Iron Based Core-Shell Structures as Versatile Materials: Magnetic Support and Solid Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Core and Shell Morphology
2.2. Design of Core-Shell Morphology
2.3. Application in Catalysis
2.3.1. Temperature-Programmed Reduction (TPR)
2.3.2. CO2 Hydrogenation
2.4. Application as Magnetic Support in Water Purification
3. Materials and Methods
3.1. Synthesis of Iron-Based Core-Shell Materials
3.2. Preparation of magPOM-SILP
3.3. Material Characterization
3.4. Catalytic Performance Test
3.5. Application as Magnetic Support Material for Water Purification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Feyen, M.; Weidenthaler, C.; Güttel, R.; Schlichte, K.; Holle, U.; Lu, A.-H.; Schüth, F. High-Temperature Stable, Iron-Based Core-Shell Catalysts for Ammonia Decomposition. Chem. Eur. J. 2011, 17, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Isaacs, M.A.; Durndell, L.J.; Parlett, C.M.; Lee, A.F.; Wilson, K. Magnetically-separable Fe3O4@SiO2@SO4-ZrO2 core-shell nanoparticle catalysts for propanoic acid esterification. Mol. Catal. 2018, 449, 137–141. [Google Scholar] [CrossRef]
- Hudson, R.; Riviere, A.; Cirtiu, C.M.; Luska, K.L.; Moores, A. Iron-iron oxide core-shell nanoparticles are active and magnetically recyclable olefin and alkyne hydrogenation catalysts in protic and aqueous media. Chem. Commun. 2012, 48, 3360–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Z.; Qin, H.; Kang, S.; Bai, J.; Wang, Z.; Li, Y.; Zheng, Z.; Li, X. Effect of graphitic carbon modification on the catalytic performance of Fe@SiO2-GC catalysts for forming lower olefins via Fischer-Tropsch synthesis. J. Colloid Interface Sci. 2018, 516, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.; Baysal, Z.; Kureti, S. Activity and Structural Changes of Fe-based Catalysts during CO2 Hydrogenation towards CH4—A Mini Review. ChemCatChem 2020, 12, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, J.; Zambrzycki, C.; Baysal, Z.; Güttel, R.; Kureti, S. Fe based core-shell model catalysts for the reaction of CO2 with H2. React. Kinet. Mech. Catal. 2020, 131, 119–128. [Google Scholar] [CrossRef]
- Kirchner, J.; Zambrzycki, C.; Güttel, R.; Kureti, S. CO2 Methanation on Fe Catalysts Using Different Structural Concepts. Chem. Ing. Tech. 2020, 92, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Strass-Eifert, A.; Sheppard, T.L.; Damsgaard, C.D.; Grunwaldt, J.-D.; Güttel, R. Stability of Cobalt Particles in and outside HZSM-5 under CO Hydrogenation Conditions Studied by Ex Situ and In Situ Electron Microscopy. ChemCatChem 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Castillo, I.F.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerff, M.; Morweiser, M.; Dillschneider, R.; Michel, A.; Menzel, K.; Posten, C. Harvesting fresh water and marine algae by magnetic separation: Screening of separation parameters and high gradient magnetic filtration. Bioresour. Technol. 2012, 118, 289–295. [Google Scholar] [CrossRef]
- Arnal, P.M.; Comotti, M.; Schüth, F. High-Temperature-Stable Catalysts by Hollow Sphere Encapsulation. Angew. Chem. Int. Ed. 2006, 45, 8224–8227. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S. The Adsorption of Gases and Vapors Vol.1: Physical Adsorption; Princeton University Press: Princeton, NJ, USA, 1943. [Google Scholar]
- Ismail, A.F.; Khulbe, K.; Matsuura, T. Gas Separation Membranes, 1st ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation—Chapter 1: Transport of Gases and Vapors in Glassy and Rubbery Polymers; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; pp. 1–47. [Google Scholar]
- Sun, C.; Mao, D.; Han, L.; Yu, J. Effect of preparation method on performance of Cu-Fe/SiO2 catalysts for higher alcohols synthesis from syngas. RSC Adv. 2016, 6, 55233–55239. [Google Scholar] [CrossRef]
- Singh, B.P.; Kumar, A.; Areizaga-Martinez, H.I.; Vega-Olivencia, C.A.; Tomar, M.S. Synthesis, characterization, and electrocatalytic ability of γ-Fe2O3 nanoparticles for sensing acetaminophen. Indian J. Pure Appl. Phys. 2017, 55, 722–728. [Google Scholar]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Zielinski, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- Ilsemann, J.; Straß-Eifert, A.; Friedland, J.; Kiewidt, L.; Thöming, J.; Bäumer, M.; Güttel, R. Cobalt@Silica core-shell catalysts for hydrogenation of CO/CO2 mixtures to methane. ChemCatChem 2019, 11, 4884–4893. [Google Scholar] [CrossRef] [Green Version]
- Loiland, J.A.; Wulfers, M.J.; Marinkovic, N.S.; Lobo, R.F. Fe/γ-Al2O3 and Fe-K/γ-Al2O3 as reverse water-gas shift catalysts. Catal. Sci. Technol. 2016, 6, 5267–5279. [Google Scholar] [CrossRef] [Green Version]
- Straß, A.; Maier, R.; Güttel, R. Continuous Synthesis of Nanostructured Co3O4@SiO2 Core-Shell Particles in a Laminar-Flow Reactor. Chem. Ing. Tech. 2017, 89, 963–967. [Google Scholar] [CrossRef]
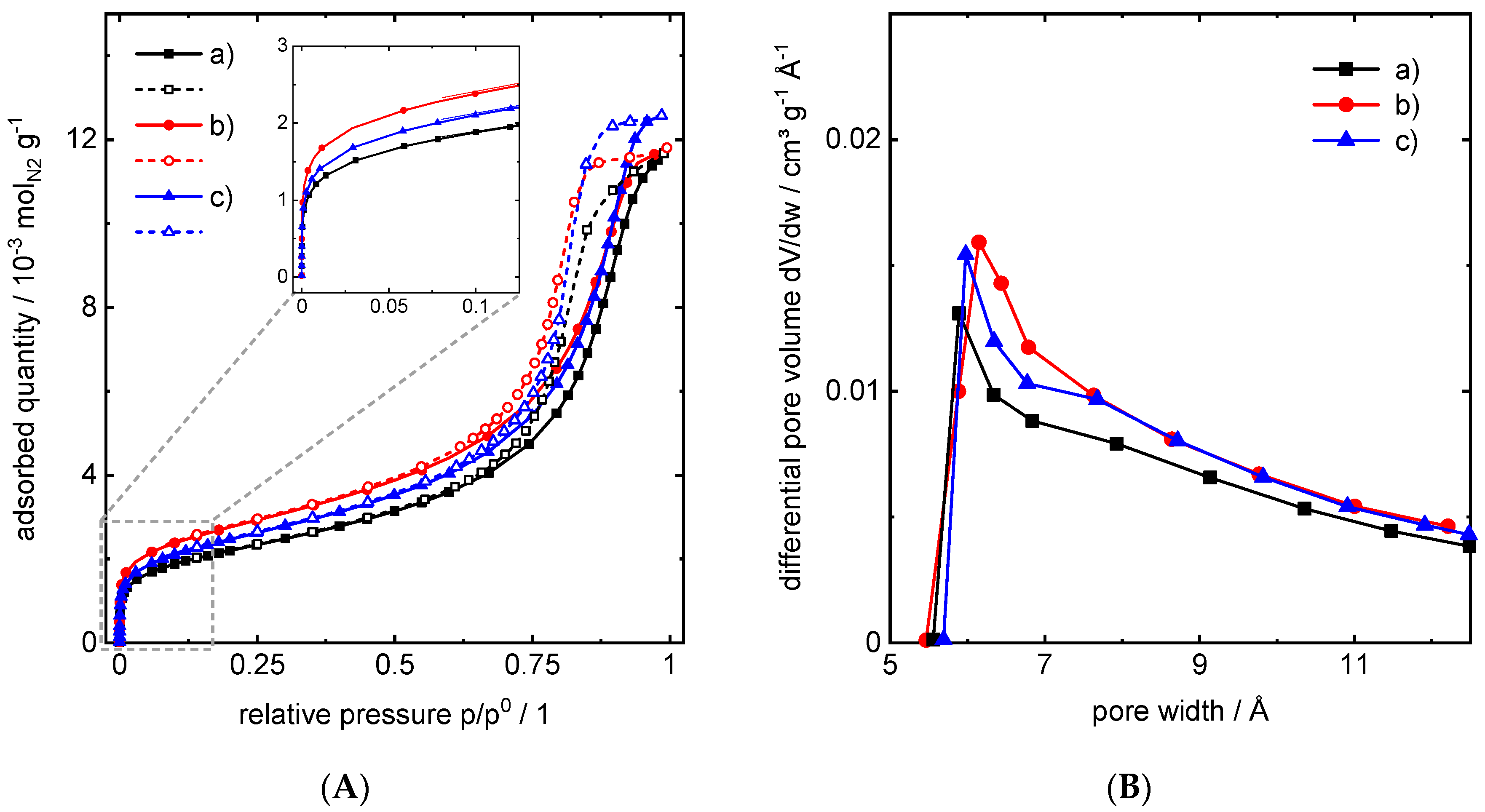


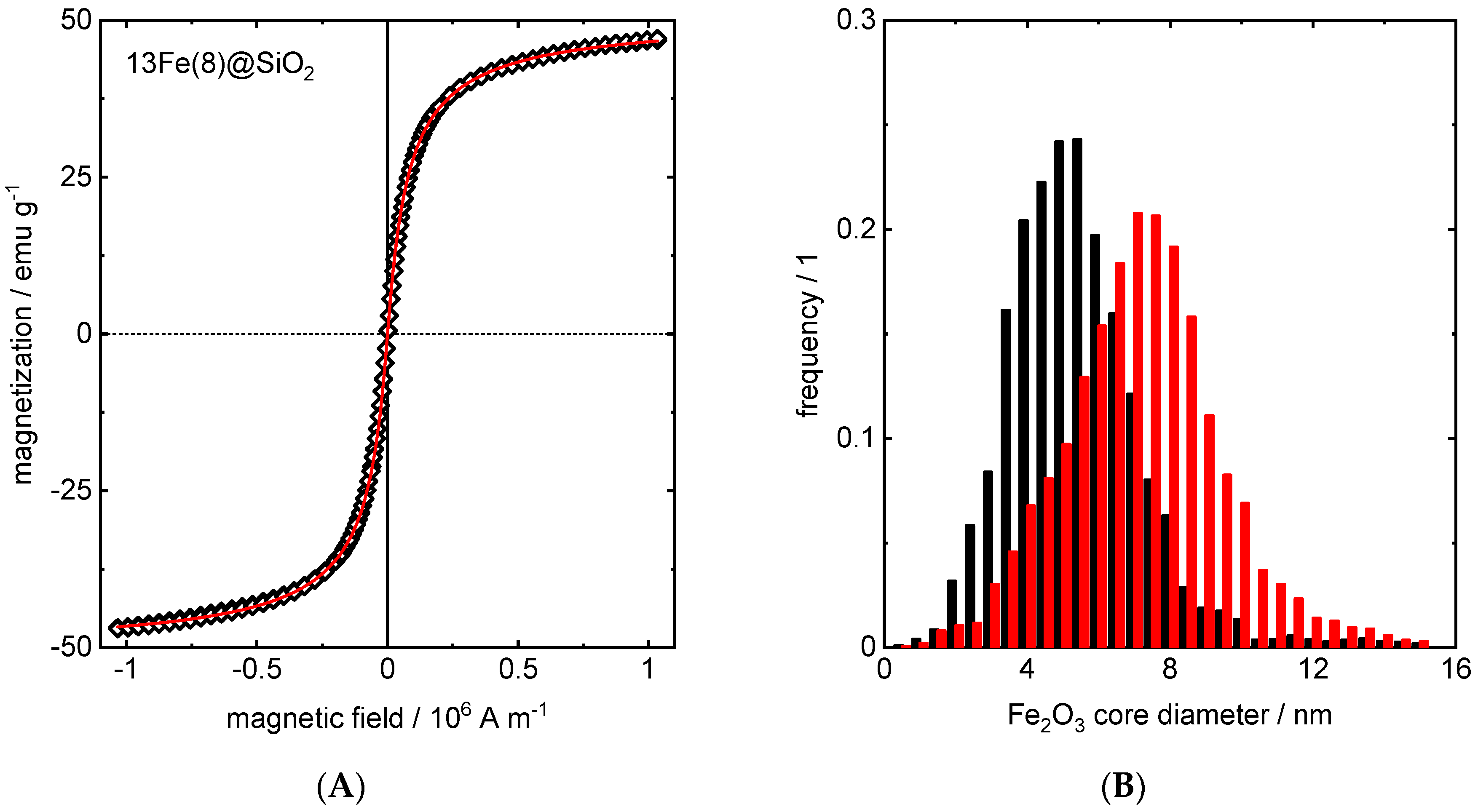

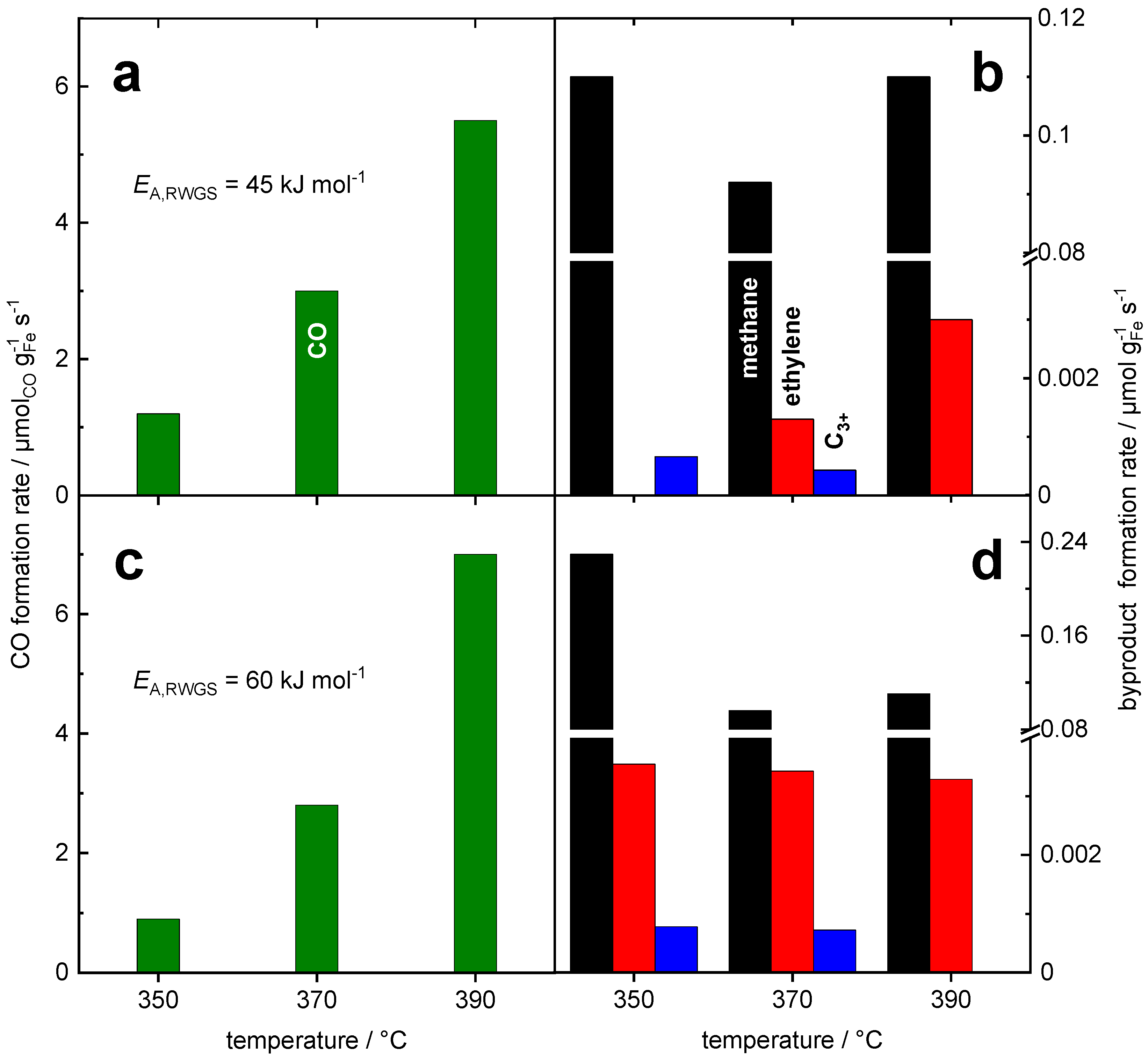

| Material | Micropore Width from HK | Interparticle Pore Width from BJH | Pore Volume from BJH Desorption | Micropore Volume from HK | Surface Area from BET |
|---|---|---|---|---|---|
| / | / | / | / | / | |
| 15Fe(4)@SiO2 | 7.8 | 13.1 | 0.39 | 0.046 | 200 |
| 15Fe(6)@SiO2 | 8.4 | 11.9 | 0.43 | 0.053 | 220 |
| 13Fe(8)@SiO2 | 7.9 | 13.4 | 0.40 | 0.046 | 180 |
| Material | Crystallite Phase | Crystallite Size | |
|---|---|---|---|
| (a) | 42Fe(6)@SiO2 | γ-Fe2O3 | 5.3 |
| (b) | 13Fe(8)@SiO2 | γ-Fe2O3 | 4.6 |
| (c) | 15Fe(6)@SiO2 | γ-Fe2O3 | 5.1 |
| (d) | 15Fe(4)@SiO2 | γ-Fe2O3 | 4.5 |
| Material | Fe-Oxide Core Size | Shell Thickness | Iron Content | Specific Fe Surface Area |
|---|---|---|---|---|
| / | / | / | ||
| 6Fe(4)@SiO2 | 4.2 ± 1.1 | 4.8 ± 0.4 | 6 | 351 |
| 15Fe(6)@SiO2 | 5.8 ± 0.9 | 3.8 ± 0.4 | 15 | 254 |
| 13Fe(8)@SiO2 | 7.8 ± 1.1 | 6.1 ± 0.6 | 13 | 189 |
| 20Fe(8)@SiO2 | 7.7 ± 1.5 | 5.1 ± 0.3 | 20 | 191 |
| Material | Fe-Oxide Core Size | Initial Magnetic Susceptibility |
|---|---|---|
| / | -/1 | |
| 6 Fe(4)@SiO2 | 5.5 ± 2.1 | 2.1 |
| 15 Fe(4)@SiO2 | 5.4 ± 1.9 | 1.9 |
| 15 Fe(6)@SiO2 | 6.0 ± 2.3 | 2.7 |
| 42 Fe(6)@SiO2 | 6.0 ± 2.0 | 2.5 |
| 13 Fe(8)@SiO2 | 7.2 ± 2.2 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrzycki, C.; Shao, R.; Misra, A.; Streb, C.; Herr, U.; Güttel, R. Iron Based Core-Shell Structures as Versatile Materials: Magnetic Support and Solid Catalyst. Catalysts 2021, 11, 72. https://doi.org/10.3390/catal11010072
Zambrzycki C, Shao R, Misra A, Streb C, Herr U, Güttel R. Iron Based Core-Shell Structures as Versatile Materials: Magnetic Support and Solid Catalyst. Catalysts. 2021; 11(1):72. https://doi.org/10.3390/catal11010072
Chicago/Turabian StyleZambrzycki, Christian, Runbang Shao, Archismita Misra, Carsten Streb, Ulrich Herr, and Robert Güttel. 2021. "Iron Based Core-Shell Structures as Versatile Materials: Magnetic Support and Solid Catalyst" Catalysts 11, no. 1: 72. https://doi.org/10.3390/catal11010072
APA StyleZambrzycki, C., Shao, R., Misra, A., Streb, C., Herr, U., & Güttel, R. (2021). Iron Based Core-Shell Structures as Versatile Materials: Magnetic Support and Solid Catalyst. Catalysts, 11(1), 72. https://doi.org/10.3390/catal11010072





