Effect of the Gallium and Vanadium on the Dibenzothiophene Hydrodesulfurization and Naphthalene Hydrogenation Activities Using Sulfided NiMo-V2O5/Al2O3-Ga2O3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
2.2. Textural Properties
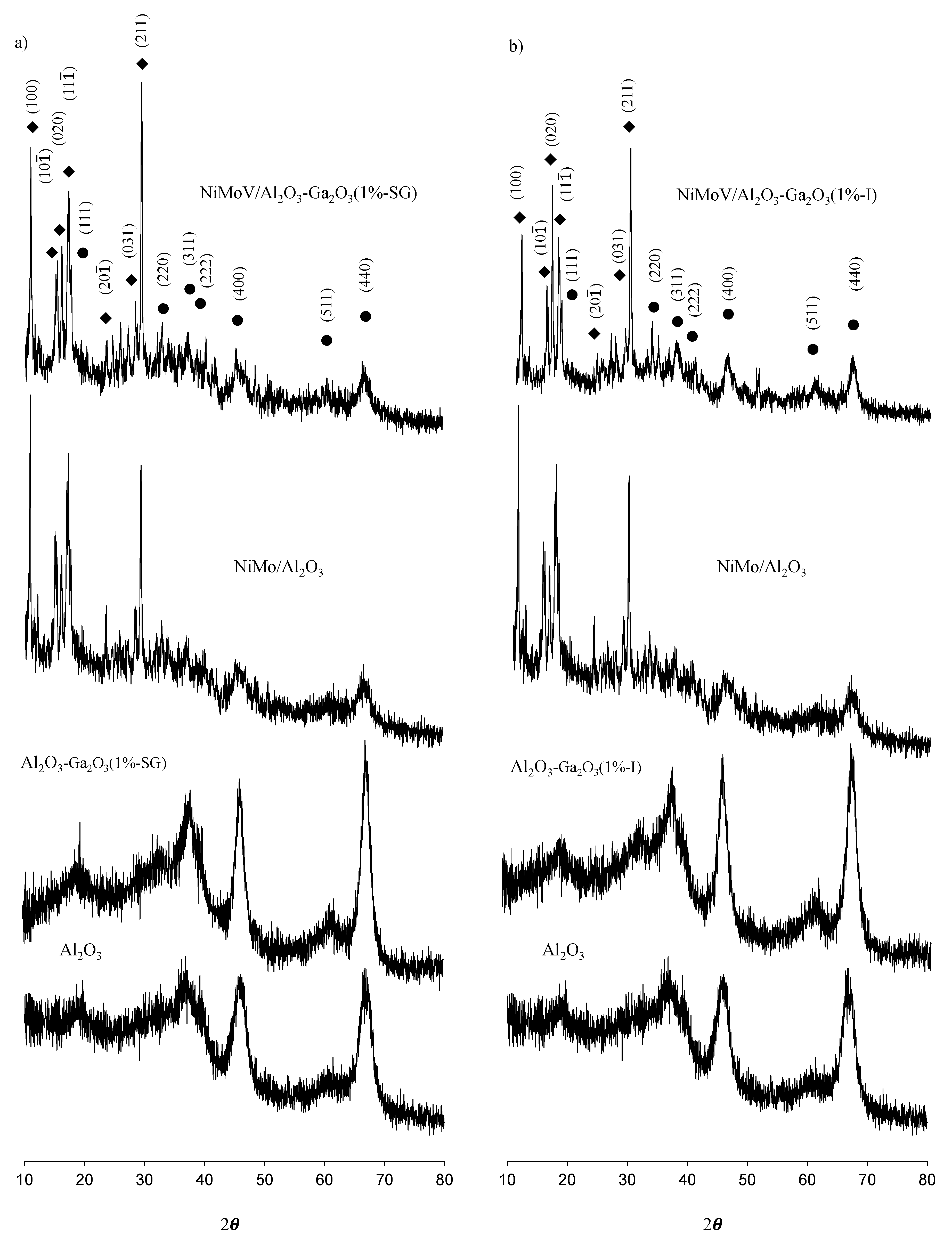
2.3. XRD Analysis
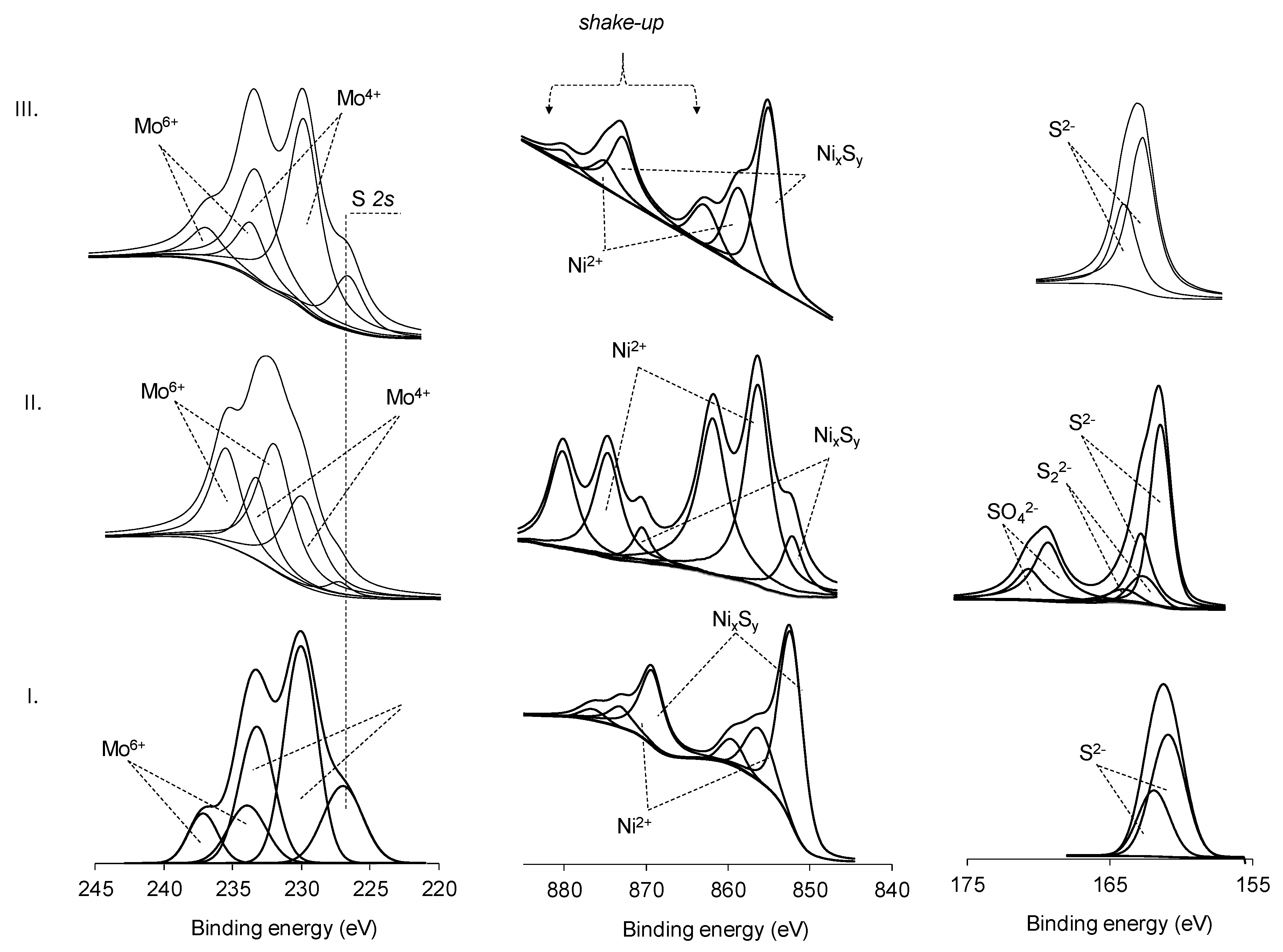
2.4. XPS Analysis
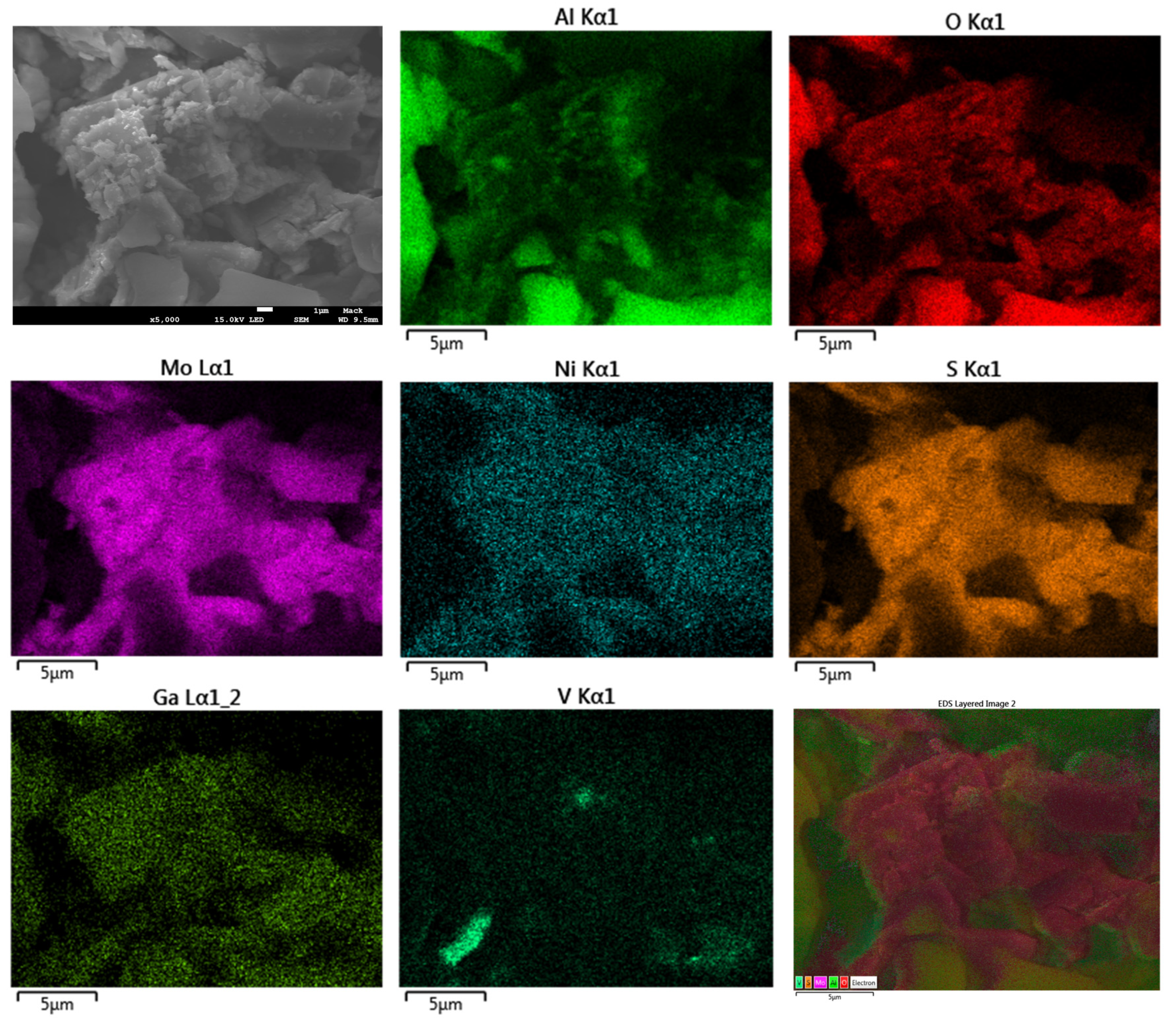
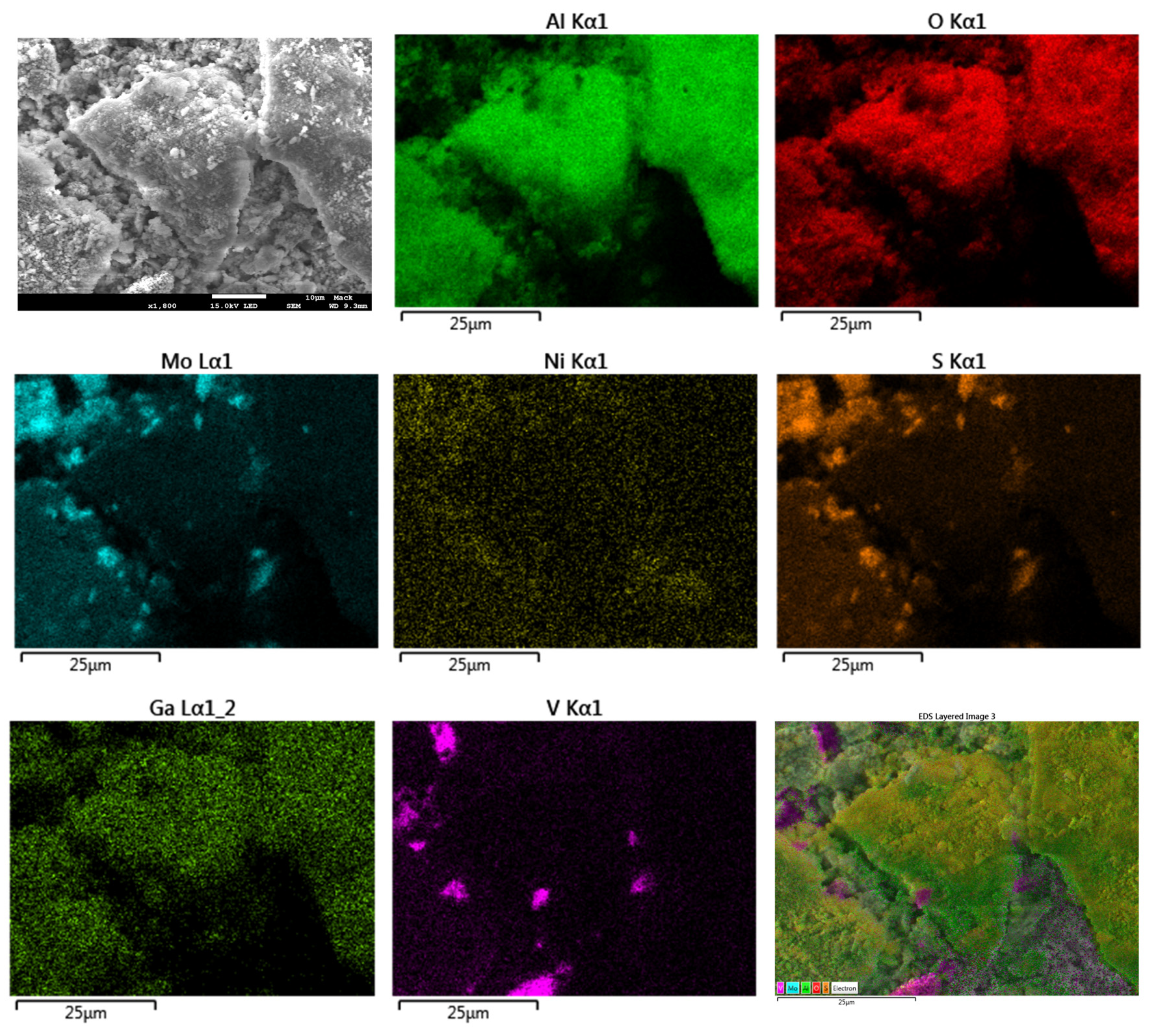
2.5. SEM Analysis with Energy Dispersive X-ray Spectroscopy and Elemental Mapping
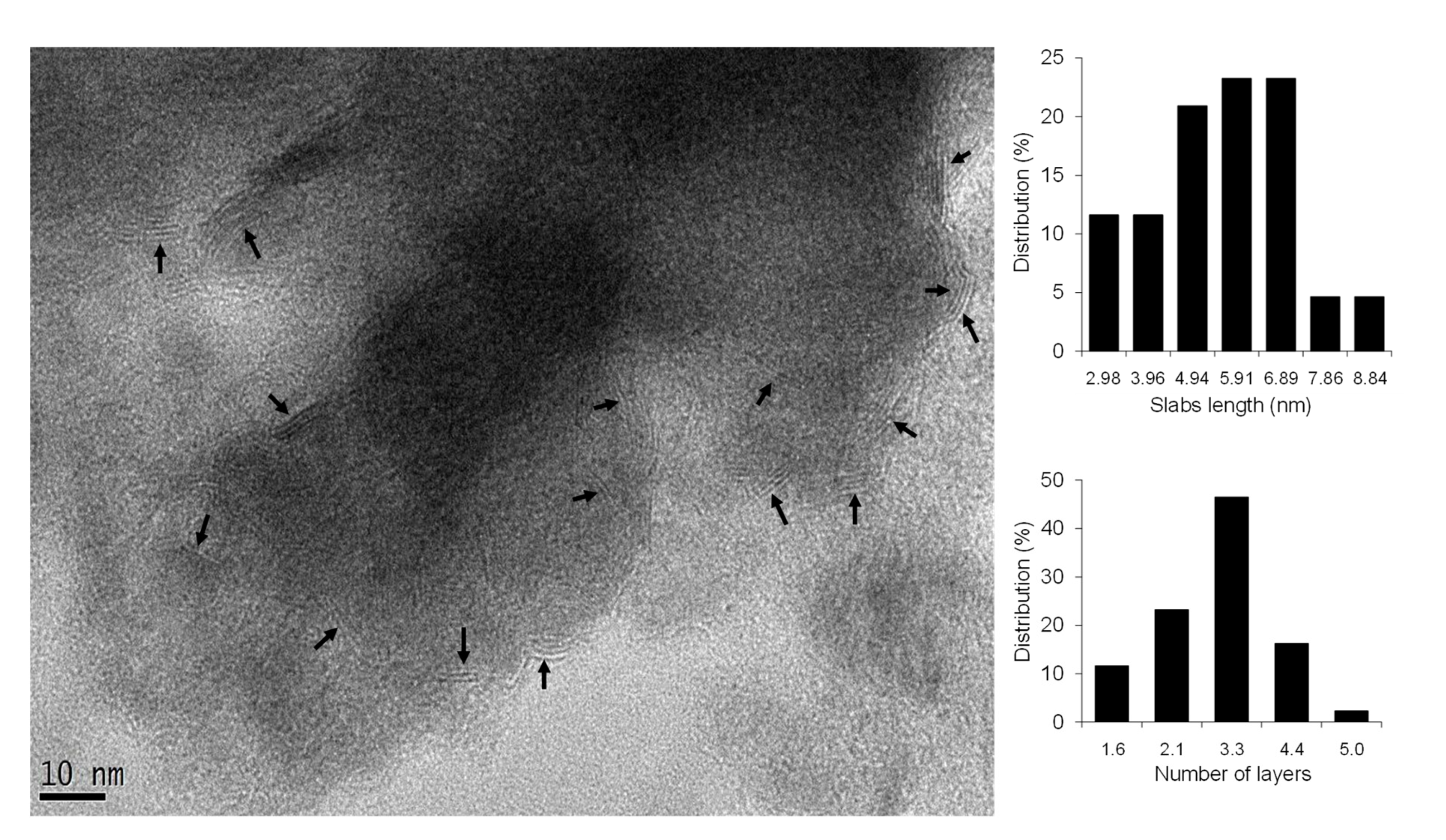
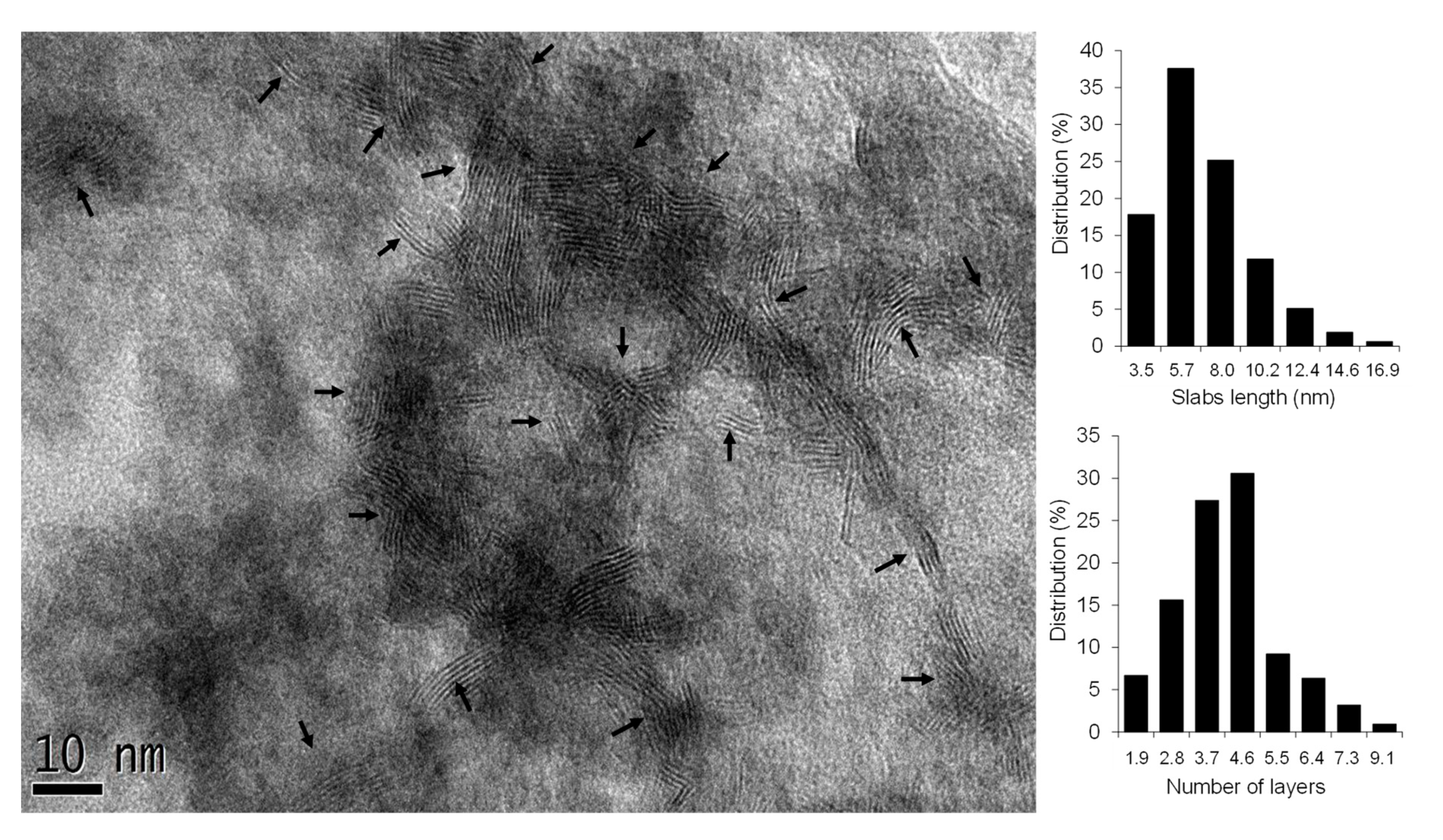
2.6. High-Resolution Transmission Electron Microscopy
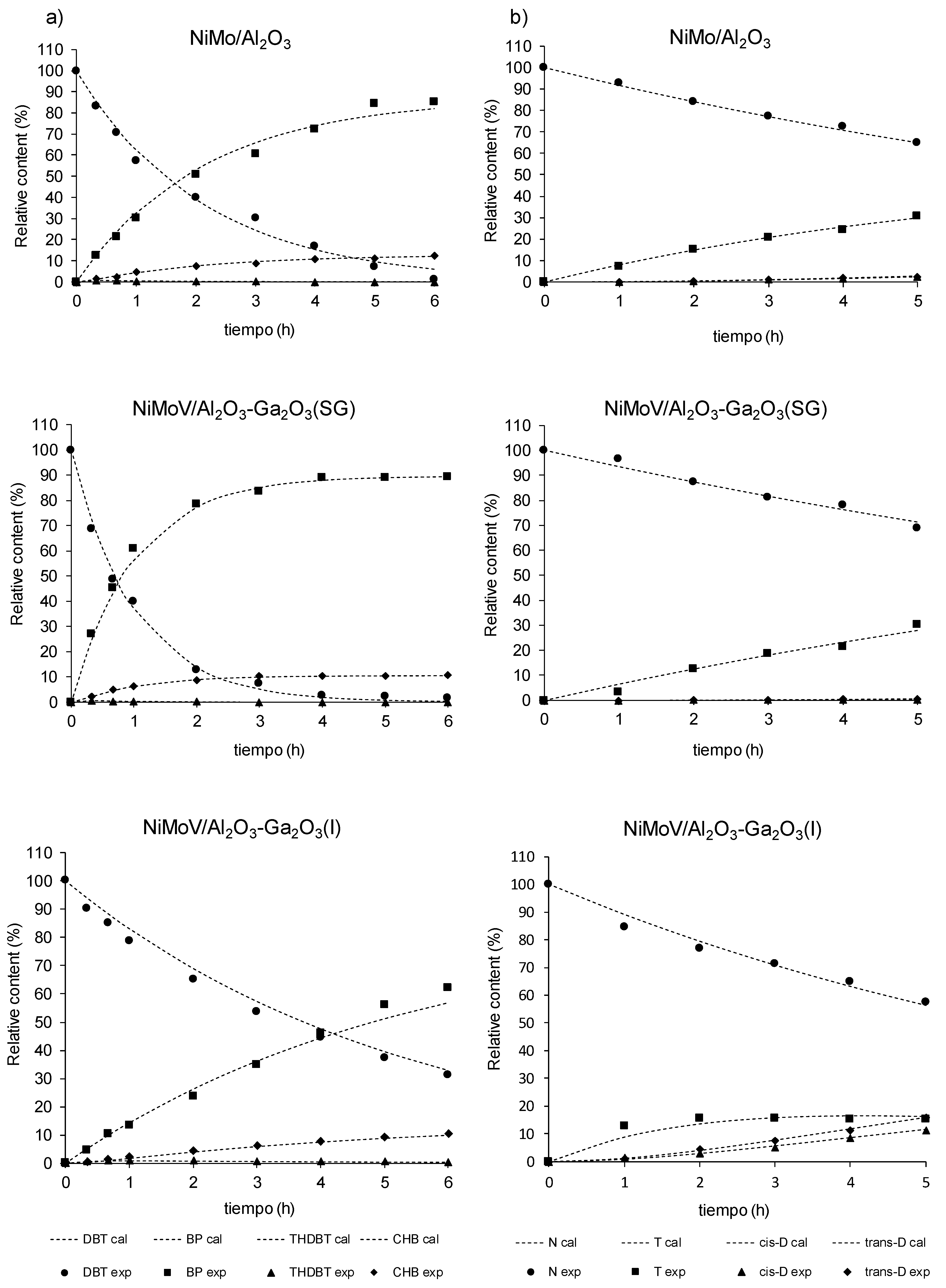
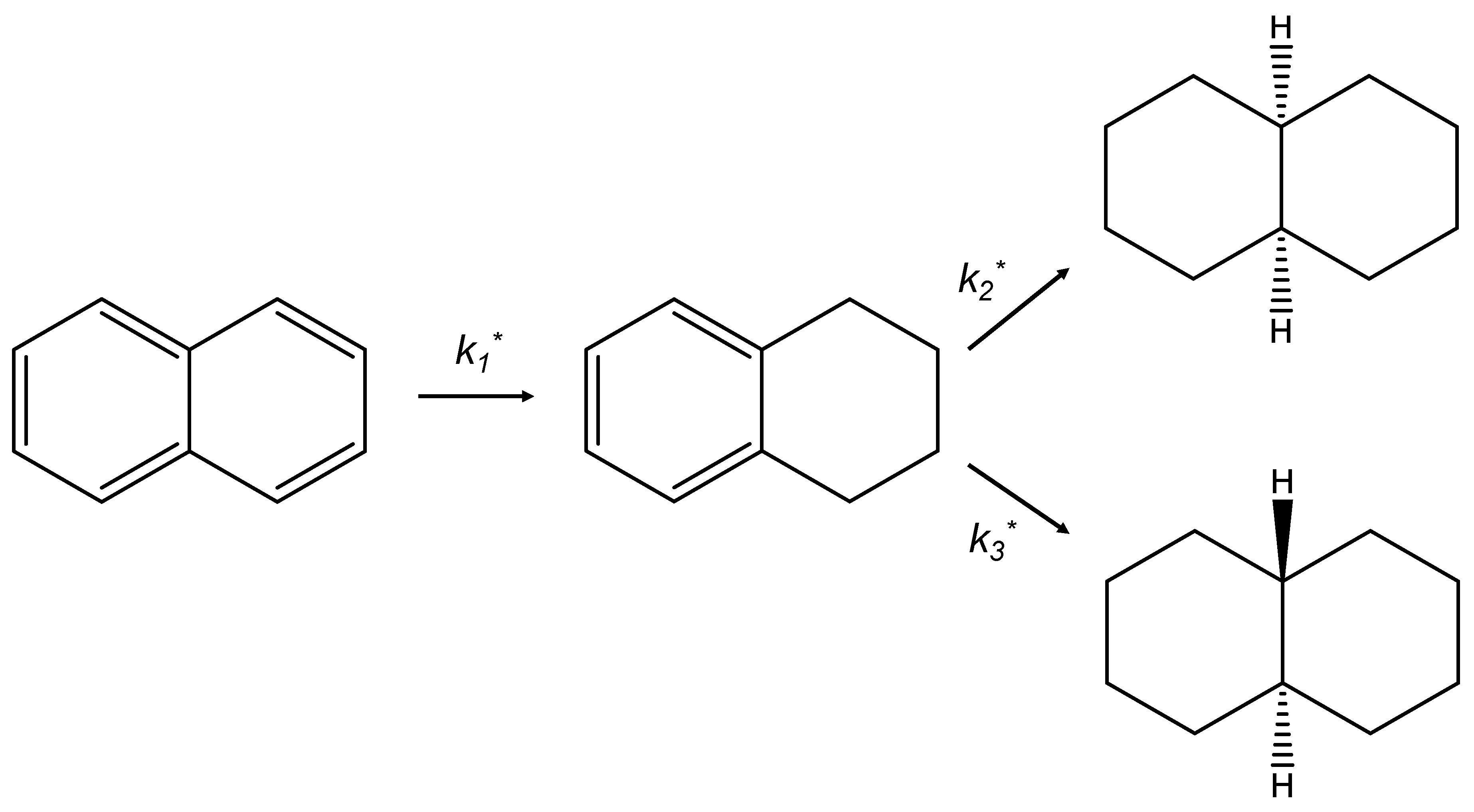
2.7. Catalytic Test
3. Materials and Methods
3.1. Preparation of Alumina Modified with Gallium
3.2. Preparation of Catalyst Precursors Supported on Modified Alumina with Gallium
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, J.R.; Boudart, M. Catalysis: Science and Technology; Springer: Berlin/Heidelberg, Germany, 1996; ISBN 978-3-642-61040-0. [Google Scholar]
- Rașeev, S.D. Thermal and Catalytic Processes in Petroleum Refining; Marcel Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-0952-5. [Google Scholar]
- Lødeng, R.; Hannevold, L.; Bergem, H.; Stöcker, M. Catalytic Hydrotreatment of Bio-Oils for High-Quality Fuel Production. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Triantafyllidis, K.S., Lappas, A.A., Stöcker, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 11; pp. 351–396. ISBN 978-0-444-56330-9. [Google Scholar]
- Debecker, D.P.; Stoyanova, M.; Rodemerck, U.; Gaigneaux, E.M. Preparation of MoO3/SiO2–Al2O3 metathesis catalysts via wet impregnation with different Mo precursors. J. Mol. Catal. Chem. 2011, 340, 65–76. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S. Active sites and support effects in hydrodesulfurization catalysts. Appl. Catal. 1986, 25, 273–293. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Siadati, M.H.; De la Rosa, M.P.; Berhault, G.; Wilcoxon, J.P.; Bearden, R.; Abrams, B.L. Catalytic Properties of Single Layers of Transition Metal Sulfide Catalytic Materials. Catal. Rev. 2006, 48, 1–41. [Google Scholar] [CrossRef]
- Babich, I. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Ministerio de Ambiente y Desarrollo Sostenible, y Ministerio de Minas y Energías. Resolución 40619 2017 pág 1-3; Resolución 90963 2014, 5.
- Gutiérrez, O.Y.; Klimova, T. Effect of the support on the high activity of the (Ni)Mo/ZrO2–SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4,6-DMDBT. J. Catal. 2011, 281, 50–62. [Google Scholar] [CrossRef]
- Rashidi, F.; Sasaki, T.; Rashidi, A.M.; Nemati Kharat, A.; Jozani, K.J. Ultradeep hydrodesulfurization of diesel fuels using highly efficient nanoalumina-supported catalysts: Impact of support, phosphorus, and/or boron on the structure and catalytic activity. J. Catal. 2013, 299, 321–335. [Google Scholar] [CrossRef]
- Chianelli, R.R. Fundamental Studies of Transition Metal Sulfide Hydrodesulfurization Catalysts. Catal. Rev. 1984, 26, 361–393. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis. In Catalysis; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–269. ISBN 978-3-642-64666-9. [Google Scholar]
- Breysse, M.; Portefaix, J.L.; Vrinat, M. Support effects on hydrotreating catalysts. Catal. Today 1991, 10, 489–505. [Google Scholar] [CrossRef]
- Palcheva, R.; Kaluža, L.; Spojakina, A.; Jirátová, K.; Tyuliev, G. NiMo/γ-Al2O3 Catalysts from Ni Heteropolyoxomolybdate and Effect of Alumina Modification by B, Co, or Ni. Chin. J. Catal. 2012, 33, 952–961. [Google Scholar] [CrossRef]
- Jirátová, K.; Kraus, M. Effect of support properties on the catalytic activity of HDS catalysts. Appl. Catal. 1986, 27, 21–29. [Google Scholar] [CrossRef]
- Saini, A.R.; Johnson, B.G.; Massoth, F.E. Studies of molybdena—Alumina catalysts XIV. Effect of Cation-Modified Aluminas. Appl. Catal. 1988, 40, 157–172. [Google Scholar] [CrossRef]
- Strohmeier, B. Surface spectroscopic characterization of the interaction between zinc ions and $gamma;-alumina. J. Catal. 1984, 86, 266–279. [Google Scholar] [CrossRef]
- Cabello, C.I.; Botto, I.L.; Thomas, H.J. Anderson type heteropolyoxomolybdates in catalysis:: 1. (NH4)3[CoMo6O24H6]·7H2O/γ-Al2O3 as alternative of Co-Mo/γ-Al2O3 hydrotreating catalysts. Appl. Catal. Gen. 2000, 197, 79–86. [Google Scholar] [CrossRef]
- Cabello, C.I.; Cabrerizo, F.M.; Alvarez, A.; Thomas, H.J. Decamolybdodicobaltate(III) heteropolyanion: Structural, spectroscopical, thermal and hydrotreating catalytic properties. J. Mol. Catal. Chem. 2002, 186, 89–100. [Google Scholar] [CrossRef]
- Altamirano, E.; de los Reyes, J.A.; Murrieta, F.; Vrinat, M. Hydrodesulfurization of 4,6-dimethyldibenzothiophene over Co(Ni)MoS2 catalysts supported on alumina: Effect of gallium as an additive. Catal. Today 2008, 133–135, 292–298. [Google Scholar] [CrossRef]
- Díaz de León, J.N.; Picquart, M.; Massin, L.; Vrinat, M.; de los Reyes, J.A. Hydrodesulfurization of sulfur refractory compounds: Effect of gallium as an additive in NiWS/γ-Al2O3 catalysts. J. Mol. Catal. Chem. 2012, 363–364, 311–321. [Google Scholar] [CrossRef]
- Cimino, A.; Lo Jacono, M.; Schiavello, M. Effect of zinc, gallium, and germanium ions on the structural and magnetic properties of nickel ions supported on alumina. J. Phys. Chem. 1975, 79, 243–249. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Pawelec, B.; Díaz de León, J.N.; de los Reyes, J.A.; Olivas, A. Effect of gallium loading on the hydrodesulfurization activity of unsupported Ga2S3/WS2 catalysts. Appl. Catal. B Environ. 2012, 111–112, 10–19. [Google Scholar] [CrossRef]
- Petre, A.L.; Auroux, A.; Gervasini, A.; Caldararu, M.; Ionescu, N.I. Calorimetric Characterization of Surface Reactivity of Supported Ga2O3 Catalysts. J. Therm. Anal. Calorim. 2001, 64, 253–260. [Google Scholar] [CrossRef]
- Dejonghe, S.; Hubaut, R.; Grimblot, J.; Bonnelle, J.P.; Des Courieres, T.; Faure, D. Hydrodemetallation of a vanadylporphyrin over sulfided NiMoγAl2O3, MoγAl2O3, and γAl2O3 catalysts—Effect of the vanadium deposit on the toluene hydrogenation. Catal. Today 1990, 7, 569–585. [Google Scholar] [CrossRef]
- Rankel, L.; Rollmann, L. Catalytic activity of metals in petroleum and their removal. Fuel 1983, 62, 44–46. [Google Scholar] [CrossRef]
- Lacroix, M.; Boutarfa, N.; Guillard, C.; Vrinat, M.; Breysse, M. Hydrogenating properties of unsupported transition metal sulphides. J. Catal. 1989, 120, 473–477. [Google Scholar] [CrossRef]
- Betancourt, P.; Rives, A.; Scott, C.E.; Hubaut, R. Hydrotreating on mixed vanadium–nickel sulphides. Catal. Today 2000, 57, 201–207. [Google Scholar] [CrossRef]
- Betancourt, P.; Marrero, S.; Pinto-Castilla, S. V–Ni–Mo sulfide supported on Al2O3: Preparation, characterization and LCO hydrotreating. Fuel Process. Technol. 2013, 114, 21–25. [Google Scholar] [CrossRef]
- Escalante, Y.; Méndez, F.J.; Díaz, Y.; Inojosa, M.; Morgado, M.; Delgado, M.; Bastardo-González, E.; Brito, J.L. MCM-41-supported vanadium catalysts structurally modified with Al or Zr for thiophene hydrodesulfurization. Appl. Petrochem. Res. 2019, 9, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Ayala-G, M.; Puello, E.; Quintana, P.; González-García, G.; Diaz, C. Comparison between alumina supported catalytic precursors and their application in thiophene hydrodesulfurization: (NH4) [NiMo6O24H6]·5H2O/γ-Al2O3 and NiMoOx/γ-Al2O3 conventional systems. RSC Adv. 2015, 5, 102652–102662. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sampieri, A.; Pronier, S.; Brunet, S.; Carrier, X.; Louis, C.; Blanchard, J.; Fajerwerg, K.; Breysse, M. Formation of heteropolymolybdates during the preparation of Mo and NiMo HDS catalysts supported on SBA-15: Influence on the dispersion of the active phase and on the HDS activity. Microporous Mesoporous Mater. 2010, 130, 130–141. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Shimada, H.; Hamada, H. Selective Reduction of NO with Propene over Ga2O3–Al2O3: Effect of Sol–Gel Method on the Catalytic Performance. J. Catal. 2000, 192, 137–148. [Google Scholar] [CrossRef]
- Ueno, A.; Suzuki, H.; Kotera, Y. Particle-size distribution of nickel dispersed on silica and its effects on hydrogenation of propionaldehyde. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens Phases 1983, 79, 127. [Google Scholar] [CrossRef]
- Puello-Polo, E.; Marquez, E.; Brito, J.L. One-pot synthesis of Nb-modified Al2O3 support for NiMo hydrodesulfurization catalysts. J. Sol-Gel Sci. Technol. 2018, 88, 90–99. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data® (ICDD®). Power Diffraction File; ICDD: Newtown Square, PA, USA, 1995. [Google Scholar]
- Galtayries, A.; Wisniewski, S.; Grimblot, J. Formation of thin oxide and sulphide films on polycrystalline molybdenum foils: Characterization by XPS and surface potential variations. J. Electron Spectrosc. Relat. Phenom. 1997, 87, 31–44. [Google Scholar] [CrossRef]
- Weber, T.; Muijsers, J.C.; van Wolput, J.H.M.C.; Verhagen, C.P.J.; Niemantsverdriet, J.W. Basic Reaction Steps in the Sulfidation of Crystalline MoO3 to MoS2, As Studied by X-ray Photoelectron and Infrared Emission Spectroscopy. J. Phys. Chem. 1996, 100, 14144–14150. [Google Scholar] [CrossRef] [Green Version]
- Aigler, J.M.; Brito, J.L.; Leach, P.A.; Houalla, M.; Proctor, A.; Cooper, N.J.; Hall, W.K.; Hercules, D.M. ESCA study of “model” allyl-based molybdenum/silica catalysts. J. Phys. Chem. 1993, 97, 5699–5702. [Google Scholar] [CrossRef]
- Le, Z.; Afanasiev, P.; Li, D.; Long, X.; Vrinat, M. Solution synthesis of the unsupported Ni–W sulfide hydrotreating catalysts. Catal. Today 2008, 130, 24–31. [Google Scholar] [CrossRef]
- Wang, X.; Ozkan, U.S. Characterization of Active Sites over Reduced Ni−Mo/Al2O3 Catalysts for Hydrogenation of Linear Aldehydes. J. Phys. Chem. B 2005, 109, 1882–1890. [Google Scholar] [CrossRef]
- Schön, G. Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium and gallium oxide. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 75–86. [Google Scholar] [CrossRef]
- Escaño, M.C.S.; Asubar, J.T.; Yatabe, Z.; David, M.Y.; Uenuma, M.; Tokuda, H.; Uraoka, Y.; Kuzuhara, M.; Tani, M. On the presence of Ga2O sub-oxide in high-pressure water vapor annealed AlGaN surface by combined XPS and first-principles methods. Appl. Surf. Sci. 2019, 481, 1120–1126. [Google Scholar] [CrossRef]
- Rakmae, S.; Osakoo, N.; Pimsuta, M.; Deekamwong, K.; Keawkumay, C.; Butburee, T.; Faungnawakij, K.; Geantet, C.; Prayoonpokarach, S.; Wittayakun, J.; et al. Defining nickel phosphides supported on sodium mordenite for hydrodeoxygenation of palm oil. Fuel Process. Technol. 2020, 198, 106236. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Jiang, F.; Chu, Y.; Nie, H. The relation between morphology of (Co)MoS2 phases and selective hydrodesulfurization for CoMo catalysts. Catal. Today 2010, 149, 35–39. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Yin, C.; Liu, B.; Li, X.; Li, Y.; Chai, Y.; Liu, Y. Low temperature catalytic hydrogenation naphthalene to decalin over highly-loaded NiMo, NiW and NiMoW catalysts. Catal. Today 2016, 276, 46–54. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Farojr, A.; Dossantos, A. Cumene hydrocracking and thiophene HDS on niobia-supported Ni, Mo and Ni–Mo catalysts. Catal. Today 2006, 118, 402–409. [Google Scholar] [CrossRef]
- Froment, G.F.; De Wilde, J.; Bischoff, K.B. Chemical Reactor Analysis and Design, 3rd ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-56541-4. [Google Scholar]
- Moulijn, J.A.; Tarfaoui, A.; Kapteijn, F. General aspects of catalyst testing. Catal. Today 1991, 11, 1–12. [Google Scholar] [CrossRef]
- Farag, H. Kinetic Analysis of the Hydrodesulfurization of Dibenzothiophene: Approach Solution to the Reaction Network. Energy Fuels 2006, 20, 1815–1821. [Google Scholar] [CrossRef]
- Vargas-Villagrán, H.; Ramírez-Suárez, D.; Ramírez-Muñoz, G.; Calzada, L.A.; González-García, G.; Klimova, y.T.E. Tuning of activity and selectivity of Ni/(Al)SBA-15 catalysts in naphthalene hydrogenation. Catal. Today 2019, S0920586119305103. [Google Scholar] [CrossRef]


| Solid | Experimental Composition (wt%)-XRF | Textural Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo | Ni | Ga2O3 | V2O5 | SBET | Sext | Smicro | Vp | Dp | |||
| (m2/g) | (cm3/g) | (nm) | |||||||||
| Al2O3 | 265 | 258 | 7 | 0.57 | 7.68 | ||||||
| Al2O3-Ga2O3(1%-SG) | 1.46 | 259 | 0.52 | 6.97 | |||||||
| Al2O3-Ga2O3(1%-I) | 1.51 | 238 | 0.45 | 6.78 | |||||||
| NiMo/Al2O3 | 25.02 | 2.38 | 6.5 | 233 | 184 | 49 | 0.26 | 7.20 | |||
| NiMoV/Al-Ga(1%-SG) | 24.02 | 2.38 | 0.99 | 6.43 | 0.31 | 6.2 | 172 | 160 | 12 | 0.37 | 7.89 |
| NiMoV/Al-Ga(1%-I) | 25.60 | 2.47 | 0.94 | 6.57 | 0.30 | 6.3 | 139 | 138 | 1 | 0.23 | 6.18 |
| Catalyst | Mo 3d5/2-3d3/2 | Ni 2p3/2-2p1/2 | S 2p3/2-2p1/2 | MoS2 Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mo4+ 229 eV (%) | Mo6+ 232.5 eV (%) | NixSy853.3 eV (%) | NiMoO4 856.1 eV (%) | S2− 161.7 eV (%) | S22− 163.1 eV (%) | SO42− 168.9 eV (%) | L (nm) | N | (fe/fc)Mo | |
| NiMo-S/Al2O3 | 3.30 | 1.00 | 0.46 | 0.14 | 6.50 | |||||
| NiMoV-S/Al-Ga(1%-SG) | 2.08 | 2.82 | 0.083 | 0.22 | 1.09 | 0.22 | 0.65 | 5.07 | 2.74 | 6.43 |
| NiMoV-S/Al-Ga(1%-I) | 2.13 | 0.57 | 0.42 | 0.18 | 3.60 | 5.94 | 3.58 | 7.79 | ||
| Catalysts | HDS Rate Constants, L/(h·mol·m2) | HYD Rate Constants, L/(h·mol·m2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| KHDS | k1* | k2* | k3* (×10−10) | k4* | HYD/DDS | k1* | k2* | k3* | |
| NiMo-S/Al2O3 | 2.47 | 2.15 | 0.314 | 5.62 | 46.2 | 0.15 | 0.454 | 0.151 | 0.164 |
| NiMoV-S/Al-Ga(1%-SG) | 7.07 | 6.34 | 0.732 | 0.112 | 79.1 | 0.12 | 0.481 | 0.022 | 0.057 |
| NiMoV-S/Al-Ga(1%-I) | 1.65 | 1.40 | 0.254 | 0.019 | 24.5 | 0.18 | 1.02 | 1.61 | 2.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puello-Polo, E.; Pájaro, Y.; Márquez, E. Effect of the Gallium and Vanadium on the Dibenzothiophene Hydrodesulfurization and Naphthalene Hydrogenation Activities Using Sulfided NiMo-V2O5/Al2O3-Ga2O3. Catalysts 2020, 10, 894. https://doi.org/10.3390/catal10080894
Puello-Polo E, Pájaro Y, Márquez E. Effect of the Gallium and Vanadium on the Dibenzothiophene Hydrodesulfurization and Naphthalene Hydrogenation Activities Using Sulfided NiMo-V2O5/Al2O3-Ga2O3. Catalysts. 2020; 10(8):894. https://doi.org/10.3390/catal10080894
Chicago/Turabian StylePuello-Polo, Esneyder, Yina Pájaro, and Edgar Márquez. 2020. "Effect of the Gallium and Vanadium on the Dibenzothiophene Hydrodesulfurization and Naphthalene Hydrogenation Activities Using Sulfided NiMo-V2O5/Al2O3-Ga2O3" Catalysts 10, no. 8: 894. https://doi.org/10.3390/catal10080894






