossRef]
- Herter, S.; McKenna, S.M.; Frazer, A.R.; Leimkuhler, S.; Carnell, A.J.; Turner, N.J. Galactose oxidase variants for the oxidation of amino alcohols in enzyme cascade synthesis. ChemCatChem 2015, 7, 2313–2317. [Google Scholar] [CrossRef]
- Zhang, W.; Fernandez Fueyo, E.; Hollmann, F.; Leemans Martin, L.; Pesic, M.; Wardenga, R.; Höhne, M.; Schmidt, S. Combining photo-organo redox- and enzyme catalysis facilitates asymmetric C-H bond functionalization. Eur. J. Org. Chem. 2019, 10, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Pedroso de Almeida, T.; Rother, D.; Hollmann, F. Towards environmentally acceptable synthesis of chiral α-hydroxy ketones via oxidase-lyase cascades. Green Chem. 2017, 19, 1226–1229. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, M.; Müller, C.R.; Domínguez de María, P. Multistep oxidase–lyase reactions: Synthesis of optically active 2-hydroxyketones by using biobased aliphatic alcohols. ChemCatChem 2013, 5, 2512–2516. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Natalia, D.; Greiner, L.; Dominguez de Maria, P. Oxidation-hydroxymethylation-reduction: A one-pot three-step biocatalytic synthesis of optically active α-aryl vicinal diols. Green Chem. 2012, 14, 94–97. [Google Scholar] [CrossRef]
- Sattler, J.H.; Fuchs, M.; Tauber, K.; Mutti, F.G.; Faber, K.; Pfeffer, J.; Haas, T.; Kroutil, W. Redox self-sufficient biocatalyst network for the amination of primary alcohols. Angew. Chem. Int. Ed. 2012, 51, 9156–9159. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Lozano, S.; Santiago-Arcos, J.; Mayoral, J.A.; López-Gallego, F. Co-immobilization and colocalization of multi-enzyme systems for the cell-free biosynthesis of aminoalcohols. ChemCatChem 2020, 12, 3030–3041. [Google Scholar] [CrossRef]
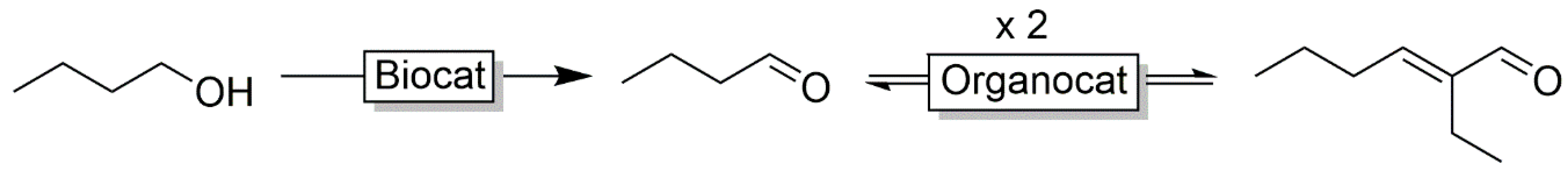
- Stewart, K.N.; Hicks, E.G.; Domaille, D.W. Merger of whole cell biocatalysis with organocatalysis upgrades alcohol feedstocks in a mild, aqueous, one-pot process. ACS Sustain. Chem. Eng. 2020, 8, 4114–4119. [Google Scholar] [CrossRef]
- Hafenstine, G.R.; Ma, K.; Harris, A.W.; Yehezkeli, O.; Park, E.; Domaille, D.W.; Cha, J.N.; Goodwin, A.P. Multicatalytic, light-driven upgrading of butanol to 2-ethylhexenal and hydrogen under mild aqueous conditions. ACS Catal. 2017, 7, 568–572. [Google Scholar] [CrossRef]
- Hafenstine, G.R.; Harris, A.W.; Ma, K.; Cha, J.N.; Goodwin, A.P. Conversion of ethanol to 2-ethylhexenal at ambient conditions using tandem, biphasic catalysis. ACS Sustain. Chem. Eng. 2017, 5, 10483–10489. [Google Scholar] [CrossRef]
- De Vitis, V.; Dall’oglio, F.; Tentori, F.; Contente, M.L.; Romano, D.; Brenna, E.; Tamborini, L.; Molinari, F. Bioprocess intensification using flow reactors: Stereoselective oxidation of achiral 1,3-diols with immobilized Acetobacter aceti. Catalysts 2019, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- De Vitis, V.; Dall’Oglio, F.; Pinto, A.; De Micheli, C.; Molinari, F.; Conti, P.; Romano, D.; Tamborini, L. Chemoenzymatic synthesis in flow reactors: A rapid and convenient preparation of captopril. ChemistryOpen 2017, 6, 668–673. [Google Scholar] [CrossRef]
- Brenna, E.; Cannavale, F.; Crotti, M.; De Vitis, V.; Gatti, F.G.; Migliazza, G.; Molinari, F.; Parmeggiani, F.; Romano, D.; Santangelo, S. Synthesis of enantiomerically enriched 2-hydroxymethylalkanoic acids by oxidative desymmetrisation of achiral 1,3-diols mediated by Acetobacter aceti. ChemCatChem 2016, 8, 3796–3803. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, R.; Borrometi, A.; Romano, A.; Sinisterra Gago, J.V.; Molinari, F. Enantioselective oxidation of (±)-2-phenyl-1-propanol to (S)-2-phenyl-1-propionic acid with Acetobacter aceti: Influence of medium engineering and immobilization. Tetrahedron Asymm. 2002, 13, 2345–2349. [Google Scholar] [CrossRef]
- Geerlof, A.; Vantol, J.B.A.; Jongejan, J.A.; Duine, J.A. Enantioselective conversions of the racemic C-3-alcohol synthons, glycidol (2,3-epoxy-1-propanol), and solketal (2,2-dimethyl-4-(hydroxymethyl)-1,3-dioxolane) by quinohemoprotein alcohol dehydrogenases and bacteria containing such enzymes. Biosci. Biotechnol. Biochem. 1994, 58, 1028–1036. [Google Scholar] [CrossRef]
- Molinari, F.; Villa, R.; Aragozzini, F.; Cabella, P.; Barbeni, M.; Squarcia, F. Multigram-scale production of aliphatic carboxylic acids by oxidation of alcohols with Acetobacter pasteurianus NCIMB 11664. J. Chem. Technol. Biotechnol. 1997, 70, 294–298. [Google Scholar] [CrossRef]
- Mihaľ, M.; Červeňanský, I.; Markoš, J.; Rebroš, M. Bioproduction of phenylacetic acid in airlift reactor by immobilized Gluconobacter oxydans. Chem. Pap. 2017, 71, 103–118. [Google Scholar] [CrossRef]
- Su, W.; Chang, Z.; Gao, K.; Wei, D. Enantioselective oxidation of racemic 1,2-propanediol to d-(-)-lactic acid by gluconobacter oxydans. Tetrahedron Asymm. 2004, 15, 1275–1277. [Google Scholar] [CrossRef]
- Sayed, M.; Dishisha, T.; Sayed, W.F.; Salem, W.M.; Temerk, H.M.; Pyo, S.-H. Enhanced selective oxidation of trimethylolpropane to 2,2-bis(hydroxymethyl)butyric acid using Corynebacterium sp. Atcc 21245. Proc. Biochem. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Sayed, M.; Dishisha, T.; Sayed, W.F.; Salem, W.M.; Temerk, H.A.; Pyo, S.-H. Selective oxidation of trimethylolpropane to 2,2-bis(hydroxymethyl)butyric acid using growing cells of Corynebacterium sp. ATCC 21245. J. Biotechnol. 2016, 221, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Perez, H.I.; Manjarrez, N.; Solis, A.; Luna, H.; Ramirez, M.A.; Cassani, J. Microbial biocatalytic preparation of 2-furoic acid by oxidation of 2-furfuryl alcohol and 2-furanaldehyde with Nocardia corallina. Afr. J. Biotechnol. 2009, 8, 2279–2282. [Google Scholar]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Knaus, T.; Tseliou, V.; Humphreys, L.D.; Scrutton, N.S.; Mutti, F.G. A biocatalytic method for the chemoselective aerobic oxidation of aldehydes to carboxylic acids. Green Chem. 2018, 20, 3931–3943. [Google Scholar] [CrossRef]
- Knaus, T.; Mutti, F.G.; Humphreys, L.D.; Turner, N.J.; Scrutton, N.S. Systematic methodology for the development of biocatalytic hydrogen-borrowing cascades: Application to the synthesis of chiral α-substituted carboxylic acids from α-substituted α,β-unsaturated aldehydes. Org. Biomol. Chem. 2015, 13, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, T.; Gröger, H.; Hummel, W. Enantioselective rearrangement coupled with water addition: Direct synthesis of enantiomerically pure saturated carboxylic acids from α,β-unsaturated aldehydes. ChemCatChem 2014, 6, 961–964. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Y.; Seet, D.; Li, Z. Regio- and stereoselective oxidation of styrene derivatives to arylalkanoic acids via one-pot cascade biotransformations. Adv. Synth. Catal. 2017, 359, 2132–2141. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.; Matos, J.R. Enantioselective oxidation of 1,2-diols to l-α-hydroxy acids using co-immobilized alcohol and aldehyde dehydrogenases as catalysts. J. Org. Chem. 1985, 50, 1992–1994. [Google Scholar] [CrossRef]
- Birmingham, W.R.; Turner, N.J. A single enzyme oxidative “cascade” via a dual-functional galactose oxidase. ACS Catal. 2018, 8, 4025–4032. [Google Scholar] [CrossRef]
- Vilím, J.; Knaus, T.; Mutti, F.G. Catalytic promiscuity of galactose oxidase: A mild synthesis of nitriles from alcohols, air, and ammonia. Angew. Chem. Int. Ed. 2018, 57, 14240–14244. [Google Scholar] [CrossRef]
- Martin, C.; Trajkovic, M.; Fraaije, M.W. Production of hydroxy acids: Selective double oxidation of diols by flavoprotein alcohol oxidase. Angew. Chem. Int. Ed. 2020, 59, 4869–4872. [Google Scholar] [CrossRef] [Green Version]
- Irwin, A.J.; Lok, K.P.; Huang, K.W.-C.; Jones, J.B. Enzymes in organic synthesis. Influence of substrate structure on rates of horse liver alcohol dehydrogenase-catalysed oxidoreductions. J. Chem. Soc. Perkin 1978, 1, 1636–1642. [Google Scholar] [CrossRef]
- Irwin, A.J.; Jones, J.B. Regiospecific and enantioselective horse liver alcohol dehydrogenase catalyzed oxidations of some hydroxycyclopentanes. J. Am. Chem. Soc. 1977, 99, 1625–1630. [Google Scholar] [CrossRef]
- Schröder, I.; Steckhan, E.; Liese, A. In situ NAD+ regeneration using 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonate) as an electron transfer mediator. J. Electroanal. Chem. 2003, 541, 109–115. [Google Scholar] [CrossRef]
- Hilt, G.; Lewall, B.; Montero, G.; Utley, J.H.P.; Steckhan, E. Efficient in-situ redox catalytic NAD(P)+ regeneration in enzymatic synthesis using transition-metal complexes of 1,10-phenanthroline-5,6-dione in its n-monomethylated derivate as catalysts. Liebigs Ann. 1997, 1997, 2289–2296. [Google Scholar] [CrossRef]
- Boratyński, F.; Janik-Polanowicz, A.; Szczepańska, E.; Olejniczak, T. Microbial synthesis of a useful optically active (+)-isomer of lactone with bicyclo[4.3.0]nonane structure. Sci. Rep. 2018, 8, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boratynski, F.; Smuga, M.; Wawrzenczyk, C. Lactones 42. Stereoselective enzymatic/microbial synthesis of optically active isomers of whisky lactone. Food Chem. 2013, 141, 419–427. [Google Scholar] [CrossRef]
- Bechi, B.; Herter, S.; McKenna, S.; Riley, C.; Leimkuhler, S.; Turner, N.J.; Carnell, A.J. Catalytic bio-chemo and bio-bio tandem oxidation reactions for amide and carboxylic acid synthesis. Green Chem. 2014, 16, 4524–4529. [Google Scholar] [CrossRef] [Green Version]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-catalyzed oxidation of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid. Angew. Chem. Int. Ed. 2014, 53, 6515–6518. [Google Scholar] [CrossRef]
- Carro, J.; Ferreira, P.; Rodriguez, L.; Prieto, A.; Serrano, A.; Balcells, B.; Arda, A.; Jimenez-Barbero, J.; Gutierrez, A.; Ullrich, R.; et al. 5-hydroxymethylfurfural conversion by fungal aryl-alcohol oxidase and unspecific peroxygenase. FEBS J. 2015, 282, 3218–3229. [Google Scholar] [CrossRef] [Green Version]
- McKenna, S.M.; Mines, P.; Law, P.; Kovacs-Schreiner, K.; Birmingham, W.R.; Turner, N.J.; Leimkühler, S.; Carnell, A.J. The continuous oxidation of HMF to FDCA and the immobilisation and stabilisation of periplasmic aldehyde oxidase (paoabc). Green Chem. 2017, 19, 4660–4665. [Google Scholar] [CrossRef]
- Cappaert, L.; Larroche, C. Oxidation of a mixture of 2-(r) and 2-(s)-heptanol to 2-heptanone by saccharomyces cerevisiae in a biphasic system. Biocatal. Biotransf. 2004, 22, 291–296. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Rocha-Martin, J.; Favela-Torres, E.; Calvo, J.; Berenguer, J.; Guisán, J.M.; López-Gallego, F. Hydrolysis and oxidation of racemic esters into prochiral ketones catalyzed by a consortium of immobilized enzymes. Biochem. Eng. J. 2016, 112, 136–142. [Google Scholar] [CrossRef]
- Albarrán-Velo, J.; Gotor-Fernández, V.; Lavandera, I. One-pot two-step chemoenzymatic deracemization of allylic alcohols using laccases and alcohol dehydrogenases. Mol. Catal. 2020, 493, 111087. [Google Scholar] [CrossRef]
- Gonzalez-Granda, S.; Mendez-Sanchez, D.; Lavandera, I.; Gotor-Fernandez, V. Laccase-mediated oxidations of propargylic alcohols. Application in the deracemization of 1-arylprop-2-yn-1-ols in combination with alcohol dehydrogenases. ChemCatChem 2020, 12, 520–527. [Google Scholar] [CrossRef]
- Albarran-Velo, J.; Lavandera, I.; Gotor-Fernandez, V. Sequential two-step stereoselective amination of allylic alcohols through the combination of laccases and amine transaminases. ChemBioChem 2020, 21, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Montero, L.; Gotor, V.; Gotor-Fernandez, V.; Lavandera, I. Stereoselective amination of racemic sec-alcohols through sequential application of laccases and transaminases. Green Chem. 2017, 19, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Sanchez, D.; Mangas-Sanchez, J.; Lavandera, I.; Gotor, V.; Gotor-Fernandez, V. Chemoenzymatic deracemization of secondary alcohols by using a TEMPO-iodine-alcohol dehydrogenase system. ChemCatChem 2015, 7, 4016–4020. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, A.; Rios-Lombardia, N.; Sattler, J.H.; Lavandera, I.; Gotor-Fernandez, V.; Kroutil, W.; Gotor, V. Deracemisation of profenol core by combining laccase/TEMPO-mediated oxidation and alcohol dehydrogenase-catalysed dynamic kinetic resolution. Catal. Sci. Technol. 2015, 5, 1443–1446. [Google Scholar] [CrossRef] [Green Version]
- Kedziora, K.; Diaz-Rodriguez, A.; Lavandera, I.; Gotor-Fernandez, V.; Gotor, V. Laccase/TEMPO-mediated system for the thermodynamically disfavored oxidation of 2,2-dihalo-1-phenylethanol derivatives. Green Chem. 2014, 16, 2448–2453. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Rodriguez, A.; Martinez-Montero, L.; Lavandera, I.; Gotor, V.; Gotor-Fernandez, V. Laccase/2,2,6,6-tetramethylpiperidinoxyl radical (TEMPO): An efficient catalytic system for selective oxidations of primary hydroxy and amino groups in aqueous and biphasic media. Adv. Synth. Catal. 2014, 356, 2321–2329. [Google Scholar] [CrossRef]
- Winter, R.T.; Fraaije, M.W. Applications of flavoprotein oxidases in organic synthesis: Novel reactivities that go beyond amine and alcohol oxidations. Curr. Org. Chem. 2012, 16, 2542–2550. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.J. Enantioselective oxidation of c-o and c-n bonds using oxidases. Chem. Rev. 2011, 111, 4073–4087. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Spadiut, O.; Pisanelli, I.; Maischberger, T.; Peterbauer, C.; Gorton, L.; Chaiyen, P.; Haltrich, D. Engineering of pyranose 2-oxidase: Improvement for biofuel cell and food applications through semi-rational protein design. J. Biotechnol. 2009, 139, 250–257. [Google Scholar] [CrossRef]
- Pisanelli, I.; Kujawa, M.; Spadiut, O.; Kittl, R.; Halada, P.; Volc, J.; Mozuch, M.D.; Kersten, P.; Haltrich, D.; Peterbauer, C. Pyranose 2-oxidase from Phanerochaete chrysosporium-expression in E. coli and biochemical characterization. J. Biotechnol. 2009, 142, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Giffhorn, F. Fungal pyranose oxidases: Occurrence, properties and biotechnical applications in carbohydrate chemistry. Appl. Microbiol. Biotechnol. 2000, 54, 727–740. [Google Scholar] [CrossRef]
- Freimund, S.; Huwig, A.; Giffhorn, F.; Köpper, S. Rare keto-aldoses from enzymatic oxidation: Substrates and oxidation products of pyranose 2-oxidase. Chem. Eur. J. 1998, 4, 2442–2455. [Google Scholar] [CrossRef]
- Tan, T.-C.; Kracher, D.; Gandini, R.; Sygmund, C.; Kittl, R.; Haltrich, D.; Hallberg, B.M.; Ludwig, R.; Divne, C. Structural basis for cellobiose dehydrogenase action during oxidative cellulose degradation. Nat. Commun. 2015, 6, 7542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K.; Nakamura, N.; Igarashi, K.; Samejima, M.; Ohno, H. Biocatalytic oxidation of cellobiose in an hydrated ionic liquid. Green Chem. 2009, 11, 351–354. [Google Scholar] [CrossRef]
- Maischberger, T.; Nguyen, T.-H.; Sukyai, P.; Kittl, R.; Riva, S.; Ludwig, R.; Haltrich, D. Production of lactose-free galacto-oligosaccharide mixtures: Comparison of two cellobiose dehydrogenases for the selective oxidation of lactose to lactobionic acid. Carbohydr. Res. 2008, 343, 2140–2147. [Google Scholar] [CrossRef]
- Ludwig, R.; Ozga, M.; Zamocky, M.; Peterbauer, C.; Kulbe, K.D.; Haltrich, D. Continuous enzymatic regeneration of electron acceptors used by flavoenzymes: Cellobiose dehydrogenase-catalyzed production of lactobionic acid as an example. Biocatal. Biotransf. 2004, 22, 97–104. [Google Scholar] [CrossRef]
- Henriksson, G.; Johansson, G.; Pettersson, G. A critical review of cellobiose dehydrogenases. J. Biotechnol. 2000, 78, 93–113. [Google Scholar] [CrossRef]
- Sha, F.; Zheng, Y.; Chen, J.; Chen, K.; Cao, F.; Yan, M.; Ouyang, P. D-tagatose manufacture through bio-oxidation of galactitol derived from waste xylose mother liquor. Green Chem. 2018, 20, 2382–2391. [Google Scholar] [CrossRef]
- Matsushita, K.; Fujii, Y.; Ano, Y.; Toyama, H.; Shinjoh, M.; Tomiyama, N.; Miyazaki, T.; Sugisawa, T.; Hoshino, T.; Adachi, O. 5-keto-d-gluconate production is catalyzed by a quinoprotein glycerol dehydrogenase, major polyol dehydrogenase, in Gluconobacter species. Appl. Environ. Microbiol. 2003, 69, 1959–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hellemond, E.W.; Vermote, L.; Koolen, W.; Sonke, T.; Zandvoort, E.; Heuts, D.P.; Janssen, D.B.; Fraaije, M.W. Exploring the biocatalytic scope of alditol oxidase from Streptomyces coelicolor. Adv. Synth. Catal. 2009, 351, 1523–1530. [Google Scholar] [CrossRef] [Green Version]
- Leferink, N.G.H.; Heuts, D.P.H.M.; Fraaije, M.W.; van Berkel, W.J.H. The growing vao flavoprotein family. Arch. Biochem. Biophys. 2008, 474, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forneris, F.; Heuts, D.P.H.M.; Delvecchio, M.; Rovida, S.; Fraaije, M.W.; Mattevi, A. Structural analysis of the catalytic mechanism and stereoselectivity in Streptomyces coelicolor alditol oxidase. Biochemistry 2008, 47, 978–985. [Google Scholar]
- Hancock, R.D.; Viola, R. Biotechnological approaches for l-ascorbic acid production. Trends Biotechnol. 2002, 20, 299–305. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Moholkar, V.S. Optimization of 1,3-dihydroxyacetone production from crude glycerol by immobilized Gluconobacter oxydans MTCC 904. Biores. Technol. 2016, 216, 1058–1065. [Google Scholar] [CrossRef]
- Ferrandi, E.E.; Bertuletti, S.; Monti, D.; Riva, S. Hydroxysteroid dehydrogenases: An ongoing story. Eur. J. Org. Chem. 2020, 2020, 4463–4473. [Google Scholar] [CrossRef]
- Stampfer, W.; Kosjek, B.; Moitzi, C.; Kroutil, W.; Faber, K. Biocatalytic asymmetric hydrogen transfer. Angew. Chem. Int. Ed. 2002, 41, 1014–1017. [Google Scholar] [CrossRef]
- Musa, M.M.; Hollmann, F.; Mutti, F.G. Synthesis of enantiomerically pure alcohols and amines via biocatalytic deracemisation methods. Catal. Sci. Technol. 2019, 9, 5487–5503. [Google Scholar] [CrossRef] [Green Version]
- Voss, C.V.; Gruber, C.C.; Kroutil, W. Deracemization of secondary alcohols through a concurrent tandem biocatalytic oxidation and reduction. Angew. Chem. Int. Ed. 2008, 47, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Voss, C.V.; Gruber, C.C.; Faber, K.; Knaus, T.; Macheroux, P.; Kroutil, W. Orchestration of concurrent oxidation and reduction cycles for stereoinversion and deracemisation of sec-alcohols. J. Am. Chem. Soc. 2008, 130, 13969–13972. [Google Scholar] [CrossRef] [PubMed]
- Tonin, F.; Otten, L.G.; Arends, I. NAD(+)-dependent enzymatic route for the epimerization of hydroxysteroids. ChemSusChem 2019, 12, 3192–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]



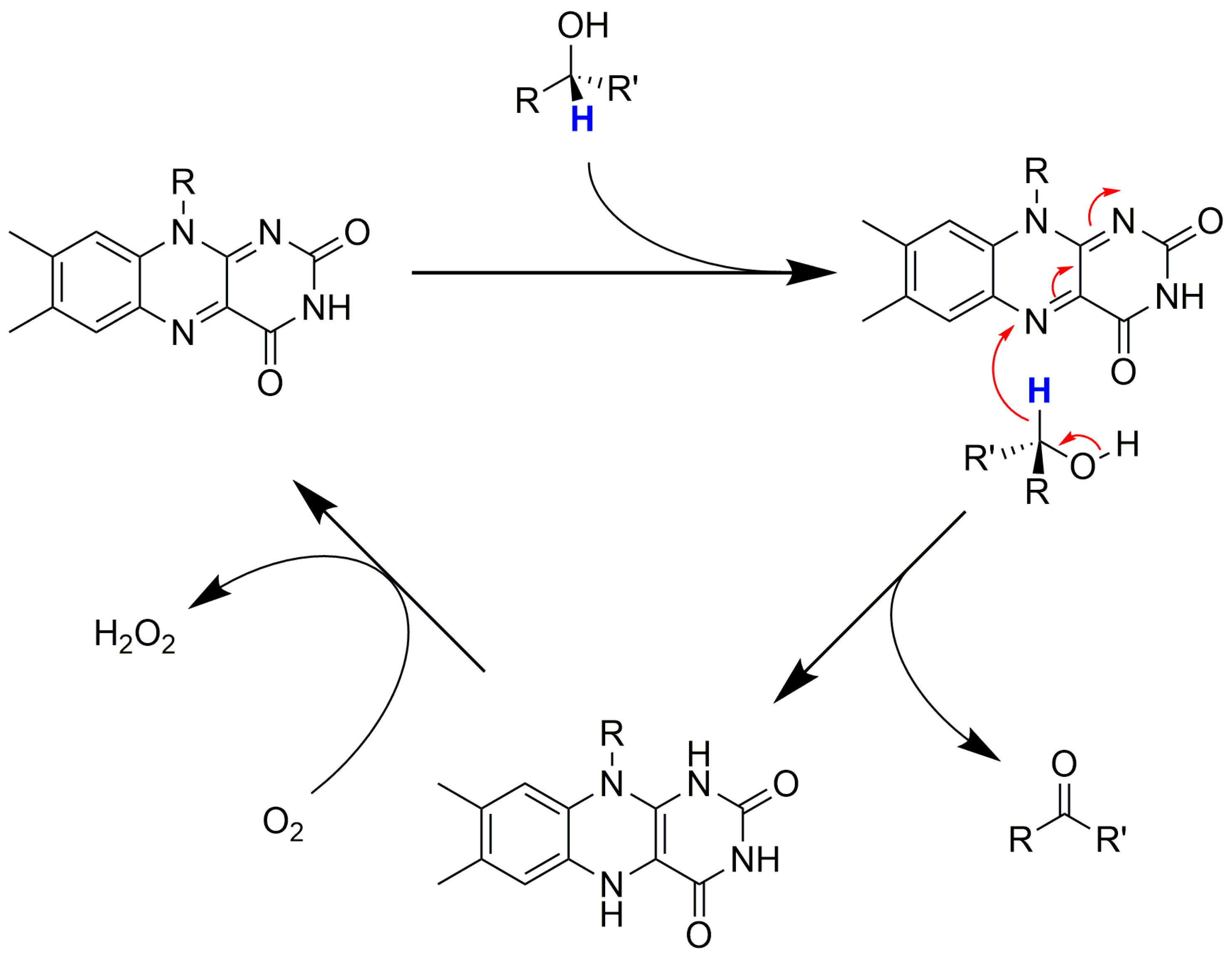


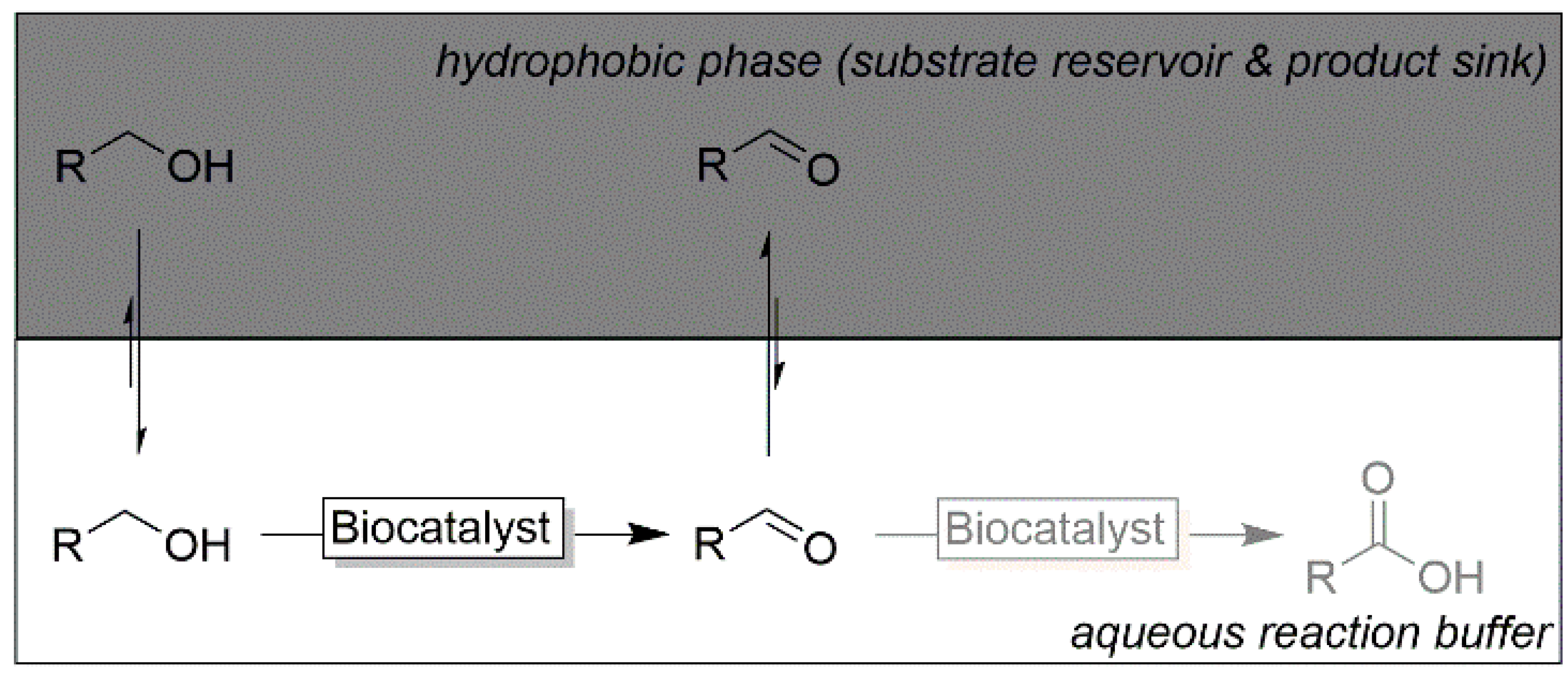
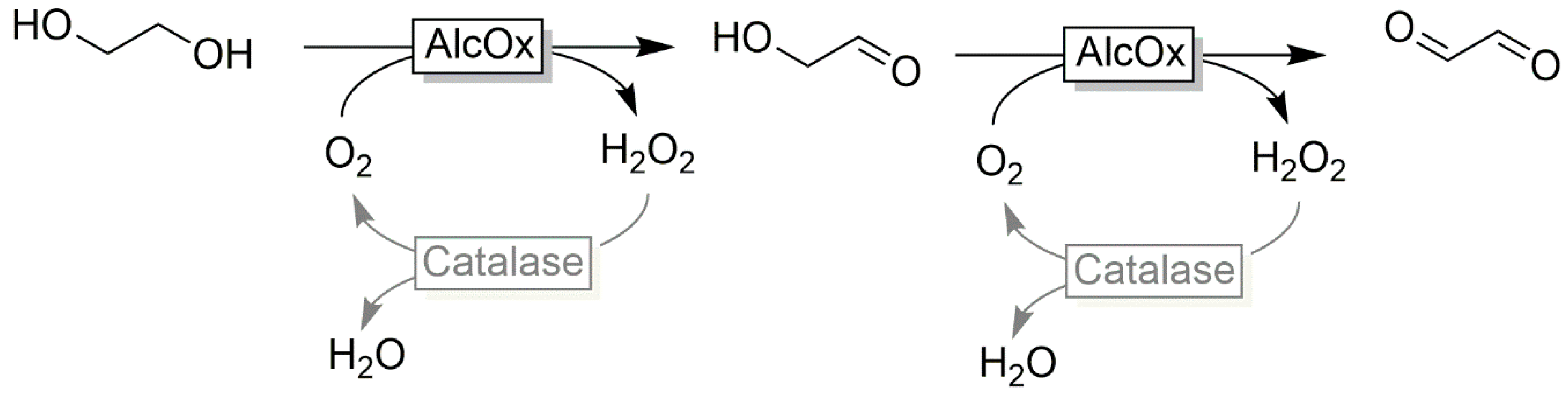

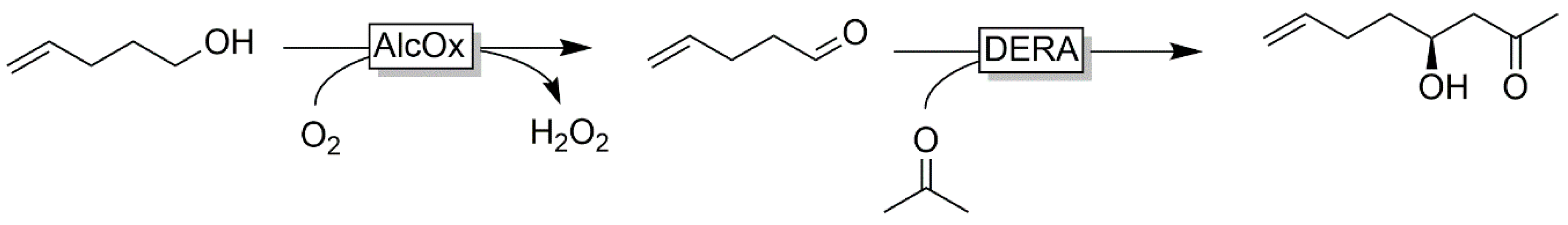



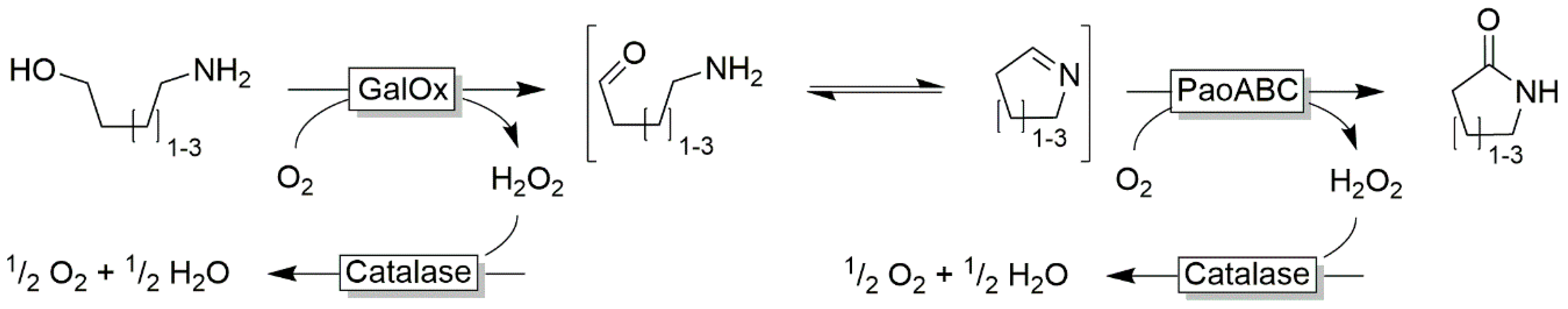
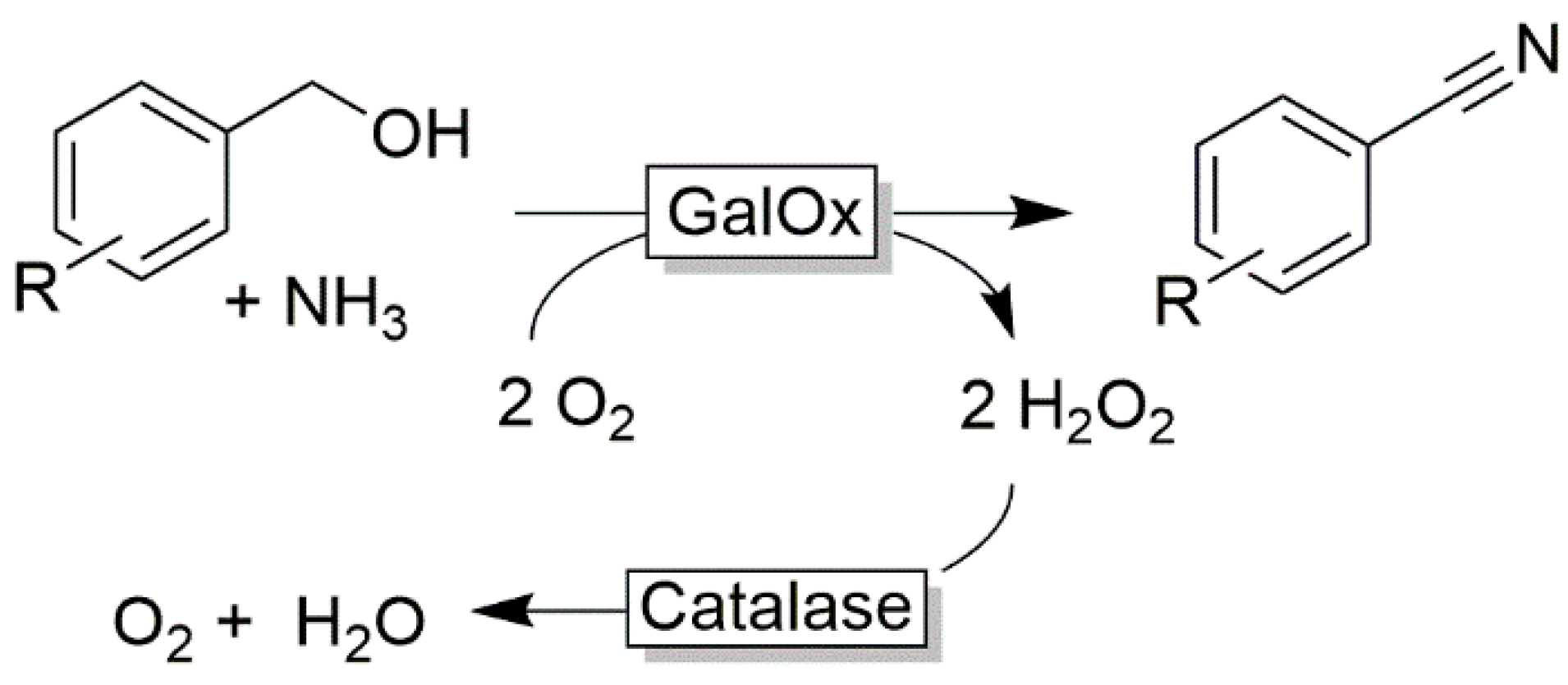



 | |||
|---|---|---|---|
| Cosubstrate | Coproduct | Catalyst | Ref. |
| O2 | H2O2 | (Modified) flavins | [72,73,74,75,76,77] |
| H2O | LMS | [65,66,67,68,69,70,71] | |
| NADH oxidase | [56,57,61,63,64,78,79,80,81] | ||
| Other oxidative enzymes | [82,83,84,85,86] | ||
 | ||
|---|---|---|
| Substrate | Acid Yield in Buffer Only [%] | Aldehyde Yield in 2LPS [%] |
 | >97 | 91 |
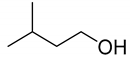 | >97 | 88 |
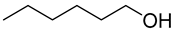 | >97 | 89 |
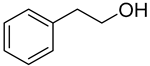 | >97 | 94 |
 | |||
|---|---|---|---|
| Catalyst | Product | Product Titer/Remarks | Ref. |
| Acetobacter |  | 23 gL−1/alginate immobilized cells | [105] |
 | 24 mM/dynamic kinetic resolution in a flow chemistry setup | [132,133,134,135] | |
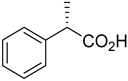 | - | - | |
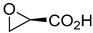 | Kinetic resolution | [136] | |
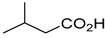 | Up to 80 g L−1 | [137] | |
| Gluconobacter | 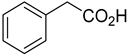 | 8 gL−1 | [138] |
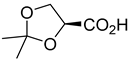 | Kinetic resolution | [136] | |
 | 20 g L−1 Kinetic resolution | [139] | |
| Corynebacterium | 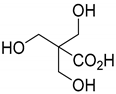 | 25 g L−1 | [140,141] |
| Norcardia |  | 9 g L−1 | [142] |
 | |||
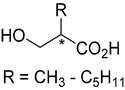
|---|---|---|---|
| Product | Biocatalyst | Yield [%] | Reference |
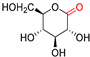 | GluOx | Up to >99 | [174] |
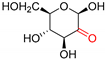 | P2O | Up to >99 | [175,176,177,178] |
| CBOx | Up to >99 | [179,180,181,182,183] | |
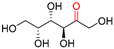 | POlDH | >99 | [184,185] |
 | AldO | >99 (10 mM) | [186,187,188] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puetz, H.; Puchľová, E.; Vranková, K.; Hollmann, F. Biocatalytic Oxidation of Alcohols. Catalysts 2020, 10, 952. https://doi.org/10.3390/catal10090952
Puetz H, Puchľová E, Vranková K, Hollmann F. Biocatalytic Oxidation of Alcohols. Catalysts. 2020; 10(9):952. https://doi.org/10.3390/catal10090952
Chicago/Turabian StylePuetz, Hendrik, Eva Puchľová, Kvetoslava Vranková, and Frank Hollmann. 2020. "Biocatalytic Oxidation of Alcohols" Catalysts 10, no. 9: 952. https://doi.org/10.3390/catal10090952
APA StylePuetz, H., Puchľová, E., Vranková, K., & Hollmann, F. (2020). Biocatalytic Oxidation of Alcohols. Catalysts, 10(9), 952. https://doi.org/10.3390/catal10090952