Enhanced Hydrogen Evolution Reaction in Surface Functionalized MoS2 Monolayers
Abstract
:1. Introduction
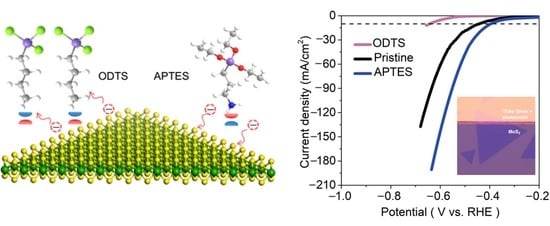
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Monolayer MoS2
3.2. Formation of Octadecyltrichlorosilane (ODTS) and (3-Aminopropyl)-Triethoxysilane (APTES) on a MoS2 Monolayer
3.3. Characterization of the Doped MoS2
3.4. Device Fabrication and Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.W.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Norskov, J.K. Tuning the MoS2 Edge-Site Activity for Hydrogen Evolution via Support Interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Jaramillo, T.F.; Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13](2-) clusters. Nat. Chem. 2014, 6, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhou, Y.; Yang, D.R.; Xu, W.X.; Wang, C.; Wang, F.B.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jorgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Biornimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Merki, D.; Hu, X.L. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef] [Green Version]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Li, G.Q.; Zhang, D.; Qiao, Q.; Yu, Y.F.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All The Catalytic Active Sites of MoS2 for Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef]
- Xie, J.F.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2015, 15, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, J.J.; Guo, H.; Chen, W.B.; Yuan, J.T.; Martinez, U.; Gupta, G.; Mohite, A.; Ajayan, P.M.; Lou, J. Unveiling Active Sites for the Hydrogen Evolution Reaction on Monolayer MoS2. Adv. Mater. 2017, 29, 1701955. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.F.; Huang, S.Y.; Li, Y.P.; Steinmann, S.N.; Yang, W.T.; Cao, L.Y. Layer-Dependent Electrocatalysis of MoS2 for Hydrogen Evolution. Nano Lett. 2014, 14, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiry, D.; Fullon, R.; Yang, J.E.; Silva, C.D.C.E.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pak, S.; Giraud, P.; Lee, Y.W.; Cho, Y.; Hong, J.; Jang, A.R.; Chung, H.S.; Hong, W.K.; Jeong, H.; et al. Thermodynamically Stable Synthesis of Large-Scale and Highly Crystalline Transition Metal Dichalcogenide Monolayers and their Unipolar n–n Heterojunction Devices. Adv. Mater. 2017, 29, 1702206. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Memaran, S.; Xin, Y.; Balicas, L.; Gutierrez, H.R. One-pot growth of two-dimensional lateral heterostructures via sequential edge-epitaxy. Nature 2018, 553, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.W.; Pak, S.; Lee, S.; Reimers, S.; Mukherjee, S.; Dudin, P.; Kim, T.K.; Cattelan, M.; Fox, N.; Dhesi, S.S.; et al. Spectral functions of CVD grown MoS2 monolayers after chemical transfer onto Au surface. Appl. Surf. Sci. 2020, 532, 147390. [Google Scholar] [CrossRef]
- Pak, S.; Jang, A.R.; Lee, J.; Hong, J.; Giraud, P.; Lee, S.; Cho, Y.; An, G.H.; Lee, Y.W.; Shin, H.S.; et al. Surface functionalization-induced photoresponse characteristics of monolayer MoS2 for fast flexible photodetectors. Nanoscale 2019, 11, 4726–4734. [Google Scholar] [CrossRef]
- Najmaei, S.; Zou, X.; Er, D.; Li, J.; Jin, Z.; Gao, W.; Zhang, Q.; Park, S.; Ge, L.; Lei, S.; et al. Tailoring the Physical Properties of Molybdenum Disulfide Monolayers by Control of Interfacial Chemistry. Nano Lett. 2014, 14, 1354–1361. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nishikawa, T.; Takenobu, T.; Mori, S.; Shimoda, T.; Mitani, T.; Shimotani, H.; Yoshimoto, N.; Ogawa, S.; Iwasa, Y. Control of carrier density by self-assembled monolayers in organic field-effect transistors. Nat. Mater. 2004, 3, 317–322. [Google Scholar] [CrossRef]
- Chakraborty, B.; Bera, A.; Muthu, D.V.S.; Bhowmick, S.; Waghmare, U.V.; Sood, A.K. Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys. Rev. B 2012, 85, 161403. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.; He, K.; Lee, C.; Lee, G.; Hone, J.; Heinz, T.F.; Shan, J. Tightly bound trions in monolayer MoS2. Nat. Mater. 2012, 12, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.-Y.Y.; Hu, P.; Zhen, L. Carrier control of MoS2 nanoflakes by functional self-assembled monolayers. ACS Nano 2013, 7, 7795–7804. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, M.S.; Shim, J.; Jeon, J.; Park, H.Y.; Jung, W.S.; Yu, H.Y.; Pang, C.H.; Lee, S.; Park, J.H. High-Performance Transition Metal Dichalcogenide Photodetectors Enhanced by Self-Assembled Monolayer Doping. Adv. Funct. Mater. 2015, 25, 4219–4227. [Google Scholar] [CrossRef]
- Pak, S.; Cho, Y.; Hong, J.; Lee, J.; Lee, S.; Hou, B.; An, G.H.; Lee, Y.W.; Jang, J.E.; Im, H.; et al. Consecutive Junction-Induced Efficient Charge Separation Mechanisms for High-Performance MoS2/Quantum Dot Phototransistors. ACS Appl. Mater. Interfaces 2018, 10, 38264–38271. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yan, M.; Zhao, K.; Liao, X.; Wang, P.; Pan, X.; Yang, W.; Mai, L. Field Effect Enhanced Hydrogen Evolution Reaction of MoS2 Nanosheets. Adv. Mater. 2017, 29, 1604464. [Google Scholar] [CrossRef]
- Pak, S.; Lee, J.; Jang, A.-R.; Kim, S.; Park, K.-H.; Sohn, J.I.; Cha, S. Strain Engineering of Contact Energy Barriers and Photoresponse Behaviors in Monolayer MoS2 Flexible Devices. Adv. Funct. Mater. 2020, 22, 2002023. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pak, S.; Lim, J.; Hong, J.; Cha, S. Enhanced Hydrogen Evolution Reaction in Surface Functionalized MoS2 Monolayers. Catalysts 2021, 11, 70. https://doi.org/10.3390/catal11010070
Pak S, Lim J, Hong J, Cha S. Enhanced Hydrogen Evolution Reaction in Surface Functionalized MoS2 Monolayers. Catalysts. 2021; 11(1):70. https://doi.org/10.3390/catal11010070
Chicago/Turabian StylePak, Sangyeon, Jungmoon Lim, John Hong, and SeungNam Cha. 2021. "Enhanced Hydrogen Evolution Reaction in Surface Functionalized MoS2 Monolayers" Catalysts 11, no. 1: 70. https://doi.org/10.3390/catal11010070