Abstract
The BiOI/NH2-MIL-125(Ti) composite photocatalyst with excellent photocatalytic performance was prepared by the solvothermal method. For the BiOI/NH2-MIL-125(Ti) (BNMT) system, the contents of NH2-MIL-125(Ti) in BNMT-4, BNMT-5, BNMT-7, BNMT-9, and BNMT-10 were 4 wt %, 5 wt %, 7 wt %, 9 wt %, and 10 wt %, respectively. XRD, XPS, SEM, and TEM characterizations indicated that BiOI/NH2-MIL-125(Ti) was successfully prepared. Brunauer, Emmett, and Teller (BET) and UV–vis diffuse reflectance spectra photoelectrochemical analysis indicated that BNMT-9 can make the specific surface area and photo absorption region larger than BiOI. In addition, the separation efficiency of photogenerated carriers was improved, and the recombination efficiency was reduced. The degradation percentages of Rhodamine B (RhB) and p-chlorophenol (P-CP) reached 99% and 90% over BNMT-9 under visible light irradiation. Additionally, the catalysts had high stability. The results of the active spices trapping experiments test indicated that h+ was the main active species. The possible degradation mechanism was proposed.
1. Introduction
Environmental pollution is one of the main problems of sustainable development in the world. Chlorophenol is a kind of toxic organic pollutant with liposolubility and bioaccumulation, which has been listed as one of the 68 kinds of water pollutants in China [1,2,3]. At present, the main methods to deal with chlorophenols in water are the biological method, microbial fuel cell method, and so on [4,5,6,7]. Photocatalysis is an effective and environmentally friendly environmental purification technology, which can decompose toxic chlorophenols into CO2, H2O, and non-toxic inorganic acids under sunlight.
Since the semiconductor photocatalyst has excellent ability to treat organic pollutants, plentiful investigations have been focused on it [8,9,10]. However, the further practical applications of traditional semiconductors represented by TiO2 and ZnO are limited due to their wide band gaps and the inadequate utilization of solar light. As a new type of non-toxic, stable photocatalyst with strong absorption of visible light, bismuth-based photocatalysts are considered cost-effective, environmentally friendly, and sustained materials. Among the numerous bismuth-based compounds, BiOI has attracted much attention for its special layered structure, appropriate bandgap, and large specific surface area [11]. BiOI can degrade many pollutants such as methyl orange (MO), methylene blue (MB), Rhodamine B (RhB), and phenol under visible light, and it also can photocatalyze the production of hydrogen and photocatalyze the sterilization [12,13,14]. However, the serious charge recombination and narrow band gap limit the practical applications of modified BiOI [15,16]. The fabrication of heterojunction was proposed to increase the properties of BiOI-based photocatalysts and improve the charge separation and migration ability [17,18,19].
Recently, an increasing interest in metal–organic frameworks (MOFs) has focused on the utilization as photocatalyst for the photo-induced removal of pollutants. MIL-125(Ti) is a highly porous crystalline titanium dicarboxylate MOFs material with good stability, large surface area, and adjustable pore structure [20]. Amino functionalized MIL-125 can change MIL-125 from ultraviolet to visible light, accelerate carrier transport, increase the specific surface area and volume ratio, and obtain better photocatalytic activity [21].
Here, NH2-MIL-125(Ti) was added to the BiOI precursor to prepare BiOI/NH2-MIL-125(Ti) composite photocatalyst (noted BNMT system), and the effect of the ratio of BiOI and NH2-MIL-125(Ti) on the performance of the composite photocatalyst was discussed in detail. The studies of X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) had shown that BiOI/NH2-MIL-125(Ti) was successfully prepared. The combination of BiOI and NH2-MIL-125(Ti) expanded the specific surface area of the complex and improved the visible light response range of the complex. The composite photocatalyst could degrade RhB and P-CP under visible light, and the catalytic performance was obviously better than that of BiOI and NH2-MIL-125(Ti), where BiOI/NH2-MIL-125(Ti)-9 wt % showed the best photocatalytic activity. Photochemical analysis showed that the transfer of electrons and holes between BiOI and NH2-MIL-125(Ti) in composite photocatalyst was highly effective, thus improving the photocatalytic performance. Cycle experiments showed that BiOI/NH2-MIL-125(Ti) (BNMT)-9 had good stability and reusability. Free radical trapping experiments indicated that h+ was the main active species, and the possible degradation mechanism was proposed.
2. Results
2.1. Characterization
2.1.1. Photocatalyst XRD Patterns
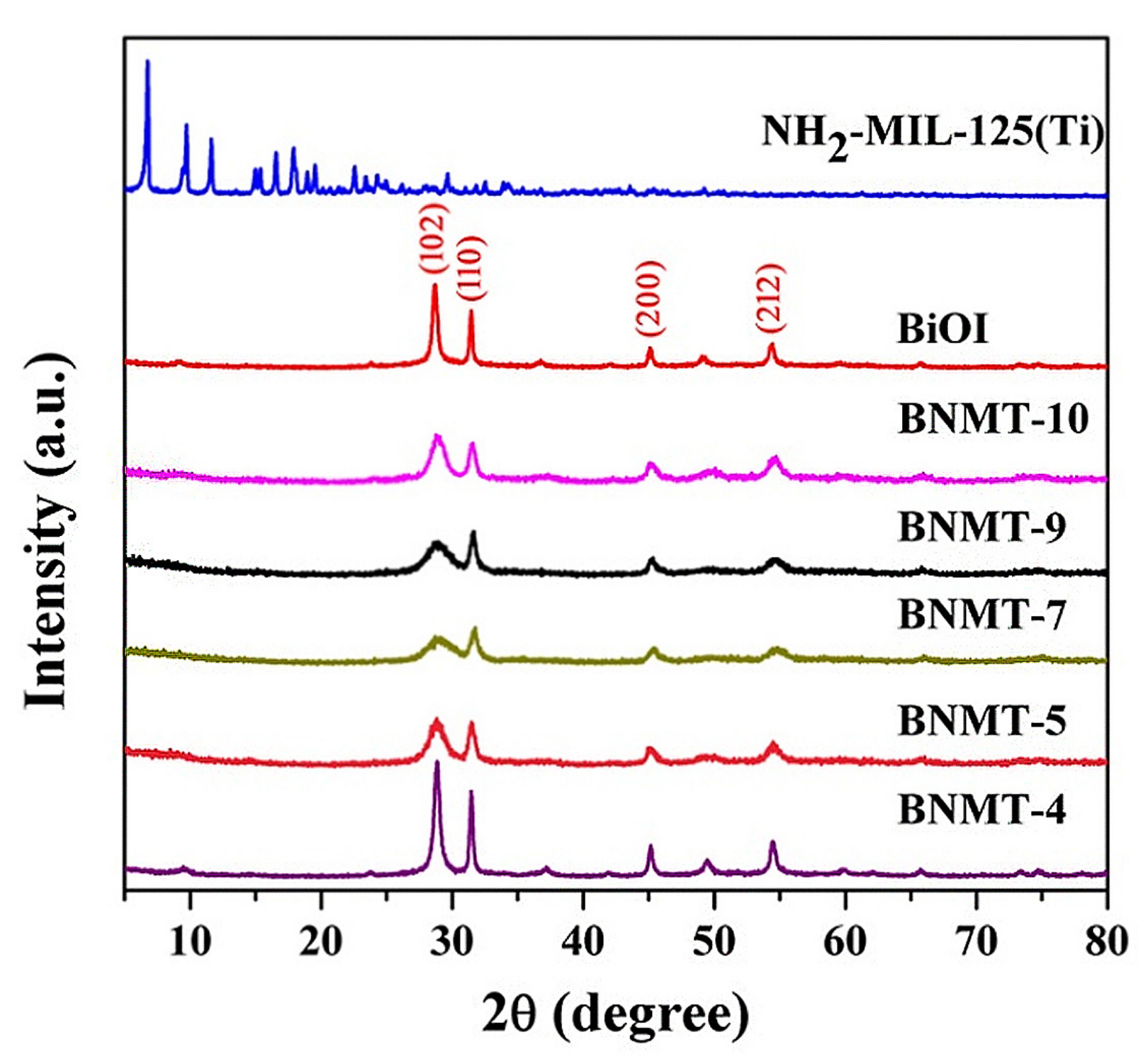
In order to explore the features of the samples, we have carried out the following characterization methods. Figure 1 showed the comparison for the XRD patterns of BiOI, NH2-MIL-125(Ti), and BiOI/NH2-MIL-125(Ti) composites with different molar ratios. The pristine BiOI showed orthorhombic phase (JCPDS No.10-0445) with well indexed (102), (110), (200), and (212) crystal planes, and the 2θ values of the four main characteristic peaks were located at 29.7˚, 31.7˚, 45.4˚, and 55.2˚, corresponding to (102), (110), (200), and (212) crystal planes of BiOI, respectively. The four peaks were sharp with high intensity, and no characteristic peaks of other substances appeared, suggesting that the crystallinity of BiOI was good, and there were no formation of by-product and change of crystal form. NH2-MIL-125(Ti) had obvious diffraction peaks at 6.8˚, 9.7˚, 11.6˚, 15.2˚, and 19.5˚, which were consistent with the MIL-125(Ti) characteristic peaks. The results showed that the presence of amino groups had no obvious effect on the structure of NH2-MIL-125(Ti) [22]. There were four obvious characteristic diffraction peaks of BiOI in the BiOI/NH2-MIL-125(Ti) complex, but there was no characteristic diffraction peak of NH2-MIL-125(Ti), which may be due to the low composition and high dispersion of NH2-MIL-125(Ti) in BiOI/NH2-MIL-125(Ti). This phenomenon has been reported in other systems [23].
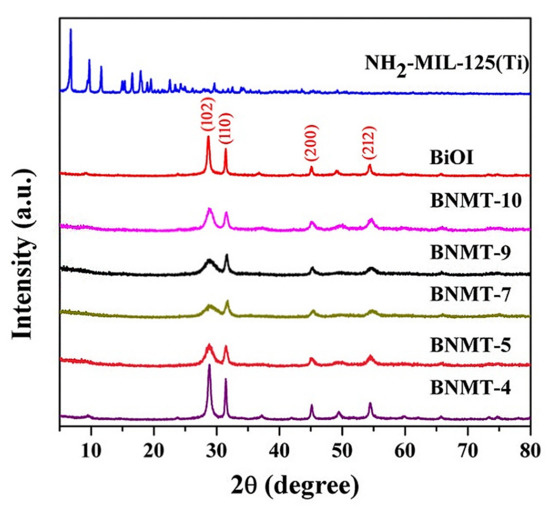
Figure 1.
XRD patterns of BiOI, NH2-MIL-125(Ti), and BiOI/NH2-MIL-125(Ti) with different composite ratios.
2.1.2. XPS Analysis of the Photocatalysts
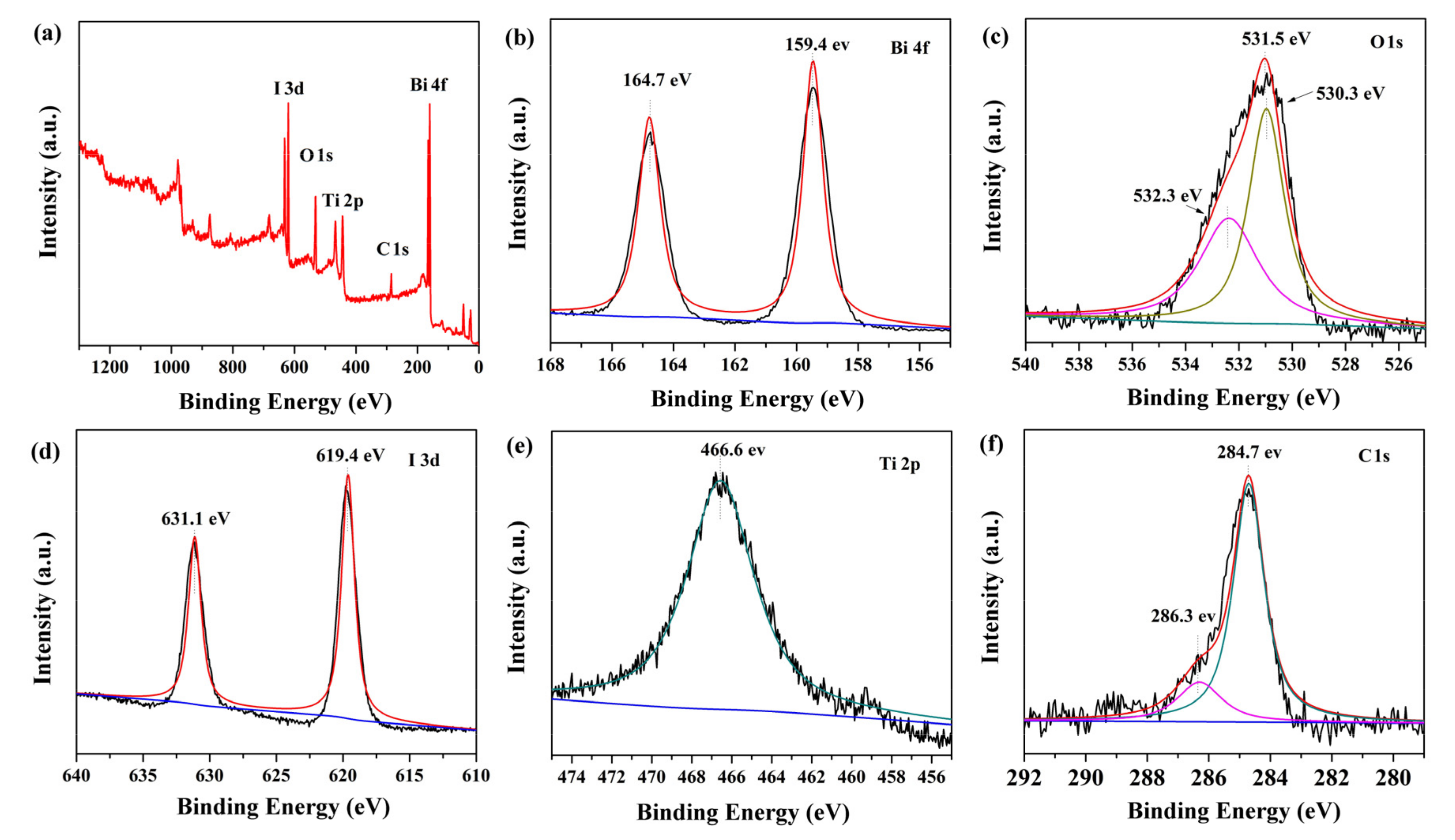
The surface chemical composition and elemental valence state of BiOI/NH2-MIL-125(Ti) were investigated by XPS measurement. The survey spectrum of BNMT-9 in Figure 2a confirmed the existence of Bi, O, I, Ti, and C in the BNMT-9 sample. Figure 2b presented Bi 4f spectrum whereby two peaks at binding energy of 164.70 eV and 159.40 eV were the split signals of Bi 4f5/2 and Bi 4f7/2, accordingly. The binding energy gap was 5.30 eV, which confirmed that Bi in the BNMT-9 was Bi3+ [24]. In Figure 2c, the O1s peak could be fitted into three peaks, which were bound up with the crystal lattice O atoms (532.3 eV), oxygen in Bi–O bond (531.5 eV), and oxygen in the titanium-oxo cluster (530.3 eV) [25]. Figure 2d showed the spectra of I 3d. The peaks located at 631.1 and 619.4 eV were assigned to I 3d3/2 and I 3d5/2 respectively. In Figure 2e, Ti 2p orbit binged to light the splitting peaks at 466.6 and 465.9 eV that lay in line with Ti 2p3/2 and Ti 2p1/2, accordingly, and this result showed the existence of Ti3+ in BNMT-9 [26]. The C 1s showed two splitting peaks at 286.3 and 284.7 eV (Figure 2f) corresponding to C=C and C–C, respectively. The above results showed that BiOI/NH2-MIL-125(Ti) was successfully prepared.
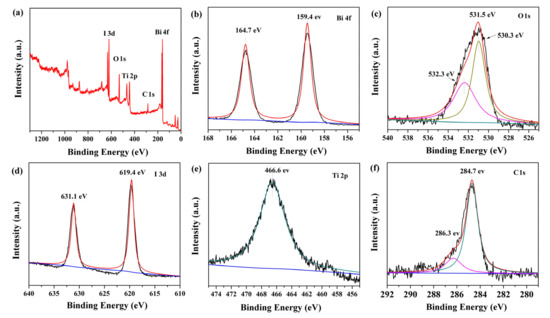
Figure 2.
The XPS spectra of BiOI/NH2-MIL-125(Ti) (BNMT)-9 sample: (a) survey scan, (b) Bi 4f spectra, (c) O 1s spectra, (d) I 3d spectra, (e) Ti 2p spectra, and (f) C 1s spectra.
2.1.3. Photocatalyst Morphologies
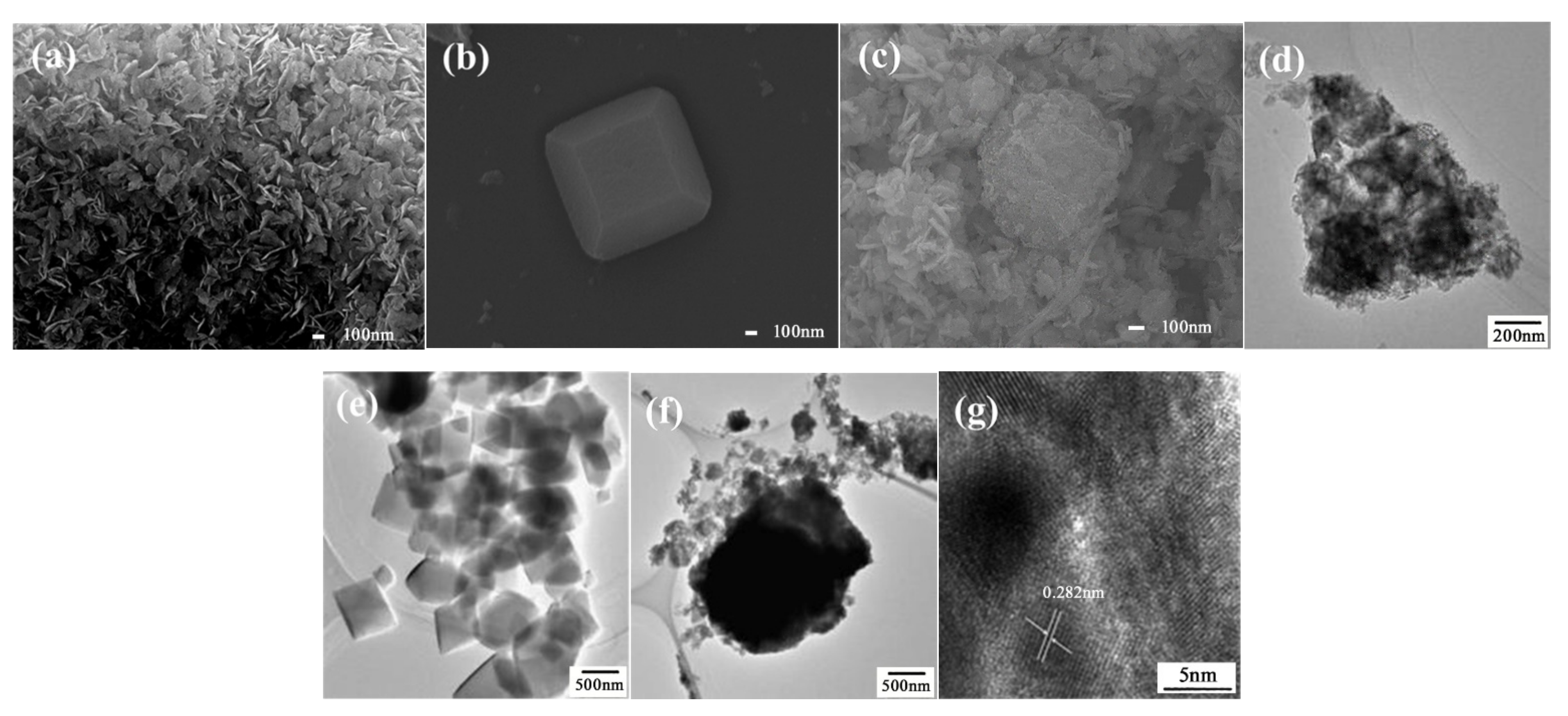
The scanning electron microscope (SEM) and transmission electron microscope (TEM) of as prepared BiOI, NH2-MIL-125(Ti), and BiOI/NH2-MIL-125(Ti) composites (take BNMT-9 as an example) are presented in Figure 3. The pure BiOI was flake-like with an average diameter of approximately 100 nm and an average thickness of about 12 nm as shown in Figure 3a,d. The SEM and TEM images of NH2-MIL-125(Ti) showed a cubic structure with a size of about 500 nm (Figure 3b,e). The SEM and TEM of BNMT-9 showed that the BiOI thin sheet wrapped on the surface of dish-like NH2-MIL-125(Ti) to form a composite photocatalyst (Figure 3c,f). At the same time, it can be found that the morphology of NH2-MIL-125(Ti) changed from a cubic structure to a dish-like shape during the synthesis of the composite photocatalyst. It may be due to the change of pH or pressure during the preparation of the composite catalysts, which was consistent with the conversion conditions between different morphologies of NH2-MIL-125(Ti) reported in previous literature [27,28]. Nevertheless, it can be seen from the results of XRD and XPS that the introduction of BiOI did not affect the crystal structure of NH2-MIL-125(Ti). In Figure 3g, BiOI in composites revealed the fringe spacing of 0.282 nm, which agreed well with (110) lattice planes in BiOI (JCPDS No. 10-0445). It could be concluded that the BiOI/NH2-MIL-125(Ti) composite photocatalyst was successfully engineered.
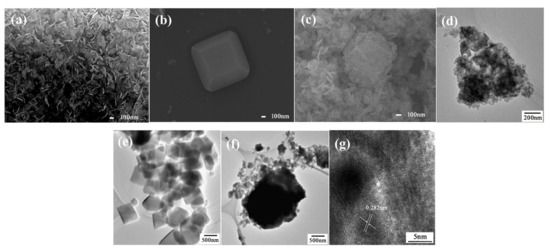
Figure 3.
SEM images of (a) BiOI, (b) NH2-MIL-125(Ti), and (c) BNMT-9. TEM images of (d) BiOI, (e) NH2-MIL-125(Ti), (f) BNMT-9, and (g) HRTEM of BNMT-9.
2.1.4. Surface Area and Pore Volume Characterization
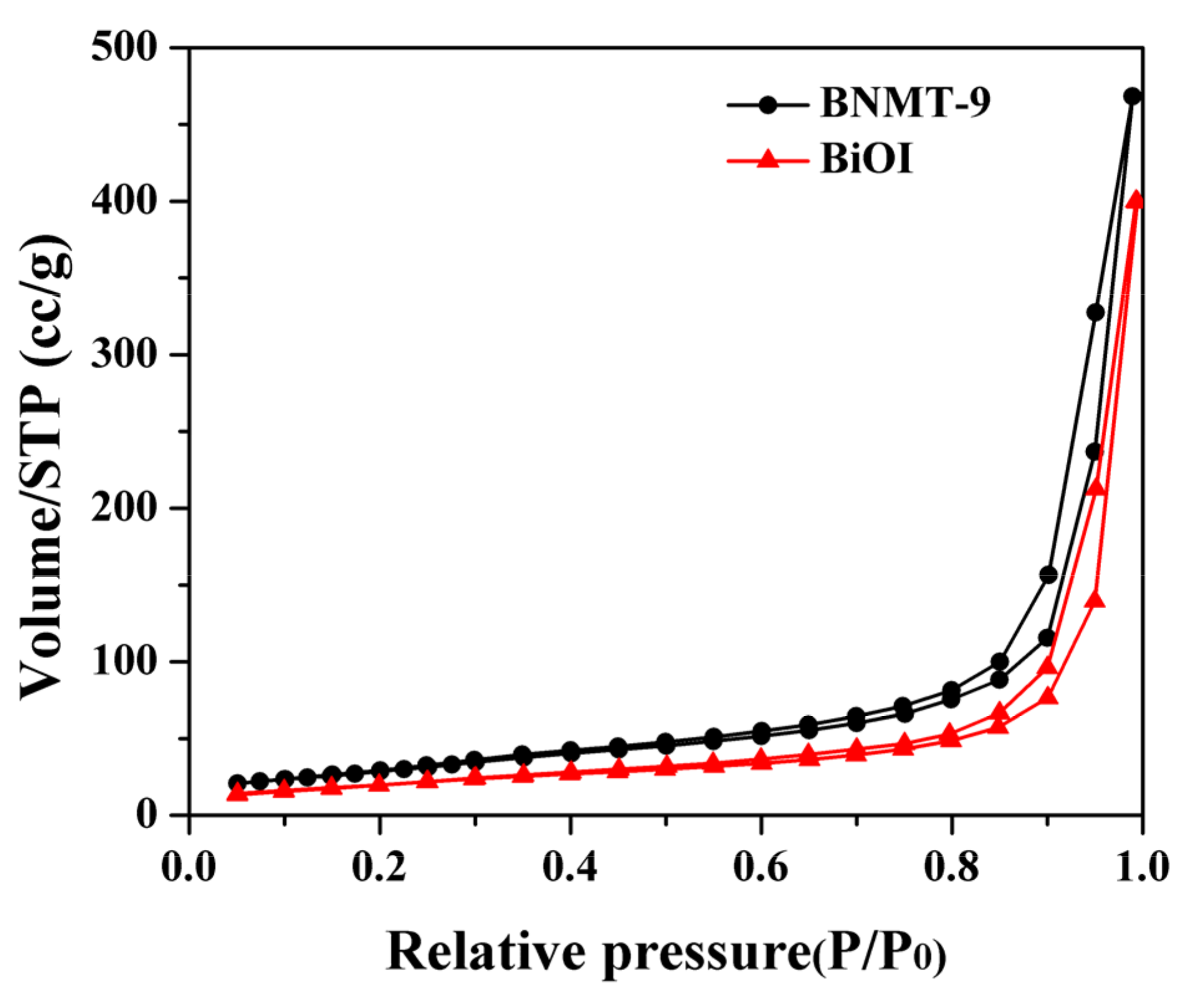
The materials surface area and pore volume were obtained by nitrogen sorption porosimetry, and the surface area was measured by the theory of Brunauer, Emmett, and Teller (BET). The results are shown in Figure 4 and Table 1. The specific surface area of BiOI and BNMT-9 were evaluated to be 75 and 112 m2/g, respectively, which indicated that the surface area of BiOI/NH2-MIL-125(Ti) could be increased through the modification of NH2-MIL-125(Ti) on BiOI. The results in Figure 4 and Table 1 showed that the BiOI and BiOI/NH2-MIL-125(Ti) samples were typical IV absorption curves with delayed H3 hysteresis loops between 0.7 and 1.0 relative pressure (P/P0), which means BiOI and BiOI/NH2-MIL-125(Ti) have mesoporous structures.
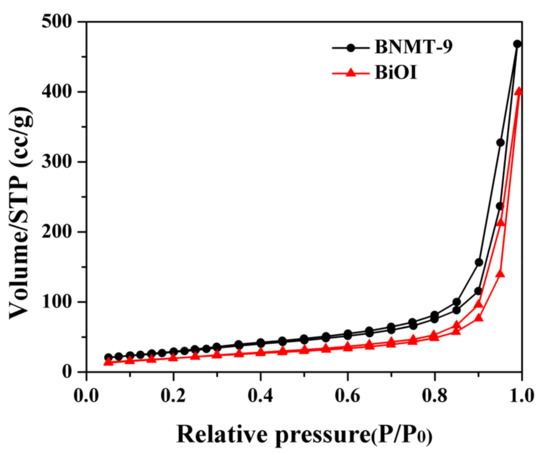
Figure 4.
N2 adsorption/desorption isotherms of BiOI and BNMT-9.

Table 1.
The Brunauer, Emmett, and Teller (BET) specific surface area and pore volume of BiOI and BNMT-9.
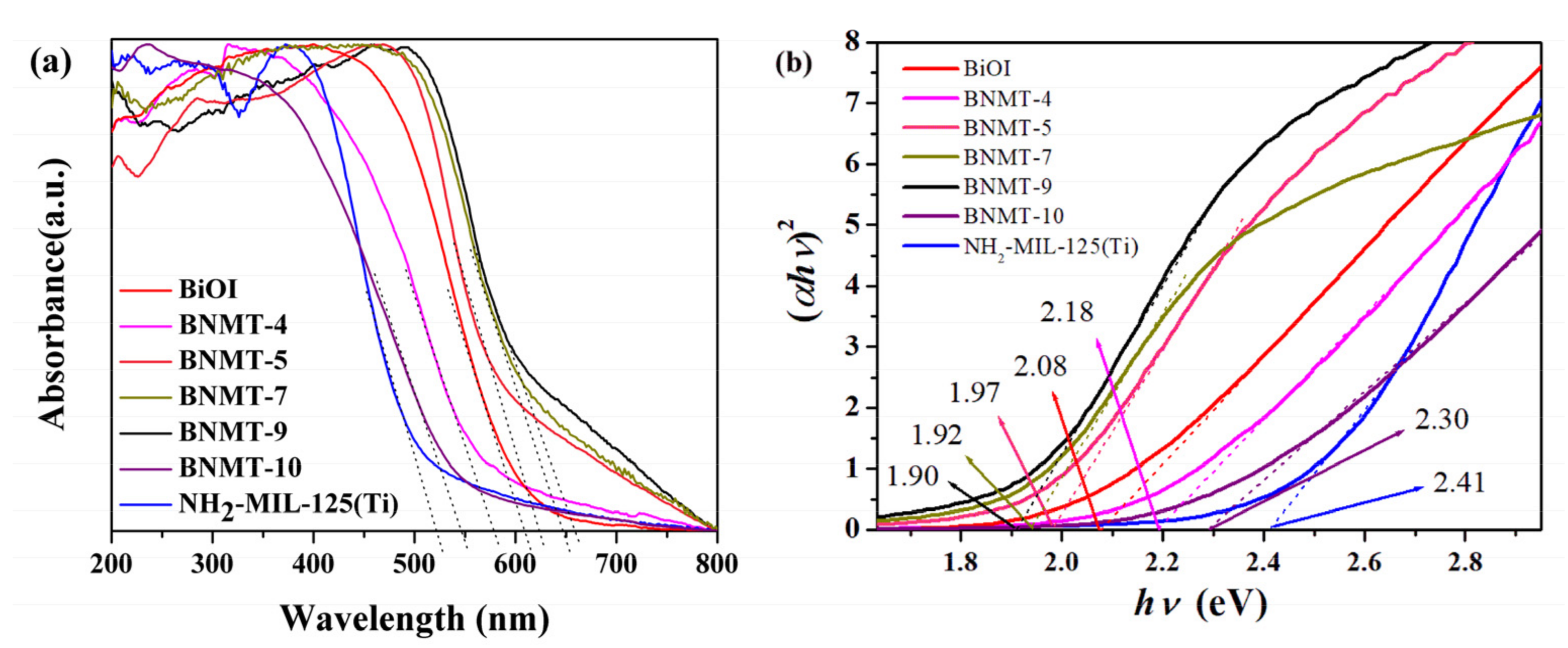
2.1.5. UV–Visible Absorption Spectra and Band Gap
The optical properties of the as-synthesized BiOI, NH2-MIL-125(Ti) and BiOI/NH2-MIL-125(Ti) composites were investigated by UV–visible diffuse reflectance spectroscopy (UV–vis). Figure 5a showed that the visible light absorption edges of all samples were in the visible range. The NH2-MIL-125(Ti) had visible light response due to the introduction of amino groups [29]. The absorption edge was about 520 nm, and the band gap value was 2.41 eV. The semiconductor BiOI had a strong visible light response with an absorption edge of about 610 nm and a band gap value of 2.08 eV. With the increase of NH2-MIL-125(Ti) recombination, the visible light response range of the composites increased first and then decreased, in which the BNMT-9 absorption range was the largest, and its absorption edge was about 660 nm. The band gaps (Eg) can be evaluated from Tauc’s plots. It could be found from Figure 5b that the band gap energy (Eg) of different samples was measured by extrapolation of the linear part of the curves calculated by plotting (αhν)2 versus hν. Accordingly, the Eg of BiOI, NH2-MIL-125(Ti), BNMT-4, BNMT-5, BNMT-7, BNMT-9, and BNMT-10 were estimated to be 2.08, 2.41, 2.18, 1.97, 1.92, 1.90, and 2.30 eV, respectively. The response range of BNMT-4 and BNMT-10 were smaller than that of BiOI but a little higher than that of BiOI/NH2-MIL-125(Ti). That meant the introduction of NH2-MIL-125(Ti) can expand the visible light response range of BiOI/NH2-MIL-125(Ti) and improve the visible light utilization ratio.
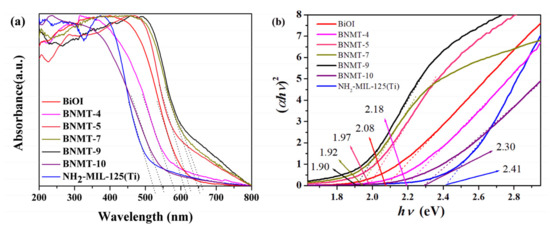
Figure 5.
(a) Solid UV–vis diffuse reflectance spectra and (b) plots of (αhv)2 versus (hv) of BiOI, NH2-MIL-125(Ti), and BiOI/NH2-MIL-125(Ti) complexes in different proportions.
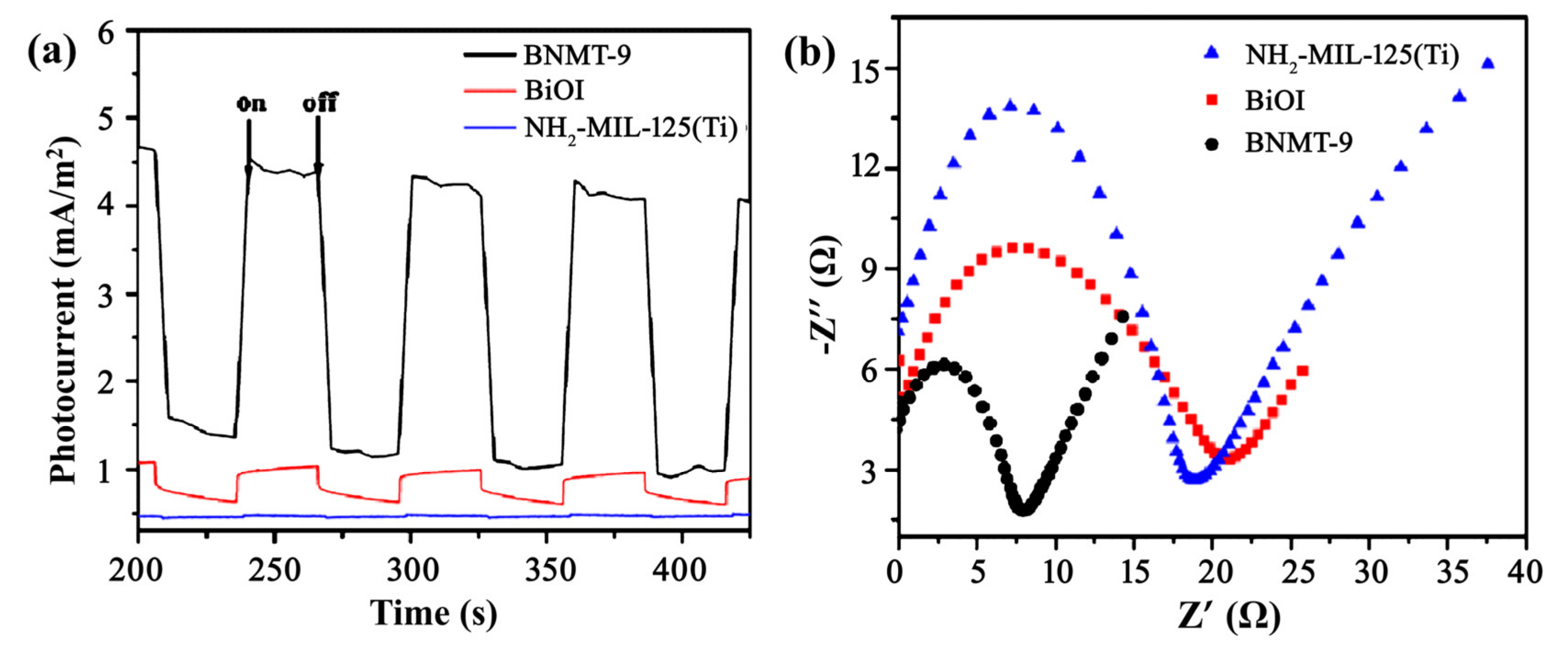
2.1.6. Electrochemical Analysis
The separation and recombination rates of photo-generated carriers in the photocatalyst were investigated by photocurrent response and impedance measurement. Figure 6a exhibited the photocurrents measured for BNMT-9, BiOI, and NH2-MIL-125(Ti). Apparent observation demonstrated that the photocurrent intensities were in the order of NH2-MIL-125(Ti) < BiOI < BNMT-9. The photocurrent intensity of the BNMT-9 was nearly 4 times as high as that of pure BiOI. This indicated that the more effective separation of photo-induced electrons and holes, the faster interfacial charge transfer occurs in the heterostructure. It can be seen from the impedance diagram (Figure 6b) that the arc radius of BNMT-9 was the smallest; thus, the photocatalytic activity of BNMT-9 is suggested to be high. According to the UV–vis diffuse reflectance spectra and photo current analysis, it was revealed that the BNMT-9 had higher photo-generated electron-hole separation efficiency and lower carrier recombination efficiency than BiOI and NH2-MIL-125(Ti). The results demonstrated that the optical property was a reason to enhance the photocatalytic performance.
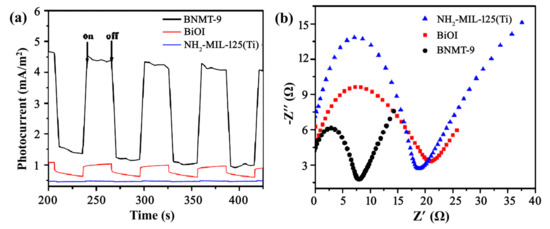
Figure 6.
(a) Photocurrent responses of BNMT-9, BiOI, and NH2-MIL-125(Ti), and (b) Impedance diagrams of BNMT-9, BiOI, and NH2-MIL-125(Ti).
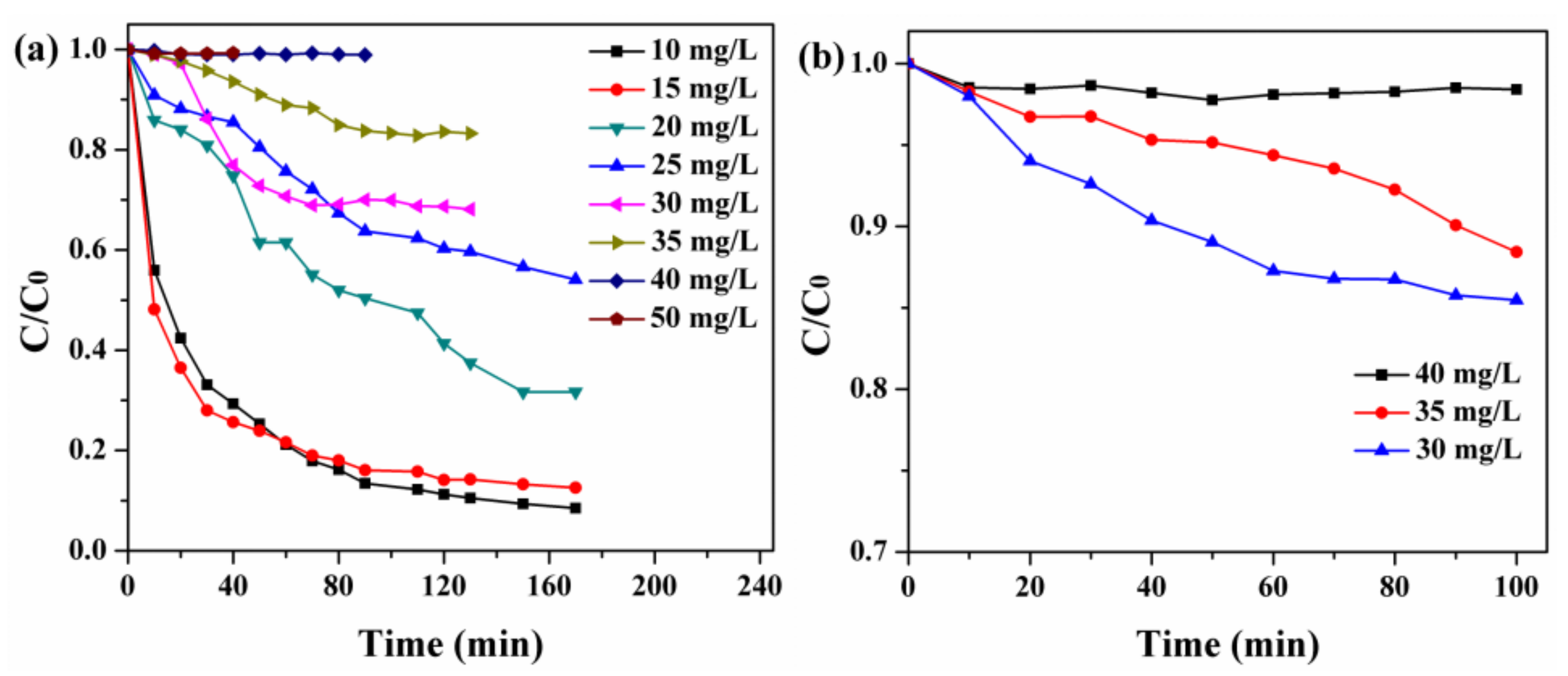
2.1.7. Analysis of Adsorption Properties
To better study the photocatalytic activity of composites, the experiments on the adsorption of RhB with varying concentrations by monomer BiOI and NH2-MIL-125(Ti) were tested to find suitable degradation concentration. The results of Figure 7a,b showed that the quick adsorption equilibriums were obtained when the initial concentration of t RhB was 40 mg/L and the amount of BiOI or NH2-MIL-125(Ti) was 40 mg.
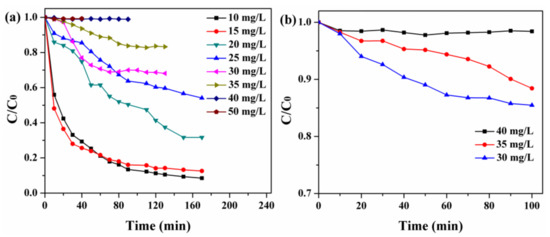
Figure 7.
(a) Adsorption of Rhodamine B (RhB) with varying concentrations over BiOI (40 mg) and (b) NH2-MIL-125(Ti) (40 mg) in 100 mL solution at room temperature.
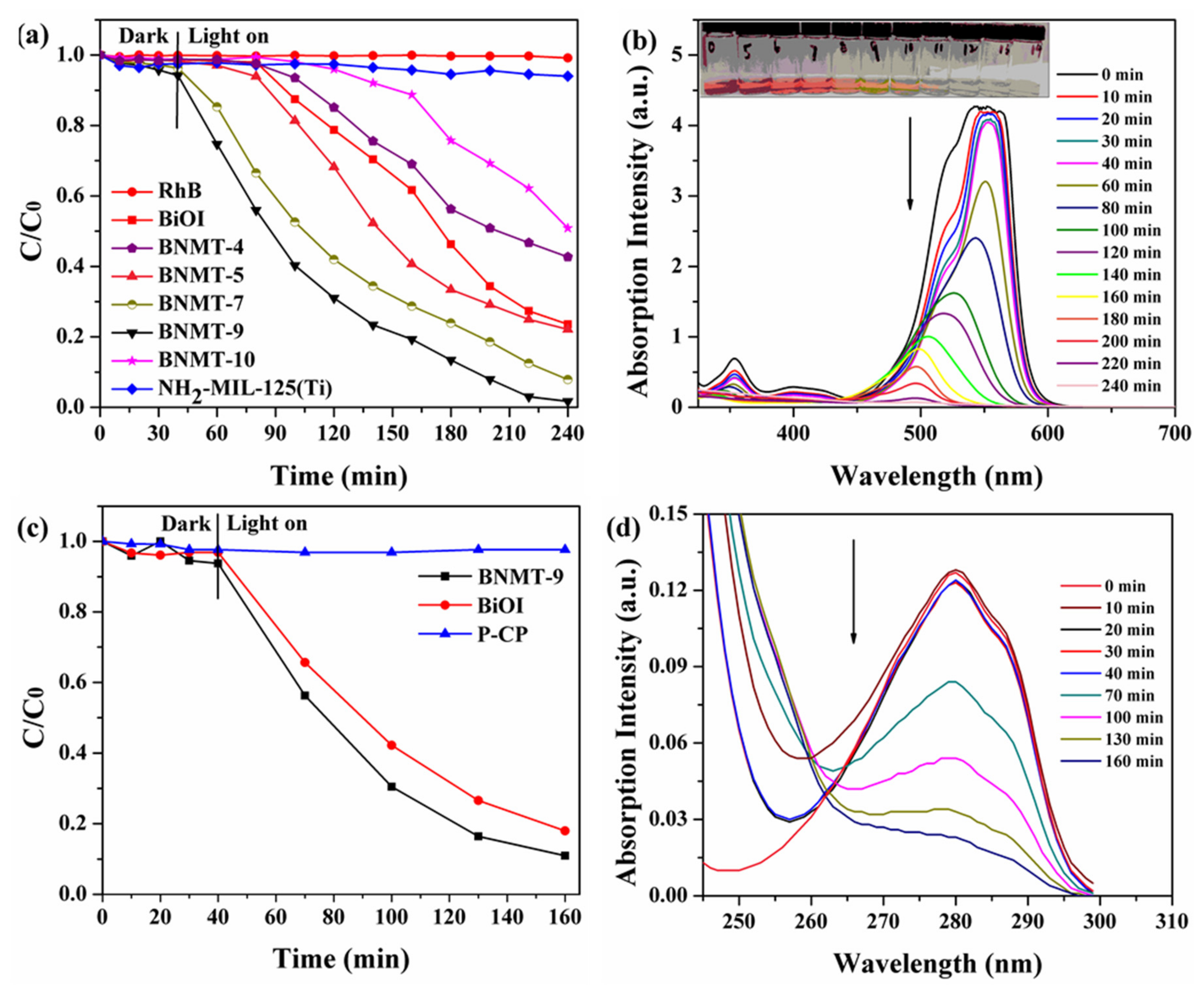
2.1.8. Photocatalytic Performance
RhB and p-chlorophenol (P-CP) were used as model organic pollutants for the purpose of demonstrating the visible light photoactivity of the as-obtained BiOI/NH2-MIL-125(Ti) composites in respect of the degradation of organic pollutants. It can be seen from Figure 8a that the catalyst reached the adsorption–desorption equilibrium after 40 min. No self-degradation of RhB occurred within 240 min irradiation (red dot line). The degradation percentage of NH2-MIL-125(Ti) was 5%, and that of BiOI was 73%. The order of degradation performance of series catalysts was RhB < NH2-MIL-125(Ti) < BNMT-10 < BNMT-4 < BiOI < BNMT-5 < BNMT-7 < BNMT-9. With the increase of NH2-MIL-125(Ti) complex amount, the degradation percentage of the composites increased first and then decreased, among which BNMT-9 exhibited the best degradation percentage, and the removal percentage was 99%. This was due to the introduction of the proper amount of NH2-MIL-125(Ti), which increased the specific surface area, created more reactive active sites, and was beneficial to the separation of photo-generated carriers and the utilization of visible light. However, when excessive NH2-MIL-125(Ti) was introduced, the proportion of active sites reached saturation, and the photocatalytic performance was no longer improved; meanwhile, the catalyst was easy to agglomerate, which was not conducive to the absorption of visible light. Furthermore, excessive NH2-MIL-125(Ti) may become the recombination center of photo-generated electron–hole pairs and reduce the activity of photocatalyst.
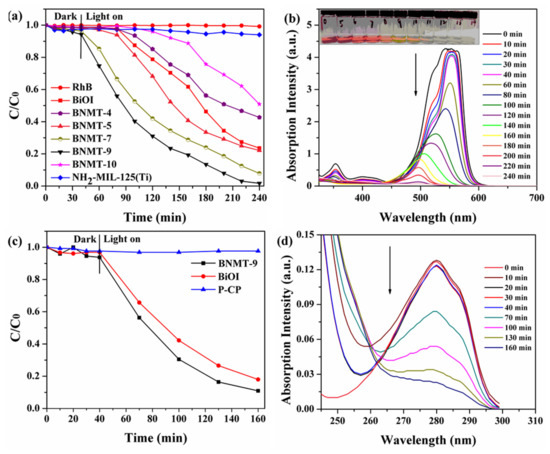
Figure 8.
Photocatalytic degradation efficiencies of (a) RhB with initial concentration of 40 mg/L and (c) P-CP with an initial concentration of 10 mg/L over different catalysts under visible-light irradiation, and time-dependent UV–vis absorption total spectra of BNMT-9 degradation (b) RhB and (d) P-CP.
UV–vis absorption total spectrometry of BNMT-9 degradation RhB was shown in Figure 8b. The absorption value decreased as the catalytic time increased, and the blue shift occurred, which may be due to the formation of intermediate produced during the RhB degradation process [30]. The absorption peak disappeared at 200 min after turning on the light, which indicated that RhB could be efficiently degraded by BNMT-9. With the increase of lighting time, RhB became lighter from the initial pink to colorless during the degradation process, which directly proved that BNMT-9 can almost completely degrade RhB.
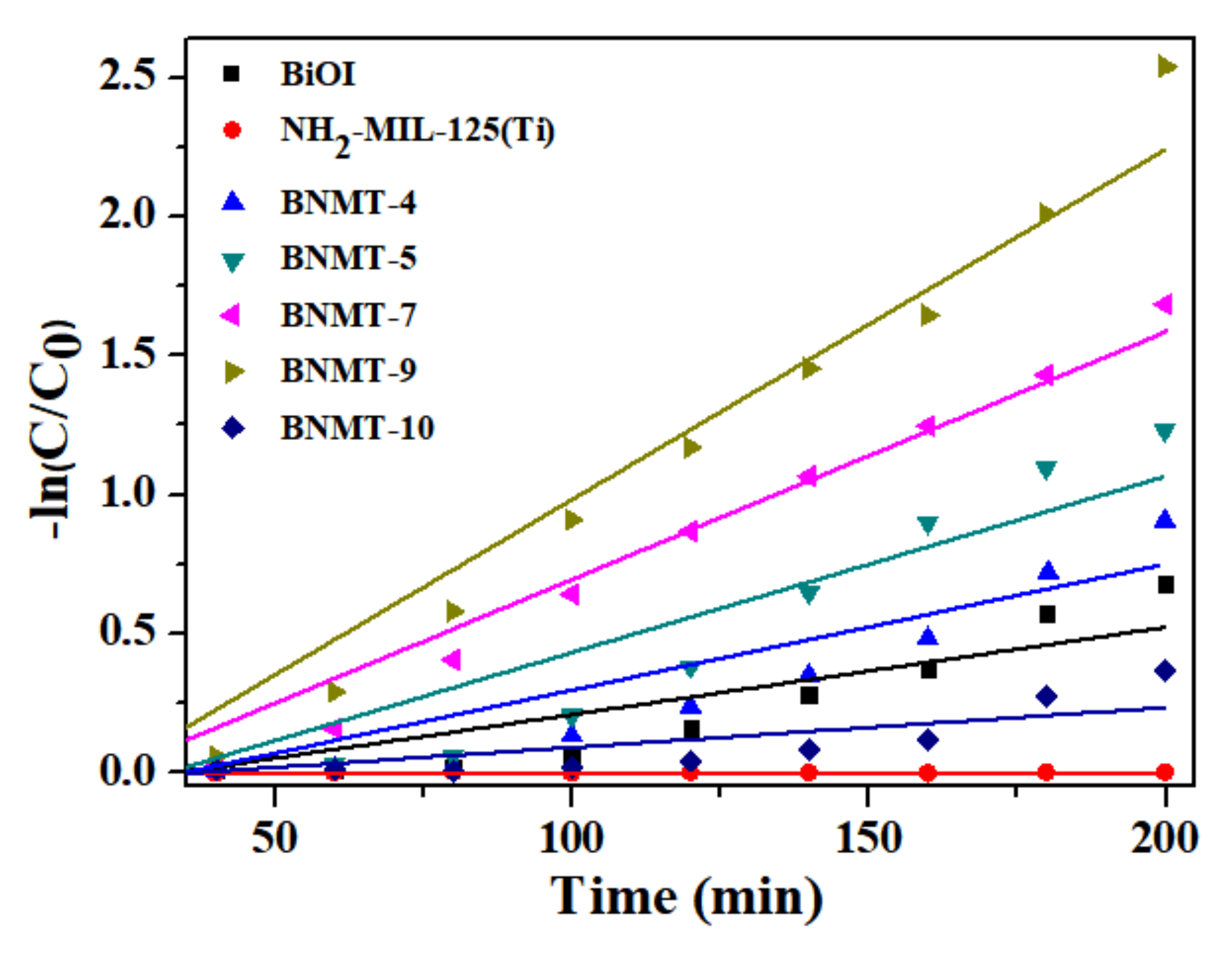
Colorless P-CP degradation experiments were carried out under visible light to avoid dye sensitization. From Figure 8c, no self-degradation of P-CP occurred within 160 min irradiation (blue triangle line). It can be clearly seen that the degradation percentage of the composites was 90% after the opening of the lamp 160 min, which was greater than BiOI. The UV–vis absorption total spectrometry of the BNMT-9 degradation of P-CP is shown in Figure 8d. The maximum absorption peak of P-CP was at 280 nm, and there is almost no concentration change within 40 min during the process of dark reaction. After turning on the lamp, the absorption peak decreased with the increase of time, and the peak became flat after 160 min. These results confirmed that the photocatalytic degradation performance of composite photocatalyst was excellent. Additionally, the reaction kinetics of RhB degradation can be further understood from the results of Figure 9. The rate constant of BNMT-9 was 0.0126 min−1, which was nearly 4.06 times that of pure BiOI (0.0031 min−1).
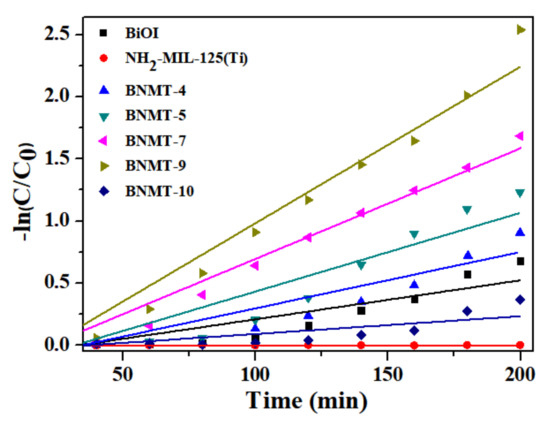
Figure 9.
The pseudo-first-order reaction kinetics of the as-prepared samples of the degradation of RhB.
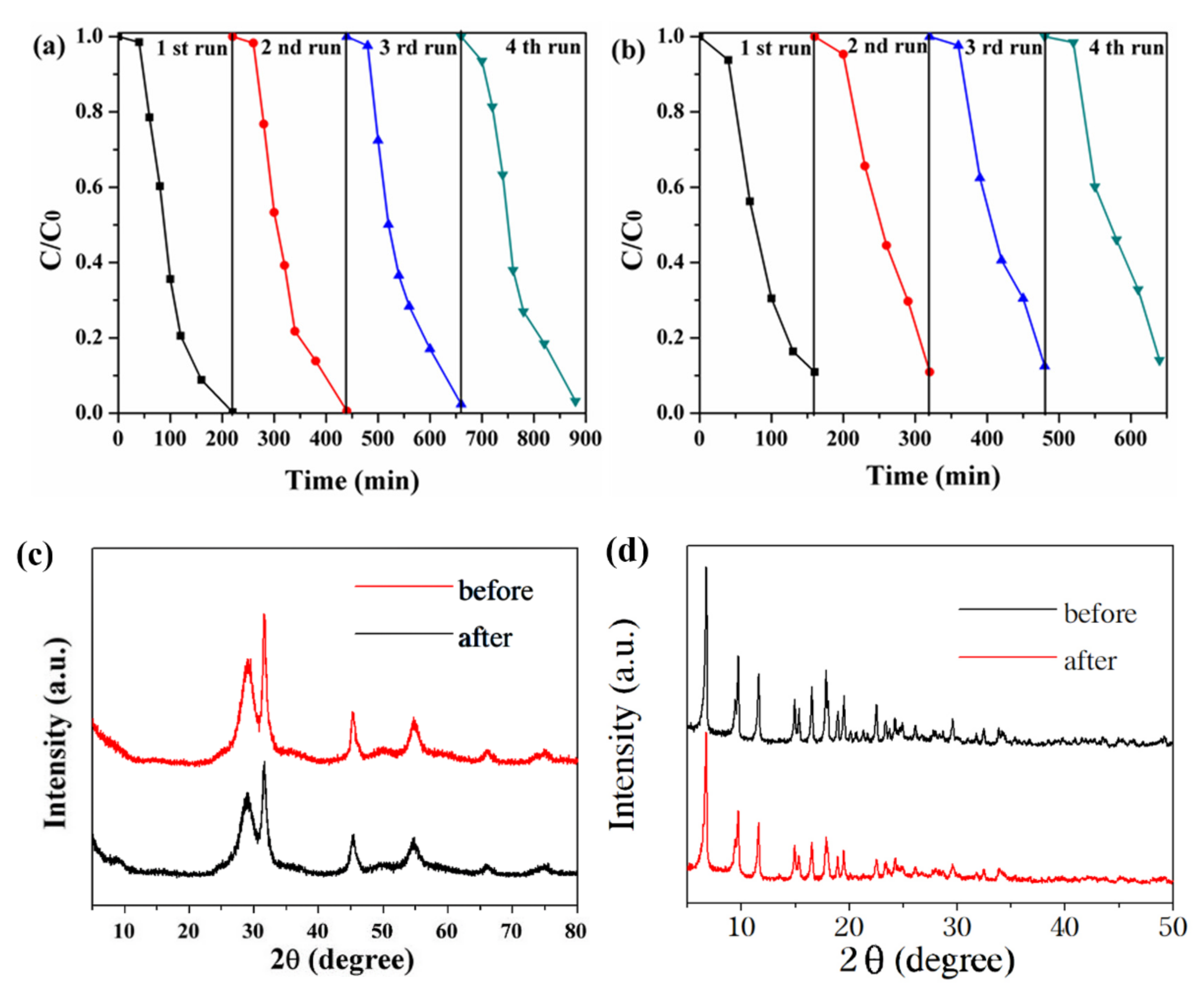
Regeneration, stability, and reusability were significant factors for the practical application of photocatalysts to dye degradation. As shown in Figure 10a, the photocatalyst exhibited excellent reusability for up to four consecutive cycles in the RhB degradation experiment, because there was little change in catalyst performance. As shown in Figure 10b, the photocatalyst was found to be reusable, and there was a slight decrease in catalytic performance after the fourth run in the P-CP degradation experiment, but the degradation percentage remained above 87%. Furthermore, the structural stability of the recycled photocatalyst was confirmed by XRD analysis, which showed no structural changes after recycling (Figure 10c,d). Hence, it was proved that the BNMT-9 composite photocatalyst had excellent stability.
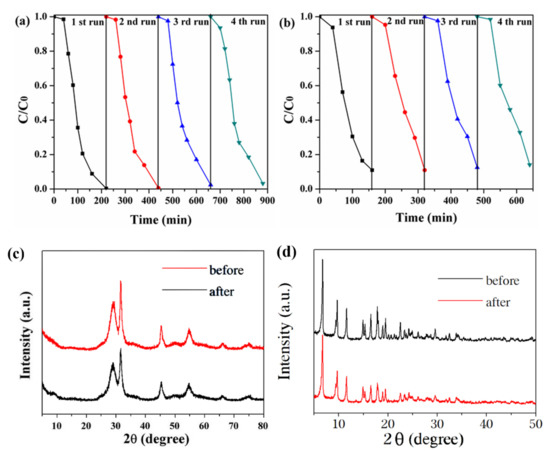
Figure 10.
Reusability of BNMT-9 for the photocatalytic degradation of (a) RhB and (b) P-CP, and (c) XRD diagram of BNMT-9 and (d) NH2-MIL-125(Ti) before and after the cyclic degradation of RhB.
2.2. Proposed Mechanism for the BiOI/NH2-MIL-125(Ti) Composite
2.2.1. Active Species Trapping Experiments
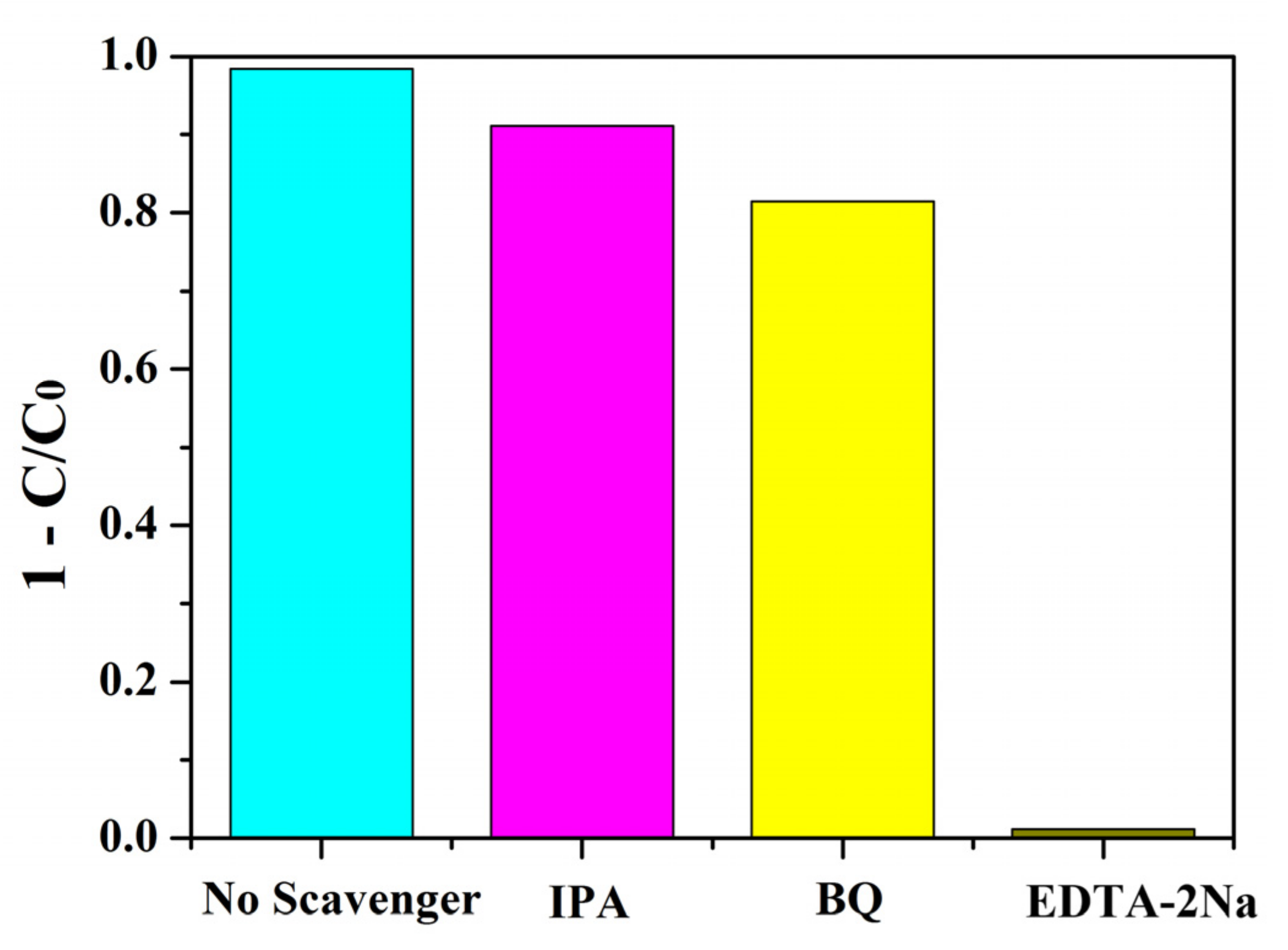
During photocatalytic reactions, the purpose of degradation and mineralization of pollutants was achieved by the transfer and transition of free radicals such as holes (h+), ·O2−, and ·OH. The addition of the corresponding radical scavengers in the trapping experiments of active species can prevent the photocatalytic reaction and judge the active species by the change of catalytic efficiency. To explore the main active species in the photocatalysis, a trapping experiment was carried out using BNMT-9 as catalyst under visible light. Ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), p-benzoquinone (BQ), and iso-propyl alcohol (IPA) were introduced in degradation reaction solution separately to trap h+, ·O2−, and ·OH, accordingly [30,31]. As depicted in Figure 11, it can be observed that the degradation of RhB was hardly affected by IPA and BQ, whereas the photocatalytic decomposition of RhB declined significantly with the addition of EDTA-2Na. The results showed that ·OH, ·O2−, and h+ were all active species, among which h+ was the main active species, which played an important role in the BNMT-9 degradation of RhB.
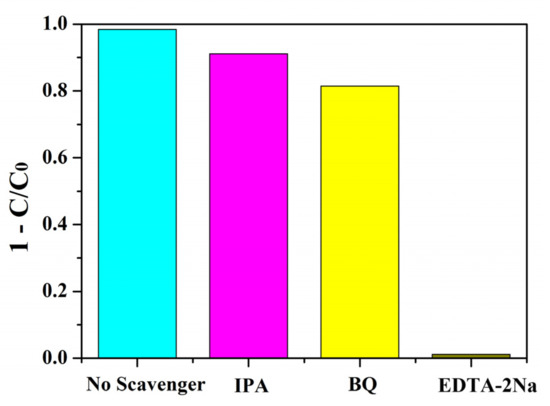
Figure 11.
Trapping experiment of active species on the degradation of RhB in the presence of BNMT-9 under visible-light irradiation.
2.2.2. Proposed Mechanism for the BiOI/NH2-MIL-125(Ti) Composite
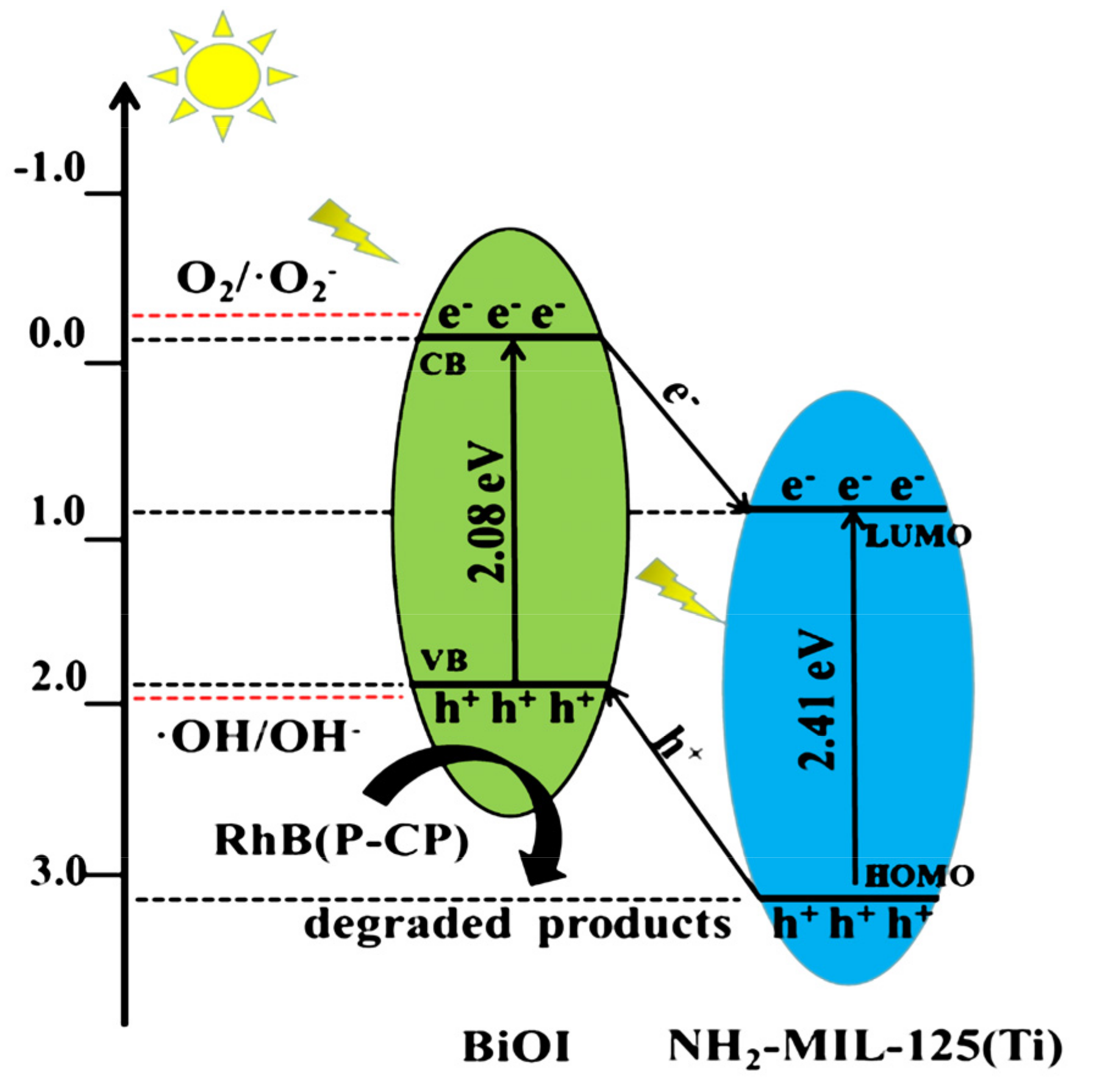
The mechanism of photocatalytic degradation of RhB and P-CP of BiOI/NH2-MIL-125(Ti) was speculated based on the experimental results of the active species trapping test. The energy band structures can be calculated by the equations [31,32,33]:
where X is the electronegativity of semiconductors, EVB stands for the valence band edge potential, ECB is the conductive band edge potential, Ee is approximately 4.5 eV, and Eg is the band gap. According to the analyses of UV–vis DRS, the Eg values of BiOI and NH2-MIL-125(Ti) were 2.08 and 2.41 eV, respectively. Based on the above formula, the ECB and EVB of BiOI were calculated to be −0.12 and 1.96 eV, and the LUMO and HOMO orbits of NH2-MIL-125(Ti) were 0.76 and 3.17 eV. Based on the above-experimental findings and previous reports, the possible mechanism in the BiOI/NH2-MIL-125(Ti) composite was depicted in Figure 12 [34,35,36]. The electrons of the BiOI transition to the LUMO orbital of NH2-MIL-125(Ti) under visible light excitation and the holes on NH2-MIL-125(Ti) transferred to the valence band of the BiOI. This process effectively inhibited the recombination of photo-generated electron–hole pairs. The conduction band potential of BiOI (−0.12 eV vs. NHE) was more positive than that of O2/·O2− (−0.33 eV vs. NHE), so the electrons in the BiOI band cannot react with O2 to produce ·O2−; at the same time, the valence band potential of BiOI (1.96 eV) was more negative than that of OH/OH− (1.99 eV vs. NHE). Therefore, h+ cannot oxidize the OH to form ·OH in water, while the h+ in NH2-MIL-125(Ti) was rapidly consumed when h+ recombines with e− in BiOI and NH2-MIL-125(Ti), so the active species that played a major role was the h+ on BiOI. Holes (h+) oxidized RhB and P-CP to decompose them into CO2, H2O, and inorganic acids, which was consistent with the results of trapping experiments and related literature [37].
EVB = X − Ee + 0.5Eg
ECB = EVB − Eg
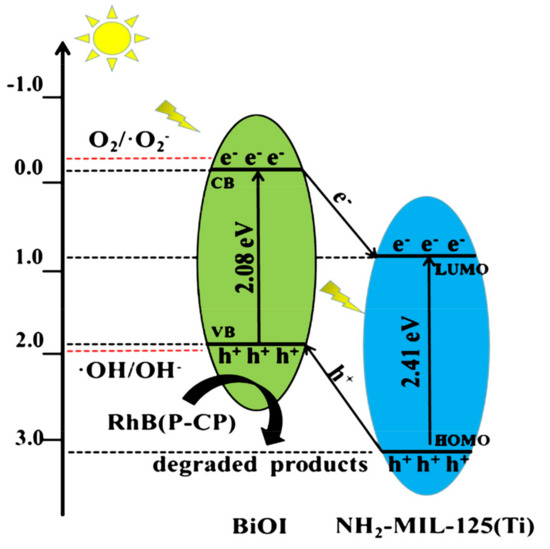
Figure 12.
Suggested mechanism for the photocatalytic degradation of RhB and P-CP by BiOI/NH2-MIL-125(Ti) under visible light.
3. Materials and Methods
3.1. Reagents and Apparatus
N, N-dimethylformamide (DMF), CH3OH, C2H5OH, and OHC2H4OH were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd., China. NH4I, aminoterephthalic acid, tetrabutyl titanate (TBT), p-benzoquinone, isopropanol, ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), Rhodamine B (RhB), and p-chlorophenol (P-CP) were purchased from Aladdin Chemical Reagents, Ltd., China. All the chemicals and reagents were of analytical grade and used as without further purification.
The crystalline structures of the materials were analyzed by powder X-ray diffraction (PXRD) (ThermoFisher, EQ (100)) with CuKa radiation (λ = 1.5406 Å) at room temperature in the range of 5–80°. X-ray photoelectron spectroscopy (XPS) measurements were characterized on a Thermo Fisher ESCALAB250XI instrument. The apparent morphology of the as-prepared products was studied by JEOL JEM-7800F scanning electron micrographs (SEM) and JEOL JEM-2100F transmission electron microscope (TEM). N2 adsorption–desorption was characterized by automatic gas adsorption instrument using Conta Autosorb-iQ analyzer. UV–vis diffuse reflectance spectra were recorded by a Shimadzu UV-2550 spectrometer. The photocatalytic activities were studied by Pofill PCX50C Discover multi-channel (automatic temperature control) photocatalytic reaction system. Photocurrents and electrochemical impedance spectroscopy (EIS) were recorded using a PGSTAT-302 N electrochemical workstation. The photocatalytic performance of the samples was tested in a reactor (GHX-3, Yangzhou University, China).
3.2. Preparation of NH2-MIL-125(Ti)
NH2-MIL-125(Ti) was prepared according to reference [22]. Specific methods were as follows. Briefly, a mixture of TBT (2.4 mL), amino terephthalic acid (2.2 g), DMF (36 mL), and methanol (4.0 mL) was subjected to solvothermal conditions in a Teflon-lined stainless-steel autoclave for 48 h at 150 °C. After reaction, the resultant precipitate was separated by centrifugation and washed 4 times with methanol to remove the residual DMF. At last, the obtained powder sample was dried at 180 °C under vacuum for two hours to remove the residual amino terephthalic acid.
3.3. Preparation of BiOI/NH2-MIL-125(Ti) (BNMT) Composite
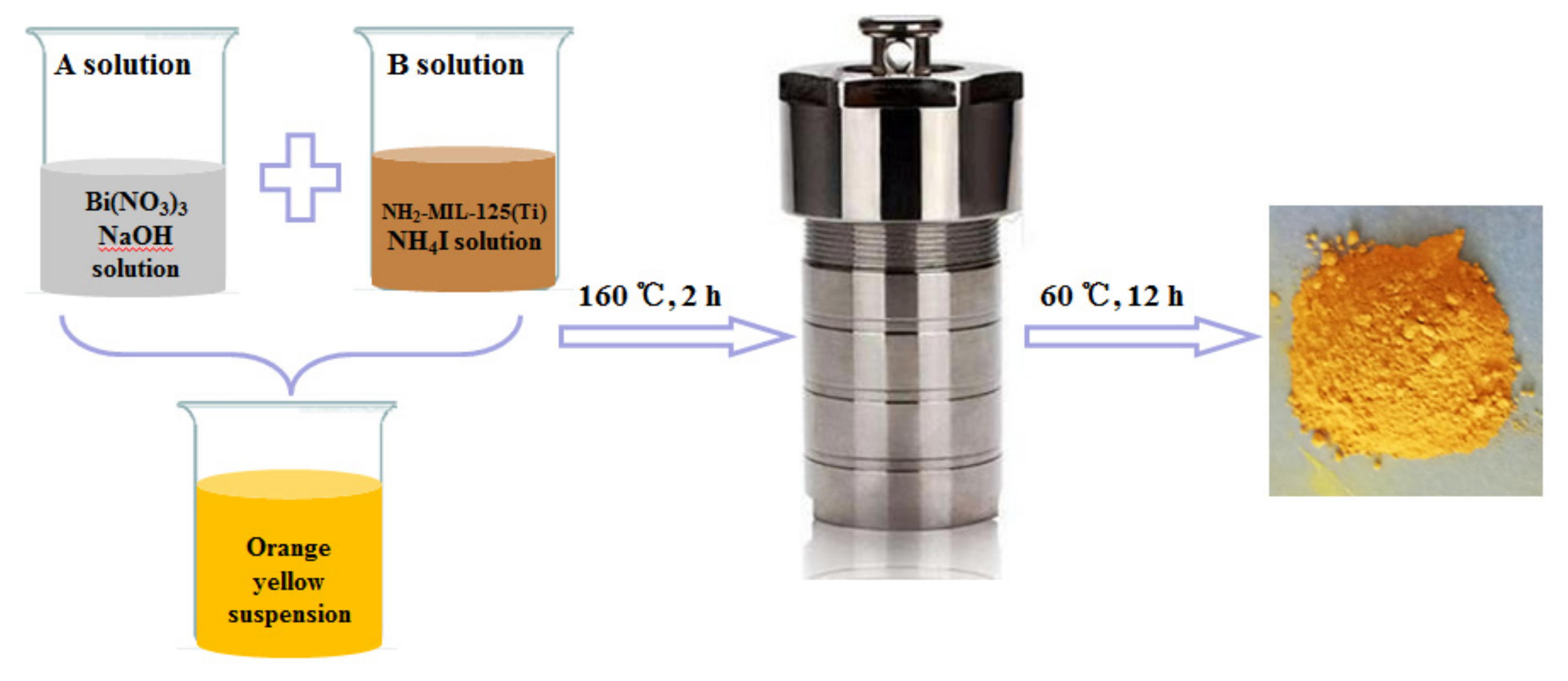
For the BNMT system, the contents of NH2-MIL-125(Ti) in BNMT-4, BNMT-5, BNMT-7, BNMT-9 and BNMT-10 were 4 wt %, 5 wt %, 7 wt %, 9 wt % and 10 wt %, respectively (Table 2). The photocatalyst was prepared by adding a certain amount of the NH2-MIL-125(Ti) to the BiOI precursor solution, as shown in Figure 13. Solution A was a white suspension obtained from 20 mL ethylene glycol solution with 4.85 g Bi(NO3)3·5H2O and 11 mL 2 mol/L NaOH. Solution B was 30 mL ethylene glycol solution with 1.44 g NH4I and 0.44 g NH2-MIL-125(Ti) solution. After vigorous magnetic stirring for 20 min at room temperature, the mixture of the two solutions was transferred into a teflon lined stainless steel autoclave (25 mL capacity) and heated at 160 °C for 2 h, and then, it was cooled to room temperature. A yellow powder was obtained after centrifugation. The solid was washed 3 times with distilled water and 3 times with methanol. Then, it was transferred to a vacuum oven and dried for 12 h at 60 °C. BiOI was synthesized under the same conditions without NH2-MIL-125(Ti) [38].

Table 2.
The list of the samples to be studied.
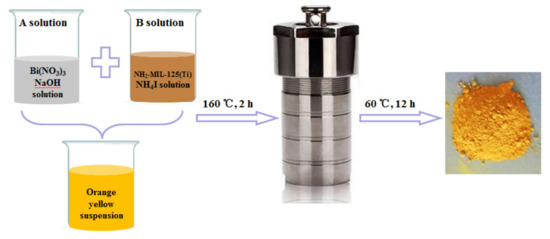
Figure 13.
The synthetic route of BiOI/NH2-MIL-125(Ti).
3.4. Photocatalytic Activity Evaluation
The photocatalytic activity of photocatalyst BiOI/NH2-MIL-125(Ti) was studied by degrading Rhodamine B (RhB) and p-chlorophenol (P-CP) under visible light illumination. Before irradiation, the 100 mL of RhB (40 mg/L) or P-CP (10 mg/L) aqueous solution was transferred to a quartz reactor, and then, 40 mg of photocatalyst was added. The mixture was stirred magnetically for 40 min in the dark to get complete adsorption equilibrium between the photocatalyst and RhB or P-CP. Then, the suspension was exposed to visible-light irradiation with a 300 W xenon lamp under magnetic stirring. The 420 nm cutoff filter was placed between the lamp and the sample to filter out ultraviolet light (λ < 420 nm). Then, 3 mL of suspension was collected at regular intervals for centrifugation. The supernatant was tested by UV–vis spectrophotometer. According to the formula A/A0 = C/C0, the change of RhB or P-CP concentration in the degradation process was determined by the change of their absorbance.
3.5. Active Species Capture Experiments
To explore the photocatalytic mechanism of the photocatalyst, radicals trapping experiments were further conducted to estimate the reactive active species generated during the irradiation of the BNMT-9 sample on the basis of degradation of RhB. During the experiments, isopropyl alcohol (IPA), benzoquinone (BQ), or ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) was added to the RhB solution to obtain concentrations of 1 mM, and all other conditions were remained the same as those used in the above-mentioned degradation of RhB experiment. IPA, BQ, and EDTA-2Na were introduced as scavengers for hydroxyl radicals (·OH), superoxide radicals (·O2−), and holes (h+), respectively.
4. Conclusions
A BiOI/NH2-MIL-125(Ti) composite photocatalyst was prepared by the solvothermal method. Series characterization and photoelectrical analysis showed that the composite photocatalysts exhibited a larger specific surface area, higher visible light utilization, and more efficient separation rate of photogenerated electrons and holes compared with BiOI and NH2-MIL-125(Ti), respectively. The content of NH2-MIL-125(Ti) had a significant effect on the photocatalytic performance of composite photocatalysts, in which the BNMT-9 exhibited the best photocatalytic activity, and the degradation percentages of RhB and P-CP reached 99% and 90%, respectively. The results of photocatalyst reusability experiments showed that the BNMT-9 composite catalyst had excellent stability. This study will provide a new scheme for the development of BiOI/MOFs composites and the degradation of chlorophenol (P-CP) and Rhodamine B (RhB).
Author Contributions
The experimental work was designed and supported by J.D., C.L., and G.C.; the catalysts were prepared by J.Z., T.Z. and R.L.; formal analysis, T.Y. and Z.L.; data curation, J.D.; writing—review and editing, D.W.; project administration, J.D. and Y.L.; funding acquisition, Y.L. and G.C. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21703078), the Project of Education Department of Jilin Province (JJKH20191015KJ), the Project of Jilin Province Development and Reform Commission (2019C044-2) and Natural Science Foundation Project of Jilin Province (20180623042TC, 20180101181JC).
Institutional Review Board Statement
Not applicable for studies over humans or animals.
Informed Consent Statement
Not applicable for studies over humans.
Data Availability Statement
Data available in a publicly accessible repository. The data presented in this study are openly available in [MDPI] at [doi:10.3390/catal11010024], reference number [38].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.; Ai, Z.H.; Zhang, L.Z. Surface structure-dependent photocatalytic O2 activation for pollutant removal with bismuth oxyhalides. Chem. Commun. 2020. [Google Scholar] [CrossRef]
- Taylor, D.; Dalgarno, S.J.; Xu, Z.T.; Vilela, F. Conjugated porous polymers: Incredibly versatile materials with far-reaching applications. Chem. Soc. Rev. 2020, 49, 3981–4042. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Kalathil, S.; Reisner, E. Semi-biological approaches to solar-to-chemical conversion. Chem. Soc. Rev. 2020, 49, 4926–4952. [Google Scholar] [CrossRef] [PubMed]
- Janicki, T.; Krupiński, M.; Długoński, J. Degradation and toxicity reduction of the endocrine disruptors nonylphenol, 4-tert-octylphenol and 4-cumylphenol by the non-ligninolytic fungus Umbelopsis isabelline. Bioresour. Technol. 2016, 200, 223–229. [Google Scholar] [CrossRef]
- Lei, Y.Q.; Wang, G.H.; Song, S.Y.; Fan, W.Q.; Pang, M.; Tang, J.K.; Zhang, H.J. Room temperature, template-free synthesis of BiOI hierarchical structures: Visible-light photocatalytic and electrochemical hydrogen storage properties. Dalton Trans. 2010, 39, 3273–3278. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Salama, W.H.; El-Badry, M.O.; Salah, H.A.; Barakat, A.Z.; Fahmy, A.S.; Mohamed, S.A. Purification and characterization of peroxidases from garden cress sprouts and their roles in lignification and removal of phenol and p-chlorophenol. J. Food Biochem. 2020. [Google Scholar] [CrossRef]
- Dong, H.J.; Zhang, X.X.; Li, J.M.; Zhou, P.J.; Yu, S.Y.; Song, N.; Liu, C.B.; Che, G.B.; Liu, C.M. Construction of morphology-controlled nonmetal 2D/3D homojunction towards enhancing photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2020, 263, 118270. [Google Scholar] [CrossRef]
- Hu, H.Y.; Lin, Y.; Hu, Y.H. Core-shell structured TiO2 as highly efficient visible light photocatalyst for dye degradation. Catal. Today 2020, 341, 90–95. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.G.; Ji, X.Y.; Xie, M.; Jiang, D.; Yan, J.; Song, Z.L.; Xu, H.; Li, H.M. Construction of polythiophene/Bi4O5I2 nanocomposites to promote photocatalytic degradation of bisphenol a. J. Alloys Compd. 2020, 823, 153773. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wang, Y.J.; Ling, Q.; Zhu, Y.F. Enhancement of full-spectrum photocatalytic activity over BiPO4/Bi2WO6 composites. Appl. Catal. B Environ. 2017, 200, 222–229. [Google Scholar] [CrossRef]
- Mera, A.C.; Moreno, Y.; Pivan, J.Y.; Peña, O.; Mansilla, H.D. Solvothermal synthesis of BiOI microspheres: Effect of the reaction time on the morphology and photocatalytic activity. J. Photochem. Photobiol. A 2014, 289, 7–13. [Google Scholar] [CrossRef]
- Lan, H.C.; Zhang, G.; Zhang, H.W.; Liu, H.J.; Liu, R.P.; Qu, J.H. Solvothermal synthesis of BiOI flower-like microspheres for efficient photocatalytic degradation of BPA under visible light irradiation. Catal Commun. 2017, 98, 9–12. [Google Scholar] [CrossRef]
- Zhu, L.F.; He, C.; Huang, Y.L.; Chen, Z.H.; Xia, D.H.; Su, M.H.; Xiong, Y.; Li, S.Y.; Shu, D. Enhanced photocatalytic disinfection of E. coli 8099 using Ag/BiOI composite under visible light irradiation. Sep. Purif. Technol. 2012, 91, 59–66. [Google Scholar] [CrossRef]
- Lin, J.N.; Hu, Z.; Li, H.; Qu, J.Q.; Zhang, M.; Liang, W.J.; Hu, S. Ultrathin nanotubes of Bi5O7I with a reduced band gap as a high-performance photocatalyst. Inorg. Chem. 2019, 58, 9833–9843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Shan, G.Q.; Dong, F.F.; Wang, C.S.; Zhu, L.Y. Glass fiber supported BiOI thinfilm fixed-bed photocatalytic reactor for water decontamination under solar light irradiation. J. Environ. Sci. 2019, 80, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.Y.; Zhang, A.C.; Zhang, D.; Feng, C.X.; Su, S.; Zhang, X.M.; Xiang, J.; Chen, G.Y.; Wang, Y. Efficient removal of Hg0 from simulated flue gas by novel magnetic Ag2WO4/BiOI/CoFe2O4 photocatalysts. Chem. Eng. J. 2019, 373, 780–791. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.W.; Zhang, Y.H.; Zhang, T.R. Achieving UV and visible-light photocatalytic activity enhancement of AgI/BiOIO3 heterostructure: Decomposition for diverse industrial contaminants and high mineralization ability. Chin. Chem. Lett. 2017, 28, 2244–2250. [Google Scholar] [CrossRef]
- Wang, J.C.; Yao, H.C.; Fan, Z.Y.; Zhang, L.; Wang, J.S.; Zang, S.Q.; Li, Z.J. Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater. Interfaces 2016, 8, 3765–3775. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti(IV) metal-organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.Z.; Wu, Y.; Zeng, G.M.; Chen, X.H.; Leng, L.J.; Wu, Z.B.; Jiang, L.B.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr (VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, X.M.; Wu, D.Y. Construction and characterization of BiOI/NH2-MIL-125 (Ti) heterostructures with excellent visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2019, 30, 3773–3781. [Google Scholar] [CrossRef]
- Dai, G.P.; Yu, J.G.; Liu, G. Synthesis and enhanced visible-light photoelectrocatalytic activity of p-n junction BiOI/TiO2 nanotube arrays. J. Phys. Chem. C 2011, 115, 7339–7346. [Google Scholar] [CrossRef]
- Chen, L.L.; Jiang, D.L.; He, T.; Wu, Z.D.; Chen, M. In-situ ion exchange synthesis of hierarchical AgI/BiOI microsphere photocatalyst with enhanced photocatalytic properties. CrystEngComm 2013, 15, 7556–7563. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ao, D. Construction of heterostructured ZnIn2S4@NH2-MIL-125(Ti) nanocomposites for visible-light-driven H2 production. Appl. Catal. B Environ. 2018, 221, 433–442. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Guo, X.W.; Li, K.Y.; Han, Y.T.; Song, C.S.; Zhang, G.L. Effects of monocarboxylic acid additives on synthesizing Metal-Organic Framework NH2-MIL-125 with controllable size and morphology. Cryst. Growth Des. 2017, 17, 6586–6595. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Guo, X.W.; Kuang, Z.C.; Li, K.Y.; Song, C.S.; Zhang, G.L. Effect of titanium ester on synthesizing NH2-MIL-125(Ti): Morphology changes from circular plate to octahedron and rhombic dodecahedron. J. Solid. State Chem. 2018, 262, 237–243. [Google Scholar] [CrossRef]
- Zhu, S.R.; Liu, P.F.; Wu, M.K.; Zhao, W.N.; Li, G.C.; Tao, K.; Yi, F.Y.; Han, L. Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light. Dalton Trans. 2016, 45, 17521–17529. [Google Scholar] [CrossRef]
- Che, H.N.; Liu, L.H.; Che, G.B.; Dong, H.J.; Liu, C.B.; Li, C.M. Control of energy band, layer structure and vacancy defect of graphitic carbon nitride by intercalated hydrogen bond effect of NO3− toward improving photocatalytic performance. Chem. Eng. J. 2019, 357, 209–219. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.G.; Zhang, Y.L.; Tang, W.H.; Cheng, X.; Liu, H.W. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B. Chem. Phys. Lett. 2016, 653, 101–107. [Google Scholar] [CrossRef]
- Hlophe, P.V.; Mahlalela, L.C.; Dlamini, L.N. A composite of platelet-like orientated BiVO4 fused with MIL-125(Ti): Synthesis and characterization. Sci. Rep. 2019, 9, 10044. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, Y.; Wu, S.; Zhu, Y.; Chen, H.; Yu, X.; Zhang, Y. Facile fabrication of BiOI/BiOCl immobilized films with improved visible light photocatalytic performance. Front. Chem. 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Fei, W.H.; Wang, H.Q.; Li, N.J.; Chen, D.Y.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. p-n Heterojunction of BiOI/ZnO nanorod arrays for piezo-photocatalytic degradation of bisphenol A in water. J. Hazard. Mater. 2020, 399, 123109. [Google Scholar] [CrossRef]
- Qin, H.M.; Wang, K.; Jiang, L.S.; Li, J.; Wu, X.Y.; Zhang, G.K. Ultrasonic-assisted fabrication of a direct Z-scheme BiOI/Bi2O4 heterojunction with superior visible lightresponsive photocatalytic performance. J. Alloys Compd. 2020, 821, 153417. [Google Scholar] [CrossRef]
- Liu, G.H.; Wang, H.Y.; Chen, D.H.; Dai, C.C.; Zhang, Z.H.; Feng, Y.J. Photodegradation performances and transformation mechanism of sulfamethoxazole with CeO2/CN heterojunction as photocatalyst. Sep. Purif. Technol. 2020, 237, 116329. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Liu, C.B.; Wang, D.D.; Yan, G.S.; Liu, B.; Che, G.B. Enhanced visible-light-induced photocatalytic degradation of tetracycline using BiOI/MIL-125(Ti) composite photocatalyst. J. Alloys Compd. 2021, 854, 157166. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.D. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity. J. Mater. Chem. 2010, 20, 5866–5870. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).