Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Pristine and Calcinated TiO2 Samples
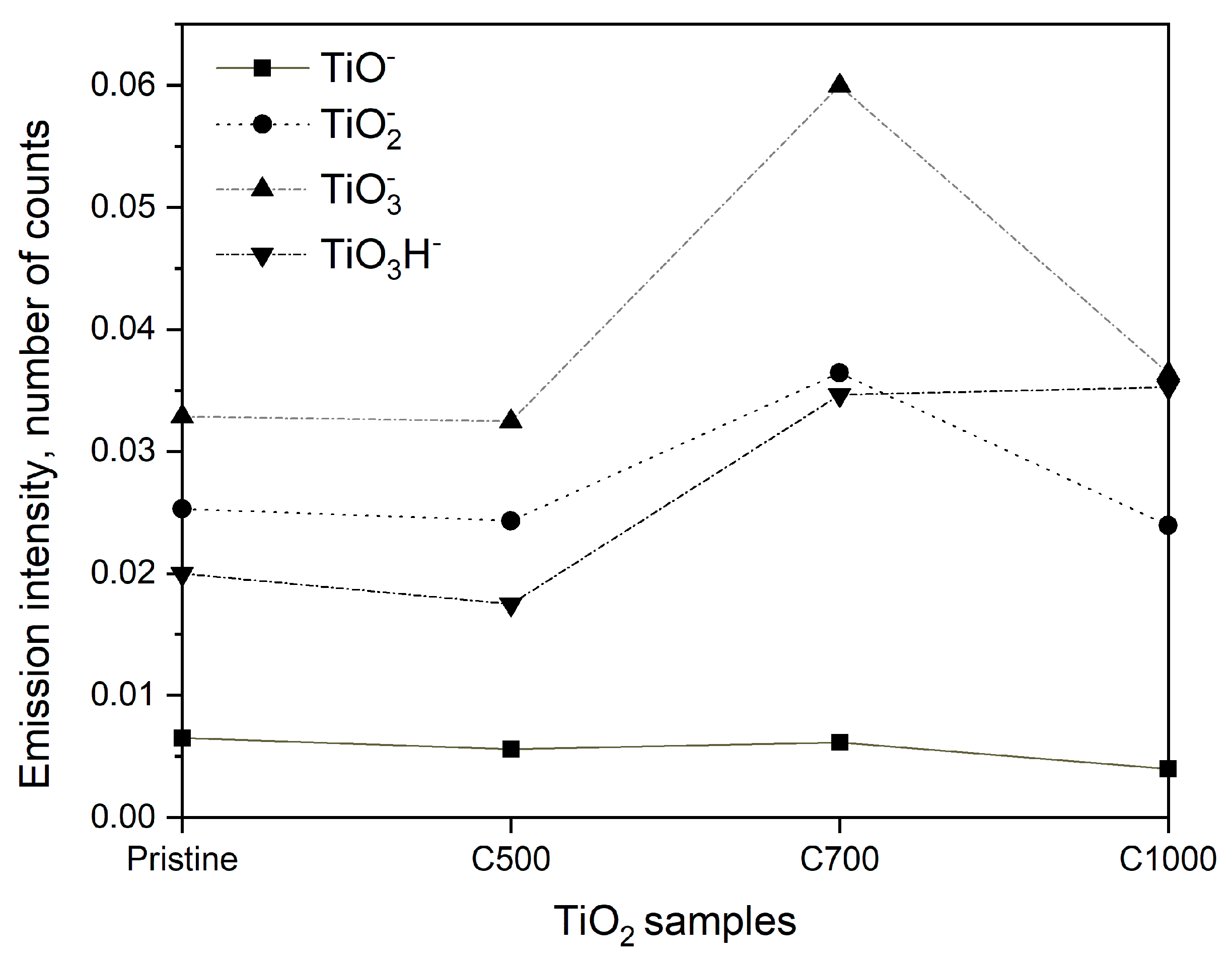
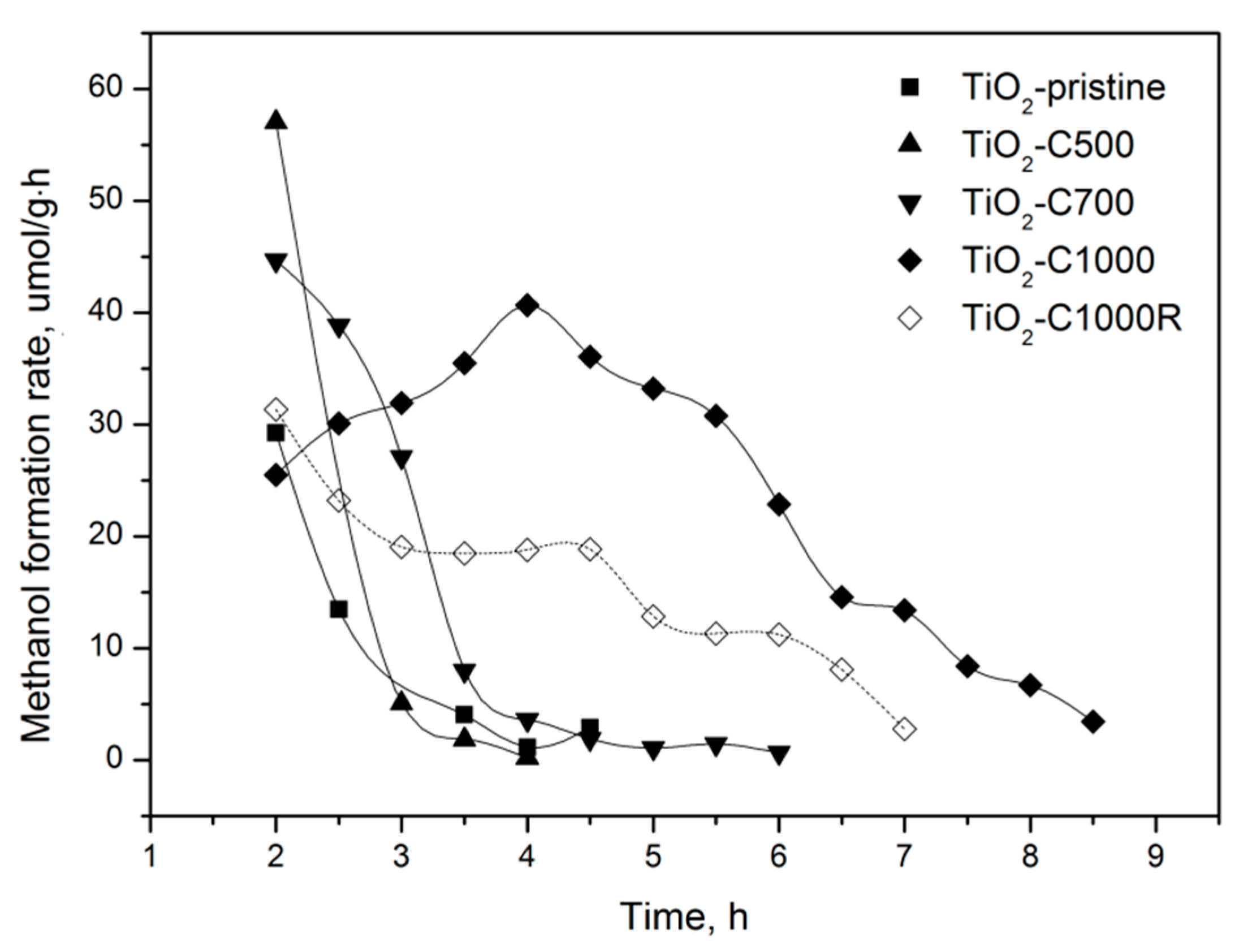
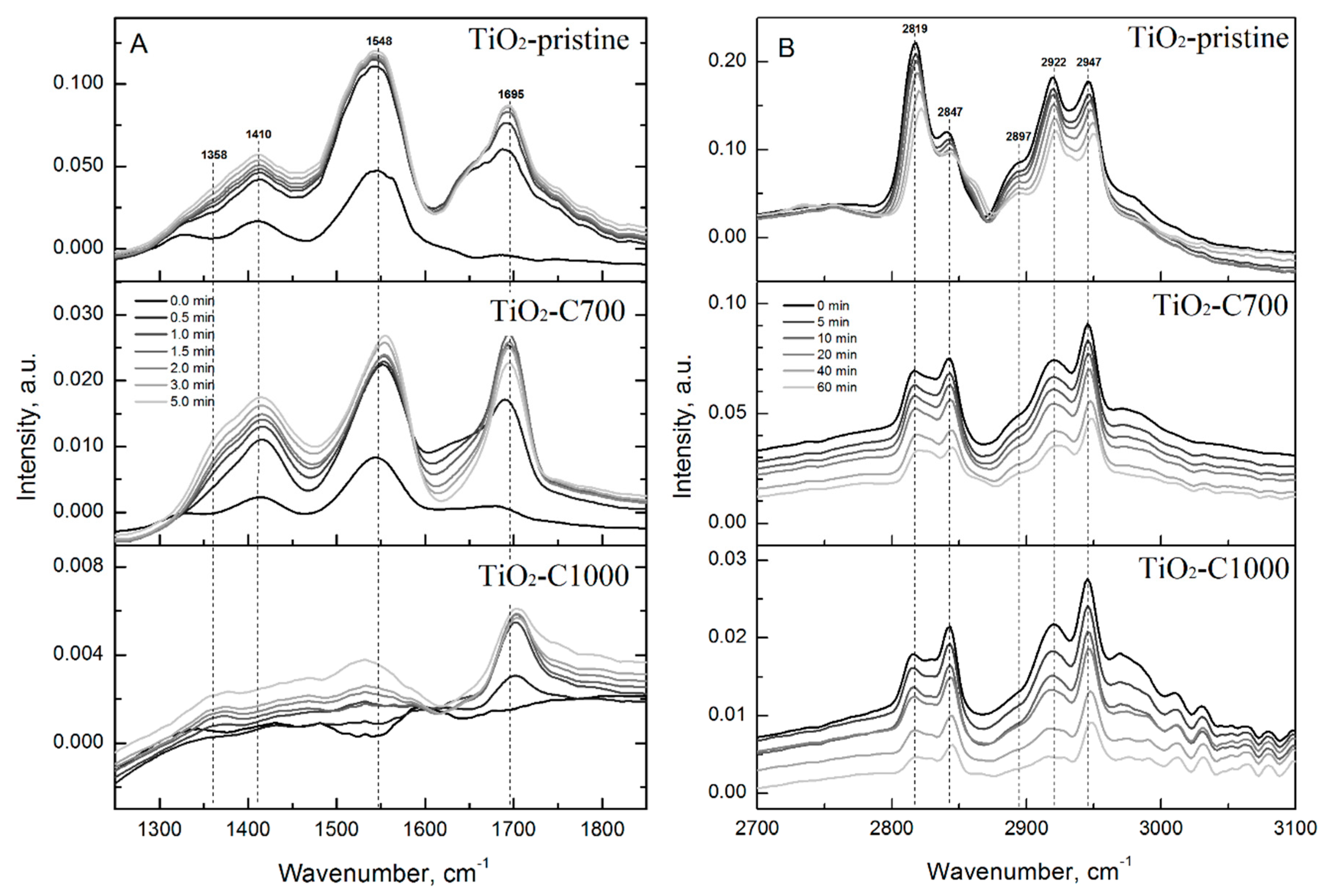
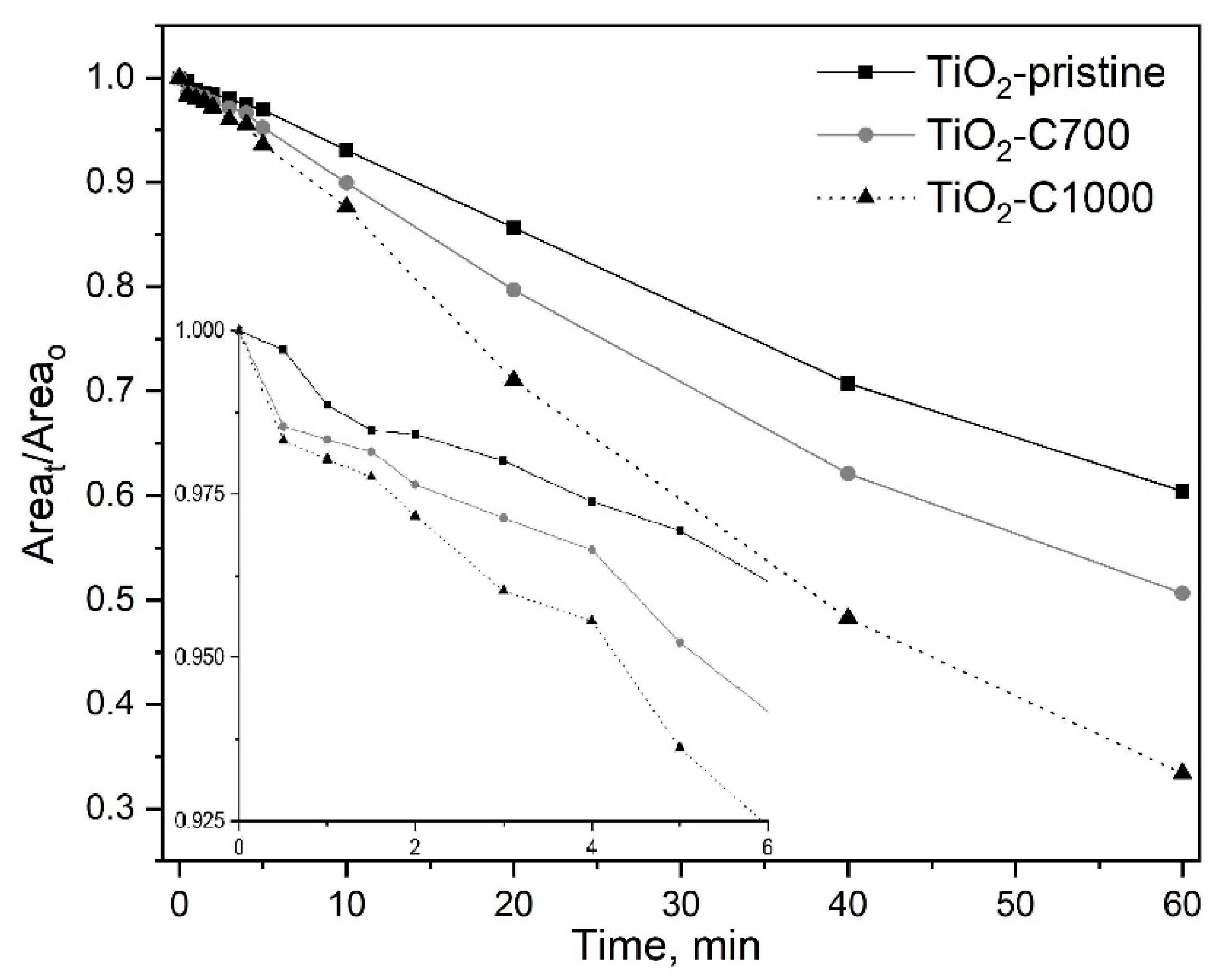
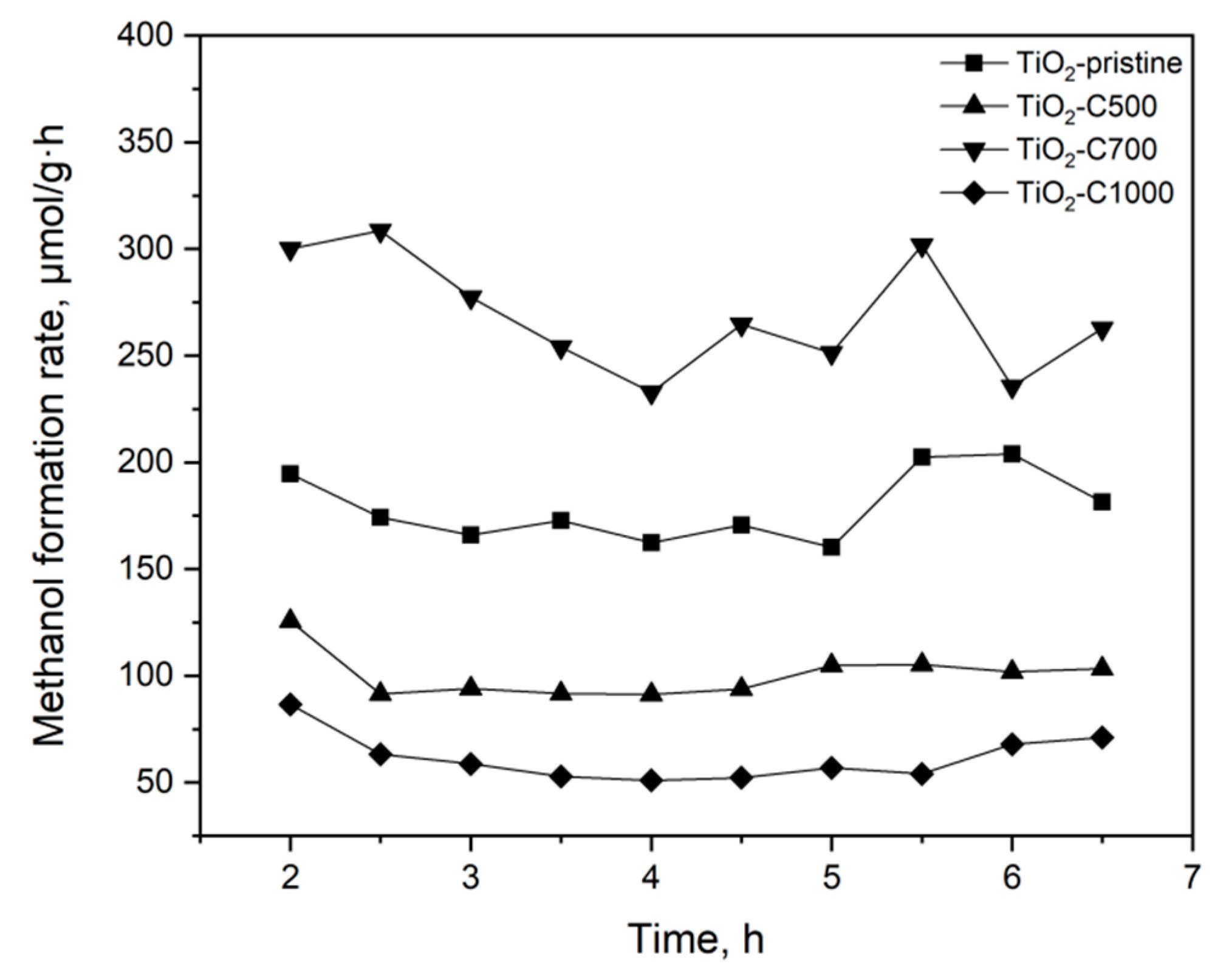
2.2. Photocatalytic and Adsorption Properties of TiO2 Samples
3. Experimental Part
3.1. Modification of TiO2
3.2. Physicochemical Characterization
3.3. Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A review on catalytic nanomaterials for volatile organic compounds VOC removal and their applications for healthy buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S.C. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D.W. Heterogeneous photocatalytic organic synthesis: State-of-the-art and future perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photocatalysis applied to organic synthesis—A green chemistry approach. Curr. Opin. Green Sustain. Chem. 2018, 10, 40–45. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; García, H. Photocatalytic CO2 Reduction to C2+ Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Sharma, N.; Das, T.; Kumar, S.; Bhosale, R.; Kabir, M.; Ogale, S.B. Photocatalytic Activation and Reduction of CO2 to CH4 over Single Phase Nano Cu3SnS4: A Combined Experimental and Theoretical Study. ACS Appl. Energy Mater. 2019, 2, 5677–5685. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-free photocatalysts for various applications in energy conversion and environmental purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kibria, G.; Mullins, C.B. Metal-free photocatalysts for hydrogen evolution. Chem. Soc. Rev. 2020, 49, 1887–1931. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis—Past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Rajendran, S.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Wong, S.P. Photocatalytic Efficiency of TiO2-Biomass Loaded Mixture for Wastewater Treatment. J. Chem. 2018, 2018, 4314969. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Ariyanti, D.; Mukhtar, S.; Ahmed, N.; Liu, Z.; Dong, J.; Gao, W. Surface modification of TiO2 for visible light photocatalysis: Experimental and theoretical calculations of its electronic and optical properties. Int. J. Mod. Phys. B 2020, 34, 1–8. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. Semicond. Photocatal. Mater. Mech. Appl. 2016. [Google Scholar] [CrossRef]
- Shifu, C.; Lei, C.; Shen, G.; Gengyu, C. The preparation of coupled WO3/TiO2 photocatalyst by ball milling. Powder Technol. 2005, 160, 198–202. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.A.; Román-Martínez, M. Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Zheng, S.; Sun, Z.; Liu, S.; Li, H. Influence of calcination temperature on the structural, adsorption and photocatalytic properties of TiO2 nanoparticles supported on natural zeolite. Powder Technol. 2015, 274, 88–97. [Google Scholar] [CrossRef]
- Yamazaki, S.; Takaki, D.; Nishiyama, N.; Yamazaki, Y. Factors affecting photocatalytic activity of TiO2. Curr. Dev. Photocatal. Photocatal. Mater. 2020, 23–38. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Pasquali, M.; Dell’Era, A.; Vecchio, S. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef]
- Yudoyono, G.; Ichzan, N.; Zharvan, V.; Daniyati, R.; Santoso, H.; Indarto, B.; Pramono, Y.H.; Zainuri, M.; Darminto, D. Effect of calcination temperature on the photocatalytic activity of TiO2 powders prepared by co-precipitation of TiCl3. AIP Conf. Proc. 2016, 1725, 020099. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.A.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef]
- Hurum, D.; Agrios, A.; Crist, S.; Gray, K.; Rajh, T.; Thurnauer, M. Probing reaction mechanisms in mixed phase TiO2 by EPR. J. Electron Spectrosc. Relat. Phenom. 2006, 150, 155–163. [Google Scholar] [CrossRef]
- Wei, Y.; Tokina, M.V.; Benderskii, A.V.; Zhou, Z.; Long, R.; Prezhdo, O.V. Quantum dynamics origin of high photocatalytic activity of mixed-phase anatase/rutile TiO2. J. Chem. Phys. 2020, 153, 044706. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Choudhury, A. Investigation of the optical property and photocatalytic activity of mixed phase nanocrystalline titania. Appl. Nanosci. 2014, 4, 839–847. [Google Scholar] [CrossRef]
- Bak, T.; Li, W.; Nowotny, J.; Atanacio, A.J.; Davis, J. Photocatalytic Properties of TiO2: Evidence of the Key Role of Surface Active Sites in Water Oxidation. J. Phys. Chem. A 2015, 119, 9465–9473. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.; Saint, C.P. Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater. 2009, 117, 233–242. [Google Scholar] [CrossRef]
- Shtyka, O.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Dubkov, S.; Gromov, D.; Maniecki, T.P. Photocatalytic Reduction of CO2 Over Me (Pt, Pd, Ni, Cu)/TiO2 Catalysts. Top. Catal. 2020, 63, 113–120. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.M.; Restrepo, G. Study of the bandgap of synthesized titanium dioxide nanoparticules using the sol-gel method and a hydrothermal treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.-H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B Environ. 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Vajda, K.; Saszet, K.; Kedves, E.; Kása, Z.; Danciu, V.; Baia, L.; Magyari, K.; Hernádi, K.; Kovács, G.; Pap, Z.; et al. Shape-controlled agglomeration of TiO2 nanoparticles. New insights on polycrystallinity vs. single crystals in photocatalysis. Ceram. Int. 2016, 42, 3077–3087. [Google Scholar] [CrossRef]
- Schaub, R.; Thostrup, P.; Lopez, N.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Besenbacher, F. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys. Rev. Lett. 2001, 87, 266104. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, F.; Li, Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Materiomics 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, K. Fabrication of antireflective hierarchical TiO2 nanostructures by moth-eye patterning of anodic anodized nanotubes. Opt. Express 2018, 26, 31490–31499. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Chen, H.-T.; Yen, F.-L.; Lu, W.-C.; Kuo, C.-W.; Wang, M.-C. Preparation of TiO2 Nanocrystallite Powders Coated with 9 mol % ZnO for Cosmetic Applications in Sunscreens. Int. J. Mol. Sci. 2012, 13, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Wu, C.Y.; Tu, K.J.; Deng, J.P.; Lo, Y.S.; Wu, C.H. Markedly enhanced surface hydroxyl groups of TiO2 nanoparticles with Superior water-dispersibility for photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; Sullivan, J.; Saied, S. Surface characterisation of plasma-nitrided titanium: An XPS study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Stadnichenko, A.I.; Muravev, V.; Koscheev, S.V.; Zaikovskii, V.I.; Aleksandrov, H.A.; Neyman, K.M.; Boronin, A.I. Study of active surface centers of Pt/CeO2 catalysts prepared using radio-frequency plasma sputtering technique. Surf. Sci. 2019, 679, 273–283. [Google Scholar] [CrossRef]
- Stadnichenko, A.I.; Svintsitskiy, D.; Kibis, L.S.; Fedorova, E.A.; Stonkus, O.; Slavinskaya, E.; Lapin, I.N.; Fakhrutdinova, E.; Svetlichnyi, V.A.; Romanenko, A.; et al. Influence of titania synthesized by pulsed laser ablation on the state of platinum during ammonia oxidation. Appl. Sci. 2020, 10, 4699. [Google Scholar] [CrossRef]



| Sample | BET SSA, m2/g | Phase Composition | Band Gap, eV | |||
|---|---|---|---|---|---|---|
| Anatase,% | Average Crystallite Size, nm | Rutile,% | Average Crystallite Size, nm | |||
| TiO2-pristine | 57 | 87.8 | 18 | 12.2 | 25 | 3.291 |
| TiO2-C500 | 56 | 84.3 | 19 | 15.7 | 30 | 3.389 |
| TiO2-C700 | 16 | - | - | 100.0 | 85 | 3.071 |
| TiO2-C1000 | 4 | - | - | 100.0 | 156 | 3.036 |
| Sample | Total Ti2p Species | O1s (eV) | O-Ti/Ti Ratio | ||
|---|---|---|---|---|---|
| O-Ti (529.9) | Ti-OH (531.5) | Total | |||
| TiO2-pristine | 29.28 | 63.09 | 7.64 | 70.73 | 2.15 |
| TiO2-C500 | 30.37 | 64.82 | 4.81 | 69.63 | 2.13 |
| TiO2-C700 | 31.03 | 57.83 | 11.13 | 68.96 | 1.86 |
| TiO2-C1000 | 29.89 | 57.26 | 12.85 | 70.11 | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtyka, O.; Shatsila, V.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Dubkov, S.; Gromov, D.; Tarasov, A.; Rogowski, J.; Stadnichenko, A.; et al. Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2. Catalysts 2021, 11, 47. https://doi.org/10.3390/catal11010047
Shtyka O, Shatsila V, Ciesielski R, Kedziora A, Maniukiewicz W, Dubkov S, Gromov D, Tarasov A, Rogowski J, Stadnichenko A, et al. Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2. Catalysts. 2021; 11(1):47. https://doi.org/10.3390/catal11010047
Chicago/Turabian StyleShtyka, Oleksandr, Viktar Shatsila, Radoslaw Ciesielski, Adam Kedziora, Waldemar Maniukiewicz, Sergey Dubkov, Dmitry Gromov, Andrey Tarasov, Jacek Rogowski, Andrey Stadnichenko, and et al. 2021. "Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2" Catalysts 11, no. 1: 47. https://doi.org/10.3390/catal11010047
APA StyleShtyka, O., Shatsila, V., Ciesielski, R., Kedziora, A., Maniukiewicz, W., Dubkov, S., Gromov, D., Tarasov, A., Rogowski, J., Stadnichenko, A., Lazarenko, P., Ryazanov, R., Szynkowska-Jóźwik, M. I., & Maniecki, T. (2021). Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2. Catalysts, 11(1), 47. https://doi.org/10.3390/catal11010047







