Effect of Pyrolysis Conditions on the Performance of Co–Doped MOF–Derived Carbon Catalysts for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results
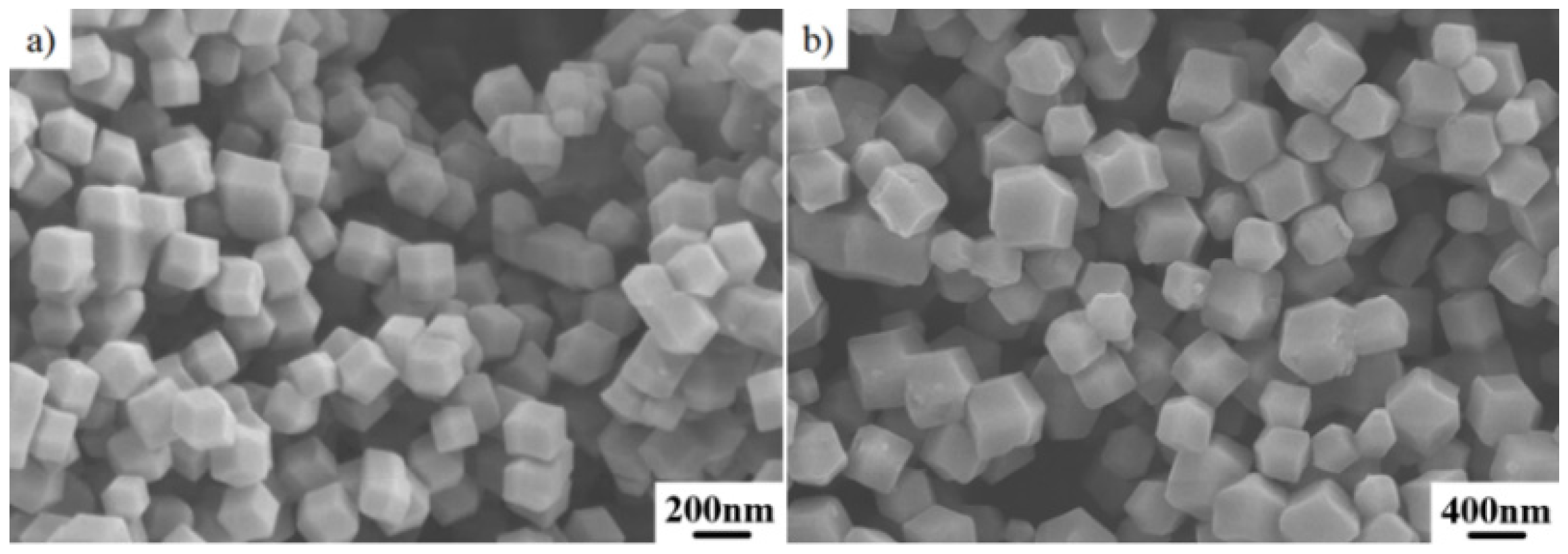
2.1. Initial Co–Doped ZIF–8 Precursor
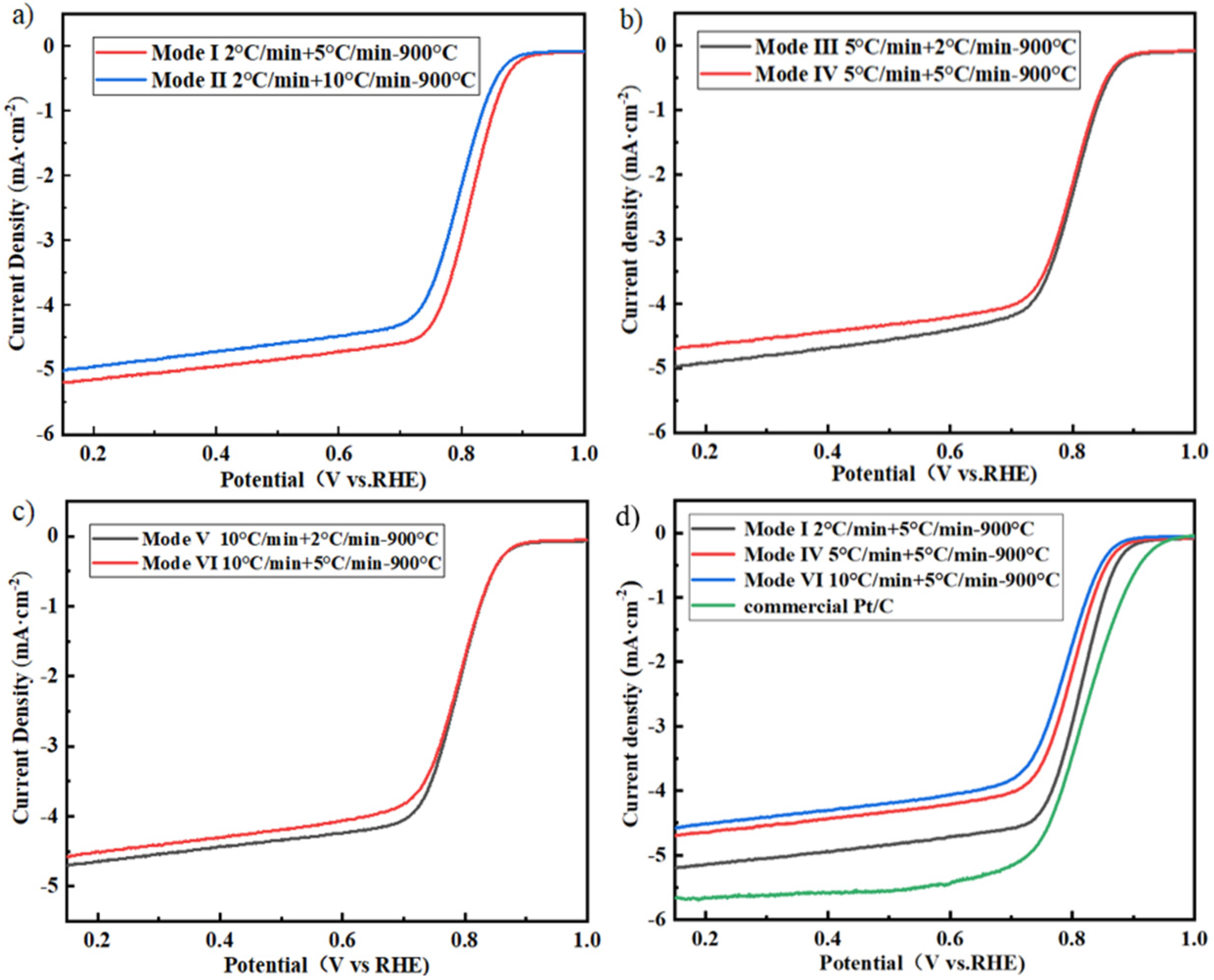
2.2. Effect of Pyrolysis Temperature and Heating Rate on the Catalyst during One–Step Pyrolysis
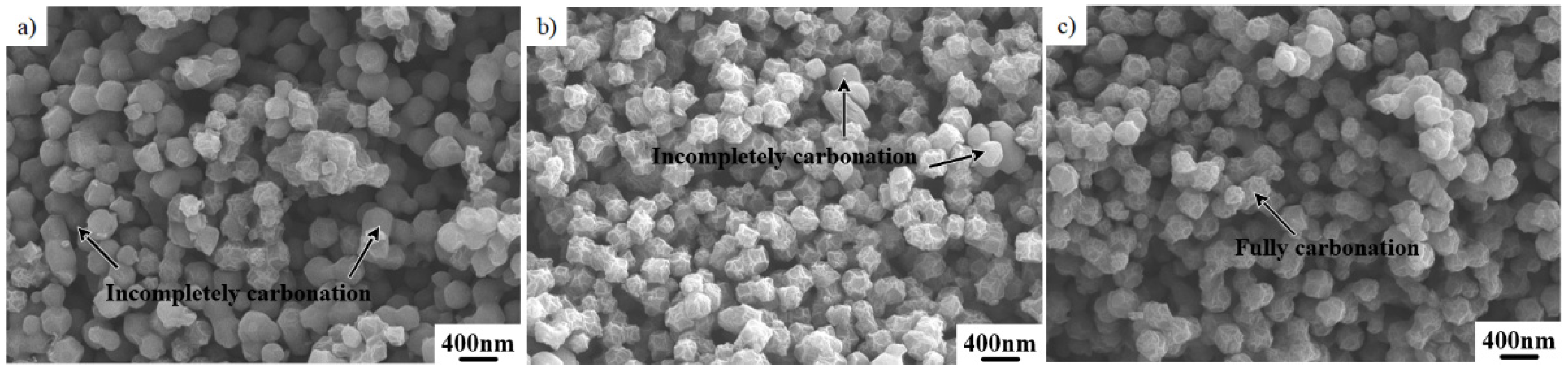
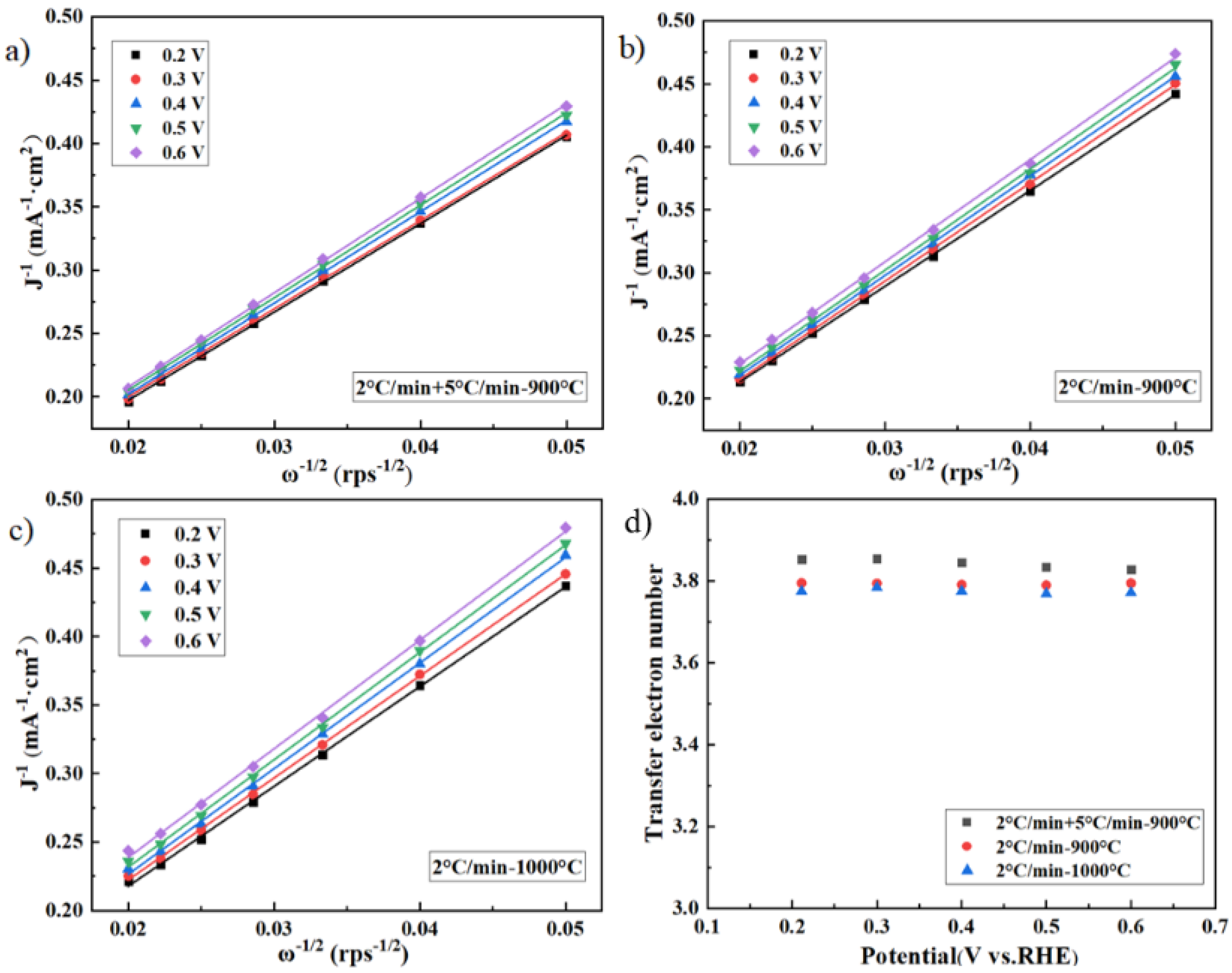
2.3. Effect of Two–Step Pyrolysis on the Performance of the Catalyst
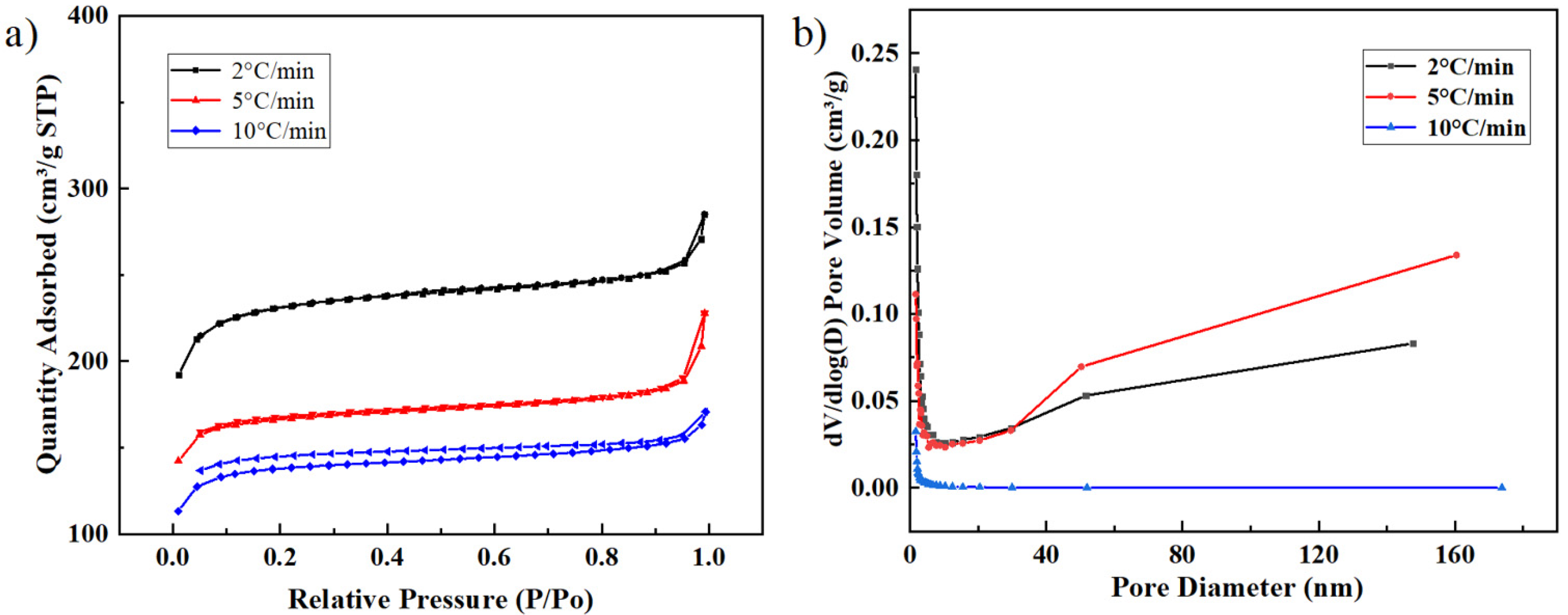
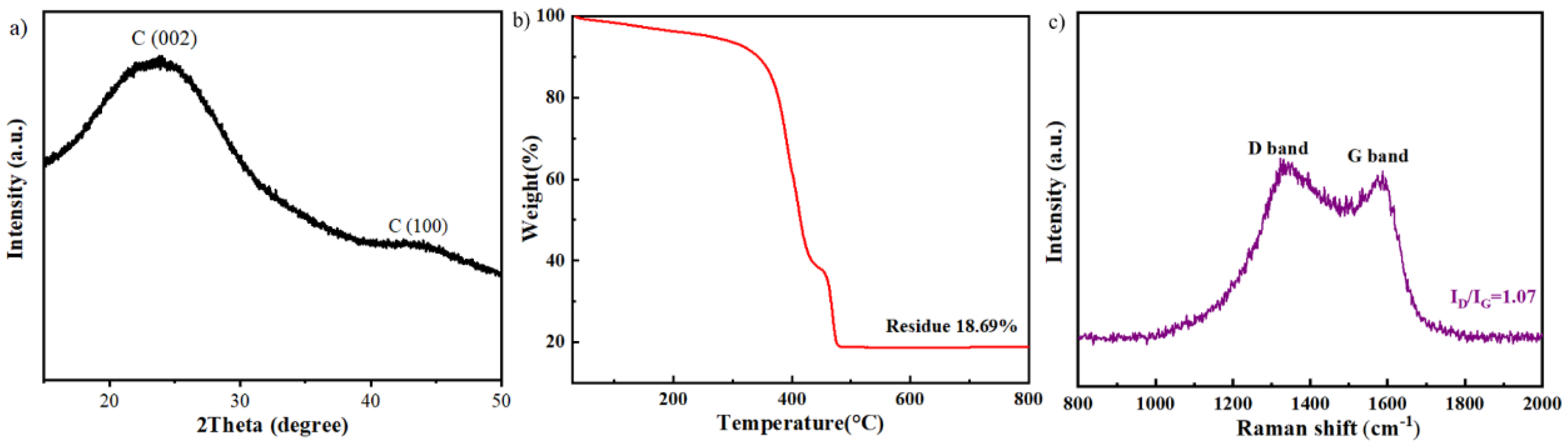
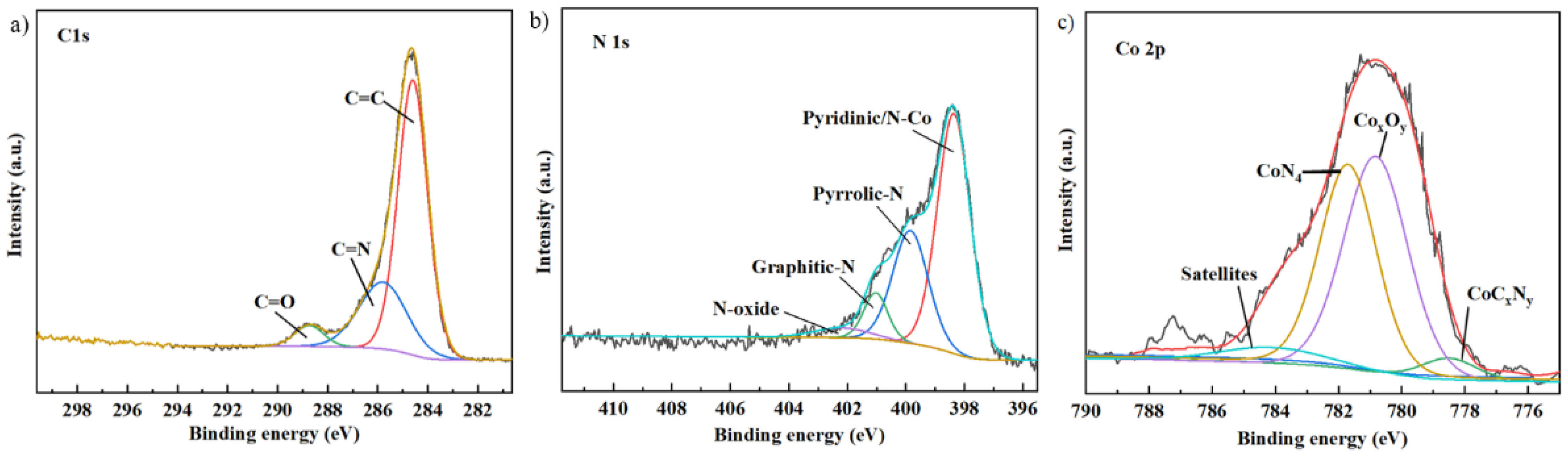
2.4. The Description of the Catalyst Carbonized at the Optimum Pyrolysis Condition
3. Materials and Methods
3.1. Materials and Equipment
3.2. The Synthesis of Co–ZIF–8 Precursor
3.3. The Pyrolysis of Co–ZIF–8 Precursor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R. Stabilization of platinum oxygen–reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [Green Version]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high–temperature proton exchange membrane fuel cell (HT–PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Zhu, C.; He, L.; Fu, S.; Dan, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three–dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The recent development of efficient Earth–abundant transition–metal nanocatalysts. Chem. Soc. Rev. 2017, 46. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Wang, H.; Lu, X.-F.; Tong, Y.-X.; Li, G.-R. Recent Progress on MOF–Derived Heteroatom–Doped Carbon–Based Electrocatalysts for Oxygen Reduction Reaction. Adv. Sci. 2018, 5, 1700515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosi, N.; Eckert, J.; Eddaoudi, M.; Vodak, D.; Kim, J.; O’Keeffe, M.; Yaghi, O. Hydrogen Storage in Microporous Metal–Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sculley, J.; Zhou, H. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Iqbal, N.; Hanif, S.; Noor, T.; Shi, X.; Zaman, N.; Haider, D.; Rizvi, S.A.M.; Kannan, A.M. MOF–Derived CuPt/NC Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2020, 10, 799. [Google Scholar] [CrossRef]
- Shaik, G.P.; Kwon, H.-J.; Lee, T.G. Highly efficient Co@NCS nanosheet electrocatalyst for oxygen reduction reaction: An environment–friendly, low–cost and sustainable electrocatalyst. Mater. Res. Bull. 2020, 128, 110873. [Google Scholar] [CrossRef]
- Zhao, D.; Shui, J.-L.; Grabstanowicz, L.; Chen, C.; Commet, S.; Lu, J.; Liu, D.-J. Highly Efficient Non-Precious Metal Electrocatalysts Prepared from One-Pot Synthesized Zeolitic Imidazolate Frameworks. Adv. Mater. 2014, 26, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, Z.; Wang, P.; Jensen, L.; Zhang, Y.; Yue, Y. Metal-Organic Framework Glass Anode with an Exceptional Cycling-Induced Capacity Enhancement for Lithium Ion Batteries. Energy 2021. [CrossRef]
- Kong, L.; Xie, C.-C.; Gu, H.; Wang, C.-P.; Zhou, X.; Liu, J.; Zhou, Z.; Li, Z.-Y.; Zhu, J.; Bu, X.-H. Thermal Instability Induced Oriented 2D Pores for Enhanced Sodium Storage. Small 2018, 14, 1800639. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Farha, O. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49. [Google Scholar] [CrossRef]
- Huang, X.-C.; Lin, Y.-Y.; Zhang, J.-P.; Chen, X.-M. Ligand-Directed Strategy for Zeolite-Type Metal–Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, G.S.; Kim, S.T.; Liu, M.; Cho, J. A Highly Efficient Electrocatalyst for the Oxygen Reduction Reaction: N-Doped Ketjenblack Incorporated into Fe/Fe3C-Functionalized Melamine Foam. Angew. Chem. 2013, 52, 1026–1030. [Google Scholar] [CrossRef]
- Kramm, U.; Lefèvre, M.; Bogdanoff, P.; Schmeißer, D.; Dodelet, J.-P. Analyzing Structural Changes of Fe–N–C Cathode Catalysts in PEM Fuel Cell by Mößbauer Spectroscopy of Complete Membrane Electrode Assemblies. J. Phys. Chem. Lett. 2014, 5, 3750–3756. [Google Scholar] [CrossRef]
- Hu, L.; Yu, F.; Wang, F.; Yang, S.; Peng, B.; Chen, L.; Wang, G.; Hou, J.; Dai, B.; Tian, Z.-Q. Overwhelming electrochemical oxygen reduction reaction of zinc-nitrogen-carbon from biomass resource chitosan via a facile carbon bath method. Chin. Chem. Lett. 2020, 31, 1207–1212. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Fang, Y.; Sun, W.; Qi, Z.; Pei, Y.; Qi, S.; Yuan, P.; Luan, X.; Goh, T.W.; et al. Metal-Organic Framework Derived Carbons: Applications as Solid Base Catalyst and Support for Pd Nanoparticles in Tandem Catalysis. Chem. Eur. J. 2017, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cullen, D.; Pan, Y.-T.; Hwang, S.; Wang, M.; Feng, Z.; Wang, J.; Engelhard, M.; Zhang, H.; He, Y.; et al. Nitrogen-Coordinated Single Cobalt Atom Catalysts for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. Adv. Mater. 2018, 30, 1706758. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Wang, Y.; Wang, Q.; Hu, G.; Mamat, X.; Zhang, S.; Wang, S. Hierarchically Ordered Porous Carbon with Atomically Dispersed FeN4 for Ultraefficient Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2020, 59, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.Q.; Yao, T.; Wu, Y.; Zheng, L.R.; Lin, Y.; Liu, W.; Ju, H.X.; Zhu, J.F.; Hong, X.; Deng, Z.X.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.; Jia, Q.; Stamatin, S.; Harrington, G.; Lyth, S.; Krtil, P.; Mukerjee, S.; et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Chen, M.J.; Cullen, D.A.; Hwang, S.; Wang, M.Y.; Li, B.Y.; Liu, K.X.; Karakalos, S.; Lucero, M.; Zhang, H.G.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Ye, Y.F.; Cai, F.; Yan, C.C.; Li, Y.S.; Wang, G.X.; Bao, X.H. Two-step pyrolysis of ZIF-8 functionalized with ammonium ferric citrate for efficient oxygen reduction reaction. J. Energy Chem. 2017, 26, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lai, Y.J.; Song, L.; Zhou, Z.Y.; Liu, J.G.; Wang, Q.; Yang, X.D.; Chen, C.; Shi, W.; Zheng, Y.P.; et al. S-Doping of an Fe/N/C ORR Catalyst for Polymer Electrolyte Membrane Fuel Cells with High Power Density. Angew. Chem. 2015, 54. [Google Scholar] [CrossRef]
- Sahraie, N.; Kramm, U.; Steinberg, J.; Zhang, Y.; Thomas, A.; Reier, T.; Paraknowitsch, J.P.; Strasser, P. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 2015, 6, 8618–8626. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Park, K.; Ni, Z.; Côté, A.; Choi, J.; Huang, R.; Uribe-Romo, F.; Chae, H.; O’Keeffe, M.; Yaghi, O. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Song, A.; Yang, W.; Yang, W.; Sun, G.; Yin, X.; Gao, L.; Wang, Y.; Qin, X.; Shao, G. Facile Synthesis of Cobalt Nanoparticles Entirely Encapsulated in Slim Nitrogen-Doped Carbon Nanotubes as Oxygen Reduction Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 3973–3981. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Deng, Y.; Dong, Y.; Wang, G.; Sun, K.; Shi, X.; Zheng, L.; Li, X.; Liao, S. Well-Defined ZIF-Derived Fe-N Codoped Carbon Nanoframes as Efficient Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 9699–9709. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jiménez, M.J.; Ye, G.; Dong Kim, N.; Samuel, E.L.G.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef]
- Cui, X.; Yang, S.; Yan, X.; Leng, J.; Shuang, S.; Ajayan, P.M.; Zhang, Z. Pyridinic-Nitrogen-Dominated Graphene Aerogels with Fe–N–C Coordination for Highly Efficient Oxygen Reduction Reaction. Adv. Funct. Mater. 2016, 26, 5708–5717. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Jiang, M.; Cao, X.; Liu, P.; Zhang, T.; Zhang, J. ZIF-8@Polyvinylpyrrolidone Nanocomposites Based N-Doped Porous Carbon for Highly Efficient Oxygen Reduction Reaction in Alkaline Solution. J. Electrochem. Soc. 2016, 163, H459–H464. [Google Scholar] [CrossRef]
- Chao, S.; Bai, Z.; Cui, Q.; Yan, H.; Wang, K.; Yang, L. Hollowed-out octahedral Co/N-codoped carbon as a highly efficient non-precious metal catalyst for oxygen reduction reaction. Carbon 2015, 82, 77–86. [Google Scholar] [CrossRef]
- Ferrandon, M.; Wang, X.; Kropf, A.; Myers, D.; Wu, G.; Johnston, C.; Zelenay, P. Stability of iron species in heat-treated polyaniline–iron–carbon polymer electrolyte fuel cell cathode catalysts. Electrochim. Acta 2013, 110, 282–291. [Google Scholar] [CrossRef]




| Heating Mode | Rate (°C/min) | Time (h) | Temperature (°C) | Rate (°C/min) | Temperature (°C) | Time (h) | Onset Potentials (VRHE) | Half–Wave Potentials (VRHE) |
|---|---|---|---|---|---|---|---|---|
| One–step pyrolysis | 2 | – | 800 | – | – | 2 | 0.86 | 0.74 |
| 2 | – | 900 | – | – | 2 | 0.97 | 0.80 | |
| 2 | – | 1000 | – | – | 2 | 0.89 | 0.79 | |
| 5 | – | 900 | – | – | 2 | 0.95 | 0.78 | |
| 10 | – | 900 | – | – | 2 | 0.94 | 0.77 | |
| Two–step pyrolysis | 2 | 1 h | 350 | 5 | 900 | 2 | 0.98 | 0.82 |
| 2 | 1 h | 350 | 10 | 900 | 2 | 0.92 | 0.79 | |
| 5 | 1 h | 350 | 2 | 900 | 2 | 0.93 | 0.78 | |
| 5 | 1 h | 350 | 5 | 900 | 2 | 0.92 | 0.78 | |
| 10 | 1 h | 350 | 2 | 900 | 2 | 0.90 | 0.77 | |
| 10 | 1 h | 350 | 5 | 900 | 2 | 0.89 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, N.; Bi, K.; Sun, W.; Wu, Q.; Li, Y.; Xu, T.; Lv, B.; Zhang, S. Effect of Pyrolysis Conditions on the Performance of Co–Doped MOF–Derived Carbon Catalysts for Oxygen Reduction Reaction. Catalysts 2021, 11, 1163. https://doi.org/10.3390/catal11101163
Cui N, Bi K, Sun W, Wu Q, Li Y, Xu T, Lv B, Zhang S. Effect of Pyrolysis Conditions on the Performance of Co–Doped MOF–Derived Carbon Catalysts for Oxygen Reduction Reaction. Catalysts. 2021; 11(10):1163. https://doi.org/10.3390/catal11101163
Chicago/Turabian StyleCui, Ning, Kexiao Bi, Wei Sun, Qianqian Wu, Yinan Li, Tiewei Xu, Binjiang Lv, and Shuling Zhang. 2021. "Effect of Pyrolysis Conditions on the Performance of Co–Doped MOF–Derived Carbon Catalysts for Oxygen Reduction Reaction" Catalysts 11, no. 10: 1163. https://doi.org/10.3390/catal11101163
APA StyleCui, N., Bi, K., Sun, W., Wu, Q., Li, Y., Xu, T., Lv, B., & Zhang, S. (2021). Effect of Pyrolysis Conditions on the Performance of Co–Doped MOF–Derived Carbon Catalysts for Oxygen Reduction Reaction. Catalysts, 11(10), 1163. https://doi.org/10.3390/catal11101163





