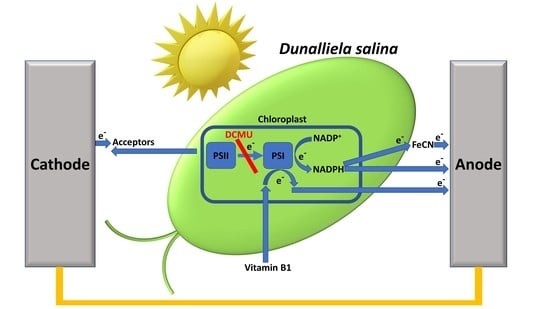
Electron Mediation and Photocurrent Enhancement in Dunalliela salina Driven Bio-Photo Electrochemical Cells
Abstract
:1. Introduction
2. Results
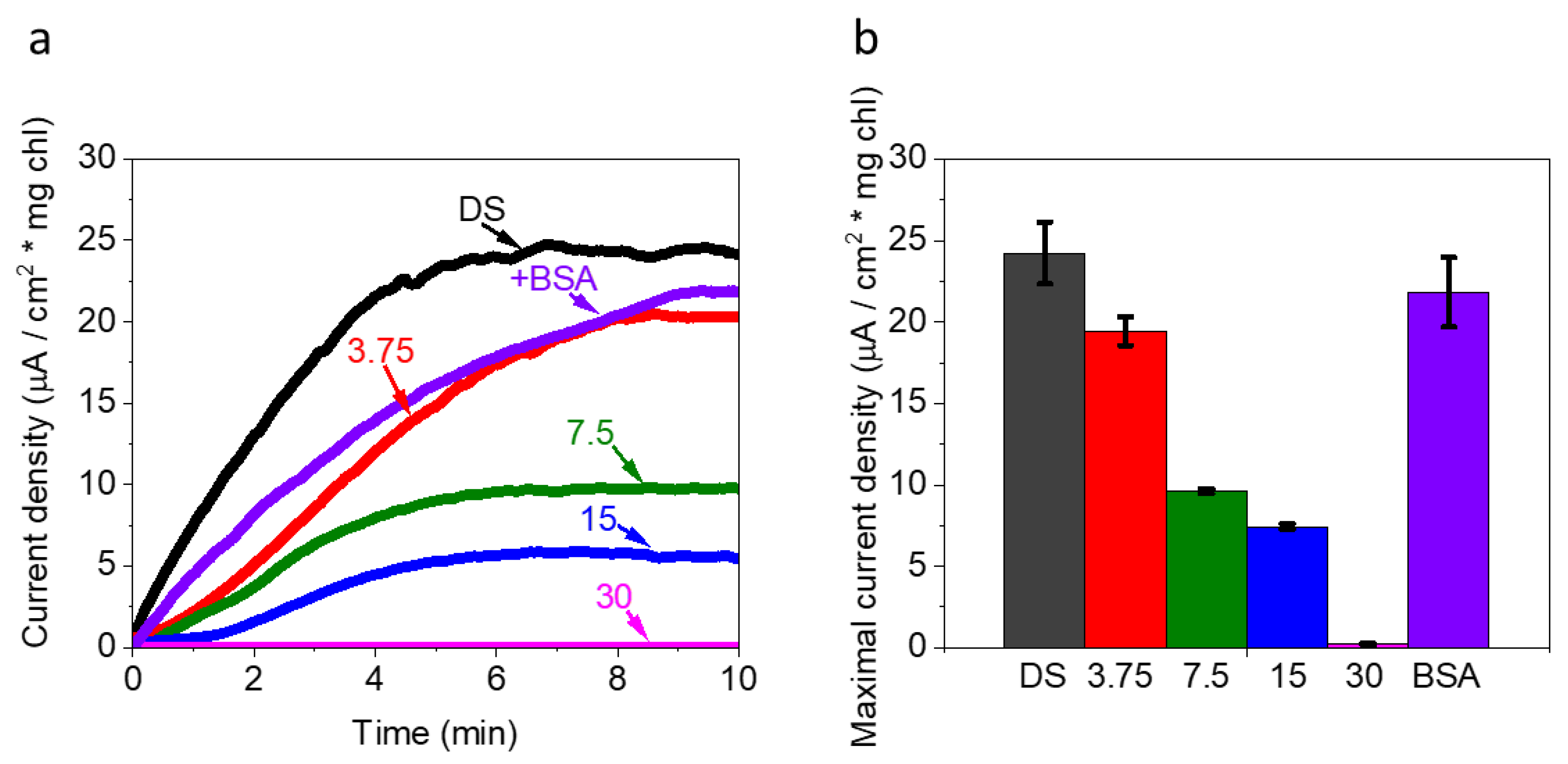
2.1. Live Ds Cells in High Ionic Strength Solution Produce Photocurrents That Originate from PSII
2.2. NAD(P)H Accumulates in the External Cellular Media of Ds
2.3. FNR Eliminates the Photocurrent Production in Ds-Based BPEC
2.4. The Addition of Exogenous NADP+, NAD+ Enhance Photocurrent Production
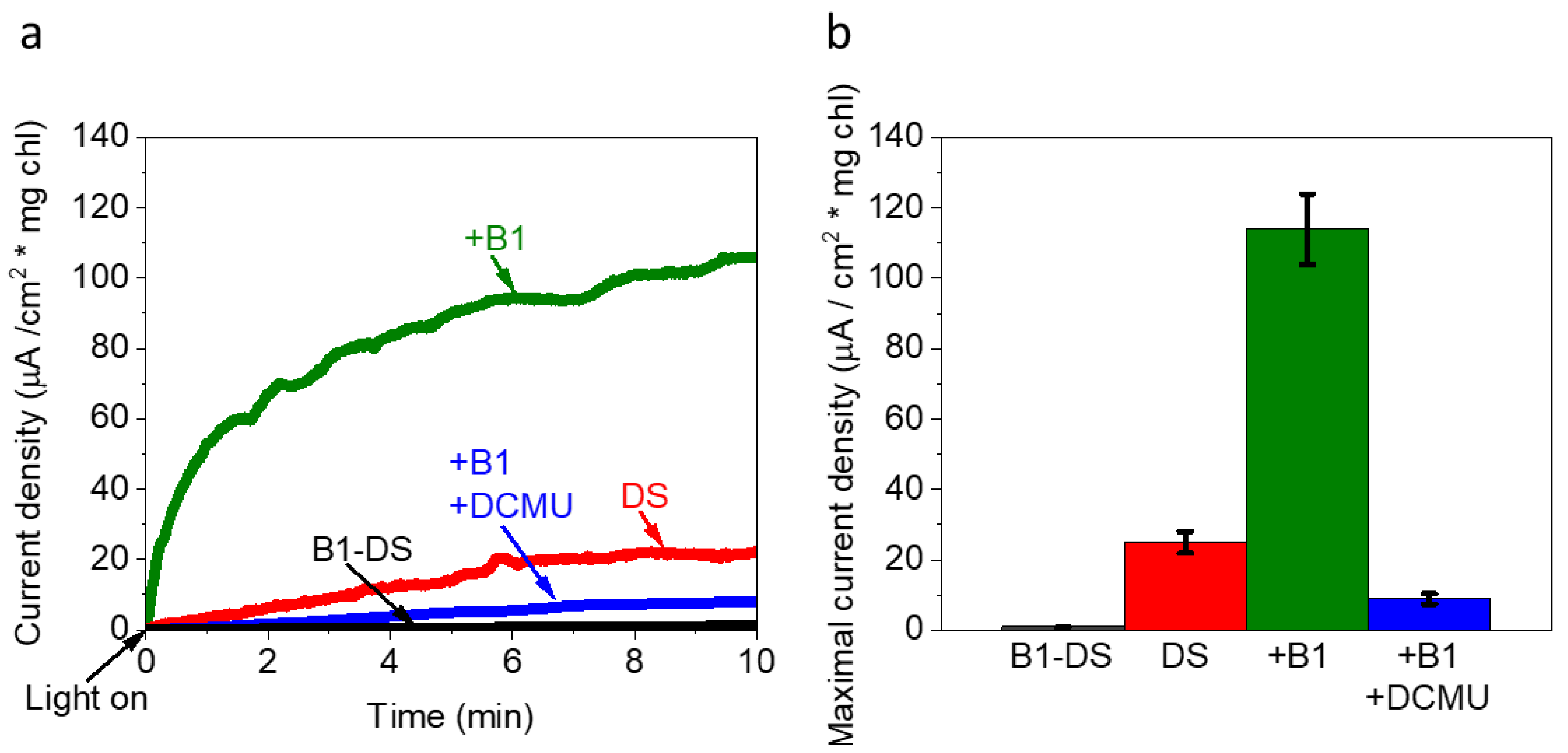
2.5. Vitamin B1 Can Mediate Electrons from the Photosynthesis Pathway to the Anode
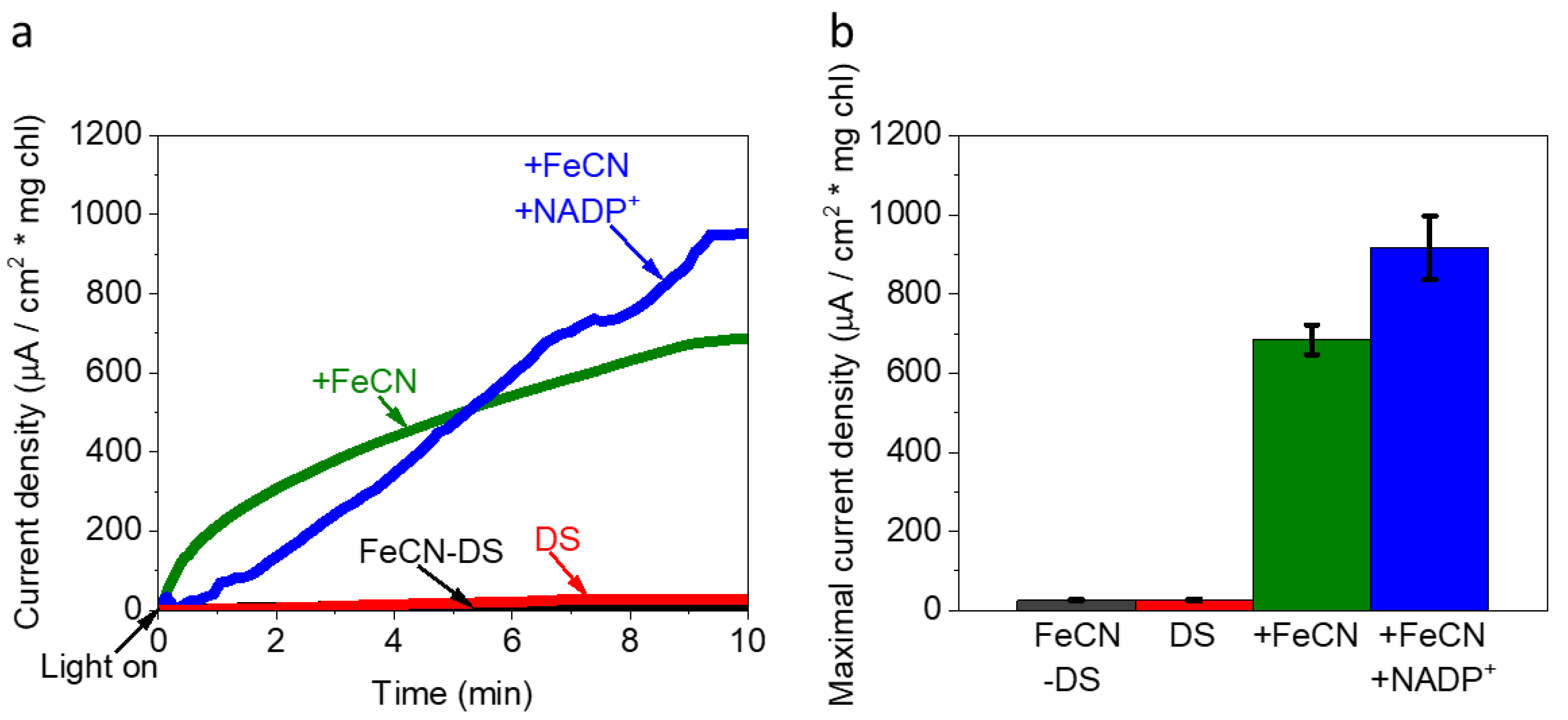
2.6. The Addition of Both FeCN and NADP+ Produces a Greater Photocurrent
3. Materials and Methods
3.1. Ds Cultivation
3.2. Samples Preparation
3.3. Chronoamperometry Measurements
3.4. Fluorescence Measurements
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, X.; Lee, H.; Choi, S. Biopower Generation in a Microfluidic Bio-Solar Panel. Sens. Actuators B Chem. 2016, 228, 151–155. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.K.; Lovley, D.R. Electricity Generation by Direct Oxidation of Glucose in Mediatorless Microbial Fuel Cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-D.; Lian, J.; Du, Z.-W.; Li, H.-R. Construction of Sugar-Based Microbial Fuel Cells by Dissimilatory Metal Reduction Bacteria. Chin. J. Biotechnol. 2006, 22, 131–137. [Google Scholar] [CrossRef]
- Ringeisen, B.R.; Henderson, E.; Wu, P.K.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.C.; Jones-Meehan, J.M. High Power Density from a Miniature Microbial Fuel Cell Using Shewanella Oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Zeikus, J. Impact of Electrode Composition on Electricity Generation in a Single-Compartment Fuel Cell Using Shewanella Putrefaciens. Appl. Microbiol. Biotechnol. 2002, 59, 58–61. [Google Scholar] [CrossRef]
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A Novel Electrochemically Active and Fe(III)-Reducing Bacterium Phylogenetically Related to Aeromonas Hydrophila, Isolated from a Microbial Fuel Cell. FEMS Microbiol. Lett. 2003, 223, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Min, B.; Cheng, S.; Logan, B.E. Electricity Generation Using Membrane and Salt Bridge Microbial Fuel Cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef]
- Fang, X.; Kalathil, S.; Reisner, E. Semi-Biological Approaches to Solar-to-Chemical Conversion. Chem. Soc. Rev. 2020, 49, 4926–4952. [Google Scholar] [CrossRef]
- Bergel, A.; Féron, D.; Mollica, A. Catalysis of Oxygen Reduction in PEM Fuel Cell by Seawater Biofilm. Electrochem. Commun. 2005, 7, 900–904. [Google Scholar] [CrossRef] [Green Version]
- Sekar, N.; Jain, R.; Yan, Y.; Ramasamy, R.P. Enhanced Photo-Bioelectrochemical Energy Conversion by Genetically Engineered Cyanobacteria. Biotechnol. Bioeng. 2016, 113, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Thirumurthy, M.A.; Hitchcock, A.; Cereda, A.; Liu, J.; Chavez, M.S.; Doss, B.L.; Ros, R.; El-Naggar, M.Y.; Heap, J.T.; Bibby, T.S.; et al. Type IV Pili-Independent Photocurrent Production by the Cyanobacterium Synechocystis Sp. PCC 6803. Front. Microbiol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heidary, N.; Kornienko, N.; Kalathil, S.; Fang, X.; Ly, K.H.; Greer, H.F.; Reisner, E. Disparity of Cytochrome Utilization in Anodic and Cathodic Extracellular Electron Transfer Pathways of Geobacter Sulfurreducens Biofilms. J. Am. Chem. Soc. 2020, 142, 5194–5203. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Richter, H.; Covalla, S.F.; Johnson, J.P.; Woodard, T.L.; Orloff, A.L.; Jia, H.; Zhang, M.; Lovley, D.R. Power Output and Columbic Efficiencies from Biofilms of Geobacter Sulfurreducens Comparable to Mixed Community Microbial Fuel Cells. Environ. Microbiol. 2008, 10, 2505–2514. [Google Scholar] [CrossRef]
- Yi, H.; Nevin, K.P.; Kim, B.-C.; Franks, A.E.; Klimes, A.; Tender, L.M.; Lovley, D.R. Selection of a Variant of Geobacter Sulfurreducens with Enhanced Capacity for Current Production in Microbial Fuel Cells. Biosens. Bioelectron. 2009, 24, 3498–3503. [Google Scholar] [CrossRef]
- Hartshorne, R.S.; Jepson, B.N.; Clarke, T.A.; Field, S.J.; Fredrickson, J.; Zachara, J.; Shi, L.; Butt, J.N.; Richardson, D.J. Characterization of Shewanella Oneidensis MtrC: A Cell-Surface Decaheme Cytochrome Involved in Respiratory Electron Transport to Extracellular Electron Acceptors. JBIC J. Biol. Inorg. Chem. 2007, 12, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Rosso, K.M.; Zachara, J.M.; Fredrickson, J.K. Mtr Extracellular Electron-Transfer Pathways in Fe(III)-Reducing or Fe(II)-Oxidizing Bacteria: A Genomic Perspective. Biochem. Soc. Trans. 2012, 40, 1261–1267. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity Production by Geobacter Sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel Cells Select for Microbial Consortia That Self-Mediate Electron Transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [Green Version]
- Simoska, O.; Sans, M.; Eberlin, L.S.; Shear, J.B.; Stevenson, K.J. Electrochemical Monitoring of the Impact of Polymicrobial Infections on Pseudomonas Aeruginosa and Growth Dependent Medium. Biosens. Bioelectron. 2019, 142, 111538. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C.; Hart, J. Comparative Study of Three Types of Microbial Fuel Cell. Enzyme Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic Substances as Electron Acceptors for Microbial Respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction; Academic Press: Cambridge, MA, USA, 2004; Volume 49, pp. 219–286. [Google Scholar] [CrossRef] [Green Version]
- White, G.F.; Shi, Z.; Shi, L.; Wang, Z.; Dohnalkova, A.C.; Marshall, M.J.; Fredrickson, J.K.; Zachara, J.M.; Butt, J.N.; Richardson, D.J.; et al. Rapid Electron Exchange between Surface-Exposed Bacterial Cytochromes and Fe(III) Minerals. Proc. Natl. Acad. Sci. USA 2013, 110, 6346–6351. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.J.; White, G.F.; Butt, J.; Richardson, D.J.; Clarke, T.A. The Crystal Structure of a Biological Insulated Transmembrane Molecular Wire. Cell 2020, 181, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.C.; Preiner, M.; Martin, W.F. Something Special about CO-Dependent CO2 Fixation. FEBS J. 2018, 285, 4181–4195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschörtner, J.; Lai, B.; Krömer, J.O. Biophotovoltaics: Green Power Generation From Sunlight and Water. Front. Microbiol. 2019, 10, 866. [Google Scholar] [CrossRef] [Green Version]
- Grattieri, M.; Beaver, K.; Gaffney, E.M.; Minteer, S.D. Tuning Purple Bacteria Salt-Tolerance for Photobioelectrochemical Systems in Saline Environments. Faraday Discuss. 2019, 215, 15–25. [Google Scholar] [CrossRef]
- Grattieri, M. Purple Bacteria Photo-Bioelectrochemistry: Enthralling Challenges and Opportunities. Photochem. Photobiol. Sci. 2020, 19, 424–435. [Google Scholar] [CrossRef]
- Grattieri, M.; Rhodes, Z.; Hickey, D.P.; Beaver, K.; Minteer, S.D. Understanding Biophotocurrent Generation in Photosynthetic Purple Bacteria. ACS Catal. 2019, 9, 867–873. [Google Scholar] [CrossRef]
- Singh, S.K.; Sundaram, S.; Sinha, S.; Rahman, M.A.; Kapur, S. Recent Advances in CO2 Uptake and Fixation Mechanism of Cyanobacteria and Microalgae. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1297–1323. [Google Scholar] [CrossRef]
- Thangam, K.R.; Santhiya, A.; Sri, S.R.A.; MubarakAli, D.; Karthikumar, S.; Kumar, R.S.; Thajuddin, N.; Soosai, M.R.; Varalakshmi, P.; Moorthy, I.G.; et al. Bio-Refinery Approaches Based Concomitant Microalgal Biofuel Production and Wastewater Treatment. Sci. Total Environ. 2021, 785, 147267. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for High-Value Products: A Way towards Green Nutraceutical and Pharmaceutical Compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Saper, G.; Kallmann, D.; Conzuelo, F.; Zhao, F.; Tóth, T.N.; Liveanu, V.; Meir, S.; Szymanski, J.; Aharoni, A.; Schuhmann, W.; et al. Live Cyanobacteria Produce Photocurrent and Hydrogen Using Both the Respiratory and Photosynthetic Systems. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shlosberg, Y.; Eichenbaum, B.; Tóth, T.N.; Levin, G.; Liveanu, V.; Schuster, G.; Adir, N. NADPH Performs Mediated Electron Transfer in Cyanobacterial-Driven Bio-Photoelectrochemical Cells. iScience 2020, 24, 101892. [Google Scholar] [CrossRef]
- Cereda, A.; Hitchcock, A.; Symes, M.D.; Cronin, L.; Bibby, T.S.; Jones, A.K. A Bioelectrochemical Approach to Characterize Extracellular Electron Transfer by Synechocystis Sp. PCC6803. PLoS ONE 2014, 9, 91484. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, H.; Kim, S. Enhanced Photocurrent Generation From a Single-Mediated Photo-Bioelectrochemical Cell Using Wild-Type Anabaena Variabilis Dispersed in Solution. ChemElectroChem 2020, 7, 4075–4083. [Google Scholar] [CrossRef]
- Pablo, F.; Buckeny, R.T.; Lim, R.P. Toxicity of Cyanide, Iron-Cyanide Complexes, and a Blast Furnace Effluent to Larvae of the Doughboy Scallop, Chlamys Asperrimus. Bull. Environ. Contam. Toxicol. 1997, 58, 93–100. [Google Scholar] [CrossRef]
- Murakawa, S.; Takahashi, T. Stimulation of Glutamate Uptake by NADH and NADPH in Intact Cells of Escherichia Coli Mutant Defective in Mg2+, Ca2+-ATPase. Agric. Biol. Chem. 1978, 42, 1803–1804. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Yang, F.; Lin, X.; Zhang, S.; Zhao, Z.K. Determining the Extremes of the Cellular NAD(H) Level by Using an Escherichia Coli NAD +-Auxotrophic Mutant. Appl. Environ. Microbiol. 2011, 77, 6133–6140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, P.; Embley, T.M.; Williams, T.A. Phylogenetic Diversity of NTT Nucleotide Transport Proteins in Free-Living and Parasitic Bacteria and Eukaryotes. Genome Biol. Evol. 2017, 9, 480–487. [Google Scholar] [CrossRef]
- Gruber, A.; Haferkamp, I. Nucleotide Transport and Metabolism in Diatoms. Biomolecules 2019, 9, 761. [Google Scholar] [CrossRef] [Green Version]
- Lovander, M.D.; Lyon, J.D.; Parr, D.L.; Wang, J.; Parke, B.; Leddy, J. Critical Review—Electrochemical Properties of 13 Vitamins: A Critical Review and Assessment. J. Electrochem. Soc. 2018, 165, G18–G49. [Google Scholar] [CrossRef]
- Gonzalez-Aravena, A.C.; Yunus, K.; Zhang, L.; Norling, B.; Fisher, A.C. Tapping into Cyanobacteria Electron Transfer for Higher Exoelectrogenic Activity by Imposing Iron Limited Growth. RSC Adv. 2018, 8, 20263–20274. [Google Scholar] [CrossRef] [Green Version]
- Marco, P.; Elman, T.; Yacoby, I. Binding of Ferredoxin NADP+ Oxidoreductase (FNR) to Plant Photosystem I. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a, b, c, and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shlosberg, Y.; Tóth, T.N.; Eichenbaum, B.; Keysar, L.; Schuster, G.; Adir, N. Electron Mediation and Photocurrent Enhancement in Dunalliela salina Driven Bio-Photo Electrochemical Cells. Catalysts 2021, 11, 1220. https://doi.org/10.3390/catal11101220
Shlosberg Y, Tóth TN, Eichenbaum B, Keysar L, Schuster G, Adir N. Electron Mediation and Photocurrent Enhancement in Dunalliela salina Driven Bio-Photo Electrochemical Cells. Catalysts. 2021; 11(10):1220. https://doi.org/10.3390/catal11101220
Chicago/Turabian StyleShlosberg, Yaniv, Tünde N. Tóth, Benjamin Eichenbaum, Lee Keysar, Gadi Schuster, and Noam Adir. 2021. "Electron Mediation and Photocurrent Enhancement in Dunalliela salina Driven Bio-Photo Electrochemical Cells" Catalysts 11, no. 10: 1220. https://doi.org/10.3390/catal11101220
APA StyleShlosberg, Y., Tóth, T. N., Eichenbaum, B., Keysar, L., Schuster, G., & Adir, N. (2021). Electron Mediation and Photocurrent Enhancement in Dunalliela salina Driven Bio-Photo Electrochemical Cells. Catalysts, 11(10), 1220. https://doi.org/10.3390/catal11101220