Design and Characterization of Ag@Cu2O-rGO Nanocomposite for the p-Nitrophenol Reduction
Abstract
:1. Introduction
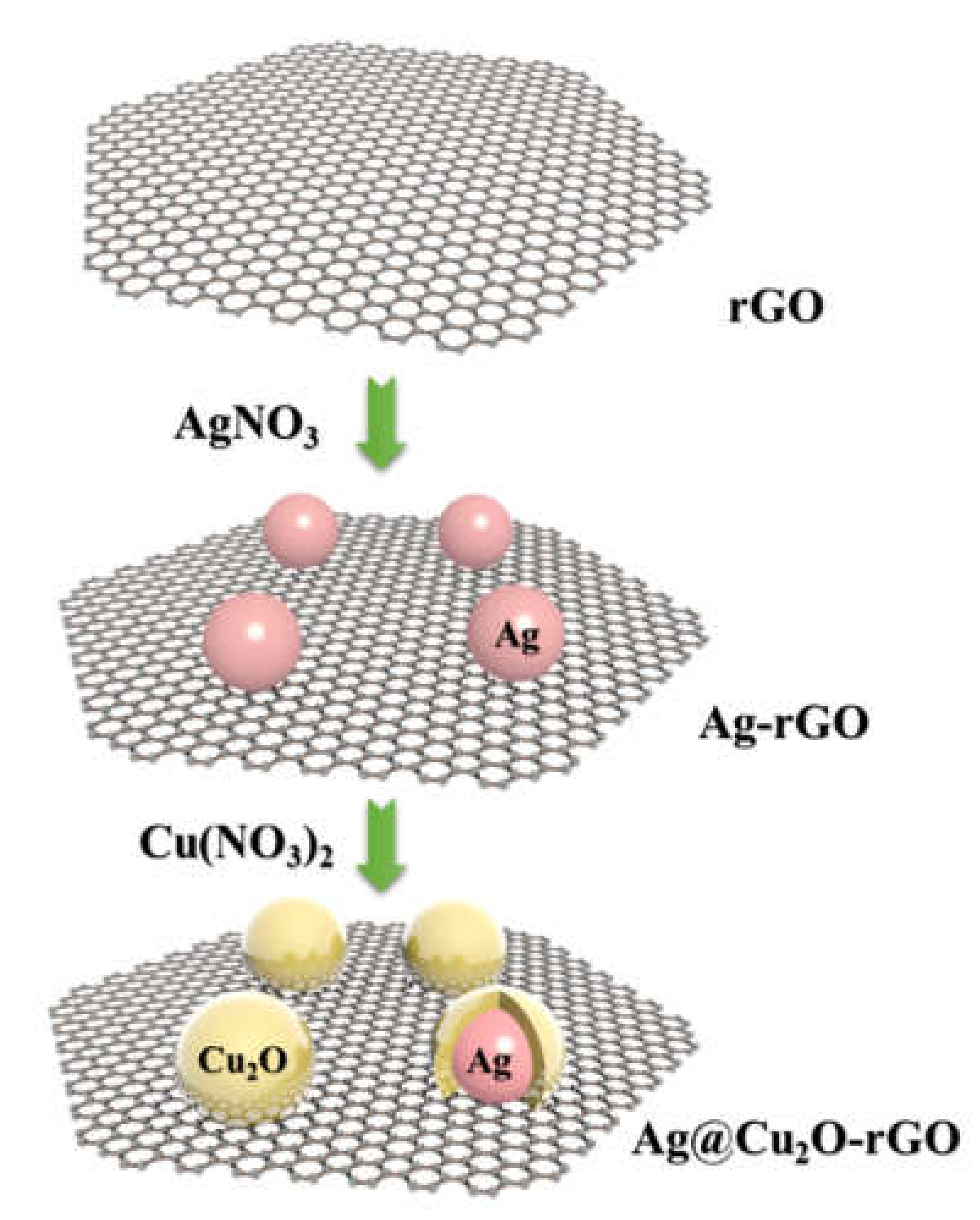
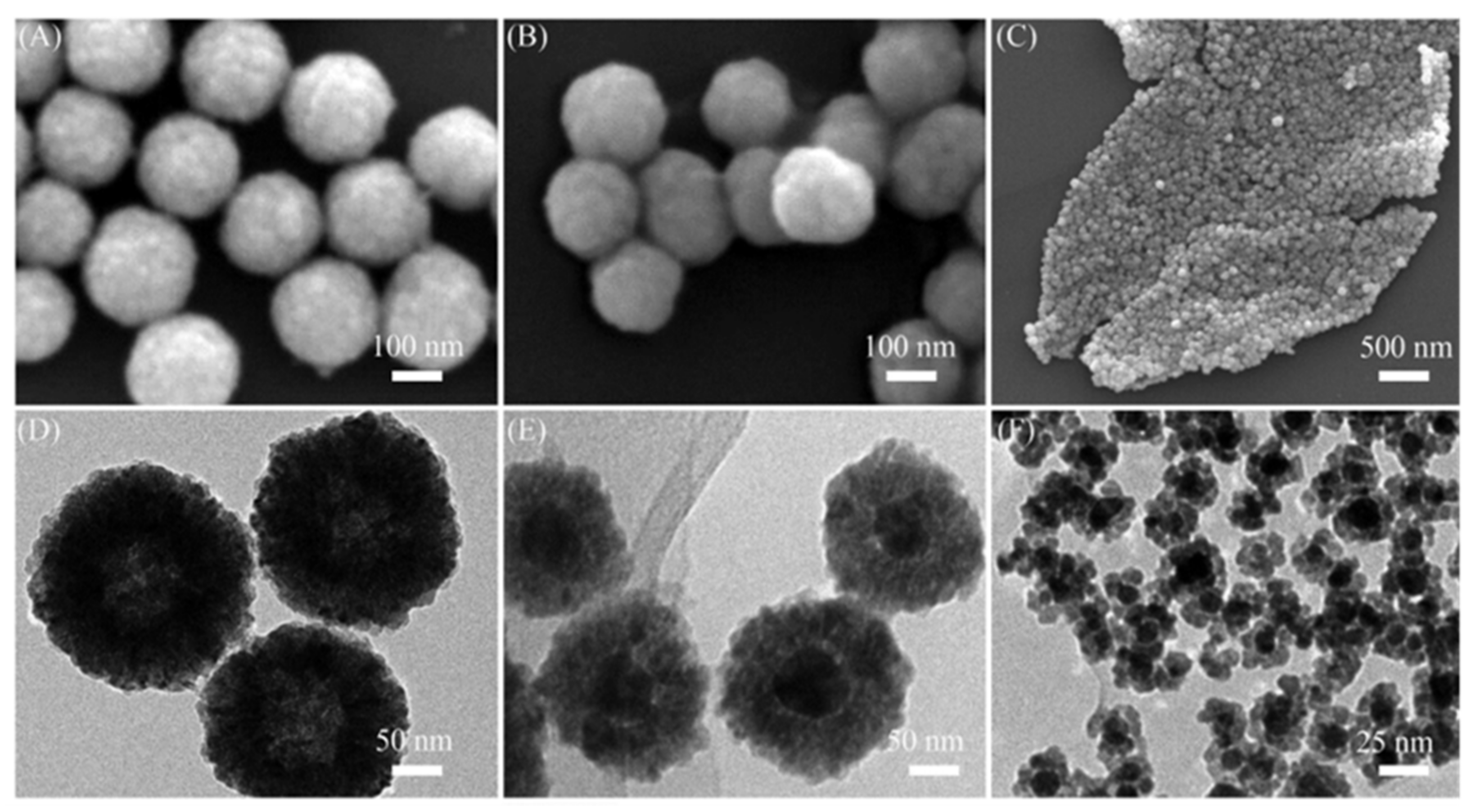
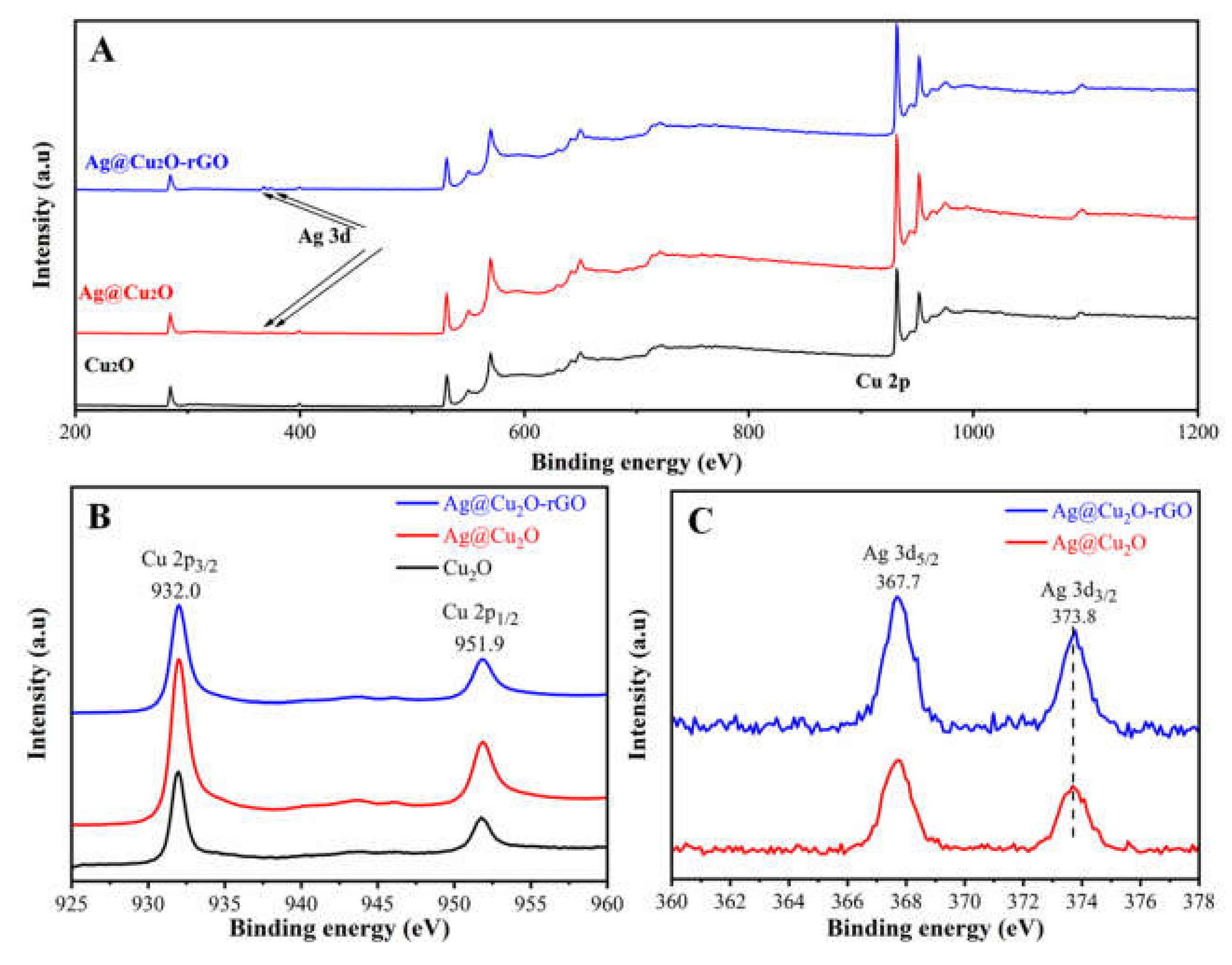
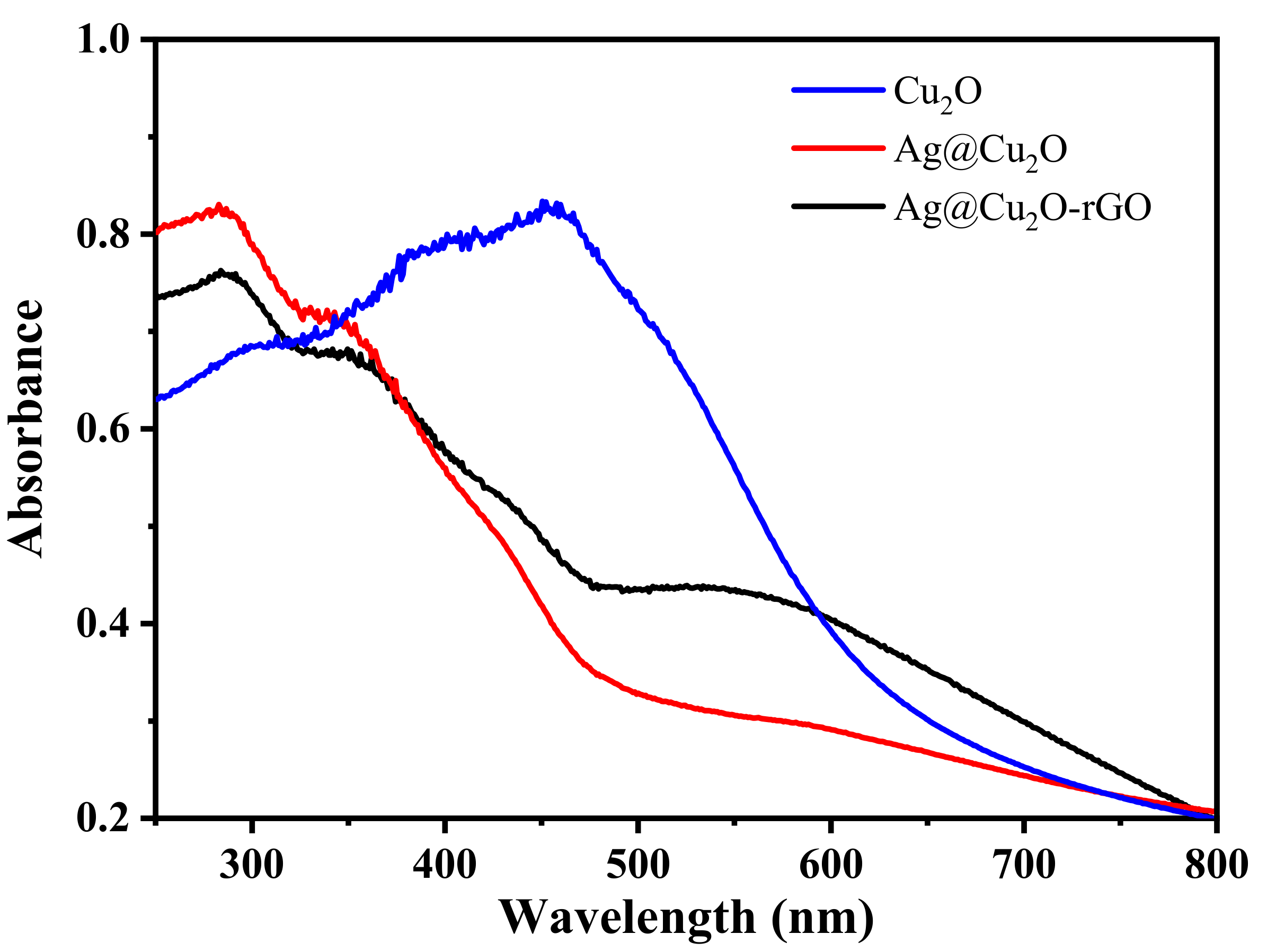
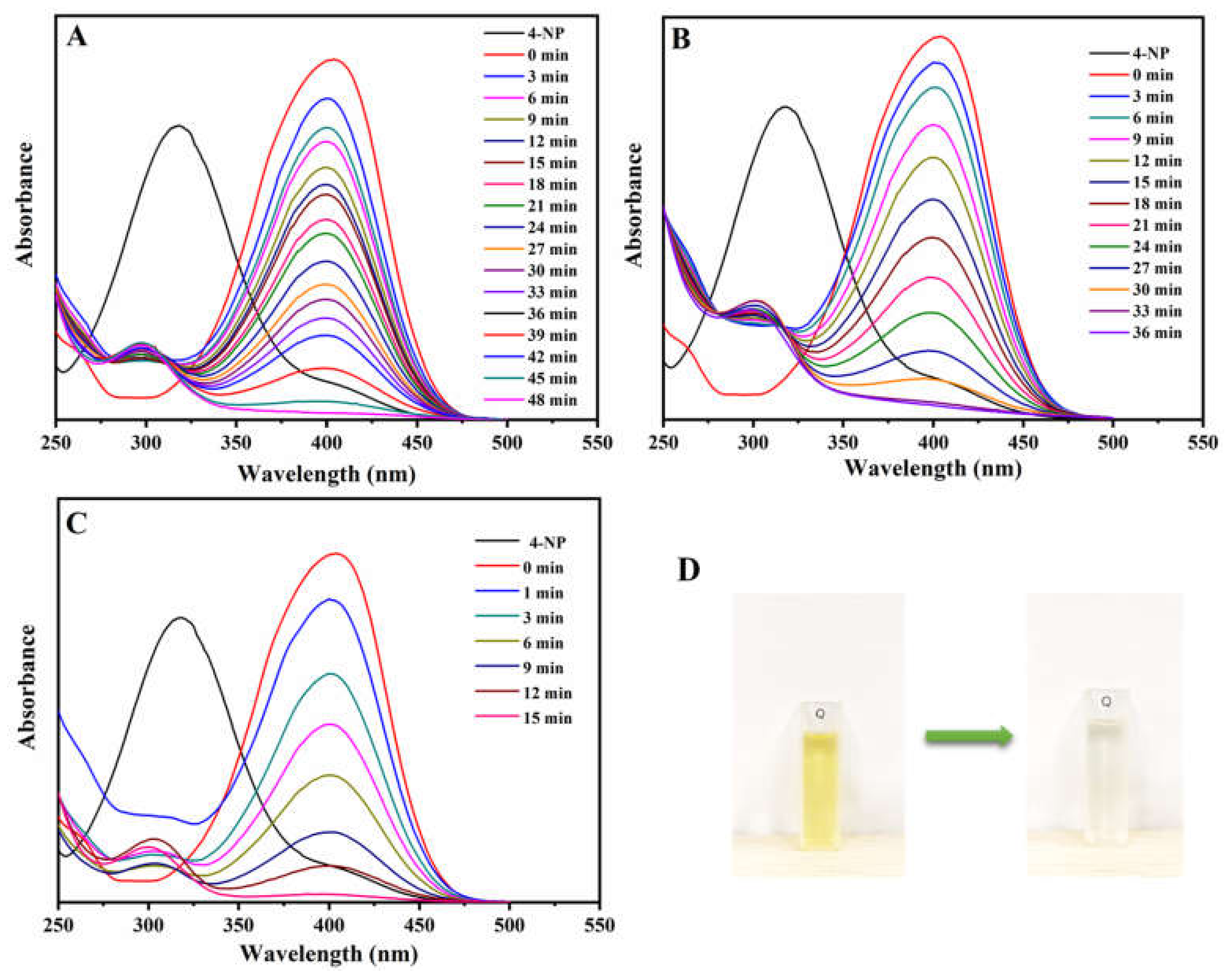
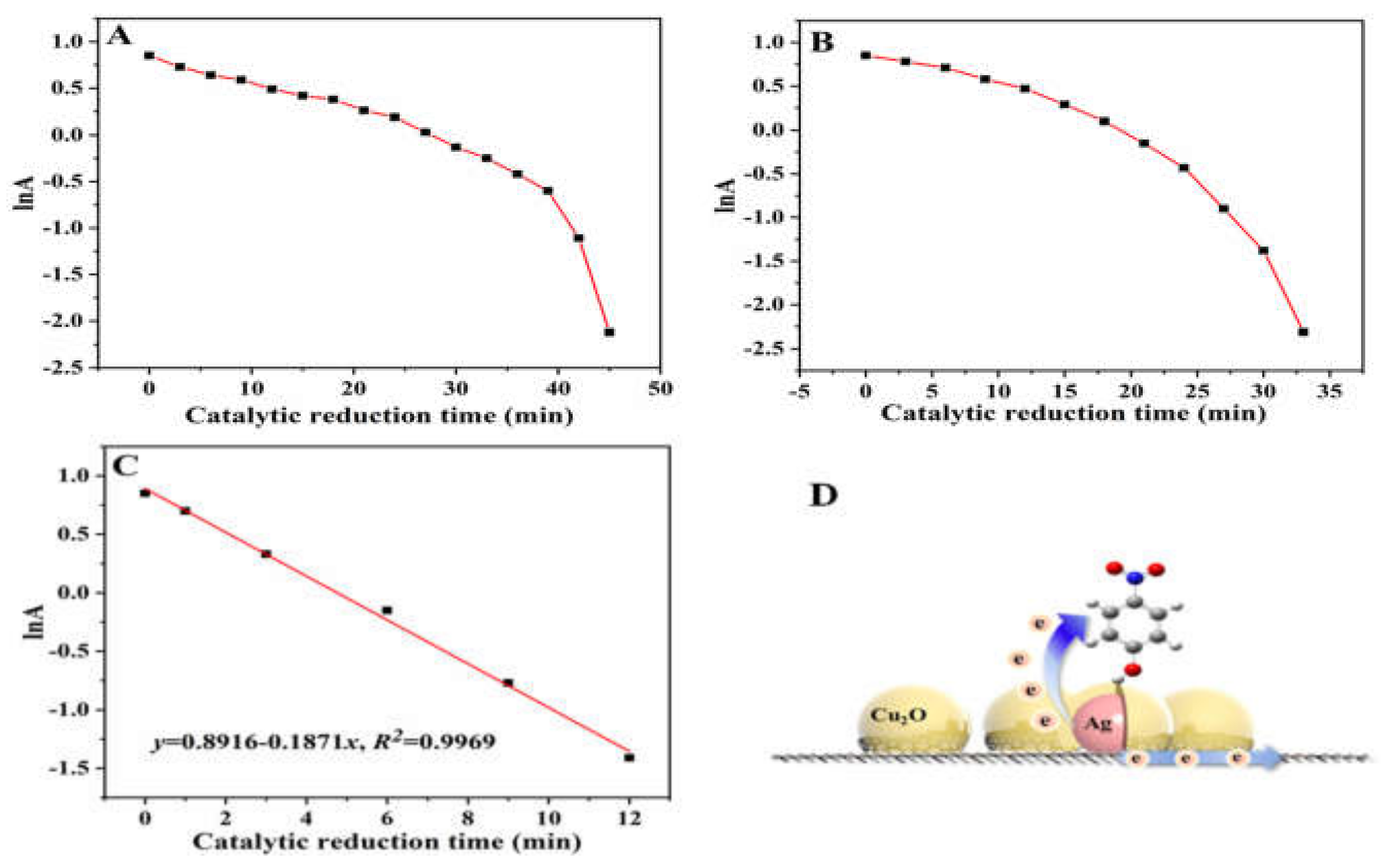
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Composite Materials
3.2.1. Synthesis of Ag@Cu2O Cell–Shell Structure
3.2.2. Synthesis of Ag@Cu2O-rGO Composite Structure
3.3. Catalytic Degradation Experiment
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yury, G. Transition metal carbides go 2D nature nanotechnology. Nat. Mater. 2015, 14, 1079–1080. [Google Scholar]
- Tian, H.W.; Liu, M.; Zheng, W.T. Constructing 2D graphitic carbon nitride nanosheets/layered MoS2/graphene ternary nanojunction with enhanced photocatalytic activity. Appl. Catal. B Environ. 2018, 225, 468–476. [Google Scholar] [CrossRef]
- Lee, Y.T.; Jeon, P.J.; Han, J.H.; Ahn, J.; Lee, H.S.; Lim, J.Y.; Choi, W.K.; Song, J.D.; Park, M.; Lm, S.; et al. Mixed-dimensional 1D ZnO-2D WSe2 van derWaals heterojunction device for photosensor. Adv. Funct. Mater. 2018, 27, 1703822–1703830. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Q.; Dan, J.; Pennycook, S.J.; Lu, X.; Zhu, H.; Xu, Q.-H.; Fan, H.J.; Ho, G.W. Ultrathin nickel boron oxide nanosheets assembled vertically on graphene: A new hybrid 2D material for enhanced photo/electro-catalysis. Mater. Horiz. 2017, 4, 885–894. [Google Scholar] [CrossRef]
- Han, C.; Lu, Y.; Zhang, J.; Ge, L.; Li, Y.; Chen, C.; Xin, Y.; Wu, L.; Fang, S. Novel PtCo alloy nanoparticle decorated 2D g-C3N4 nanosheets with enhanced photocatalytic activity for H2 evolution under visible light irradiation. J. Mater. Chem. A 2015, 3, 23274–23282. [Google Scholar] [CrossRef]
- Fu, X.-L.; Fang, H.; Liu, F.-R.; Ren, S.-W.; Cao, J.-T.; Liu, Y.-M. Electrochemiluminescence energy resonance transfer in 2D/2D heterostructured g-C3N4/MnO2 for glutathione detection. Biosens. Bioelectron. 2019, 129, 72–78. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. UV-activated MoS2 based fast and reversible NO2 sensor at room temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef]
- Tu, Z.; Guy, G.; Mohsen, A.; Haag, R. Multivalent interactions between 2D nanomaterials and biointerfaces. Adv. Mater. 2018, 30, 1706709–1706735. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Singh, A.; Khare, P.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Pollutant soot for pollutant dye degradation: Soluble graphene nanosheets for visible light induced photodegradation of methylene blue. ACS Sustain. Chem. Eng. 2017, 5, 8860–8869. [Google Scholar] [CrossRef]
- Miller, O.D.; Ilic, O.; Christensen, T.; Reid, M.T.H.; Atwater, H.A.; Joannopoulos, J.D.; Soljačić, M.; Johnson, S.G. Limits to the optical response of graphene and two-dimensional materials. Nano Lett. 2017, 17, 5408–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkin, P.; Daeneke, T.; Wang, Y.; Carey, B.J.; Berean, K.J.; Clark, R.M.; Ou, J.Z.; Trinchi, A.; Cole, I.S.; Kalantar-Zadeh, K. 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 2016, 4, 13563–13571. [Google Scholar] [CrossRef]
- Li, Z.-X.; Yang, B.-L.; Zou, K.-Y.; Kong, L.; Yue, M.-L.; Duan, H.-H. Novel porous carbon nanosheet derived from a 2D Cu-MOF: Ultrahigh porosity and excellent performances in the supercapacitor cell. Carbon 2019, 144, 540–548. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Yun, J.M.; Kang, M.; Lee, S.; Jung, Y.-S.; Hwang, K.; Heo, Y.-J.; Kim, J.-E.; Kang, R.; Kim, D.-Y. 2D/2D vanadyl phosphate (VP) on reduced graphene oxide as a hole transporting layer for efficient organic solar cells. Org. Electron. 2018, 59, 92–98. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A platform for surface-enhanced raman spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef]
- Zhu, A.; Qiao, L.; Jia, Z.; Tan, P.; Liu, Y.; Ma, Y.; Pan, J. C-S bonds induced ultrafine SnS2 dots/ porous g-C3N4 sheets 0D/2D heterojunction: Synthesis and photocatalytic mechanism investigation. Dalton Trans. 2017, 46, 17032–17040. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, D. Covalent organic frameworks for heterogeneous catalysis: Principle, current status, and challenges. ACS Cent. Sci. 2020, 6, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, M.; Zhang, S.; Liu, X.; Wang, F. Development of functional black phosphorus nanosheets with remarkable catalytic and antibacterial performance. Nanoscale 2018, 10, 10428–10435. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Draxl, C. Hybrid organic-inorganic perovskites as promising substrates for Pt single-atom catalysts. Phys. Rev. Lett. 2019, 122, 046101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Liu, M.M.; Zhao, Y.; Kou, Q.; Wang, Y.; Liu, Y.; Zhang, Y.; Yang, J.; Jung, Y.M. Enhanced catalyst activity by decorating of Au on Ag@Cu2O nanoshell. Appl. Surf. Sci. 2018, 435, 72–78. [Google Scholar] [CrossRef]
- Li, Y.; Cain, J.D.; Hanson, E.D.; Murthy, A.A.; Hao, S.; Shi, F.; Li, Q.; Wolverton, C.; Chen, X.; Dravid, V.P. Au@MoS2 core-shell heterostructures with strong light-matter interactions. Nano Lett. 2016, 16, 7696–7702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Han, D.L.; Pang, Z.Y.; Sun, Y.; Wang, Y.; Zhang, Y.; Yang, J.; Chen, L. Charge transfer in an ordered Ag/Cu2S/4-MBA system based on surface-enhanced Raman scattering. J Phys. Chem. C 2018, 122, 5599–5605. [Google Scholar] [CrossRef]
- Zhu, W.; Crozier, K.B. Quantum mechanical limit to plasmonic enhancement as observed by surface-enhanced Raman scattering. Nat. Commun. 2014, 5, 5228–5235. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, F.; Song, X.; Yin, Z.; Bu, Y. Construction of reduced graphene oxide-supported Ag-Cu2O composites with hierarchical structures for enhanced photocatalytic activities and recyclability. J. Mater. Chem. A 2015, 3, 5923–5933. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Ma, X.; Jin, J.; Ji, W.; Wang, X.; Song, W.; Zhao, B.; He, C. Fabrication of Ag-Cu2O/reduced graphene oxide nanocomposites as SERS substrates for in situ monitoring of peroxidase-like catalytic reaction and biosensing. ACS Appl. Mater. Int. 2017, 9, 19074–19081. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Qian, W.; El-Sayed, M.A. Following charge separation on the nanoscale in Cu2O-Au nanoframe hollow nanoparticles. Nano Lett. 2011, 11, 3285–3289. [Google Scholar] [CrossRef]
- Huang, J.; Vongehr, S.; Tang, S.; Lu, H.; Shen, J.; Meng, X. Ag dendrite-based Au/Ag bimetallic nanostructures with strongly enhanced catalytic activity. Langmuir 2009, 25, 11890–11896. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Guo, S.; Chen, L. Design and Characterization of Ag@Cu2O-rGO Nanocomposite for the p-Nitrophenol Reduction. Catalysts 2021, 11, 43. https://doi.org/10.3390/catal11010043
Song C, Guo S, Chen L. Design and Characterization of Ag@Cu2O-rGO Nanocomposite for the p-Nitrophenol Reduction. Catalysts. 2021; 11(1):43. https://doi.org/10.3390/catal11010043
Chicago/Turabian StyleSong, Chao, Shuang Guo, and Lei Chen. 2021. "Design and Characterization of Ag@Cu2O-rGO Nanocomposite for the p-Nitrophenol Reduction" Catalysts 11, no. 1: 43. https://doi.org/10.3390/catal11010043




