The Effect of Strontium Doping on LaFeO3 Thin Films Deposited by the PLD Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure of the Targets
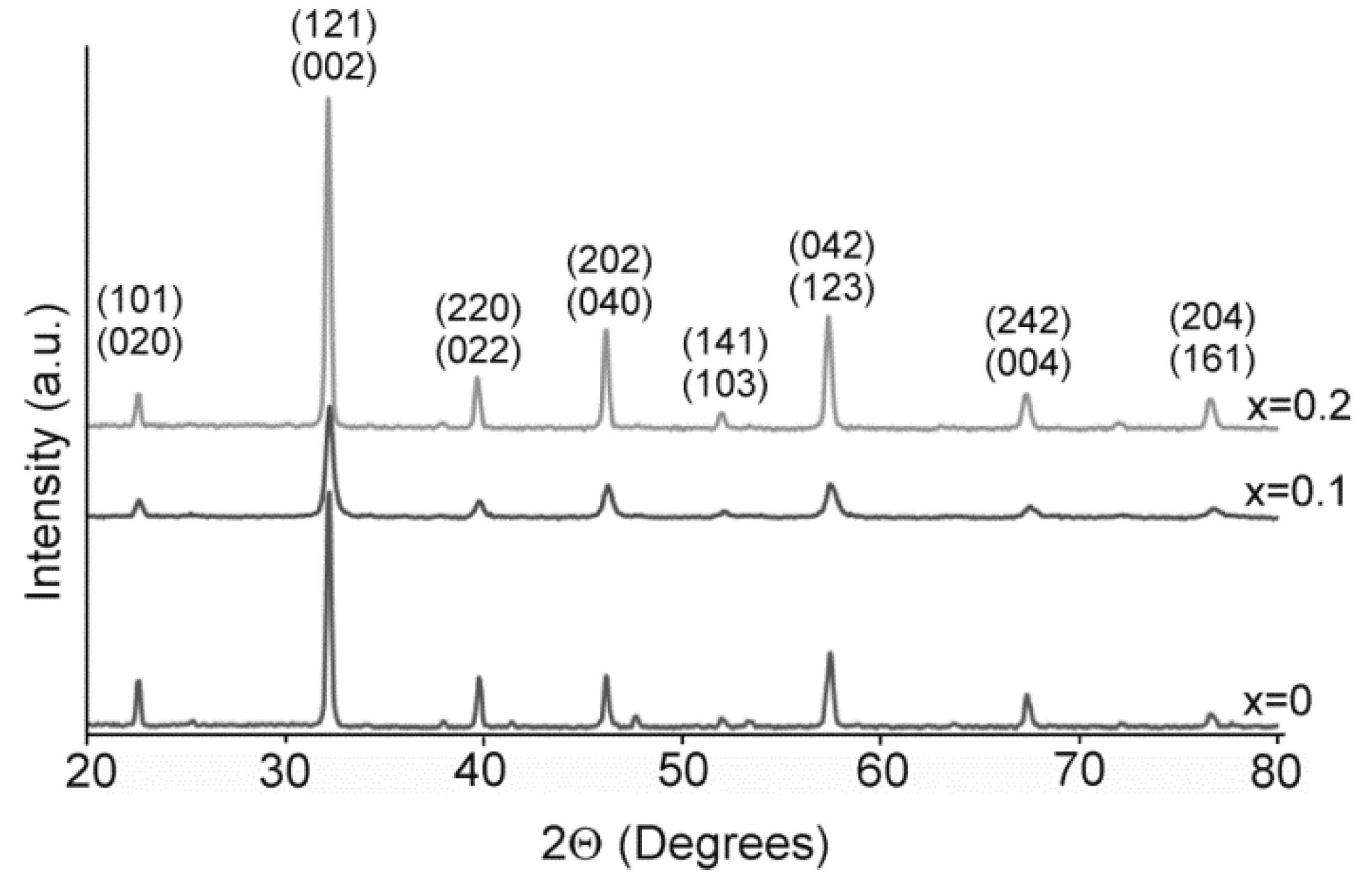
2.2. Structure and Morphology Characterization of Thin Films
2.3. Gas-Sensing Properties
- (a)
- electroneutrality formed Fe4+,(La1−x3+Srx2+)(Fe1−x3+Fex4+)O3
- (b)
- oxygen vacancies compensation, ▯—oxygen vacancies,(La1−x3+Srx2+)Fe(O3−x/2▯x/2)
- (c)
- compound compensation (both electroneutrality and oxygen vacancies compensation) which occur in substituted perovskites oxides,(La1−x3+Srx2+)(Fe1−y3+Fey4+) (O3− (x−y)/2▯(x−y)/2) [29].
3. Materials and Methods
3.1. Deposition of the Thin Films
3.2. Characterization of the Thin Films and Targets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fergus, J.W. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Liu, X.; Ji, H.; Gu, Y.; Xu, M. Preparation and acetone sensitive characteristics of nano-LaFeO3 semiconductor thin films by polymerization complex method. Mater. Sci. Eng. B 2006, 133, 98–101. [Google Scholar] [CrossRef]
- Sukumar, M.; Kennedy, L.J.; Vijaya, J.J.; Al-Najar, B.; Bououdina, M. Facile synthesis of Fe3+ doped La2CuO4/LaFeO3 perovskite nanocomposites: Structural, optical, magnetic and catalytic properties. Mater. Sci. Semicond. Process. 2019, 100, 225–235. [Google Scholar] [CrossRef]
- Furfori, S.; Russo, N.; Fino, D.; Saracco, G.; Specchia, V. NO SCR reduction by hydrogen generated in line on perovskite-type catalysts for automotive diesel exhaust gas treatment. Chem. Eng. Sci. 2010, 65, 120–127. [Google Scholar] [CrossRef]
- Patel, F.; Patel, S. La1−xSrxCoO3 (x = 0, 0.2) perovskites type catalyst for carbon monoxide emission control from auto-exhaust. Proc. Eng. 2013, 51, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Liang, Y. Synthesis of three-dimensionally ordered macroporous LaFeO3 with enhanced methanol gas sensing properties. Sens. Actuators B Chem. 2015, 209, 706–713. [Google Scholar] [CrossRef]
- Gwon, O.; Yoo, S.; Shin, J.; Kim, G. Optimization of La1−xSrxCoO3−δ perovskite cathodes for intermediate temperature solid oxide fuel cells through the analysis of crystal structure and electrical properties. Int. J. Hydrogen Energy 2017, 39, 20806–20811. [Google Scholar] [CrossRef]
- Cao, X.; Zhi, L.; Jia, Y.; Li, Y.; Zhao, K.; Cui, X.; Ci, L.; Ding, K.; Wei, J. Enhanced efficiency of perovskite solar cells by introducing controlled chloride incorporation into MAPbI3 perovskite films. Electrochim. Acta 2018, 275, 1–7. [Google Scholar] [CrossRef]
- Dhilip, M.; Aparna Devi, N.; Stella Punitha, J.; Anbarasu, V.; Saravana Kumar, K. Conventional synthesis and characterization of cubically ordered La2FeMnO6 double perovskite compound. Vacuum 2019, 167, 16–20. [Google Scholar] [CrossRef]
- Jaouali, I.; Hamrouni, H.; Moussa, N.; Nsib, M.F.; Centeno, M.A.; Bonavita, A.; Neri, G.; Leonardi, S.G. LaFeO3 ceramics as selective oxygen sensors at mild temperature. Ceram. Int. 2018, 44, 4183–4189. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Valmor, K.G.; Mastelaro, R.; Ponpandian, N. Surface morphology-dependent room-temperature LaFeo3 nanostructure thin films as selective NO2 gas sensor prepared by radio frequency magnetron sputtering. Appl. Mater. Interfaces 2014, 6, 13917–13927. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yang, X.; Lin, J.; Guo, Z.; He, Y. The structure and magnetic properties of magnesium-substituted LaFeO3 perovskite negative electrode material by citrate sol-gel. Int. J. Hydrogen Energy 2018, 43, 12720–12729. [Google Scholar] [CrossRef]
- Murade, P.A.; Sangawar, V.S.; Chaudhari, G.N.; Kapse, V.D.; Bajpeyee, A.U. Acetone gas-sensing performance of Sr-doped nanostructured LaFeO3 semiconductor prepared by citrate sol–gel route. Curr. Appl. Phys. 2011, 11, 451–456. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, S.K.; Sachdev, K. Effect of precursors on the morphology and surface area of LaFeO3. Ceram. Int. 2019, 452, 7217–7225. [Google Scholar] [CrossRef]
- Pecchi, G.; Jiliberto, M.G.; Buljan, A.; Delgado, E.J. Relation between defects and catalytic activity of calcium doped LaFeO3 perovskite. Solid State Ion. 2011, 187, 27–32. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Pei, J.; Chen, Y.; Li, L.; Xie, J.; Hu, J. Sensing performances to low concentration acetone for palladium doped LaFeO3 sensors. J. Rare Earths 2016, 37, 704–710. [Google Scholar] [CrossRef]
- Cyza, A.; Kopia, A.; Cieniek, L.; Kusiński, J. Structural characterization of Sr doped LaFeO3 thin films prepared by pulsed electron deposition method. Mater. Today Proc. 2016, 3, 2707–2712. [Google Scholar] [CrossRef]
- Kumar, M.; Srikanth, S.; Ravikumar, B.; Alex, T.C.; Das, S.K. Synthesis of pure and Sr-doped LaGaO3. LaFeO3 and LaCoO3 and Sr. Mg-doped LaGaO3 for ITSOFC application using different wet chemical routes. Mater. Chem. Phys. 2009, 113, 803–815. [Google Scholar] [CrossRef]
- Phokha, S.; Hunpratup, S.; Pinitsoontorn, S.; Putasaeng, B.; Rujirawat, S.; Maensiri, S. Structure, magnetic, and dielectric properties of Ti-doped LaFeO3 ceramics synthesized by polymer pyrolysis method. Mater. Res. Bull. 2015, 67, 118–125. [Google Scholar] [CrossRef]
- Nafsin, N.; Castro, R.H.R. Direct measurements of quasi-zero grain boundary energies in ceramics. J. Mater. Res. 2017, 32, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Premper, J.; Sander, D.; Kirschner, J. In situ stress measurements during pulsed laser deposition of BaTiO3 and SrTiO3 atomic layers on Pt(001). Appl. Surf. Sci. 2015, 335, 44–49. [Google Scholar] [CrossRef]
- Stämmler, S.; Merkle, R.T.; Stuhlhofer, B.; Logvenov, G.; Hahn, K.; van Aken, P.A.; Maier, J. Phase constitution. Sr distribution and morphology of self-assembled La-Sr-Co-O composite films prepared by PLD. Solid State Ion. 2017, 303, 172–180. [Google Scholar] [CrossRef]
- Kopia, A.; Kowalski, K.; Leroux, C.; Gavarri, J.R. Influence of the substrate on the structure stability LaLuO3 thin films deposited by PLD method. Vacuum 2016, 134, 120–129. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Tong, Y.; Zou, H.; Peng, J.; Zheng, X. Mixed-potential-type gas sensors based on Pt/YSZ film/LaFeO3 for detecting NO2. J. Electron. Mater. 2017, 46, 6895–6900. [Google Scholar] [CrossRef]
- Jędrusik, M. Microstructure and Sensing Properties of LaMO3 (M = Co. Fe) Thin Films. Ph.D. Thesis, AGH Univerisity of Science and Technology, Cracow, Poland, 9 December 2019. [Google Scholar]
- Chen, Y.; Qin, H.i.; Wang, X.; Li, L.; Hu, J. Acetone sensing properties and mechanism of nano-LaFeO3 thick-films. Sens. Actuators B 2016, 235, 56–66. [Google Scholar] [CrossRef]
- Wang, C.; Rong, Q.; Zhang, Y.; Hua, J.; Zia, B.; Zhu, Z.; Zhang, J.; Liu, Q. Molecular imprinting Ag-LaFeO3 spheres for highly sensitive acetone gas detection. Mater. Res. Bull. 2019, 109, 265–272. [Google Scholar] [CrossRef]
- Wei, W.; Guo, S.; Chen, C.; Sun, L.; Chen, Y.; Guo, W.; Ruan, S. High sensitive and fast formaldehyde gas sensor based on Ag-doped LaFeO3 nanofibers. J. Alloy. Compd. 2017, 695, 1122–1127. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Sun, K.; Hu, J.; Qin, H. Nanocrystalline Gd1–xCaxFeO3 sensors for detection of methanol gas. J. Rare Earths 2017, 35, 690–696. [Google Scholar]
- Maziarz, W. TiO2/SnO2 and TiO2/CuO thin film nano-heterostructures as gas sensors. Appl. Surface Sci. 2019, 480, 361–370. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Sun, L.; Hu, J. CO2 sensing properties and mechanism of nanocrystalline LaFeO3 sensor. Sens. Actuators B 2013, 188, 965–971. [Google Scholar] [CrossRef]











| Content Sr | LaFeO3 [T = 30 °C] | x = 0 | x = 0.1 | x = 0.2 |
|---|---|---|---|---|
| Orthorhombic Pmna 62 a = c γ = 90° | ||||
| Cell parameters | ||||
| a [nm] | 0.5554 | 0.5566 | 0.5520 | 0.5521 |
| b [nm] | 0.7853 | 0.7856 | 0.7859 | 0.7837 |
| c [nm] | 0.5556 | 0.5556 | 0.5544 | 0.5545 |
| V [nm3] | 0.2420 | 0.243 | 0.241 | 0.239 |
| Crystallites size D [nm] | - | 36 ± 3 | 20 ± 3 | 18 ± 3 |
| Ra [nm] | - | 2.27 | 5.57 | 7.97 |
| Rmax [nm] | - | 15.9 | 32.5 | 54 |
| Rt | 6.3 | 14 | 15 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyza, A.; Cieniek, Ł.; Moskalewicz, T.; Maziarz, W.; Kusiński, J.; Kowalski, K.; Kopia, A. The Effect of Strontium Doping on LaFeO3 Thin Films Deposited by the PLD Method. Catalysts 2020, 10, 954. https://doi.org/10.3390/catal10090954
Cyza A, Cieniek Ł, Moskalewicz T, Maziarz W, Kusiński J, Kowalski K, Kopia A. The Effect of Strontium Doping on LaFeO3 Thin Films Deposited by the PLD Method. Catalysts. 2020; 10(9):954. https://doi.org/10.3390/catal10090954
Chicago/Turabian StyleCyza, Anna, Łukasz Cieniek, Tomasz Moskalewicz, Wojciech Maziarz, Jan Kusiński, Kazimierz Kowalski, and Agnieszka Kopia. 2020. "The Effect of Strontium Doping on LaFeO3 Thin Films Deposited by the PLD Method" Catalysts 10, no. 9: 954. https://doi.org/10.3390/catal10090954





