Reductive Dechlorination of Chloroacetamides with NaBH4 Catalyzed by Zero Valent Iron, ZVI, Nanoparticles in ORMOSIL Matrices Prepared via the Sol-Gel Route
Abstract
1. Introduction
2. Results and Discussion
2.1. 1.0 M ZVI-NPs Ethanolic Suspension
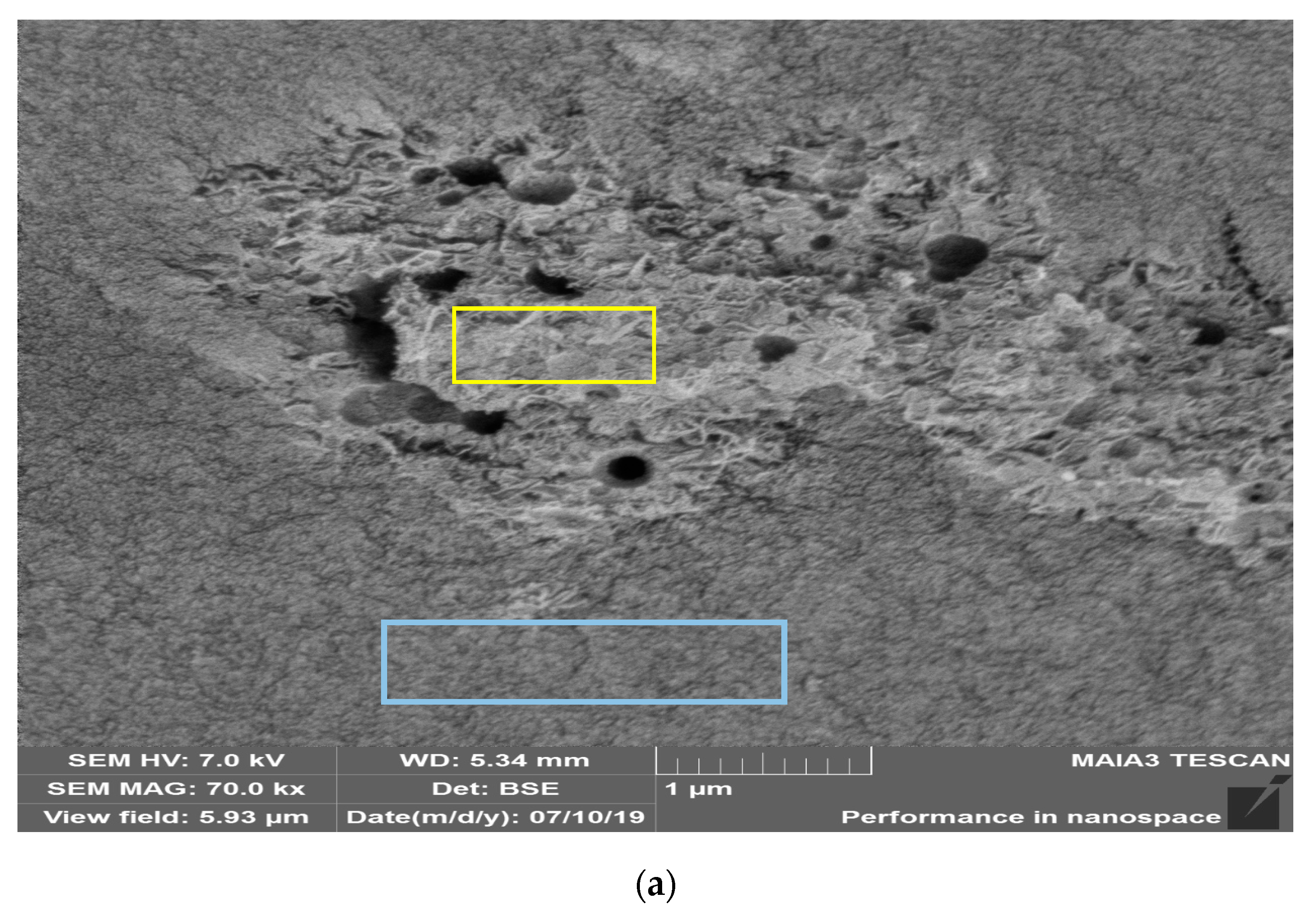
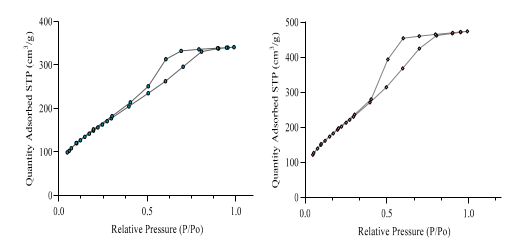
2.2. Catalyst Characterization
2.3. Catalytic Reductive Dehalogenation
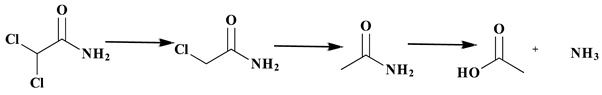
2.3.1. Dehalogenation of Di- and Mono-Chloroacetamides
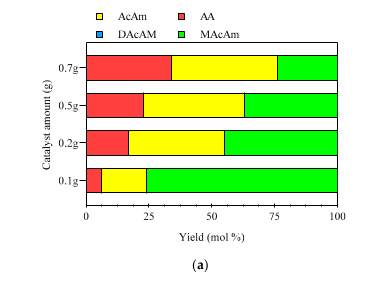
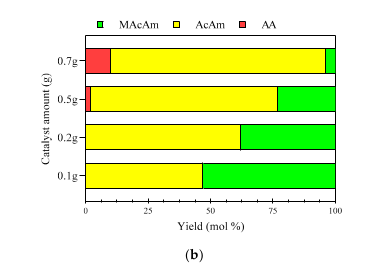
2.3.2. Catalyst Dosing Dehalogenation Experiments
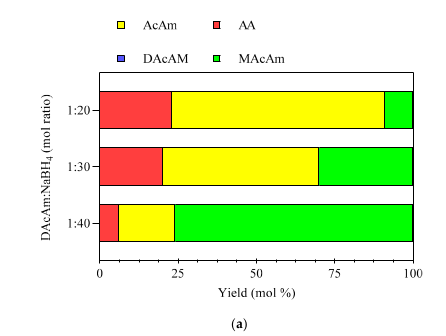
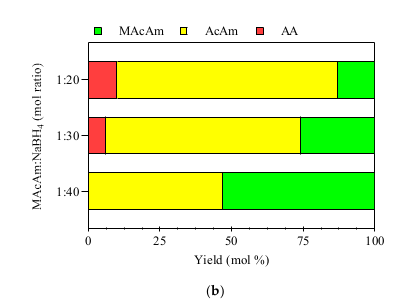
2.3.3. NaBH4 Dosing Dechlorination Experiments
2.3.4. Catalyst Stability Test
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Preparation of ZVI-NP Suspension
3.2.2. ZVI@ORMOSIL Synthesis via the Sol-Gel Route
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dragstedt, C.A. The halogenated hydrocarbons: Their toxicity and potential dangers. AMA Arch. Intern. Med. 1956, 97, 261–262. [Google Scholar] [CrossRef]
- Kimura, S.Y.; Vu, T.N.; Komaki, Y.; Plewa, M.J.; Mariñas, B.J. Acetonitrile, and N-Chloroacetamide formation from the reaction of Acetaldehyde and Monochloramine. Environ. Sci. Technol. 2015, 49, 9954–9963. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.T.; McGuire, C.M.; Mubarak, M.S.; Peters, D.G. Electroreductive remediation of halogenated environmental pollutants. Chem. Rev. 2016, 116, 15198–15234. [Google Scholar] [CrossRef]
- Borojovich, E.J.C.; Bar-Ziv, R.; Oster-Golberg, O.; Sebbag, H.; Zinigrad, M.; Meyerstein, D.; Zidki, T. Halo-organic pollutants: The effect of an electrical bias on their decomposition mechanism on porous iron electrodes. Appl. Catal. B 2017, 210, 255–262. [Google Scholar] [CrossRef]
- Letourneau, D.R.; Gill, C.G.; Krogh, E.T. Photosensitized degradation kinetics of trace halogenated contaminants in natural waters using membrane introduction mass spectrometry as an in-situ reaction monitor. Photochem. Photobiol. Sci. 2015, 14, 2108–2118. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Drzewicz, P.; Pańta, P.; Głuszewski, W.; Nałecz-Jawecki, G.; Sawicki, J.; Sampa, M.H.O.; Oikawa, H.; Borrely, S.I.; Czaplicka, M.; et al. Radiolytic degradation and toxicity changes in g-irradiated solutions of 2,4-dichlorophenol. Radiat. Phys. Chem. 2002, 65, 357–366. [Google Scholar] [CrossRef]
- Kar, P.; Mishra, B.G. Hydrodehalogenation of halogenated organic contaminants from aqueous sources by Pd nanoparticles dispersed in the micropores of pillared clays under transfer hydrogenation condition. J. Clust. Sci. 2014, 25, 1463–1478. [Google Scholar] [CrossRef]
- Heveling, J. Heterogeneous Catalytic chemistry by example of industrial applications. J. Chem. Educ. 2012, 89, 1530–1536. [Google Scholar] [CrossRef]
- Zidki, T.; Bar-Ziv, R.; Green, U.; Cohen, H.; Meisel, D.; Meyerstein, D. The effect of the nano-silica support on the catalytic reduction of water by gold, silver and platinum nanoparticles–nanocomposite reactivity. Phys. Chem. Chem. Phys. 2014, 16, 15422–15429. [Google Scholar] [CrossRef] [PubMed]
- Zidki, T.; Cohen, H.; Meyerstein, D.; Meisel, D. Effect of silica-supported silver nanoparticles on the dihydrogen yields from irradiated aqueous solutions. J. Phys. Chem. C 2007, 111, 10461–10466. [Google Scholar] [CrossRef]
- Adhikary, J.; Meistelman, M.; Burg, A.; Shamir, D.; Meyerstein, D.; Albo, Y. Reductive dehalogenation of monobromo-and tribromoacetic acid by sodium borohydride catalyzed by gold nanoparticles entrapped in sol-gel matrices follows different pathways. Eur. J. Inorg. Chem. 2017, 11, 1510–1515. [Google Scholar] [CrossRef]
- Meistelman, M.; Adhikary, J.; Burg, A.; Shamir, D.; Gershinsky, G.; Meyerstein, D.; Albo, Y. Ag0 and Au0 nanoparticles encapsulated in sol-gel matrices as catalysts in reductive de-halogenation reactions. Chim. Oggi. 2017, 35, 23–26. [Google Scholar]
- Adhikary, J.; Meyerstein, D.; Marks, V.; Meistelman, M.; Gershinsky, G.; Burg, A.; Shamir, D.; Kornweitz, H.; Albo, Y. Sol-gel entrapped Au0-and Ag0-nanoparticles catalyze reductive de- halogenation of halo-organic compounds by BH4−. Appl. Catal. B-Environ. 2018, 239, 450–462. [Google Scholar] [CrossRef]
- Trabelsi, K.; Meistelman, M.; Ciriminna, R.; Albo, Y.; Pagliaro, M. Effective and green removal of trichloroacetic acid from disinfected water. Materials 2020, 13, 827–828. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable catalysis. Chemicals 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. New trends in sustainable nanocatalysis: Emerging use of earth abundant metals. Curr. Opin. Green Sust. 2017, 7, 39–45. [Google Scholar] [CrossRef]
- MacCrehan, W.A.; Bedner, M.; Helz, G.R. Making chlorine greener: Performance of alternative dechlorination agents in wastewater. Chemosphere 2005, 60, 381–388. [Google Scholar] [CrossRef]
- Ibrahem, A.K.; Moghny, T.A.; Mustafa, Y.M.; Maysour, N.E.; El-Din El-Dars, F.M.S.; Hassan, R.F. Degradation of trichloroethylene contaminated soil by zero-valent iron nanoparticles. ISRN Soil Sci. 2012, 1–10. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Tiwari, S.; Mohan, D.; Pandey, A.; Solanki, P.R. Nanoscale zero-valent iron for aqueous lead removal. Adv. Mat. Proc. 2017, 2, 235–241. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Rasika Dias, H.V.; Kharissova, O.V.; Jiménez-Pérez, V.M.; Pérez, B.O.; Flores, B.M. Iron-containing nanomaterials: Synthesis, properties, and environmental applications. RSC Adv. 2012, 2, 9325–9358. [Google Scholar] [CrossRef]
- Guler, U.A. Removal of tetracycline from aqueous solutions using nanoscale zero valent iron and functional pumice modified nanoscale zero valent iron. J. Environ. Eng. Landsc. 2017, 25, 223–233. [Google Scholar] [CrossRef]
- Zha, S.; Cheng, Y.; Gao, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem. Eng. J. 2014, 255, 141–148. [Google Scholar] [CrossRef]
- Shea, P.J.; Machacek, T.A.; Comfort, S.D. Accelerated remediation of pesticide-contaminated soil with zerovalent iron. Environ. Pollut. 2004, 132, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Li, X.; Bond, T.; Gao, N.; Bin, X.; Wang, Q.; Ding, S. Copper increases reductive dehalogenation of haloacetamides by zero-valent iron in drinking water: Reduction efficiency and integrated toxicity risk. Water Res. 2016, 107, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chu, W.; Wei, H.; Zhao, H.; Xu, B.; Gao, N.; Yin, D. Reductive dechlorination of haloacetamides in drinking water by Cu/Fe bimetal. Sep. Purif. Technol. 2018, 203, 226–232. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Chu, W.; Li, X.; Wei, H.; Gao, N. Weak magnetic field accelerates chloroacetamide removal by zero-valent iron in drinking water. Chem. Eng. J. 2019, 358, 40–47. [Google Scholar] [CrossRef]
- Bouazizi, N.; Vieillard, J.; Bargougui, R.; Couvrat, N.; Thoumire, O.; Morin, S.; Ladam, G.; Mofaddel, N.; Brun, N.; Azzouz, A.; et al. Entrapment and stability of iron nanoparticles within APTES modified graphene oxide sheets with improved catalytic activity. J. Alloy. Compd. 2019, 771, 1090–1102. [Google Scholar] [CrossRef]
- Sravanthi, K.; Ayodhya, D.; Swamy, P.Y. Green synthesis, characterization, and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles. Mater. Sci. Energy Technol. 2019, 2, 298–307. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl. Catal. B-Environ. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Meyerstein, D.; Adhikary, J.; Burg, A.; Shamir, D.; Albo, A. Zero-valent iron nanoparticles entrapped in SiO2 sol-gel matrices: A catalyst for the reduction of several pollutants. Catal. Commun. 2020, 133, 1–5. [Google Scholar] [CrossRef]
- Plewa, M.J.; Muellner, M.G.; Richardson, S.D.; Fasano, F.; Buettner, K.M.; Woo, Y.; McKague, A.B.; Wagner, E.D. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: An emerging class of nitrogenous drinking water disinfection byproducts. Environ. Sci. Technol. 2008, 42, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gao, N.; Yin, D.; Krasner, S.W. Formation, and speciation of nine haloacetamides, an emerging class of nitrogenous DBPs, during chlorination or chloramination. J. Hazard. Mater. 2013, 260, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Li, D.; Gao, N.; Templeton, M.R.; Tan, C.; Gao, Y. The control of emerging haloacetamide DBP precursors with UV/persulfate treatment. Water Res. 2015, 72, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Andrade, A.L.; Fabris, J.D.; Ardisson, J.D.; Valente, M.A.; Ferreira, J.M.F. Effect of Tetramethylammonium Hydroxide on nucleation, surface modification and growth of magnetic nanoparticles. J. Nanomater. 2012, 15, 1–10. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1–19. [Google Scholar] [CrossRef]
- Grosman, A.; Ortega, C. Capillary condensation in porous materials hysteresis and interaction mechanism without pore blocking/percolation process. Langmuir 2008, 24, 3977–3986. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Santos, A.M.; Vasconcelos, W.L. Obtention of nanostructured silica glass by sol-gel process with incorporation of lead compounds. Mater. Res. 1999, 2, 201–204. [Google Scholar] [CrossRef]
- Lenza, R.F.S.; Vasconcelos, W.L. Preparation of silica by sol-gel method using formamide. Mater. Res. 2001, 4, 189–194. [Google Scholar] [CrossRef]
- Rusonik, I.; Cohen, H.; Meyerstein, D. Cu (I) (2,5,8,11-tetramethyl-2,5,8,11-tetraazadodecane) as a catalyst for Ullmann’s reaction. J. Chem. Soc. Dalton Trans. 2003, 10, 2024–2028. [Google Scholar] [CrossRef]
- Zidki, T.; Cohen, H.; Meyerstein, D. Reactions of alkyl-radicals with gold and silver nanoparticles in aqueous solutions. Phys. Chem. Chem. Phys. 2006, 8, 3552–3556. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ziv, R.; Zidki, T.; Zilbermann, I.; Yardeni, G.; Meyerstein, D. Effect of hydrogen pretreatment of platinum nanoparticles on their catalytic properties: Reactions with alkyl radicals–a mechanistic study. Chem. Cat. Chem. 2016, 8, 2761–2764. [Google Scholar] [CrossRef]
- Solorzano, L. Determination of ammonia in natural waters by the phenol hypochlorite. Method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Park, G.; Oh, H.; Ahn, S. Improvement of the ammonia analysis by the phenate method in water and wastewater. B Korean Chem. Soc. 2009, 30, 2032–2038. [Google Scholar] [CrossRef]
- Rhine, E.D.; Sims, G.K.; Mulvaney, R.L.; Pratt, E.J. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci. Soc. Am. J. 1998, 62, 473–480. [Google Scholar] [CrossRef]
- Kimble, K.W.; Walker, J.P.; Finegold, D.N.; Asher, S.A. Progress toward the development of a point-of-care photonic crystal ammonia sensor. Anal. Bioanal. Chem. 2006, 385, 678–685. [Google Scholar] [CrossRef]



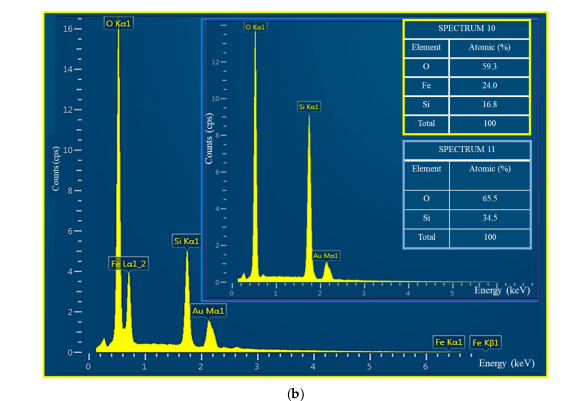

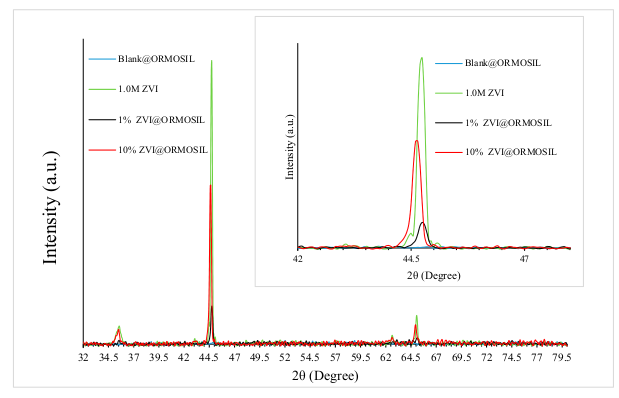


| Substance | 2θ | Miller Index | File No. |
|---|---|---|---|
| Fe0 | 44.7° | 1,1,0 | 04-007-9753 |
| Fe0 | 65.1° | 2,0,0 | |
| Fe3O4 | 35.4° | 3,1,1 | 04-005-4319 |
| Fe3O4 | 62.5° | 4,0,0 |
| Material | Dp (nm) |
|---|---|
| 1.0 M susp. | 44.9 |
| 10% ZVI@ORMOSIL | 38.8 |
| 1% ZVI@ORMOSIL | 34.7 |
| Sample | Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Blank@ORNOSIL | 574 | 0.53 | 4.7 |
| 1.0% ZVI@ORMOSIL | 751 | 0.73 | 4.7 |
| Starting Subs. | Medium | DAcAm (%) | MAcAm (%) | AcAm (%) | AA (%) |
|---|---|---|---|---|---|
| DAcAm | H2O | 0 | 76 | 18 | 6 |
| MAcAm | 0 | 53 | 47 | 0 | |
| DAcAm/MAcAm, (1/1) | 0 | 65 | 31 | 3 | |
| DAcAm | EtOH:H2O 8:2 | 22 | 63 | 10 | 5 |
| MAcAm | 0 | 84 | 16 | 0 | |
| DAcAm/MAcAm, (1/1) | 12 | 75 | 13 | 3 | |
| DAcAm | ACN:H2O 8:2 | 46 | 52 | 2 | 0 |
| MAcAm | 0 | 80 | 20 | 0 | |
| DAcAm/MAcAm, (1/1) | 23 | 66 | 11 | 0 | |
| DAcAm | 2-PrOH:H2O 8:2 | 60 | 40 | 0 | 0 |
| MAcAm | 0 | 90 | 10 | 0 | |
| DAcAm/MAcAm, (1/1) | 30 | 65 | 5 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meistelman, M.; Meyerstein, D.; Bardea, A.; Burg, A.; Shamir, D.; Albo, Y. Reductive Dechlorination of Chloroacetamides with NaBH4 Catalyzed by Zero Valent Iron, ZVI, Nanoparticles in ORMOSIL Matrices Prepared via the Sol-Gel Route. Catalysts 2020, 10, 986. https://doi.org/10.3390/catal10090986
Meistelman M, Meyerstein D, Bardea A, Burg A, Shamir D, Albo Y. Reductive Dechlorination of Chloroacetamides with NaBH4 Catalyzed by Zero Valent Iron, ZVI, Nanoparticles in ORMOSIL Matrices Prepared via the Sol-Gel Route. Catalysts. 2020; 10(9):986. https://doi.org/10.3390/catal10090986
Chicago/Turabian StyleMeistelman, Michael, Dan Meyerstein, Amos Bardea, Ariela Burg, Dror Shamir, and Yael Albo. 2020. "Reductive Dechlorination of Chloroacetamides with NaBH4 Catalyzed by Zero Valent Iron, ZVI, Nanoparticles in ORMOSIL Matrices Prepared via the Sol-Gel Route" Catalysts 10, no. 9: 986. https://doi.org/10.3390/catal10090986
APA StyleMeistelman, M., Meyerstein, D., Bardea, A., Burg, A., Shamir, D., & Albo, Y. (2020). Reductive Dechlorination of Chloroacetamides with NaBH4 Catalyzed by Zero Valent Iron, ZVI, Nanoparticles in ORMOSIL Matrices Prepared via the Sol-Gel Route. Catalysts, 10(9), 986. https://doi.org/10.3390/catal10090986









