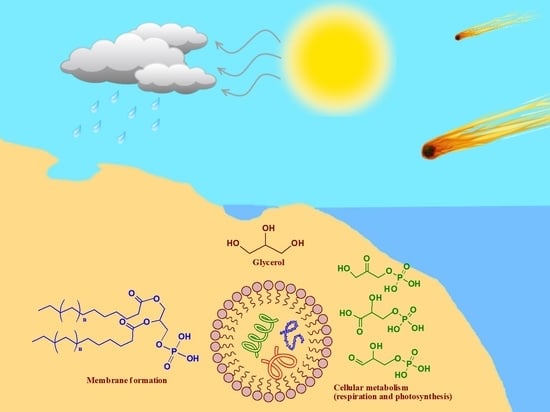
The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life
Abstract
:1. Introduction
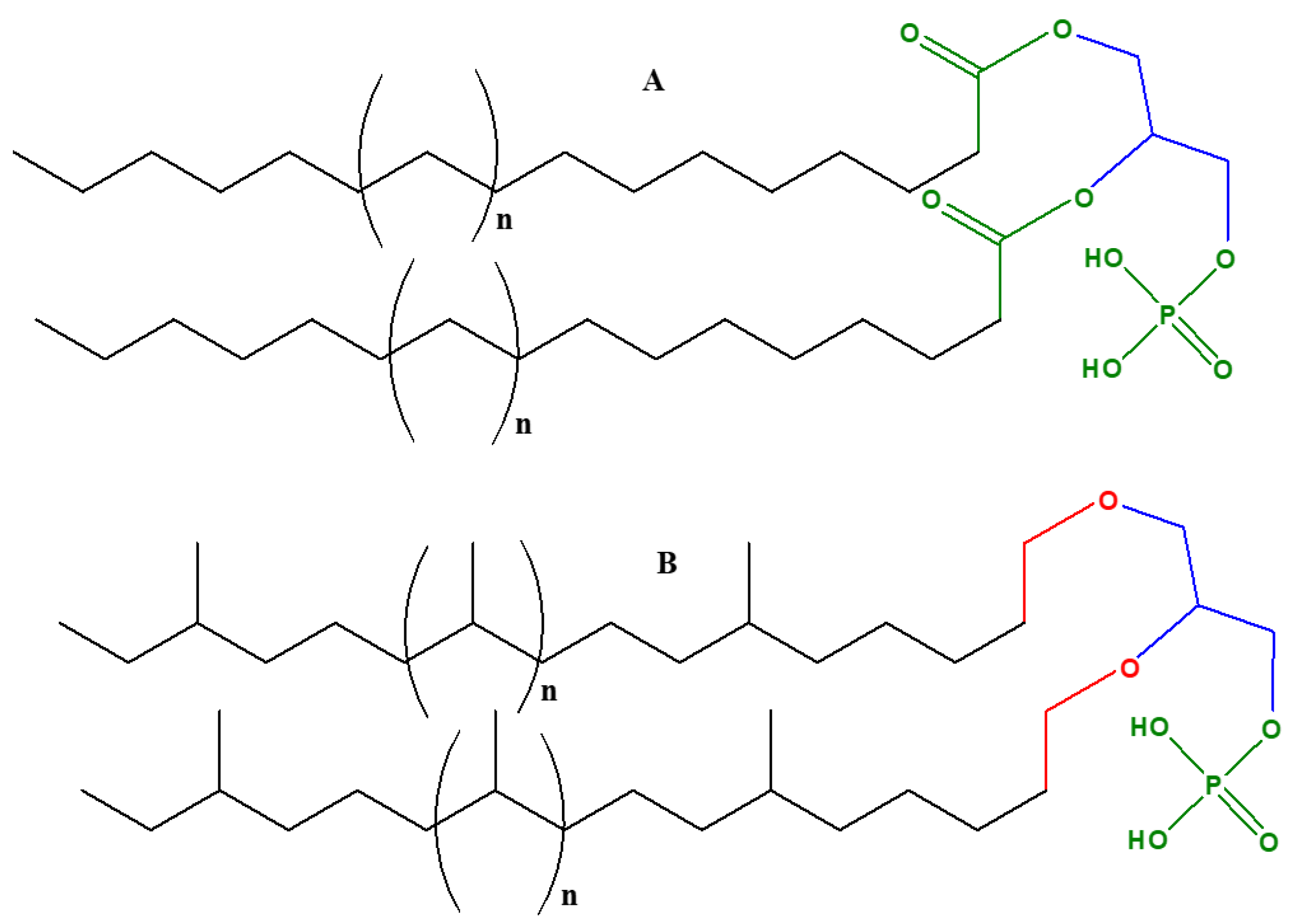
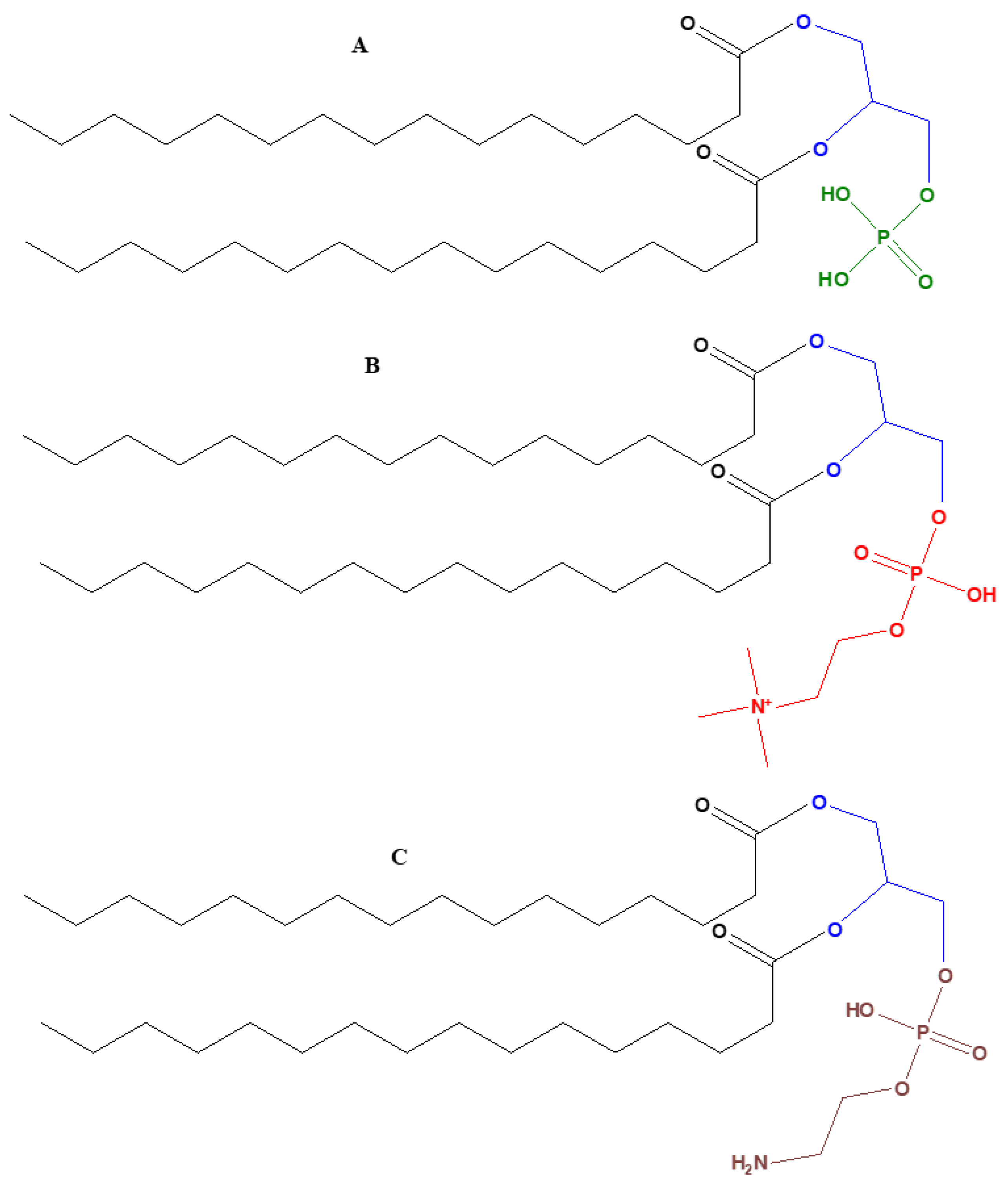
2. The Varieties of Glycerol Used by Modern Biochemistry
- (1)
- Their phospholipids are comprised of sn-glycerol-1-phosphate
- (2)
- The hydrophobic side chain is built of isoprenoid units
- (3)
3. Significance of Glycerol in the Biochemistry of Extremophiles
3.1. Role of Glycerol in the Biochemistry of Microalgae, Archaea and Other Organisms
3.2. Plausible Commercial Synthesis of Glycerol from Algae and Other Organisms
3.3. Glycerol and its Phosphorylated Derivatives as Solutes in Thermophiles
4. Prebiotic Origin of Glycerol
Extraterrestrial Sources of Glycerol
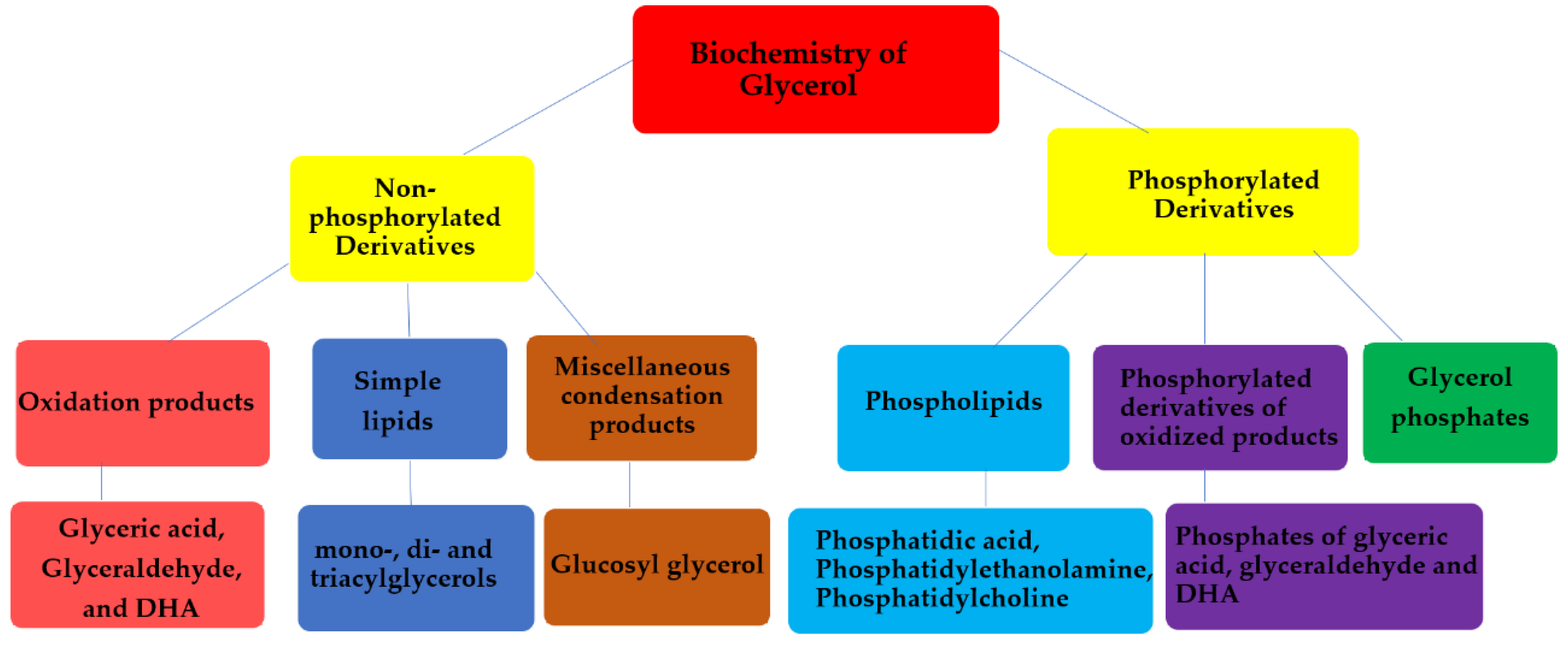
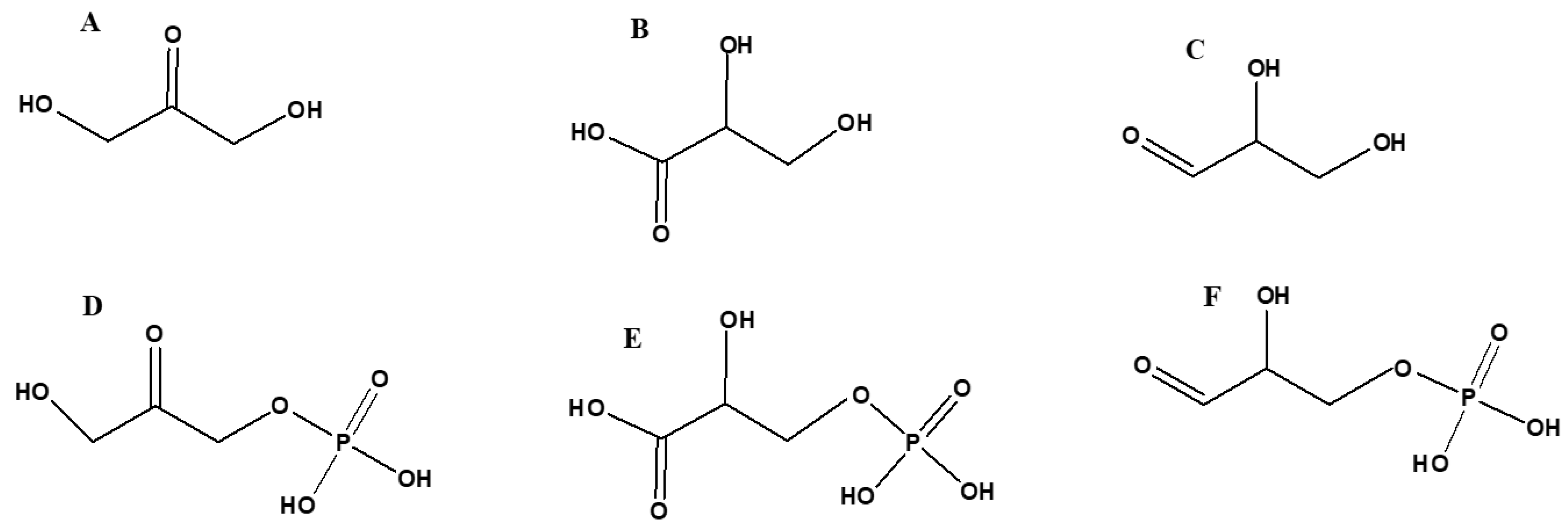
5. Biochemical Derivatives of Glycerol and their Prebiotic Origin
5.1. Acylglycerols
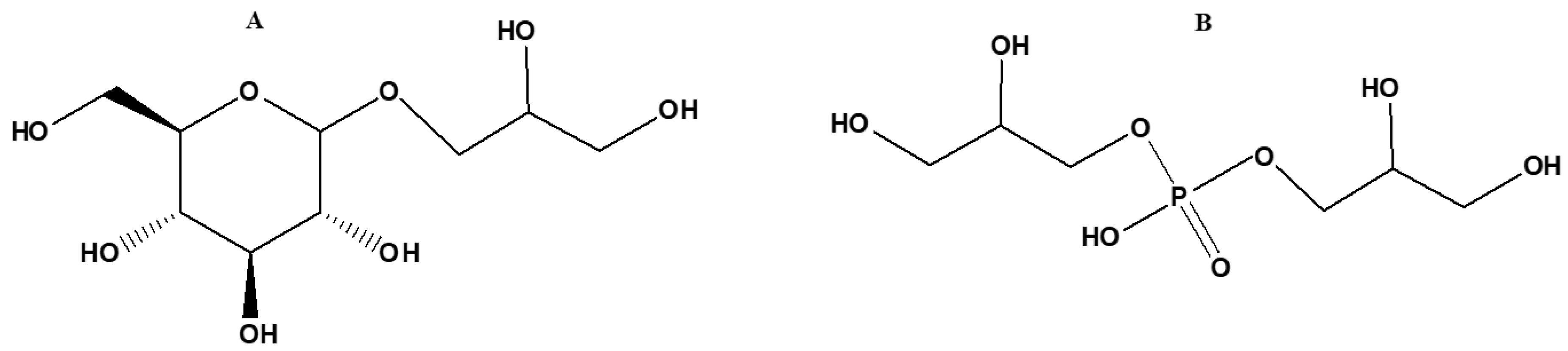
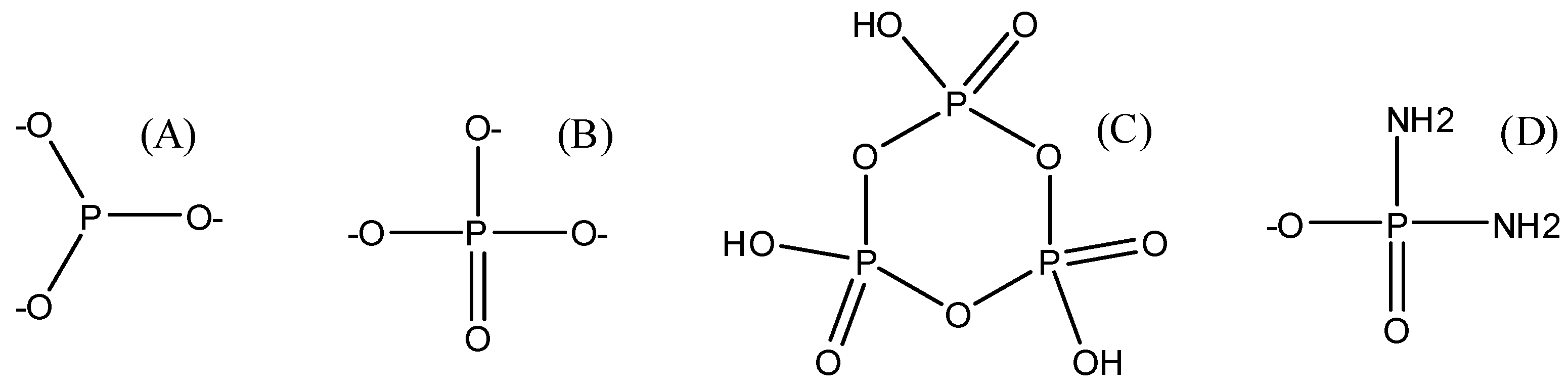
5.2. Glycerol Phosphates
5.3. Minerals and Condensation Agents in the Prebiotic Syntheses of Glycerol Phosphates
5.4. Role of Non-Aqueous Solvents in the Prebiotic Syntheses of Glycerol Phosphates
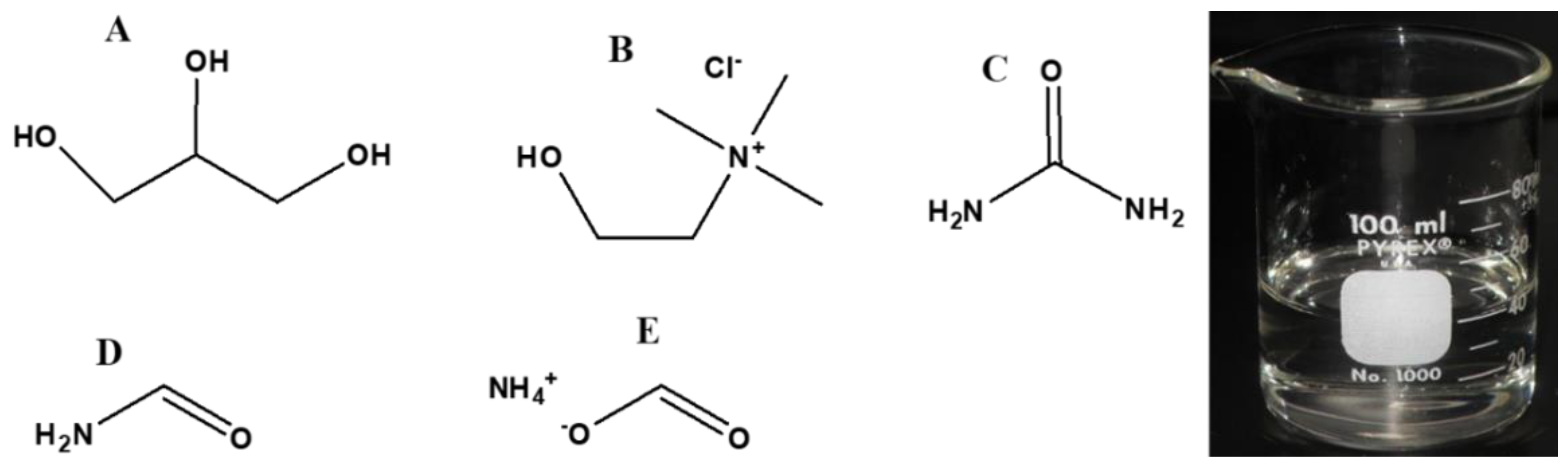
5.5. Formamide as a Prebiotic Solvent
5.6. Deep Eutectic Solvents
6. Extraterrestrial Sources of Glycerol Phosphates
7. Glycerol Phosphates as ‘Self-Replicating’ Molecules
8. Plausible Prebiotic Syntheses of Phospholipids on the Early Earth
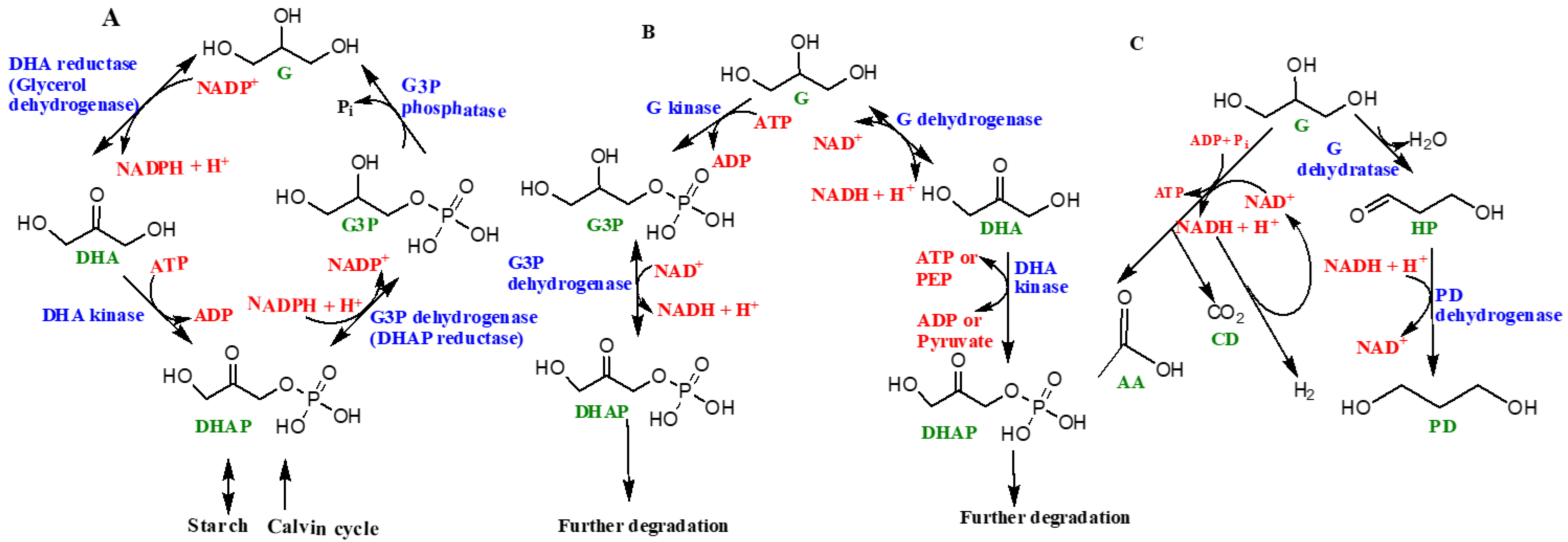
9. A Few More Key Derivatives of Glycerol Relevant to Origin of Life, Biochemistry, and Modern Life
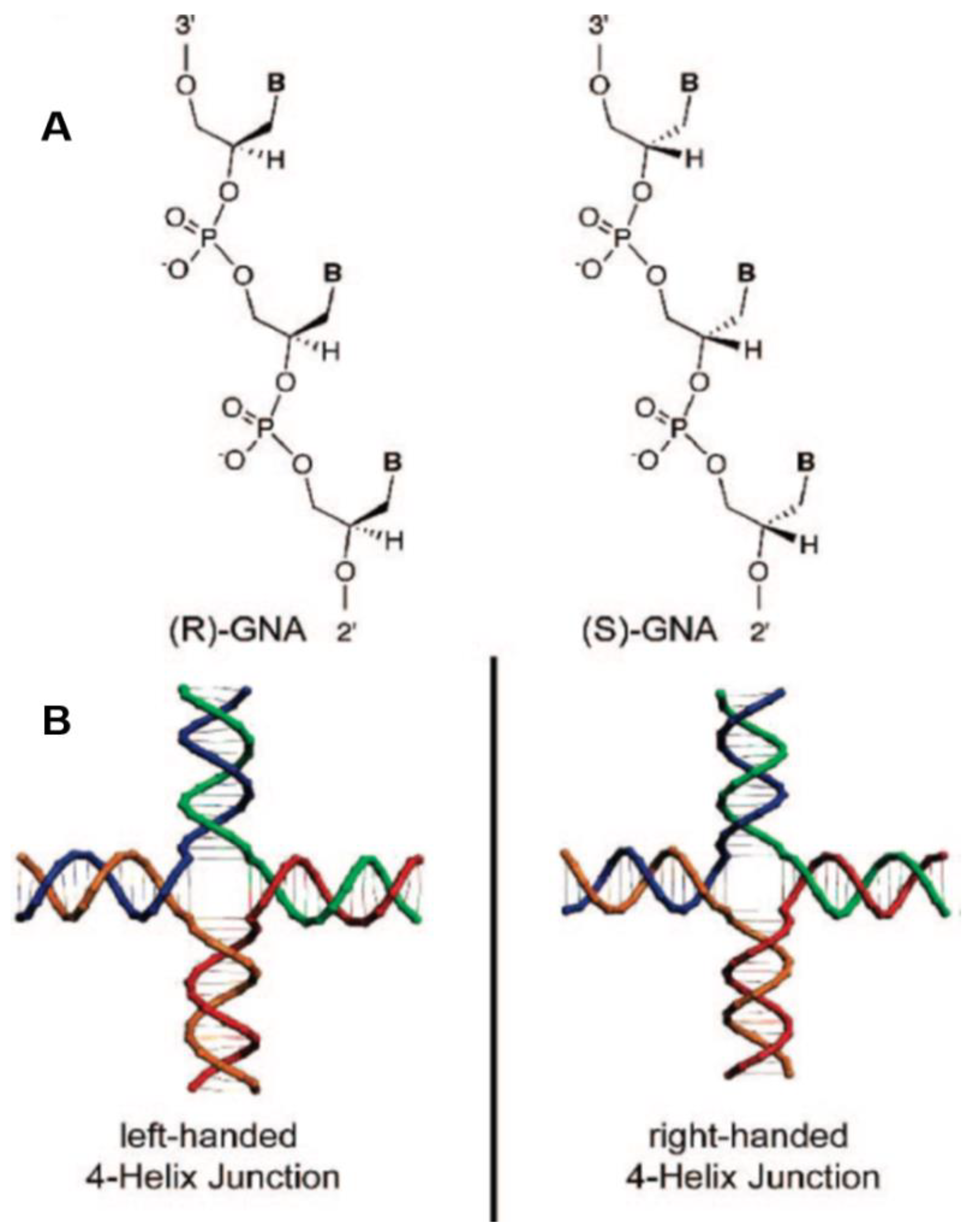
10. A Potential Prebiotic role for Glycerol in Nucleic Acids
11. Recapitulation and Closing Thoughts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary of Terms
| Aminonitrile | An organic compound that contains both an amino and a nitrile functional group. Relevant compounds such as α-amino nitriles are important intermediates in Strecker type synthesis of amino acids. |
| Cenancestor | The most recent ancestor (organism) from which living beings have evolved. |
| Chiral molecules | Tetrahedral organic centers (usually carbon) that have different arrangements of functional groups, resulting in 3D differences in structure. |
| Diamidophosphate | A type of high-energy condensed phosphate that has been known to efficiently phosphorylate organics such as sugars, alcohols, and other compounds. |
| Enantiomers | Chiral molecules that are mirror image of each other. |
| Eukaryotes and prokaryotes | Eukaryotes are organisms in which nucleus and genetic material is confined within the boundaries of a nuclear membrane whereas prokaryotes do not have a well-defined definite nuclear membrane. |
| GDPH | Also known as ‘Glycerol phosphate dehydrogenase’, this is an enzyme that catalyzes the reversible conversion of dihydroxyacetone phosphate to sn-glycerol-3-phosphate. This process involves a redox process. G1DPH catalyzes the glycerol-1-phosphate formation in primitive organisms such as archaea while G3DPH catalyzes the formation of glycerol-3-phosphate in modern organisms and in many bacteria. |
| Heterotrophic bacteria | Bacteria that cannot photosynthesize. |
| Isomers | Organic compounds having same molecular but different structural formulas. |
| Kiliani–Fischer type synthesis | A method of synthesizing monosaccharides. Cyanide (e.g., NaCN) is added via nucleophilic addition to the carbonyl group of a sugar, resulting in an increase in carbon number of the sugar. |
| Light and Dark reactions | The first stage of photosynthesis is to trap light energy and to form ATP and NADPH from light (commonly referred as ‘light reactions’). The second stage synthesizes glucose by CO2 fixation and utilizing stored chemical energy is called the ‘dark reaction’. |
| Lipolysis | The hydrolytic cleavage of ester bonds in the triglycerides to generate fatty acids and glycerol. |
| Mafic and ultramafic rocks | These are SiO2-poor igneous rocks: Mafic rocks are dominated with plagioclase and pyroxene and ultramafic rocks are rich in olivine and pyroxene. |
| Strecker type synthesis | An organic chemical reaction that produces amino acids by the reaction of an aldehyde with ammonium chloride and cyanide. |
| Racemic mixture | A mixture containing equal amounts of right and left-handed molecules. |
| Symbiosis | A long-term association between two living organisms which could either be beneficial or harmful, i.e., mutualism (association benefits both) or parasitism (one benefits, other is harmed). |
| The Embden-Meyerhof-Parnas pathway | The biochemical pathway that allows the consumption of glucose to generate ATP, NADH, and many other biosynthetic precursors, e.g., 3-phosphoglycerate or pyruvate. |
References
- Robergs, R.A.; Griffin, S.E. Glycerol Biochemistry, Pharmacokinetics and Clinical and Practical Applications. Sports Med. 1998, 26, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J.; et al. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430–E439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, M.; Khan, K.; Calder, G.; Kurpad, A. Glycerol exchange across the human forearm assessed by a Combination of tracer and arteriovenous exchange techniques. Clin. Sci. 1993, 84, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Bortz, W.M.; Paul, P.; Haff, A.C.; Holmes, W.L. Glycerol turnover and oxidation in man. J. Clin. Investig. 1972, 51, 1537–1546. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Taylor, K. Glycerol kinase activities from vertebrates and invertebrates. Biochem. J. 1969, 112, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.S.B.; Nahata, M.C.; Hilty, M.D. Glycerol: A review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1981, 1, 147–160. [Google Scholar] [CrossRef]
- Lin, E.C. Glycerol utilization and its regulation in mammals. Annu. Rev. Biochem. 1977, 46, 765–795. [Google Scholar] [CrossRef]
- Oscai, L.B.; Essig, D.A.; Palmer, W.K. Lipase regulation of muscle triglyceride hydrolysis. J. Appl. Physiol. 1990, 69, 1571–1577. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Rotondo, F.; Ho-Palma, A.C.; Remesar, X.; Fernández-López, J.A.; Romero, M.D.M.; Alemany, M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci. Rep. 2017, 7, 8983. [Google Scholar] [CrossRef] [Green Version]
- Jansson, P.A.; Larsson, A.; Smith, U.; Lönnroth, P. Glycerol production in subcutaneous adipose tissue of lean and obese humans. J. Clin. Investig. 1992, 89, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Langin, D. Control of fatty acid and glycerol release in adipose tissue lipolysis. Comptes Rendus Biol. 2006, 329, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Bombeck, C.T.; Sigel, B.; Tauber, J.W.; Jeffay, H. Glycerokinase activity in human adipose-tissue as related to obesity. Int. J. Obesity 1984, 8, 609–622. [Google Scholar]
- Ross, B.D.; Hems, R.; Krebs, H.A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem. J. 1967, 102, 942–951. [Google Scholar] [CrossRef] [Green Version]
- Roca, P.; Sáinz, F.; González, M.; Alemany, M. Energetic components in the unincubated egg fractions of several avian species. Comp. Biochem. Physiol. B 1982, 72, 439–443. [Google Scholar] [CrossRef]
- Saito, H.; Posas, F. Response to hyperosmotic stress. Genetics 2012, 192, 289–318. [Google Scholar] [CrossRef] [Green Version]
- Engelking, L.R. Gluconeogenesis. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 225–230. [Google Scholar]
- Driedzic, W.R.; Short, C.E. Relationship between food availability, glycerol and glycogen levels in low-temperature challenged rainbow smelt Osmerus mordax. J. Exp. Biol. 2007, 210, 2866–2872. [Google Scholar] [CrossRef] [Green Version]
- Ewart, K.V.; Fletcher, G.L. Isolation and characterization of antifreeze proteins from smelt (Osmerus mordax) and Atlantic herring (Clupea harengus harengus). Can. J. Zool. 1990, 68, 1652–1658. [Google Scholar] [CrossRef]
- Raymond, J.A. Glycerol is a colligative antifreeze in some northern fishes. J. Exp. Zool. 1992, 262, 347–352. [Google Scholar] [CrossRef]
- Klein, M.; Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Glycerol metabolism and transport in yeast and fungi: Established knowledge and ambiguities. Environ. Microbiol. 2017, 19, 878–893. [Google Scholar] [CrossRef] [Green Version]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Xiberras, J.; Klein, M.; Nevoigt, E. Glycerol as a substrate for Saccharomyces cerevisiae based bioprocesses—Knowledge gaps regarding the central carbon catabolism of this ‘non-fermentable’ carbon source. Biotechnol. Adv. 2019, 37, 107378. [Google Scholar] [CrossRef] [PubMed]
- Nevoigt, E.; Stahl, U. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1997, 21, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, A. Metabolic surprises in Saccharomyces cerevisiae during adaptation to saline conditions: Questions, some answers and a model. FEMS Microbiol. Lett. 2000, 182, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Fang, H.; Rao, Z.; Shen, W.; Zhuge, B.; Wang, Z.; Zhuge, J. Cloning and characterization of a NAD+-dependent glycerol-3-phosphate dehydrogenase gene from Candida glycerinogenes, an industrial glycerol producer. FEMS Yeast Res. 2008, 8, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Cedric, B.; Jörg, S. Glycerol metabolism and its implication in virulence in Mycoplasma. FEMS Microbiol. Rev. 2017, 41, 640–652. [Google Scholar]
- Córdova, A.; Notz, W.; Barbas, C.F., 3rd. Direct organocatalytic aldol reactions in buffered aqueous media. Chem. Commun. 2002, 24, 3024–3025. [Google Scholar]
- Clough, S.R. Glyceraldehyde. In Encyclopedia of Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 448–449. [Google Scholar]
- Björkman, O. Comparative studies on photosynthesis in higher plants. In Photophysiology Current Topics in Photobiology and Photochemistry; Giese, A.C., Ed.; Academic Press: Cambridge, MA, USA, 1973; Volume 8, pp. 1–63. [Google Scholar]
- Chozhavendhan, S.; Devi, G.K.; Bharathiraja, B.; Kumar, R.P.; Elavazhagan, S. Assessment of crude glycerol utilization for sustainable development of biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts. Technology, Advances, Life Cycle Assessment, and Economics; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 195–212. [Google Scholar]
- Fayolle, D.; Altamura, E.; D’Onofrio, A.; Madanamothoo, W.; Fenet, B.; Mavelli, F.; Buchet, R.; Stano, P.; Fiore, M.; Strazewski, P. Crude phosphorylation mixtures containing racemic lipid amphiphiles self-assemble to give stable primitive compartments. Sci. Rep. 2017, 7, 18106. [Google Scholar] [CrossRef] [Green Version]
- Weis, R.M.; McCollen, H.M. Two-dimensional chiral crystals of phospholipids. Nature 1984, 310, 47–49. [Google Scholar] [CrossRef]
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. 2012, 10, 507–515. [Google Scholar] [CrossRef]
- Peretó, J.; López-García, P.; Moreira, D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004, 29, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kates, M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog. Chem. Fats Other Lipds 1977, 15, 301–342. [Google Scholar] [CrossRef]
- Kate, M. Membrane lipids of archaea. In New Comprehensive Biochemistry; Kates, M., Kushner, D.J., Matheson, A.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 26, pp. 261–295. [Google Scholar]
- Koga, Y. Thermal Adaptation of the Archaeal and Bacterial Lipid Membranes. Archaea 2012, 2012, 789652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Caforio, A.; Driessen, A.J. Biosynthesis of archaeal membrane ether lipids. Front. Microbiol. 2014, 5, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, Y.; Kyuragi, T.; Nishihara, M. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycero-phosphate backbones caused the separation of the two lines of descent. J. Mol. Evol. 1998, 46, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. Ser. B 2003, 358, 59–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wächtershäuser, G. From pre-cells to eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Fiore, M.; Buchet, R. Symmetry Breaking of Phospholipids. Symmetry 2020, 12, 1488. [Google Scholar] [CrossRef]
- Rampelotto, P.H. Extremophiles and Extreme Environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context. Front. Microbiol. 2019, 10, 780. [Google Scholar]
- Javaux, E.J. Extreme life on Earth—Past, present and possibly beyond. Res. Microbiol. 2006, 157, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Glycerol metabolism in hypersaline environments. Environ. Microbiol. 2017, 19, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Craigie, J.S.; McLachlan, J. Glycerol as a photosynthetic product in Dunaliella tertiolecta Butcher. Can. J. Bot. 1964, 42, 777–778. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. The Role of Glycerol in the Osmotic Regulation of the Halophilic Alga Dunaliella parva. Plant Physiol. 1973, 51, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A. Adaptation of the unicellular alga Dunaliella parva to a saline environment. J. Phycol. 1975, 11, 50–54. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Brown, A.D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch. Microbiol. 1974, 96, 37–52. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Kessly, D.S.; Brown, A.D. The salt relations of Dunaliella. Further observations on glycerol production and its regulation. Arch Microbiol. 1977, 113, 131–138. [Google Scholar] [CrossRef]
- Brown, A.D. Microbial water stress. Bacteriol. Rev. 1976, 40, 803–846. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Avron, M. NADP specific dihydroxyacetone reductase from Dunaliella parva. FEBS Lett. 1973, 29, 153–155. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A.; Sussman, I.; Avron, M. Glycerol production by Dunaliella. In New Trends in Research and Utilization of Solar Energy through Biological Systems; Mislin, H., Bachofen, R., Eds.; Springer: Basel, Switzerland, 1982; Volume 38, pp. 49–52. [Google Scholar]
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol. Biochem. 2007, 45, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.S.; Rainey, F.A.; Nobre, M.F. The Prokaryotes. In The Genus Thermus and Relatives; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 7, pp. 797–812. [Google Scholar]
- Falb, M.; Müller, K.; Königsmaier, L.; Oberwinkler, T.; Horn, P.; von Gronau, S.; Gonzalez, O.; Pfeiffer, F.; Bornberg-Bauer, E.; Oesterhelt, D. Metabolism of halophilic archaea. Extremophiles 2008, 12, 177–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawal, N.; Kelkar, S.M.; Altekar, W. Alternative routes of carbohydrate metabolism in halophilic archaebacteria. Indian J. Biochem. Biophys. 1988, 25, 674–686. [Google Scholar] [PubMed]
- Patil, Y.; Junghare, M.; Muller, N. Fermentation of glycerol by Anaerobium acetethylicum and its potential use in biofuel production. Microb. Biotechnol. 2017, 10, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Junghare, M.; Pester, M.; Müller, N.; Schink, B. Characterization and phylogeny of Anaerobium acetethylicum gen. nov., sp. nov., a strictly anaerobic gluconate-fermenting bacterium isolated from a methanogenic bioreactor. Int. J. Syst. Evol. Microbiol. 2015, 65, 3289–3296. [Google Scholar] [CrossRef]
- Hidalgo, M.; Puerta-Fernández, E. Fermentation of glycerol by a newly discovered anaerobic bacterium: Adding value to biodiesel production. Microb. Biotechnol. 2017, 10, 528–530. [Google Scholar] [CrossRef]
- Stevenson, A.; Cray, J.A.; Williams, J.P.; Santos, R.; Sahay, R.; Neuenkirchen, N.; McClure, C.D.; Grant, I.R.; Houghton, J.D.; Quinn, J.P.; et al. Is there a common water-activity limit for the three domains of life? ISME J. 2015, 9, 1333–1351. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, A.; Hamill, P.G.; Medina, Á.; Kminek, G.; Rummel, J.D.; Dijksterhuis, J.; Timson, D.J.; Magan, N.; Leong, S.L.; Hallsworth, J.E. Glycerol enhances fungal germination at the water-activity limit for life. Environ. Microbiol. 2017, 19, 947–967. [Google Scholar] [CrossRef] [Green Version]
- Norkrans, B.; Kylin, A. Regulation of the potassium to sodium ratio and of the osmotic potential in relation to salt tolerance in yeasts. J. Bacteriol. 1969, 100, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Pasteur, M.L. Production constante de glycérine dans la fermentation alcoolique. C. R. Acad. Sci. 1858, 46, 857. (In French) [Google Scholar]
- Semkiv, M.V.; Ruchala, J.; Dmytruk, K.V.; Sibirny, A.A. 100 Years Later, What Is New in Glycerol Bioproduction? Trends Biotechnol. 2020, 38, 907–916. [Google Scholar] [CrossRef]
- Santos, H.; da Costa, M.S. Compatible solutes of organisms that live in hot saline environments. Environ. Microbiol. 2002, 4, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfredsson, G.A.; Kristjánsson, J.K.; Hjörleifsdottir, S.; Stetter, K.O. Rhodothermus marinus, gen. nov. sp. nov., a thermophilic halophilic bacterium from submarine hot springs in Iceland. J. Gen. Microbiol. 1988, 134, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Ventosa, A.; Nieto, J.J.; Oren, A. Biology of aerobic moderately halophilic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 504–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrei, A.; Banciu, H.L.; Oren, A. Living with salt: Metabolic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol. Lett. 2012, 330, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, D.T. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiol. Rev. 2000, 24, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Benaroudj, N.; Hee Lee, H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.; da Costa, M.S. Organic solutes from thermophiles and hyperthermophiles. Meth. Enzymol. 2001, 334, 302–315. [Google Scholar]
- Martins, L.O.; Huber, R.; Huber, H.; Stetter, K.O.; da Costa, M.S.; Santos, H. Organic solutes in hyperthermophilic archaea. Appl. Environ. Microbiol. 1997, 63, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Lamosa, P.; Burke, A.; Peist, R.; Huber, R.; Liu, M.-Y.; Silva, G.; Rodrigues-Pousada, C.; LeGall, J.; Maycock, C.; Santos, H. Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from the hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 2000, 66, 1974–1979. [Google Scholar] [CrossRef] [Green Version]
- da Costa, M.S.; Santos, H.; Galinski, E.A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 1998, 61, 117–153. [Google Scholar]
- Borges, N.; Ramos, A.; Raven, N.D.; Sharp, R.J.; Santos, H. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 2002, 6, 209–216. [Google Scholar] [CrossRef]
- McCollom, T.M.; Seewald, J.S. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 2007, 107, 382–401. [Google Scholar] [CrossRef] [PubMed]
- Bassez, M.-P. Is high-pressure water the cradle of life? J. Phys. Condens. Matter 2003, 15, L353. [Google Scholar] [CrossRef]
- Lopez, A.; Fiore, M. Investigating prebiotic protocells for a comprehensive understanding of the origins of life: A prebiotic systems chemistry perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef] [PubMed]
- McCollom, T.M.; Simoneit, B.R.T. Abiotic formation of hydrocarbons and oxygenated compounds during thermal decomposition of iron oxalate. Orig. Life Evol. Biosph. 1999, 29, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Baross, J.A.; Delaney, J.R. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 2002, 30, 385–491. [Google Scholar] [CrossRef] [Green Version]
- Westall, F.; Hickman-Lewis, K.; Hinman, N.; Gautret, P.; Campbell, K.A.; Bréhéret, J.G.; Foucher, F.; Hubert, A.; Sorieul, S.; Dass, A.V.; et al. A hydrothermal-sedimentary context for the origin of life. Astrobiology 2018, 18, 259–293. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Matsuda, Y.; Sakazawa, C.; Matsuura, T. Formose reactions II. The photochemical formose reaction. Bull. Chem. Soc. Jpn. 1977, 50, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.K.; Schutte, W.; Greenberg, J.M.; Ferris, J.P.; Briggs, R.; Connor, S.; Van de Bult, C.P.; Baas, F. Photochemical reactions in interstellar grains photolysis of CO, NH3, and H2O. Orig. Life Evol. Biosph. 1985, 16, 21–40. [Google Scholar] [CrossRef]
- Briggs, R.; Ertem, G.; Ferris, J.P.; Greenberg, J.M.; McCain, P.J.; Mendoza-Gomez, C.X.; Schutte, W. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Orig. Life Evol. Biosph. 1992, 22, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Bredehöft, J.H.; Meierhenrich, U.J.; d’Hendecourt, L.; Thiemann, W.H. Urea, Glycolic acid, and glycerol in an oganic residue produced by ultraviolet irradiation of interstellar/pre-cometary ice analogs. Astrobiology 2010, 10, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R.I.; Maity, S.; Jones, B.M. Synthesis of prebiotic glycerol in interstellar ices. Angew. Chem. Int. Ed. 2015, 54, 195–200. [Google Scholar] [CrossRef]
- Meinert, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; d’Hendecourt, L.S.; Meierhenrich, U.J. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Civiš, S.; Szabla, R.; Szyja, B.M.; Smykowski, D.; Ivanek, O.; Knížek, A.; Kubelík, P.; Šponer, J.; Ferus, M.; Šponer, J.E. TiO2-catalyzed synthesis of sugars from formaldehyde in extraterrestrial impacts on the early Earth. Sci. Rep. 2016, 6, 23199. [Google Scholar] [CrossRef]
- Fedoseev, G.; Chuang, K.-J.; Qasim, D.; Linnartz, H.; Ioppolo, S.; van Dishoeck, E.F.; Linnartz, H. Formation of glycerol through hydrogenation of CO ice under prestellar core conditions. Astrophys. J. 2017, 842, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cronin, J.R.; Chang, S. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorite. In The Chemistry of Life’s Origins; Greenberg, J.M., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 209–258. [Google Scholar]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cooper, G.W.; Flynn, G.J. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 625–651. [Google Scholar]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef]
- Eichberg, J.; Sherwood, E.; Epps, D.E.; Oró, J. Cyanamide mediated syntheses under plausible primitive earth conditions. IV. The synthesis of acylglycerols. J. Mol. Evol. 1977, 10, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Odom, D.G.; Lahav, N.; Chang, S. Prebiotic nucleotide oligomerization in a fluctuating environment: Effects of kaolinite and cyanamide. J. Mol. Evol. 1979, 12, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tsanakopoulou, M.; Sutherland, J.D. Cyanamide as a prebiotic phosphate activating agent–catalysis by simple 2-oxoacid salts. Chem. Comm. 2017, 53, 11893–11896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Ohri, A.; Sharma, P. Could Purines Be Formed from Cyanamide and Cyanoacetylene in a Prebiotic Earth Environment? ACS Omega 2019, 4, 12771–12781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Österberg, R.; Orgel, L.E.; Lohrmann, R. Further studies of urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973, 2, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.I.; Simoneit, B.R.T. Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig. Life Evol. Biosph. 2006, 36, 93–108. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoeit, B.R.T.; Deamer, D.W. Abiotic formation of acylglycerols under simulated hydrothermal conditions and self-assembly properties of such lipid products. Adv. Space Res. 2007, 40, 1649–1658. [Google Scholar]
- Rushdi, A.I.; Simoneit, B.R.T. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400 °C. Orig. Life Evol. Biosph. 2001, 31, 103–118. [Google Scholar] [CrossRef]
- Fiore, M.; Strazewski, P. Prebiotic lipidic amphiphiles and condensing agents on the early Earth. Life 2016, 6, 17. [Google Scholar] [CrossRef]
- Oro, J. Chemical synthesis of lipids and the origin of life. J. Biol. Phys. 1995, 20, 135–147. [Google Scholar] [CrossRef]
- Maurer, S.E.; Deamer, D.W.; Boncella, J.M.; Monnard, P.A. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 2009, 9, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Epps, D.E.; Nooner, D.W.; Eichberg, J.; Sherwood, E.; Oró, J. Cyanamide mediated synthesis under plausible primitive earth conditions. VI. The synthesis of glycerol and glycerophosphates. J. Mol. Evol. 1979, 14, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Ge, T.; Yingwu, W.; Chao, H.; Zhan, S.; Hongming, Y.; Shouhua, F. Resolving the enigma of prebiotic C-O-P bond formation: Prebiotic hydrothermal synthesis of important biological phosphate esters. Heteroat. Chem. 2010, 21, 161–167. [Google Scholar]
- Gull, M.; Cafferty, B.J.; Hud, N.V.; Pasek, M.A. Silicate-promoted phosphorylation of glycerol in non-aqueous solvents: A prebiotically plausible route to organophosphates. Life 2017, 7, 29. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Burcar, B.; Pasek, M.A.; Gull, M.; Cafferty, B.J.; Velasco, F.; Hud, N.V. Darwin’s warm little pond: A one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed. 2016, 55, 13249–13253. [Google Scholar] [CrossRef]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2018, 10, 212–217. [Google Scholar] [CrossRef]
- Gull, M.; Pasek, M.A. Is struvite a prebiotic mineral? Life 2013, 3, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Pasek, M.A.; Kee, T.P. On the origin of phosphorylated biomolecules. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 57–84. [Google Scholar]
- Schoffstall, A.M. Prebiotic phosphorylation of nucleosides in formamide. Orig. Life Evol. Biosph. 1976, 7, 399–412. [Google Scholar] [CrossRef]
- Schoffstall, A.M.; Mahone, S.M. Formate ester formation in amide solutions. Orig. Life Evol. Biosph. 1988, 18, 389–396. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kim, H.J.; Hutter, D.; Benner, S.A. Abiotic regioselective phosphorylation of adenosine with borate in formamide. Astrobiology 2015, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Pino, S.; Costanzo, G.; Di Mauro, E. From formamide to RNA: The roles of formamide and water in the evolution of chemical information. Res. Microbiol. 2009, 160, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.Y.; Krasavin, E.; Di Mauro, E. Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, 2746–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasek, M.A. Thermodynamics of Prebiotic Phosphorylation. Chem. Rev. 2019, 120, 4690–4706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira, V.K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; Agostino, C.D.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Austin, S.M.; Waddell, T.G. Prebiotic synthesis of vitamin B6- type compounds. Orig. Life Evol. Biosph. 1999, 29, 287–296. [Google Scholar] [CrossRef]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.L.; Schlesinger, G. Prebiotic syntheses of vitamin coenzymes: I. cysteamine and 2-mercaptoethane-sulfonic acid (coenzyme M). J. Mol. Evol. 1993, 36, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.L.; Burcar, B.T.; Hud, N.V.; Febrian, R.; Mehta, C.; Bracher, P.J.; Atlas, Z.D.; Pasek, M.A. The prebiotic provenance of semi-aqueous solvents. Orig. Life Evol. Biosph. 2020, 50, 1–14. [Google Scholar] [CrossRef]
- Schwartz, A.W. Phosphorus in prebiotic chemistry-an update and a note on plausibility. In Handbook of Astrobiology; Kolb, V., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 355–359. [Google Scholar]
- Gull, M.; Omran, A.; Feng, T.; Pasek, M.A. Silicate-, magnesium ion-, and urea-induced prebiotic phosphorylation of uridine via pyrophosphate; revisiting the hot drying water pool scenario. Life 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Gull, M. Prebiotic Phosphorylation Reactions on the Early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Pasek, M.A.; Gull, M.; Herschy, B. Phosphorylation on the early earth. Chem. Geol. 2017, 475, 149–170. [Google Scholar] [CrossRef]
- Hazen, R.M. Evolution of minerals. Sci. Am. 2010, 302, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A. Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 853–858. [Google Scholar] [CrossRef] [Green Version]
- Pasek, M.A.; Lauretta, D. Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig. Life Evol. Biosph. 2008, 38, 5–21. [Google Scholar] [CrossRef]
- Bryant, D.E.; Greenfield, D.; Richard, D.; Benjamin, W.; Johnson, R.G.; Herschy, B.; Smith, C.; Pasek, M.A.; Telford, R.; Scowen, I.; et al. Hydrothermal modification of the Sikhote-Alin iron meteorite under low pH geothermal environments. A plausibly prebiotic route to activated phosphorus on the early Earth. Geochim. Cosmochim. Acta 2013, 109, 90–112. [Google Scholar] [CrossRef]
- Pasek, M.A.; Lauretta, D.S. Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 2005, 5, 515–535. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 10089–10094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.L. Model of early self-replication based on covalent complementarity for a copolymer of glycerate-3-phosphate and glycerol-3-phosphate. Orig. Life Evol. Biosph. 1989, 19, 79–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walde, P. Surfactant assemblies and their various possible roles for the origin(s) of life. Orig. Life Evol. Biosph. 2006, 36, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef]
- Epps, D.E.; Sherwood, E.; Eichberg, J.; Oró, J. Cyanamide mediated syntheses under plausible primitive earth conditions V. The synthesis of phosphatidic acids. J. Mol. Evol. 1978, 11, 279–292. [Google Scholar] [CrossRef]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of Phosphatidylcholine under possible primitive earth conditions. J. Mol. Evol. 1982, 18, 196–202. [Google Scholar] [CrossRef]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of phosphatidylethanolamine under possible primitive earth conditions. J. Mol. Evol. 1987, 25, 1–6. [Google Scholar] [CrossRef]
- Bonfio, C.; Caumes, C.; Duffy, C.D.; Patel, B.H.; Percivalle, C.; Tsanakopoulou, M.; Sutherland, J.D. Length-selective synthesis of acylglycerol-phosphates through energy-dissipative cycling. J. Am. Chem. Soc. 2019, 141, 3934–3939. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Ricardo, A.; Illangkoon, H.; Kim, M.J.; Carrigan, M.A.; Frye, F.; Benner, S.A. Synthesis of Carbohydrates in Mineral-Guided Prebiotic Cycles. J. Am. Chem. Soc. 2011, 133, 9457–9468. [Google Scholar] [CrossRef]
- Simonov, A.N.; Pestunova, O.P.; Matvienko, L.G.; Snytnikov, V.N.; Snytnikova, O.A.; Tsentalovich, Y.P.; Parmon, V.N. Possible prebiotic synthesis of monosaccharides from formaldehyde in presence of phosphates. Adv. Space. Res. 2007, 40, 1634–1640. [Google Scholar] [CrossRef]
- Breslow, R.; Ramalingam, V.; Appayee, C. Catalysis of glyceraldehyde synthesis by primary or secondary amino acids under prebiotic conditions as a function of pH. Orig. Life Evol. Biosph. 2013, 43, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Cheng, Z.-L. L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 5723–5725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steer, A.M.; Bia, N.; Smith, D.K.; Clarke, P.A. Prebiotic synthesis of 2-deoxy-D-ribose from interstellar building blocks promoted by amino esters or amino nitriles. Chem. Commun. 2017, 53, 10362–10365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.L. Prebiotic sugar synthesis: Hexose and hydroxy acid synthesis from glyceraldehyde catalyzed by iron (III) hydroxide oxide. J. Mol. Evol. 1992, 35, 1–6. [Google Scholar] [CrossRef]
- Kolb, V.; Orgel, L.E. Phosphorylation of glyceric acid in aqueous solution using trimetaphosphate. Orig. Life Evol. Biosph. 1996, 26, 7–13. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Guntha, S.; Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 2000, 39, 2281–2285. [Google Scholar] [CrossRef]
- Coggins, A.; Powner, M. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 2017, 9, 310–317. [Google Scholar] [CrossRef]
- Imbault, A.L.; Gong, J.; Farnood, R. Photocatalytic production of dihydroxyacetone from glycerol on TiO2 in acetonitrile. RSC Adv. 2020, 10, 4956–4968. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.L. The triose model: Glyceraldehyde as a source of energy and monomers for prebiotic condensation reactions. Orig. Life Evol. Biosph. 1987, 17, 107–119. [Google Scholar] [CrossRef]
- Weber, A.L.; Hsu, V. Energy-rich glyceric acid oxygen esters: Implications for the origin of glycolysis. Orig. Life Evol. Biosph. 1990, 20, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.P.; Gladstone, G.R.; Yung, Y. Photochemical production of formaldehyde in Earth’s primitive atmosphere. Science 1980, 210, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Weber, A. Nonenzymatic formation of “energy-rich” lactoyl and glyceroyl thioesters from glyceraldehyde and a thiol. J. Mol. Evol. 1984, 20, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deamer, D.W. The first living systems: A bioenergetic perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, C.; Crowe, M.A.; Sutherland, J.D. Two-step potentially prebiotic synthesis of α-d-cytidine-5′-phosphate from D-glyceraldehyde-3-phosphate. J. Am. Chem. Soc. 2007, 129, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Tse, E.; Blackmond, D.G. A route to enantiopure RNA precursors from nearly racemic starting materials. Nat. Chem. 2011, 3, 704–706. [Google Scholar] [CrossRef]
- Orgel, L.E. The implausibility of metabolic cycles on the prebiotic Earth. PLoS Biol. 2008, 6, 005–0012. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.S.; McCullum, E.O.; Chaput, J.C. Synthesis of two mirror image 4-helix junctions derived from glycerol nucleic acid. J. Am. Chem. Soc. 2008, 130, 5846–5847. [Google Scholar] [CrossRef]
- Liu, H.; Gao, J.; Lynch, S.R.; Saito, Y.D.; Maynard, L.; Kool, E.T. A four-base paired genetic helix with expanded size. Science 2003, 302, 868–871. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-W.; Zhang, S.; McCullum, E.O.; Chaput, J.C. Experimental Evidence That GNA and TNA were not sequential polymers in the prebiotic evolution of RNA. J. Mol. Evol. 2007, 65, 289–295. [Google Scholar] [CrossRef]
- Karri, P.; Punna, V.; Kim, K.; Krishnamurthy, R. Base-pairing properties of a structural isomer of glycerol nucleic acid. Angew. Chem. Int. Ed. 2013, 52, 5840–5844. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Punna, V.; Karri, P.; Krishnamurthy, R. Synthesis of phosphoramidites of isoGNA, an isomer of glycerol nucleic acid. Beilstein J. Org. Chem. 2014, 10, 2131–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Kasamatsu, T.; Kaneko, T.; Saito, T. Production of Organic Compounds in Interstellar Space. In Exobiology: Matter, Energy, and Information in the Origin and Evolution of Life in the Universe; Chela-Flores, J., Raulin, F., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 213–216. [Google Scholar]
- Ehrenfreund, P.; Spaans, M.; Holm, N.G. The evolution of organic matter in space. Philos. Trans. R. Soc. A 2011, 369, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S. Complex organics in space from Solar System to distant galaxies. Astron. Astrophys. Rev. 2016, 24, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Hirakawa, N.; Matsubara, Y.; Yamashita, S.; Okuchi, T.; Asahina, K.; Tanaka, R.; Suzuki, N.; Naraoka, H.; Takano, Y.; et al. Precometary organic matter: A hidden reservoir of water inside the snow line. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Cami, J. Cosmic carbon chemistry: From the interstellar medium to the early Earth. Cold Spring Harb. Perspect. Biol. 2010, 2, a002097. [Google Scholar] [CrossRef] [Green Version]
- Mason, B. Organic matter from space. Sci. Am. 1963, 208, 43–49. [Google Scholar] [CrossRef]
- Oró, J.; Mills, T. Chemical evolution of primitive solar system bodies. Adv. Space Res. 1989, 9, 105–120. [Google Scholar] [CrossRef]
- Pohorille, A. From organic molecules in space to the origins of life and back. Adv. Space Res. 2002, 30, 1509–1520. [Google Scholar] [CrossRef]
- Cockell, C.S. The origin and emergence of life under impact bombardment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1845–1856. [Google Scholar] [CrossRef] [Green Version]
- Osinski, G.R.; Cockell, C.S.; Pontefract, A.; Sapers, H.M. The Role of Meteorite Impacts in the Origin of Life. Astrobiology 2020, 20, 1121–1149. [Google Scholar] [CrossRef] [PubMed]
- Fegley, B.; Prinn, R.G.; Hartman, H.; Watkins, G.H. Chemical effects of large impacts on the Earth’s primitive atmosphere. Nature 1986, 319, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.P.; Borucki, W.J. Organic synthesis in experimental impact shocks. Science 1997, 276, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P. Catalysis and prebiotic RNA synthesis. Orig. Life Evol. Biosph. 1993, 23, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P. Prebiotic synthesis on minerals: Bridging the prebiotic and RNA worlds. Biol. Bull. 1999, 196, 311–314. [Google Scholar] [CrossRef]
- Mattia Bizzarri, B.; Botta, L.; Pérez-Valverde, M.I.; Saladino, R.; Di Mauro, E.; García-Ruiz, J.M. Silica Metal Oxide Vesicles Catalyze Comprehensive Prebiotic Chemistry. Chemistry 2018, 24, 8126–8132. [Google Scholar] [CrossRef]
- Dalai, P.; Sahai, N. Mineral-lipid interactions in the origins of life. Trends Biochem. Sci. 2019, 44, 331–341. [Google Scholar] [CrossRef]
- Jheeta, S.; Joshi, P.C. Prebiotic RNA synthesis by montmorillonite catalysis. Life 2014, 4, 318–330. [Google Scholar] [CrossRef]
- Cleaves, H.J., II; Michalkova, S.A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef]
- Schoonen, M.; Smirnov, A.; Cohn, C. A perspective on the role of minerals in prebiotic synthesis. AMBIO 2004, 33, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Petit, S.; Cockell, C.S.; Albani, A.E.; Beaufort, D. The Fe-rich clay microsystems in basalt-komatiite lavas: Importance of Fe-smectites for pre-biotic molecule catalysis during the Hadean eon. Orig. Life Evol. Biosph. 2010, 40, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Siegel, B.Z.; Siegel, S.M. Enzyme-mimicking properties of silicates and other minerals. Adv. Space Res. 1981, 1, 27–36. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M. Clays and other minerals in prebiotic processes. Orig. Life Evol. Biosph. 1984, 14, 307–314. [Google Scholar] [CrossRef]
- Ertem, G. Montmorillonite, oligonucleotides, RNA and origin of life. Orig. Life Evol. Biosph. 2004, 34, 549–570. [Google Scholar] [CrossRef]
- Cockell, C.S.; Lee, P.; Broady, P.; Lim, D.S.S.; Osinski, J.; Parnell, J.; Koeberl, C.; Pesonen, L.; Salminen, J. Effects of asteroid and comet impacts on habitats for lithophytic organisms—A synthesis. Meteorit. Planet. Sci. 2005, 40, 1901–1916. [Google Scholar] [CrossRef]
- Cockell, C.S.; Lee, P. The biology of impact craters—A review. Biol. Rev. 2002, 77, 279–310. [Google Scholar] [CrossRef]



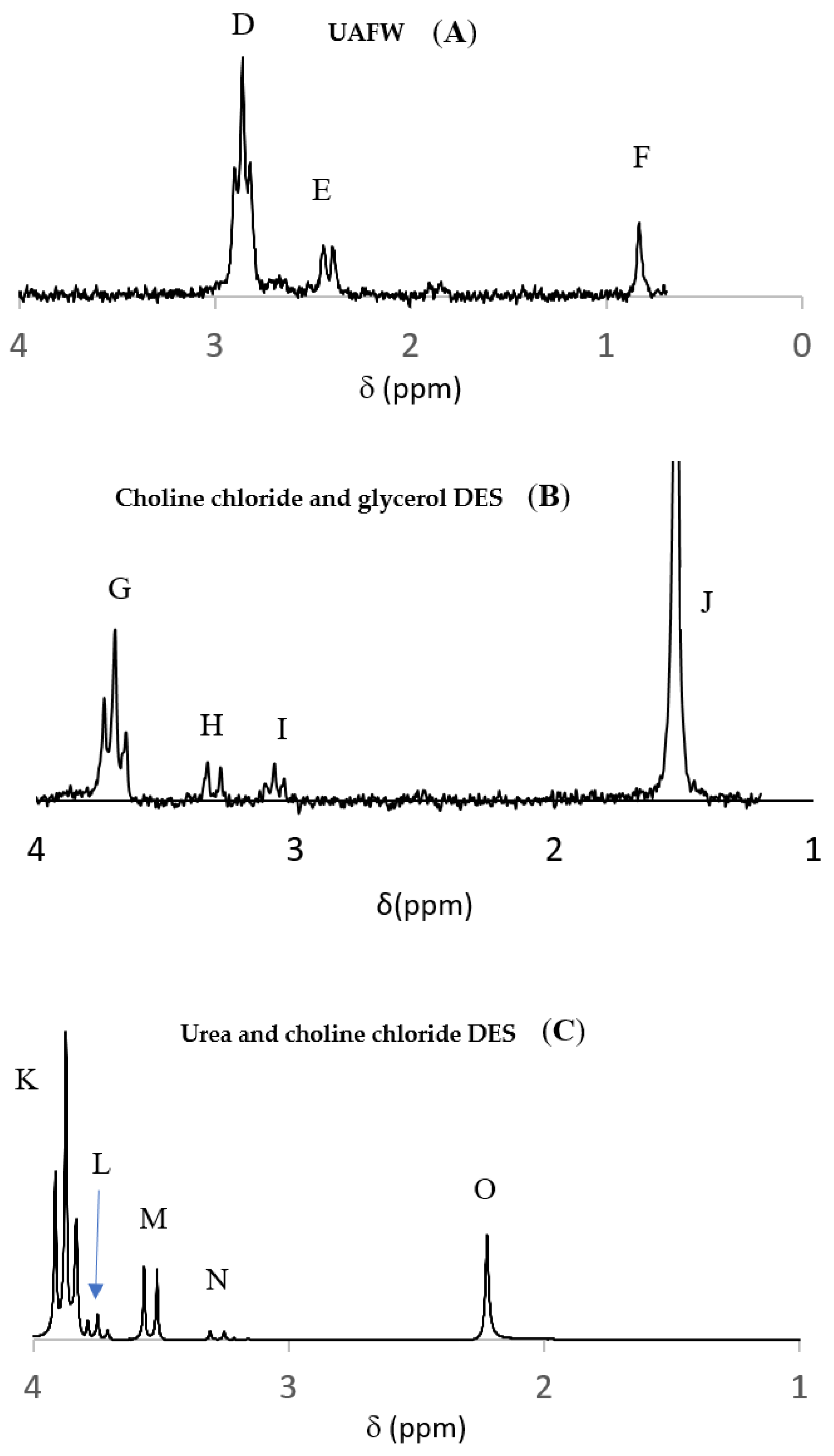


| 1 P Source | Catalyst/Condensation Agent | Reaction Conditions | 2 Yields | Ref. |
|---|---|---|---|---|
| NH4H2PO4 | urea/NH4Cl/cyanamide | heat at 85 °C, 16 h | 30% | [111] |
| H3PO4 | wide range of clays & minerals | hydrothermal conditions, 100–200 °C | 1% | [112] |
| TMP, NaH2PO4 | Silicates | formamide, DES of glycerol & choline chloride, 85 °C | 10–90% | [113] |
| NaH2PO4 monetite, H3PO3, struvite | - | DES of choline chloride & urea, heat 60–80 °C | 10–65% | [114] |
| Na2HPO4, P minerals | - | UAFW, wet-dry cycles, 65 or 80 °C | 10–50% | [115] |
| DAP | imidazole | room temp-50 °C, paste reactions | 12–60% | [116] |
| struvite, monetite | - | 75 °C, heating leading to dryness | 28% | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gull, M.; Pasek, M.A. The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life. Catalysts 2021, 11, 86. https://doi.org/10.3390/catal11010086
Gull M, Pasek MA. The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life. Catalysts. 2021; 11(1):86. https://doi.org/10.3390/catal11010086
Chicago/Turabian StyleGull, Maheen, and Matthew A. Pasek. 2021. "The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life" Catalysts 11, no. 1: 86. https://doi.org/10.3390/catal11010086
APA StyleGull, M., & Pasek, M. A. (2021). The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life. Catalysts, 11(1), 86. https://doi.org/10.3390/catal11010086