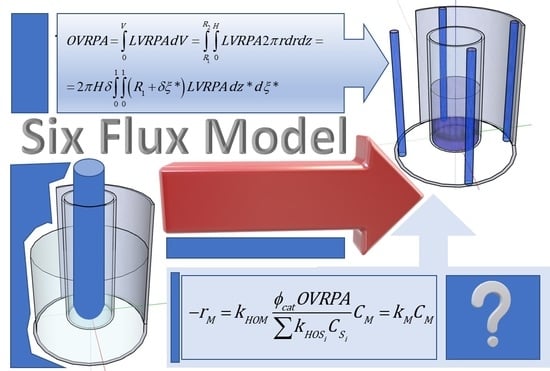
Six Flux Model for the Central Lamp Reactor Applied to an External Four-Lamp Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. LVRPA and OVRPA
2.2. Kinetics of Photocatalytic Oxidation of Some Organic Contaminants
Determination of the Intrinsic Rate Constant of Photocatalytic Oxidation
- Case of first-order kinetics
- Case of Langmuir kinetics
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Beltrán, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Mehrjouei, M.; Muller, S.; Moller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Bidga, R.J. Considering Fenton’s chemistry for wastewater treatment. Chem. Eng. Prog. 1995, 91, 62–67. [Google Scholar]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, L.G.; Dionysiou, D.D. (Eds.) Photocatalysis, Vol. 1: Fundamentals and Perspectives; RSC: London, UK, 2016. [Google Scholar]
- Li Puma, G.; Khor, J.N.; Brucato, A. Modelling of an annular photocatalytic reactor for water purification: Oxidation of pesticides. Environ. Sci. Technol. 2004, 38, 3737–3745. [Google Scholar] [CrossRef]
- Cassano, A.E.; Martin, C.A.; Brandi, R.J.; Alfano, O.M. Photoreactor analysis and design: Fundamentals and applications. Ind. Eng. Chem. Res. 1995, 34, 2155–2201. [Google Scholar] [CrossRef]
- Zalazar, C.S.; Romero, R.L.; Martin, C.A.; Cassano, A.E. Photocatalytic intrinsic reaction kinetics I: Mineralization of dichloroacetic acid. Chem. Eng. Sci. 2005, 60, 5240–5254. [Google Scholar] [CrossRef]
- Zazueta, A.L.L.; Destaillats, H.; Li Puma, G. Radiation field modeling and optimization of a compact and modular multi-plate photocatalytic reactor (MPPR) for air/water purification by Monte Carlo method. Chem. Eng. J. 2013, 217, 475–485. [Google Scholar] [CrossRef]
- Alvarado-Rolon, O.; Natividad, R.; Ramirez-Garcia, J.; Orozco-Velazco, J.; Hernandez-Servin, J.A.; Ramirez-Serrano, A. Kinetic modelling of paracetamol degradation by photocatalysis: Incorporating the competition for photons by the organic molecule and the photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 4121, 113252. [Google Scholar] [CrossRef]
- Moreira, J.; Serrano, B.; Ortiz, A.; de Lasa, H. Evaluation of Photon Absorption in an Aqueous TiO2 Slurry Reactor Using Monte Carlo Simulations and Macroscopic Balance. Ind. Eng. Chem. Res. 2010, 49, 10524–10534. [Google Scholar] [CrossRef]
- Brucato, A.; Cassano, A.E.; Grisafi, F.; Montante, G.; Rizzuti, L.; Vella, G. Estimating radiant fields in flat heterogeneous photoreactors by the six-flux model. AIChE J. 2006, 52, 3882–3890. [Google Scholar] [CrossRef]
- Li Puma, G.; Brucato, A. Dimensionless analysis of slurry photocatalytic reactors using two-flux and six-flux radiation absorption–scattering models. Cat. Today 2007, 122, 78–90. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F.; Oropesa, A. Ozone and photocatalytic processes to remove the antibiotic sulfamethoxazole from water. Water Res. 2008, 42, 3799–3808. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Beltrán, F.J. TiO2 photocatalytic oxidation of a mixture of emerging contaminants: A kinetic study independent of radiation absorption based on the direct-indirect model. Chem. Eng. J. 2018, 339, 369–380. [Google Scholar] [CrossRef]
- Rivas, J.; Solis, R.R.; Gimeno, O.; Sagasti, J. Photocatalytic elimination of aqueous 2-methyl-4-chlorophenoxyacetic acid in the presence of commercial and nitrogen-doped TiO2. Int. J. Environ. Sci. Technol. 2015, 12, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Rivas, F.J.; Hidalgo, A.; Solís, R.R.; Tierno, M. Photocatalysis in an external four-lamp reactor: Modelling and validation—dichloroacetic acid photo-oxidation in the presence of TiO2. Int. J. Environ. Sci. Technol. 2019, 16, 6705–6716. [Google Scholar] [CrossRef]
- Encinas, A.; Rivas, F.J.; Beltrán, F.J.; Oropesa, A. Combination of black-light photocatalysis and ozonation for emerging contaminants degradation in secondary effluents. Chem. Eng. Technol. 2013, 36, 492–499. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibañez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A. Free Radical and Direct Ozone Reaction Competition to Remove Priority and Pharmaceutical Water Contaminants with Single and Hydrogen Peroxide Ozonation Systems. Ozone Sci. Eng. 2018, 40, 251–265. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Checa, M.; Rivas, F.J.; García-Araya, J.F. Modeling the Mineralization Kinetics of Visible Led Graphene Oxide/Titania Photocatalytic Ozonation of an Urban Wastewater Containing Pharmaceutical Compounds. Catalysts 2020, 10, 1256. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, Z.; Zhao, S.; Wang, H.; Yang, L. Enhanced ozonation of dichloroacetic acid in aqueous solution using nanometer ZnO powders. J. Environ. Sci. 2010, 22, 1527–1533. [Google Scholar] [CrossRef]
- Benitez, F.J.; Acero, J.L.; Real, F.J.; Roman, S. Oxidation of MCPA and 2,4-D by UV Radiation, Ozone, and the Combinations UV/H2O2 and O3/H2O2. Environ. Sci. Health Part B 2004, 39, 393–409. [Google Scholar] [CrossRef]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.Y.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hsu, Y.H. Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts 2021, 11, 966. [Google Scholar] [CrossRef]
- Zertal, A.; Molnár-Gábor, D.; Malouki, M.A.; Sehili, T.; Boule, P. Photocatalytic transformation of 4-chloro-2-methylphenoxyacetic acid (MCPA) on several kinds of TiO2. Appl. Catal. B Environ. 2004, 49, 83–89. [Google Scholar] [CrossRef]
- Rodriguez, R.; Blesa, M.A.; Regazzoni, A.E. Surface Complexation at the TiO2 (anatase)/Aqueous Solution Interface: Chemisorption of Catechol. J. Colloid Interface Sci. 1996, 177, 122. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Casas, J.A.; Carbajo, J.; Faraldos, M.; Bahamonde, A. Influence of TiO2 optical parameters in a slurry photocatalytic reactor: Kinetic modelling. Appl. Catal. B Environ. 2017, 200, 164–173. [Google Scholar] [CrossRef]







| Compound | Reference for Results | kHOM × 10−9, M−1s−1 | Reference for kHOM |
|---|---|---|---|
| Dichloroacetic acid, DCA | [21] | 3.9 | [29] |
| 2-methyl-4-chlorophenoxyacetic acid MCPA | [20] | 6.6 | [30] |
| Metoprolol (MTP) | [22] | 7.3 | [31] |
| Diclofenac (DCF) | [22] | 7.5 | [32] |
| Ketorolac (KTR) | [22] | 7.3 | [31] |
| Compound | CM0, mgL−1 | Reaction Time, min | kd, min−1 | kHOM/SF d, M−1 | SFd, s−1 |
|---|---|---|---|---|---|
| DCA a | 5 | 25 | 6.47 × 10−2 | 9.00 × 104 | 4.33 × 104 |
| 20 | 50 | 2.70 × 10−2 | 3.76 × 104 | 1.04 × 105 | |
| 50 | 50 | 1.98 × 10−2 | 2.76 × 104 | 1.42 × 105 | |
| 70 | 50 | 1.69 × 10−2 | 2.35 × 104 | 1.66 × 105 | |
| MCPA b | 5 | 20 | 1.116 × 10−1 | 1.29 × 106 | 5.13 × 103 |
| KTR c | 0.05 | 15 | 5.47 × 10−2 | 1.10 × 106 | 6.67 × 103 |
| 20 | 60 | 3.3 × 10−3 | 6.61 × 104 | 1.10 × 105 | |
| DCF c | 0.05 | 15 | 6.11 × 10−2 | 1.22 × 106 | 6.13 × 103 |
| 20 | 60 | 3.4 × 10−3 | 6.81 × 104 | 1.10 × 105 | |
| MTP c | 0.05 | 15 | 4.62 × 10−2 | 0.95 × 106 | 7.89 × 103 |
| 20 | 60 | 2.9 × 10−3 | 5.81 × 104 | 1.26 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán, F.J.; Rivas, J.; Garcia-Araya, J.-F. Six Flux Model for the Central Lamp Reactor Applied to an External Four-Lamp Reactor. Catalysts 2021, 11, 1190. https://doi.org/10.3390/catal11101190
Beltrán FJ, Rivas J, Garcia-Araya J-F. Six Flux Model for the Central Lamp Reactor Applied to an External Four-Lamp Reactor. Catalysts. 2021; 11(10):1190. https://doi.org/10.3390/catal11101190
Chicago/Turabian StyleBeltrán, Fernando J., Javier Rivas, and Juan-Fernando Garcia-Araya. 2021. "Six Flux Model for the Central Lamp Reactor Applied to an External Four-Lamp Reactor" Catalysts 11, no. 10: 1190. https://doi.org/10.3390/catal11101190