Utilization of Clay Materials as Support for Aspergillus japonicus Lipase: An Eco-Friendly Approach
Abstract
:1. Introduction
2. Results and Discussion
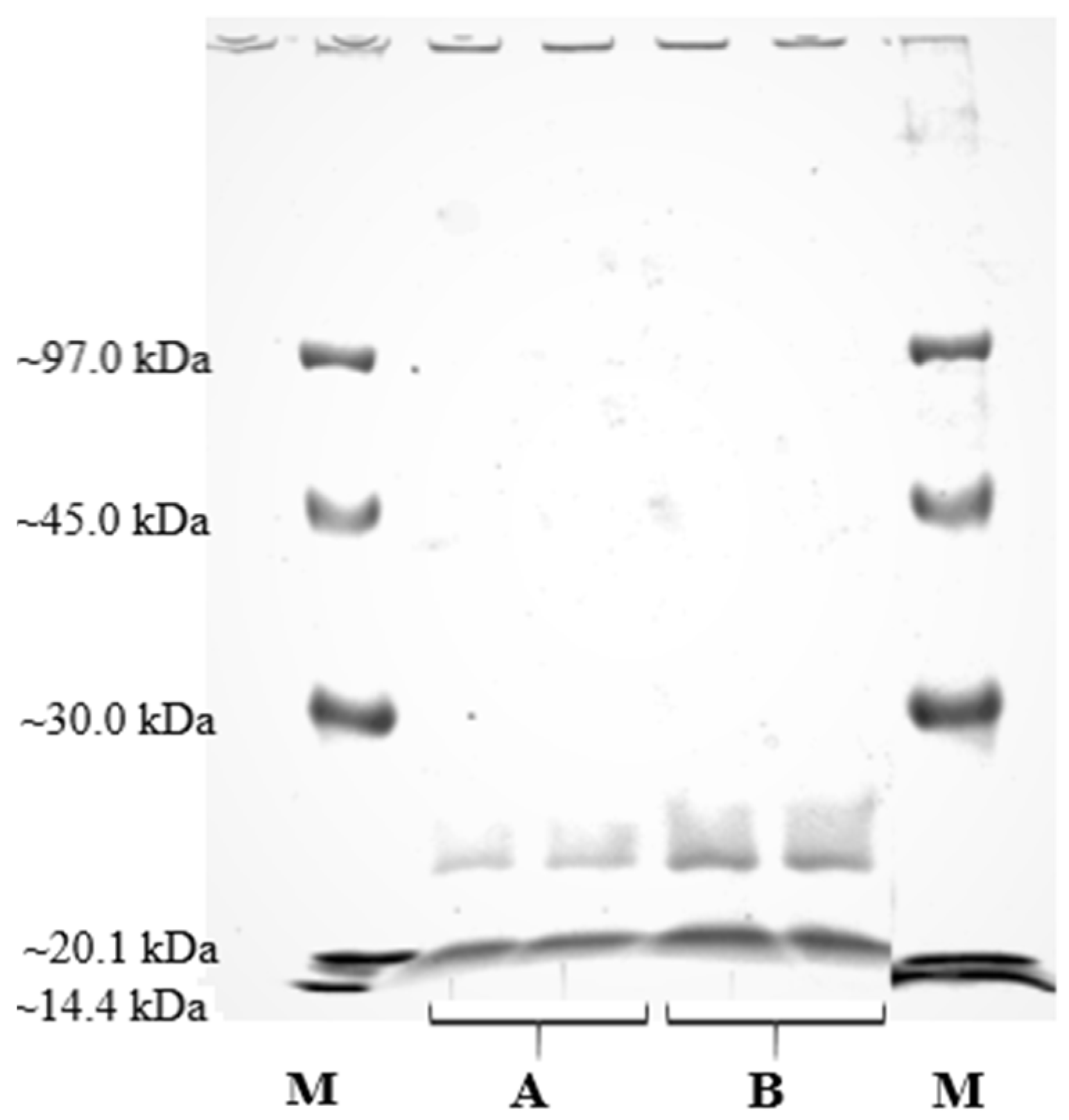
2.1. Production of Lipase by A. japonicus
2.2. Characterization of Free A. japonicus Lipase
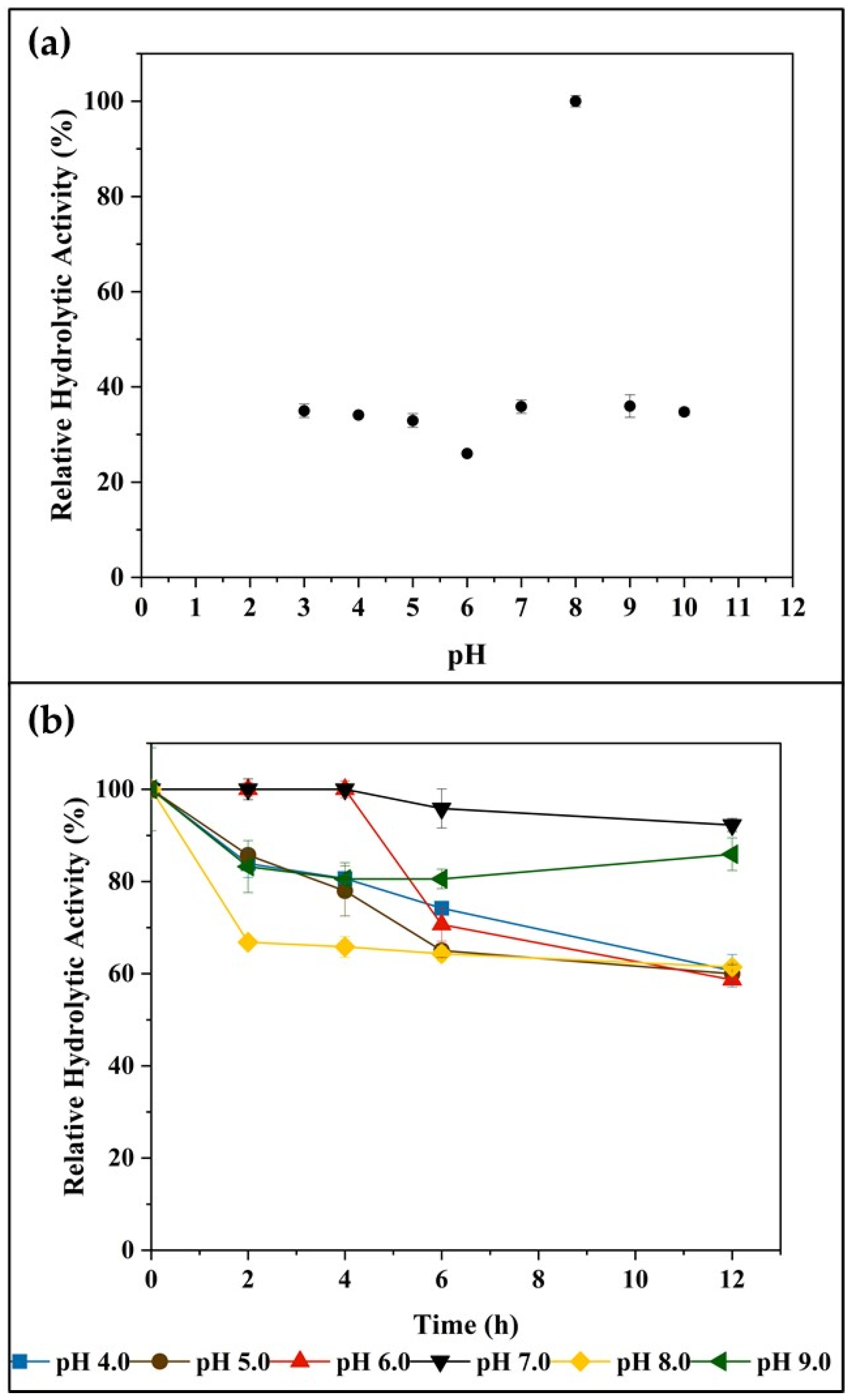
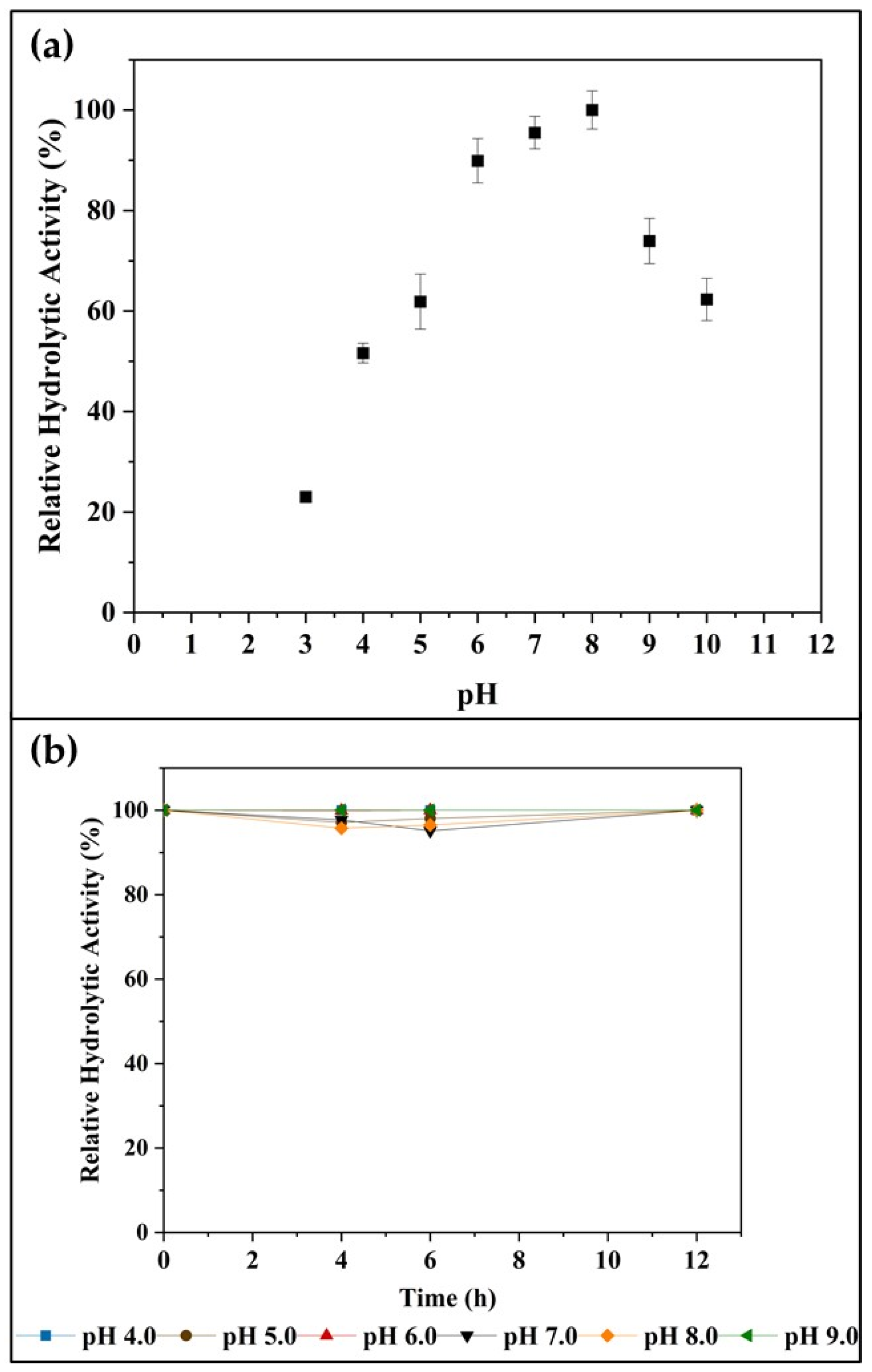
2.2.1. pH Effects on Enzyme Activity and Stability
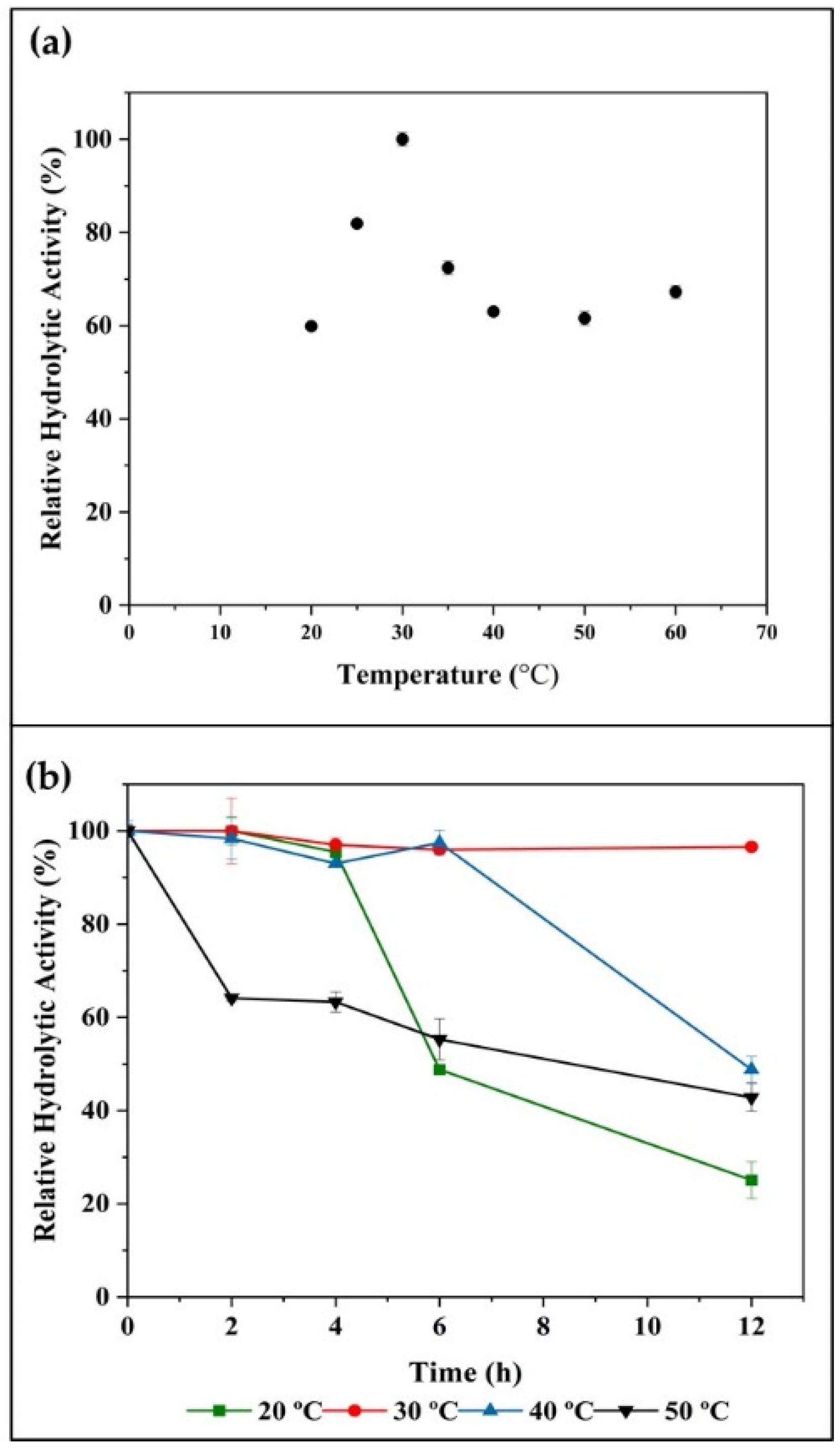
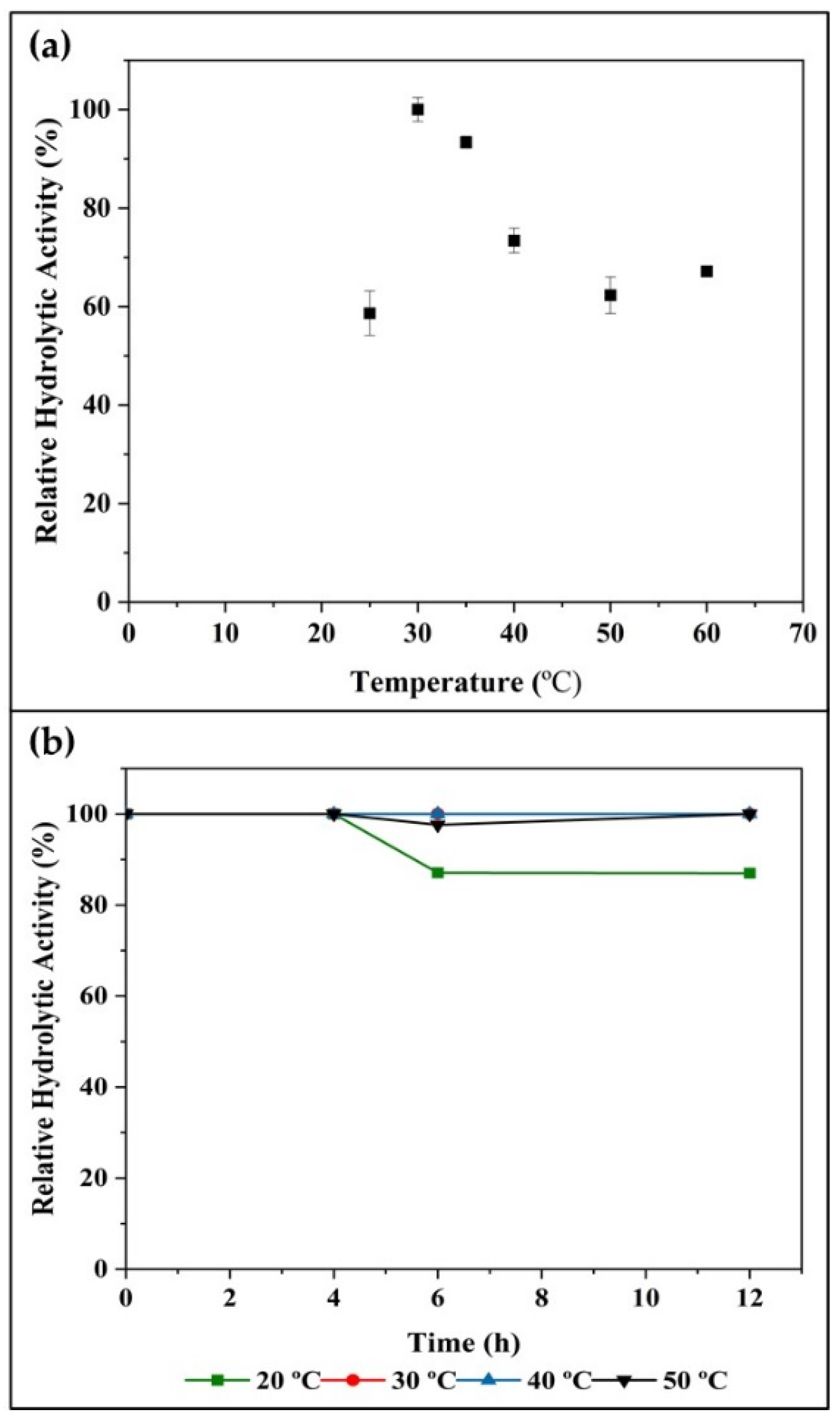
2.2.2. Temperature Effects on Enzyme Activity and Stability
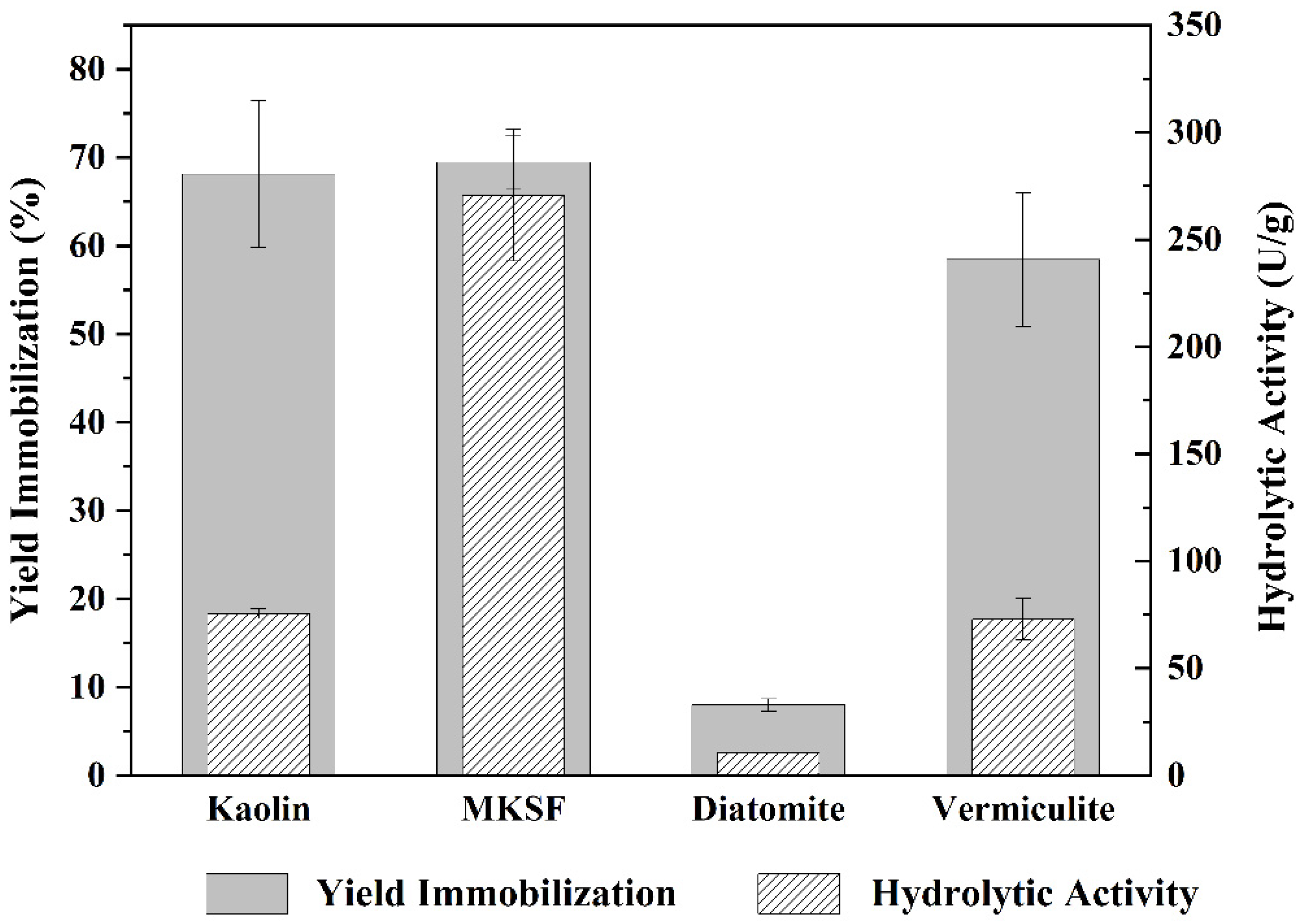
2.3. Immobilization of A. japonicus Lipase Using Different Supports
2.4. Characterization of A. japonicus Lipase Immobilized on MKSF
2.4.1. Effect of pH and Temperature on Immobilized Lipase Activity
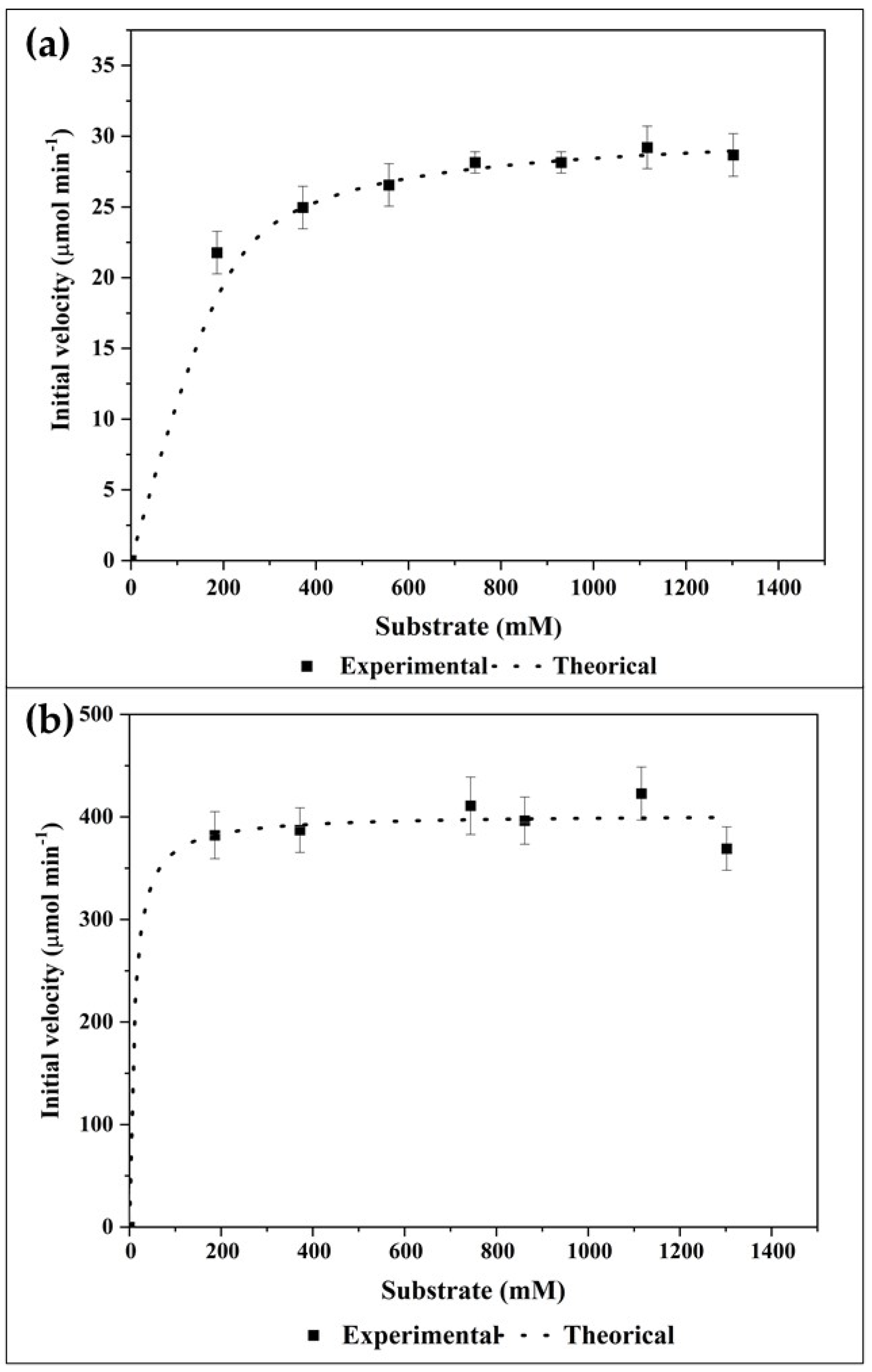
2.4.2. Kinetic Parameters of Free and Immobilized Lipase
3. Materials and Methods
3.1. Material
3.2. Microorganism
3.3. Production of Aspergillus japonicus Lipase by Submerged Culture
3.4. Analytical Methods
3.4.1. Determination of Hydrolytic Enzyme Activity
3.4.2. Determination of Protein Content
3.4.3. SDS-PAGE Analysis
3.4.4. Lipase Immobilization Using Different Supports
3.5. Characterization of Free and Immobilized Microbial Lipase
3.5.1. Determination of Optimum pH and Temperature
3.5.2. Determination of Kinetic Parameters
3.5.3. Stability of the Free and Immobilized Microbial Lipase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seddigi, Z.S.; Malik, M.S.; Ahmed, S.A.; Babalghith, A.O.; Kamal, A. Lipases in asymmetric transformations: Recent advances in classical kinetic resolution and lipase–metal combinations for dynamic processes. Coord. Chem. Rev. 2017, 348, 54–70. [Google Scholar] [CrossRef]
- Sankar, S.; Ponnuraj, K. Less explored plant lipases: Modeling and molecular dynamics simulations of plant lipases in different solvents and temperatures to understand structure-function relationship. Int. J. Biol. Macromol. 2020, 164, 3546–3558. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Rafiee, F.; Rezaee, M. Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef]
- Microbial Lipase Market—Growth, Trends and Forecasts (2018–2023). Available online: https://www.marketsandmarkets.com/Market-Reports/microbial-lipase-market-248464055.html (accessed on 9 August 2021).
- Geoffry, K.; Achur, R.N. Screening and production of lipase from fungal organisms. Biocatal. Agric. Biotechnol. 2018, 14, 241–253. [Google Scholar] [CrossRef]
- Pandey, N.; Dhakar, K.; Jain, R.; Pandey, A. Temperature dependent lipase production from cold and pH tolerant species of Penicillium. Mycosphere 2016, 7, 1533–1545. [Google Scholar] [CrossRef]
- Meghwanshi, G.K.; Vashishtha, A. Biotechnology of fungal lipases. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 383–411. ISBN 9789811303937. [Google Scholar]
- Basheer, S.M.; Chellappan, S.; Beena, P.; Sukumaran, R.K.; Elyas, K.; Chandrasekaran, M. Lipase from marine Aspergillus awamori BTMFW032: Production, partial purification and application in oil effluent treatment. New Biotechnol. 2011, 28, 627–638. [Google Scholar] [CrossRef]
- Melani, N.; Tambourgi, E.B.; Silveira, E. Lipases: From Production to Applications. Sep. Purif. Rev. 2020, 49, 143–158. [Google Scholar] [CrossRef]
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368. [Google Scholar] [CrossRef]
- Contesini, F.J.; Calzado, F.; Madeira, J.V.; Rubio, M.V.; Zubieta, M.P.; de Melo, R.R.; Gonçalves, T.A. Aspergillus Lipases: Biotechnological and Industrial Application. In Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2017; pp. 639–666. [Google Scholar]
- Remonatto, D.; de Oliveira, J.V.; Guisan, J.M.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via Alcoholysis of Sunflower Oil by Eversa Lipases Immobilized on Hydrophobic Supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Vescovi, V.; Kopp, W.; Guisán, J.M.; Giordano, R.L.; Mendes, A.A.; Tardioli, P.W. Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino- glutaraldehyde spacer arms. Process. Biochem. 2016, 51, 2055–2066. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Pascacio, V.G.T.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzym. Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; López-Gallego, F.; Bolivar, J.M.; Rocha-Martín, J.; Fernandez-Lorente, G. The Science of Enzyme Immobilization. In Methods in Molecular Biology; Guisan, J., Bolivar, J., López-Gallego, F., Rocha-Martín, J., Eds.; Publisher: Humana, NY, USA, 2020; Volume 2100, pp. 1–26. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.-T.; Shi, Y.-P. “Recent advances on support materials for lipase immobilization and applicability as biocatalysts in inhibitors screening methods”—A review. Anal. Chim. Acta 2020, 1101, 9–22. [Google Scholar] [CrossRef]
- Etcheverry, M.; Cappa, V.; Trelles, J.; Zanini, G. Montmorillonite-alginate beads: Natural mineral and biopolymers based sorbent of paraquat herbicides. J. Environ. Chem. Eng. 2017, 5, 5868–5875. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, J.A.; Jiménez-Gómez, C.P. Catalytic Applications of Clay Minerals and Hydrotalcites. Catalysts 2021, 11, 68. [Google Scholar] [CrossRef]
- Naik, S.; Scholin, J.; Goss, B. Stabilization of phytase enzyme on montmorillonite clay. J. Porous Mater. 2016, 23, 401–406. [Google Scholar] [CrossRef]
- Viana, A.C.; Ramos, I.G.; Mascarenhas, A.J.S.; dos Santos, E.L.; Sant’Ana, A.E.G.; Goulart, H.F.; Druzian, J.I. Release of aggregation pheromone rhynchophorol from clay minerals montmorillonite and kaolinite. J. Therm. Anal. Calorim. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Tanasković, S.J.; Jokić, B.; Grbavčić, S.; Drvenica, I.; Prlainović, N.; Luković, N.; Knežević-Jugović, Z. Immobilization of Candida antarctica lipase B on kaolin and its application in synthesis of lipophilic antioxidants. Appl. Clay Sci. 2017, 135, 103–111. [Google Scholar] [CrossRef]
- Pereira, F.A.; Sousa, K.S.; Cavalcanti, G.R.; França, D.B.; Queiroga, L.N.; dos Santos, I.M.G.; Fonseca, M.; Jaber, M. Green biosorbents based on chitosan-montmorillonite beads for anionic dye removal. J. Environ. Chem. Eng. 2017, 5, 3309–3318. [Google Scholar] [CrossRef] [Green Version]
- Długosz, O.; Banach, M. Kinetic, isotherm and thermodynamic investigations of the adsorption of Ag+ and Cu2+ on vermiculite. J. Mol. Liq. 2018, 258, 295–309. [Google Scholar] [CrossRef]
- Mohammadi, M.; Heshmati, M.K.; Sarabandi, K.; Fathi, M.; Lim, L.-T.; Hamishehkar, H. Activated alginate-montmorillonite beads as an efficient carrier for pectinase immobilization. Int. J. Biol. Macromol. 2019, 137, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, G.; Duan, X.; Liang, X.; Meng, J.; Liang, J. Environmental Applications of Diatomite Minerals in Removing Heavy Metals from Water. Ind. Eng. Chem. Res. 2019, 58, 11638–11652. [Google Scholar] [CrossRef]
- Brião, G.D.V.; da Silva, M.G.C.; Vieira, M.G.A. Efficient and Selective Adsorption of Neodymium on Expanded Vermiculite. Ind. Eng. Chem. Res. 2021, 60, 4962–4974. [Google Scholar] [CrossRef]
- Song, X.; Li, C.; Chai, Z.; Zhu, Y.; Yang, Y.; Chen, M.; Ma, R.; Liang, X.; Wu, J. Application of diatomite for gallic acid removal from molasses wastewater. Sci. Total Environ. 2021, 765, 142711. [Google Scholar] [CrossRef]
- Vallova, S.; Plevova, E.; Smutna, K.; Sokolova, B.; Vaculikova, L.; Valovicova, V.; Hundakova, M.; Praus, P. Removal of analgesics from aqueous solutions onto montmorillonite KSF. J. Therm. Anal. Calorim. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X.; Xu, C.Y. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Appl. Clay Sci. 2013, 80–81, 443–452. [Google Scholar] [CrossRef]
- Szabó, T.; Mitea, R.; Leeman, H.; Premachandra, G.S.; Johnston, C.T.; Szekeres, M.; Dékány, I.; Schoonheydt, R.A. Adsorption of protamine and papain proteins on saponite. Clays Clay Miner. 2008, 56, 494–504. [Google Scholar] [CrossRef]
- Sanjay, G.; Sugunan, S. Enhanced pH and thermal stabilities of invertase immobilized on montmorillonite K-10. Food Chem. 2006, 94, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, S.; Sugunan, S. Enzymes immobilized on montmorillonite K 10: Effect of adsorption and grafting on the surface properties and the enzyme activity. Appl. Clay Sci. 2007, 35, 67–75. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Li, J.; Sheng, G.; Chen, H. Comparative Study on Lipases Immobilized onto Bentonite and Modified Bentonites and Their Catalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 9030–9037. [Google Scholar] [CrossRef]
- Da Silva, V.C.F.; Contesini, F.; Carvalho, P.D.O. Enantioselective behavior of lipases from Aspergillus niger immobilized in different supports. J. Ind. Microbiol. Biotechnol. 2009, 36, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Benamia, F.; Benouis, S.; Belafriekh, A.; Semache, N.; Rebbani, N.; Djeghaba, Z. Efficient Candida rugosa lipase immobilization on Maghnite clay and application for the production of (1R)-(−)-Menthyl acetate. Chem. Pap. 2017, 71, 785–793. [Google Scholar] [CrossRef]
- Scherer, R.P.; Dallago, R.L.; Penna, F.G.; Bertella, F.; de Oliveira, D.; de Oliveira, J.V.; Pergher, S.B. Influence of process parameters on the immobilization of commercial porcine pancreatic lipase using three low-cost supports. Biocatal. Agric. Biotechnol. 2012, 1, 290–294. [Google Scholar] [CrossRef]
- Babavatan, E.O.; Yildirim, D.; Peksel, A.; Binay, B. Immobilization ofRhizomucor mieheilipase onto montmorillonite K-10 and polyvinyl alcohol gel. Biocatal. Biotransform. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Tu, N.; Shou, J.; Dong, H.; Huang, J.; Li, Y. Improved Catalytic Performance of Lipase Supported on Clay/Chitosan Composite Beads. Catalysts 2017, 7, 302. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Duan, Y.-D.; Wang, Q.-E.; Cheng, H.-M. Preparation of Immobilized Lipase on Silica Clay as a Potential Biocatalyst on Synthesis of Biodiesel. Catalysts 2020, 10, 1266. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Scherer, R.P.; Silva, M.F.; Golunski, S.; Pergher, S.; Oliveira, D.; Oliveira, J.V.; Treichel, H. Natural montmorillonite as support for the immobilization of inulinase from Kluyveromyces marxianus NRRL Y-7571. Biocatal. Agric. Biotechnol. 2012, 1, 284–289. [Google Scholar] [CrossRef]
- Contesini, F.J.; Lopes, D.B.; Macedo, G.; Nascimento, M.D.G.; de Carvalho, P. Aspergillus sp. lipase: Potential biocatalyst for industrial use. J. Mol. Catal. B Enzym. 2010, 67, 163–171. [Google Scholar] [CrossRef]
- Sita, K.K.; Narasimha, R.M.; Karanam, S.K.; Medicherla, N.R. Enhanced lipase production by mutation induced Aspergillus japonicus. Afr. J. Biotechnol. 2008, 7, 2064–2067. [Google Scholar] [CrossRef]
- Bharti, M.K.; Khokhar, D.; Pandey, A.K.; Gaur, A.K. Purification and Characterization of Lipase from Aspergillus japonicas: A Potent Enzyme for Biodiesel Production. Natl. Acad. Sci. Lett. 2013, 36, 151–156. [Google Scholar] [CrossRef]
- Sobha, K.; Lalitha, N.; Pavani, N.; Praharshini, R.; Pradeep, D. Statistical optimization of Factors influencing the activity and the kinetics of partially purified Lipase produced from Aspergillus japonicus (MTCC 1975) cultured in protein enriched medium. Int. J. Chem. Tech. Res. 2014, 6, 4817–4831. [Google Scholar]
- Souza, L.T.A.; Oliveira, J.S.; Dos Santos, V.L.; Régis, W.C.B.; Santoro, M.M.; Resende, R.R. Lipolytic Potential of Aspergillus japonicus LAB01: Production, Partial Purification, and Characterisation of an Extracellular Lipase. BioMed Res. Int. 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakash, A.; Ebenezer, P. Optimization of Aspergillus japonicus lipase production by Response Surface Methodology. J. Acad. Ind. Res. 2012, 1, 23–30. [Google Scholar]
- Jayaprakash, A.; Ebenezer, P. Investigation on extracellular lipase production by Aspergillus japonicus isolated from the paper nest of Ropalidia marginata. Indian J. Sci. Technol. 2010, 3, 113–117. [Google Scholar] [CrossRef]
- Rani, N.V.K.M.E.; Kannan, R.R.G.N.D. Lipase Production using Aspergillus japonicus MF-1 through Biotransformation of Agro-Waste and Medicinal Oil Effluent. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2005–2020. [Google Scholar] [CrossRef] [Green Version]
- Colla, L.M.; Ficanha, A.M.M.; Rizzardi, J.; Bertolin, T.E.; Reinehr, C.; Costa, J.A.V. Production and Characterization of Lipases by Two New Isolates of Aspergillus through Solid-State and Submerged Fermentation. BioMed Res. Int. 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Soni, S.; Vohra, R.; Gupta, L.; Gupta, J. Purification and characterisation of a thermostable alkaline lipase from a new thermophilic Bacillus sp. RSJ-1. Process. Biochem. 2002, 37, 1075–1084. [Google Scholar] [CrossRef]
- Tacin, M.V.; Massi, F.P.; Fungaro, M.H.P.; Teixeira, M.F.S.; de Paula, A.V.; Santos-Ebinuma, V. Biotechnological valorization of oils from agro-industrial wastes to produce lipase using Aspergillus sp. from Amazon. Biocatal. Agric. Biotechnol. 2019, 17, 369–378. [Google Scholar] [CrossRef]
- Mandari, V.; Nema, A.; Devarai, S.K. Sequential optimization and large scale production of lipase using tri-substrate mixture from Aspergillus niger MTCC 872 by solid state fermentation. Process. Biochem. 2020, 89, 46–54. [Google Scholar] [CrossRef]
- Utami, T.S.; Hariyani, I.; Alamsyah, G.; Hermansyah, H. Production of dry extract extracellular lipase from Aspergillus niger by solid state fermentation method to catalyze biodiesel synthesis. Energy Procedia 2017, 136, 41–46. [Google Scholar] [CrossRef]
- Das, A.; Shivakumar, S.; Bhattacharya, S.; Shakya, S.; Swathi, S.S. Purification and characterization of a surfactant-compatible lipase from Aspergillus tamarii JGIF06 exhibiting energy-efficient removal of oil stains from polycotton fabric. 3 Biotech 2016, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Saxena, R.; Davidson, W.; Sheoran, A.; Giri, B. Purification and characterization of an alkaline thermostable lipase from Aspergillus carneus. Process. Biochem. 2003, 39, 239–247. [Google Scholar] [CrossRef]
- Gomes, F.M.; De Paula, A.V.; Silva, G.D.S.; De Castro, H.F. Determinação das propriedades catalíticas em meio aquoso e orgânico da lipase de Candida rugosa imobilizada em celulignina quimicamente modificada por carbonildiimidazol. Química Nova 2006, 29, 710–718. [Google Scholar] [CrossRef]
- Mala, J.G.S.; Takeuchi, S. Understanding Structural Features of Microbial Lipases—An Overview. Anal. Chem. Insights 2008, 3, ACI.S551-19. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Quiquampoix, H.; Staunton, S.; Baron, M.-H.; Ratcliffe, R.G. Interpretation of the pH dependence of protein adsorption on clay mineral surfaces and its relevance to the understanding of extracellular enzyme activity in soil. Colloids Surf. A Physicochem. Eng. Asp. 1993, 75, 85–93. [Google Scholar] [CrossRef]
- De Oliveira, M.F.; Johnston, C.T.; Premachandra, G.S.; Teppen, B.J.; Li, H.; Laird, D.A.; Zhu, D.; Boyd, S.A. Spectroscopic Study of Carbaryl Sorption on Smectite from Aqueous Suspension. Environ. Sci. Technol. 2005, 39, 9123–9129. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Froment, L.; Hesseler, M.; Serban, S. New highly robust divinyl benzene/acrylate polymer for immobilization of lipase CALB. Eur. J. Lipid Sci. Technol. 2013, 115, 468–472. [Google Scholar] [CrossRef]
- Basso, A.; Hesseler, M.; Serban, S. Hydrophobic microenvironment optimization for efficient immobilization of lipases on octadecyl functionalised resins. Tetrahedron 2016, 72, 7323–7328. [Google Scholar] [CrossRef]
- Lorente, F.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process. Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Sani, F.; Mokhtar, N.F.; Shukuri, M.; Ali, M.; Noor, R.; Raja, Z.; Rahman, A. Enhanced Performance of Immobilized Rhizopus oryzae Lipase on Coated Porous Polypropylene Support with Additives. Catalysts 2021, 11, 303. [Google Scholar] [CrossRef]
- Mulinari, J.; Oliveira, J.V.; Hotza, D. Lipase immobilization on ceramic supports: An overview on techniques and materials. Biotechnol. Adv. 2020, 42, 107581. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.E.; Reetz, M.T. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998, 16, 396–403. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, O.-M.; Lee, Y.Y.; Phuah, E.-T.; Akoh, C.C. Lipase/Esterase: Properties and Industrial Applications. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academia Press: Waltham, MA, USA, 2019; pp. 158–167. [Google Scholar]
- Nascimento, P.A.M.; Picheli, F.P.; Lopes, A.; Pereira, J.F.B.; Santos-Ebinuma, V.D.C. Effects of cholinium-based ionic liquids on Aspergillus niger lipase: Stabilizers or inhibitors. Biotechnol. Prog. 2019, 35, e2838. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Muñoz, G.; Lorente, F.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19-20, 279–286. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Lorente, F.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A Single Step Purification, Immobi-lization, and Hyperactivation of Lipases via Interfacial Adsorption on Strongly Hydrophobic Supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Tacin, M.V.; Costa-Silva, T.A.; de Paula, A.V.; Palomo, J.M.; Santos-Ebinuma, V.D.C. Microbial lipase: A new approach for a heterogeneous biocatalyst. Prep. Biochem. Biotechnol. 2021, 51, 749–760. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, G.; Jiang, X.; Ma, J.; Zhang, H.; Song, H. Active biocatalysts based on Candida rugosa lipase immobilized in vesicular silica. Process. Biochem. 2012, 47, 953–959. [Google Scholar] [CrossRef]
- Balcão, V.; Paiva, A.L.; Malcata, F. Bioreactors with immobilized lipases: State of the art. Enzym. Microb. Technol. 1996, 18, 392–416. [Google Scholar] [CrossRef]
- Tang, A.; Zhang, Y.; Wei, T.; Wu, J.; Li, Q.; Liu, Y. Immobilization of Candida cylindracea Lipase by Covalent Attachment on Glu-Modified Bentonite. Appl. Biochem. Biotechnol. 2018, 187, 870–883. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, S.; Lan, D.; Wang, X.; Liu, J.; Khan, F.I.; Wang, Y. Crystal structure of a lipase from Streptomyces sp. strain W007—Implications for thermostability and regiospecificity. FEBS J. 2017, 284, 3506–3519. [Google Scholar] [CrossRef] [Green Version]
- Lozzi, I.; Calamai, L.; Fusi, P.; Bosetto, M.; Stotzky, G. Interaction of horseradish peroxidase with montmorillonite homoionic to Na+ and Ca2+: Effects on enzymatic activity and microbial degradation. Soil Biol. Biochem. 2001, 33, 1021–1028. [Google Scholar] [CrossRef]
- Sanjay, G.; Sugunan, S. Acid activated montmorillonite: An efficient immobilization support for improving reusability, storage stability and operational stability of enzymes. J. Porous Mater. 2008, 15, 359–367. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, L.; Li, G.; Zhang, P.; Li, D.; Huang, F.; Wei, Q. Activity of Laccase Immobilized on TiO2-Montmorillonite Complexes. Int. J. Mol. Sci. 2013, 14, 12520–12532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, W.G., Jr.; Pacheco, T.F.; Gao, S.; Martins, P.A.; Guisán, J.M.; Caetano, N.S. 1 Sugarcane Bagasse Saccharification by Enzymatic Hydrolysis. Catalysts 2021, 11, 340. [Google Scholar] [CrossRef]
- Carvalho, T.; Pereira, A.D.S.; Bonomo, R.C.F.; Franco, M.; Finotelli, P.V.; Amaral, P.F. Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: Adsorption isotherms and thermodynamic approach. Int. J. Biol. Macromol. 2020, 160, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Soares, C.M.F.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 1999, 77–79, 745–757. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remonatto, D.; Ferrari, B.R.; Bassan, J.C.; Mussagy, C.U.; de Carvalho Santos-Ebinuma, V.; Veloso de Paula, A. Utilization of Clay Materials as Support for Aspergillus japonicus Lipase: An Eco-Friendly Approach. Catalysts 2021, 11, 1173. https://doi.org/10.3390/catal11101173
Remonatto D, Ferrari BR, Bassan JC, Mussagy CU, de Carvalho Santos-Ebinuma V, Veloso de Paula A. Utilization of Clay Materials as Support for Aspergillus japonicus Lipase: An Eco-Friendly Approach. Catalysts. 2021; 11(10):1173. https://doi.org/10.3390/catal11101173
Chicago/Turabian StyleRemonatto, Daniela, Bárbara Ribeiro Ferrari, Juliana Cristina Bassan, Cassamo Ussemane Mussagy, Valéria de Carvalho Santos-Ebinuma, and Ariela Veloso de Paula. 2021. "Utilization of Clay Materials as Support for Aspergillus japonicus Lipase: An Eco-Friendly Approach" Catalysts 11, no. 10: 1173. https://doi.org/10.3390/catal11101173
APA StyleRemonatto, D., Ferrari, B. R., Bassan, J. C., Mussagy, C. U., de Carvalho Santos-Ebinuma, V., & Veloso de Paula, A. (2021). Utilization of Clay Materials as Support for Aspergillus japonicus Lipase: An Eco-Friendly Approach. Catalysts, 11(10), 1173. https://doi.org/10.3390/catal11101173







