The Effect of Preparation Method of Ni-Supported SiO2 Catalysts for Carbon Dioxide Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
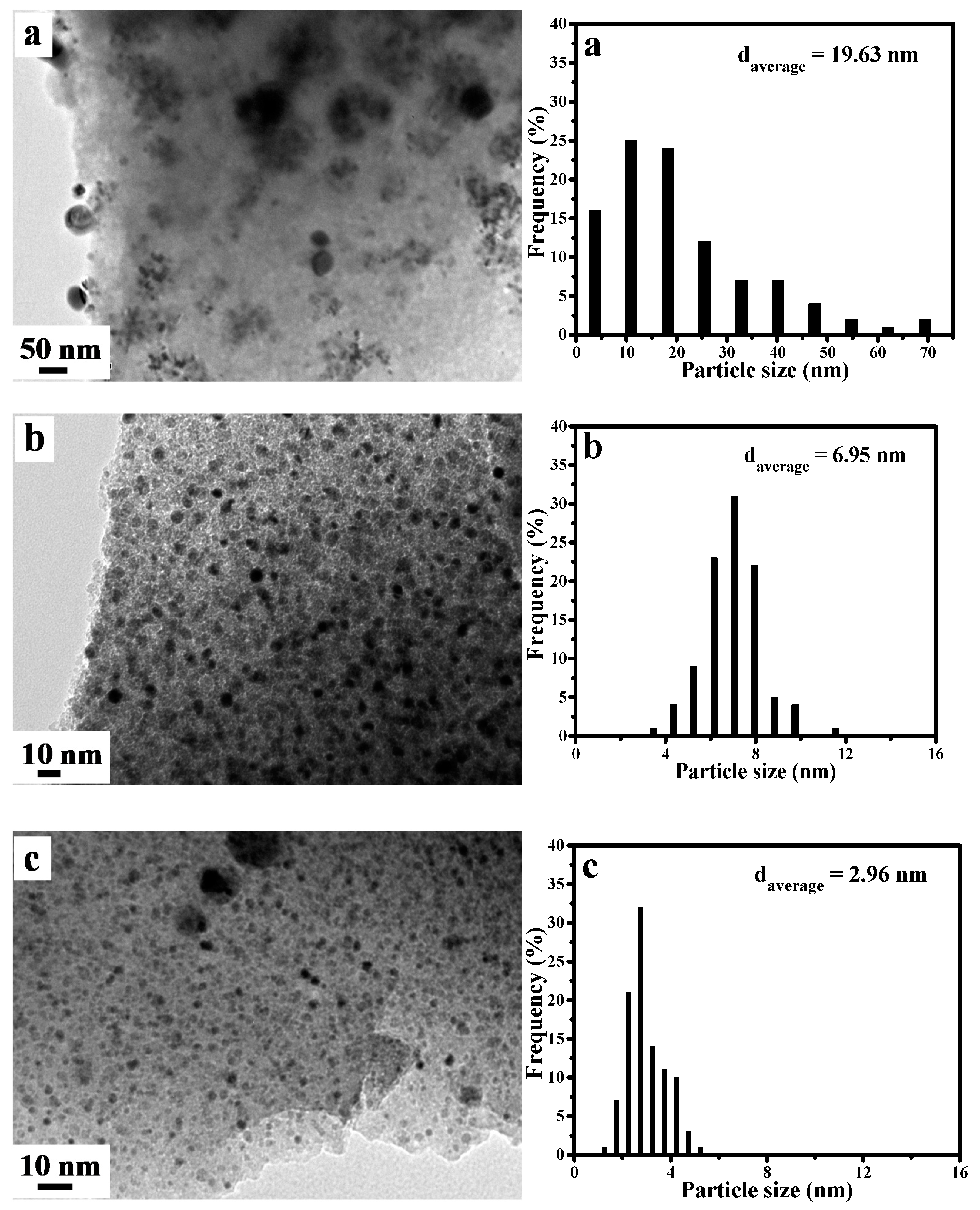
2.1. Textural Properties of Ni-Based Catalysts
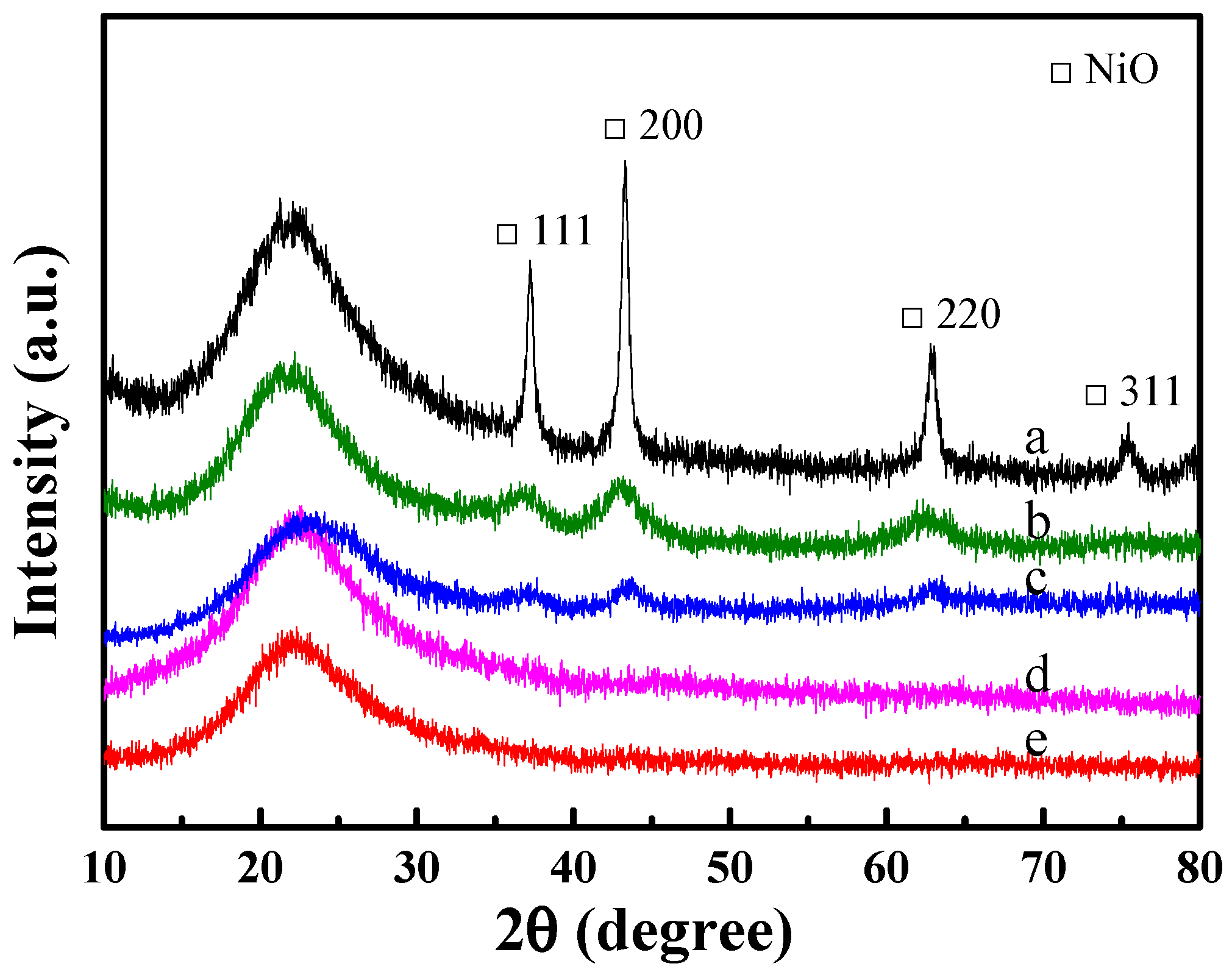
2.2. Structural Properties of Ni-Based Catalysts
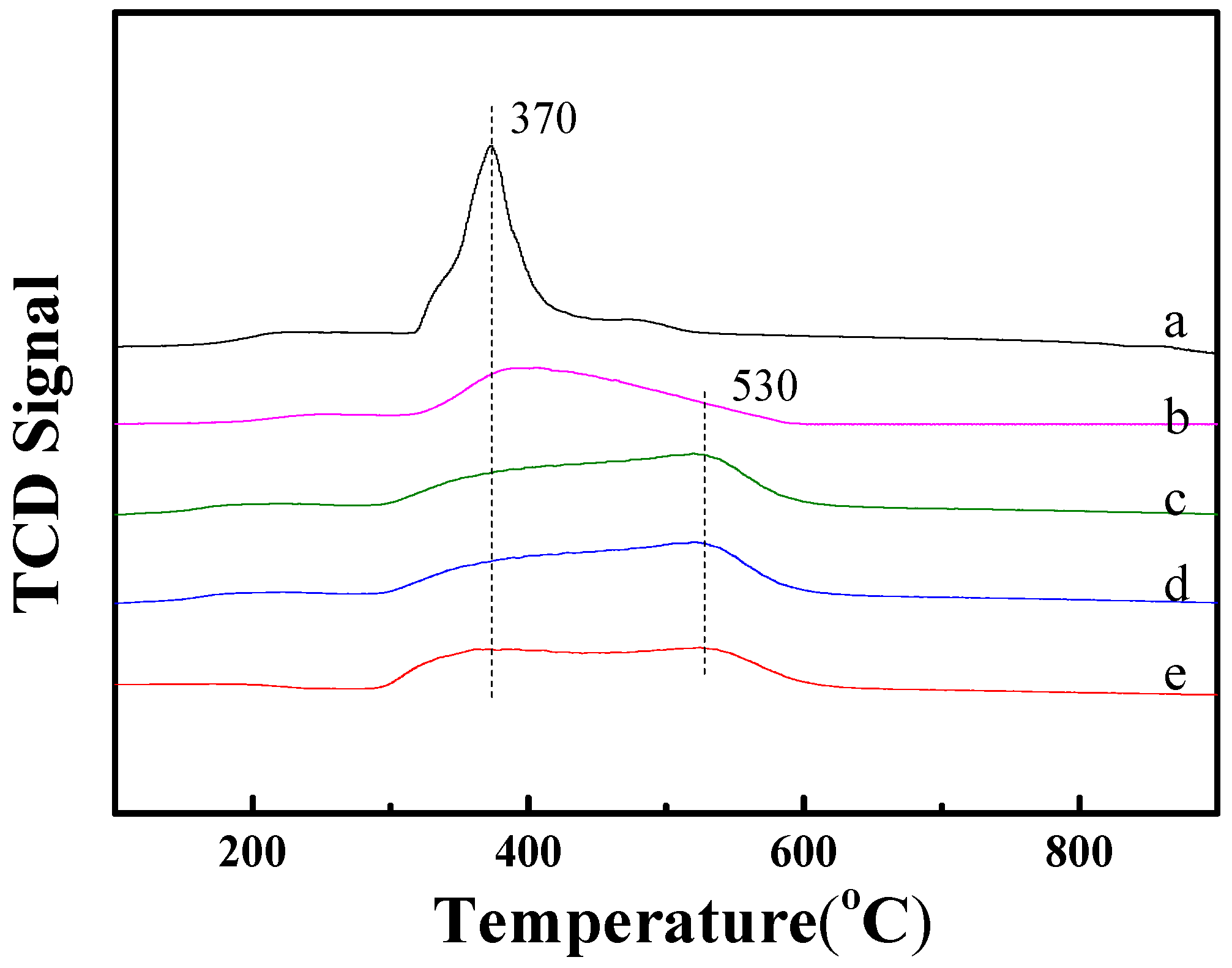
2.3. Reduction Ability of Ni-Based Catalysts
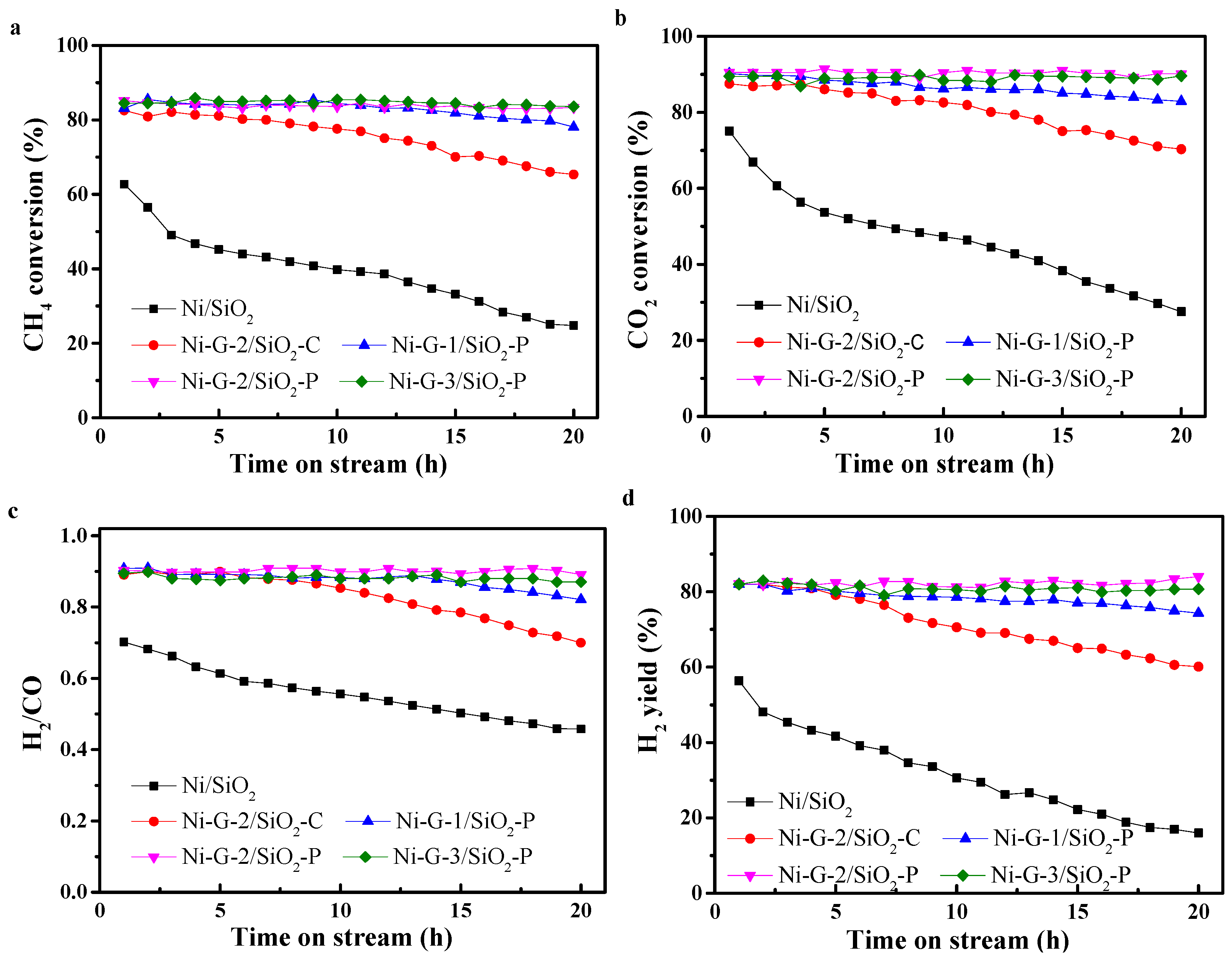
2.4. Catalytic Activity and Stability Tests
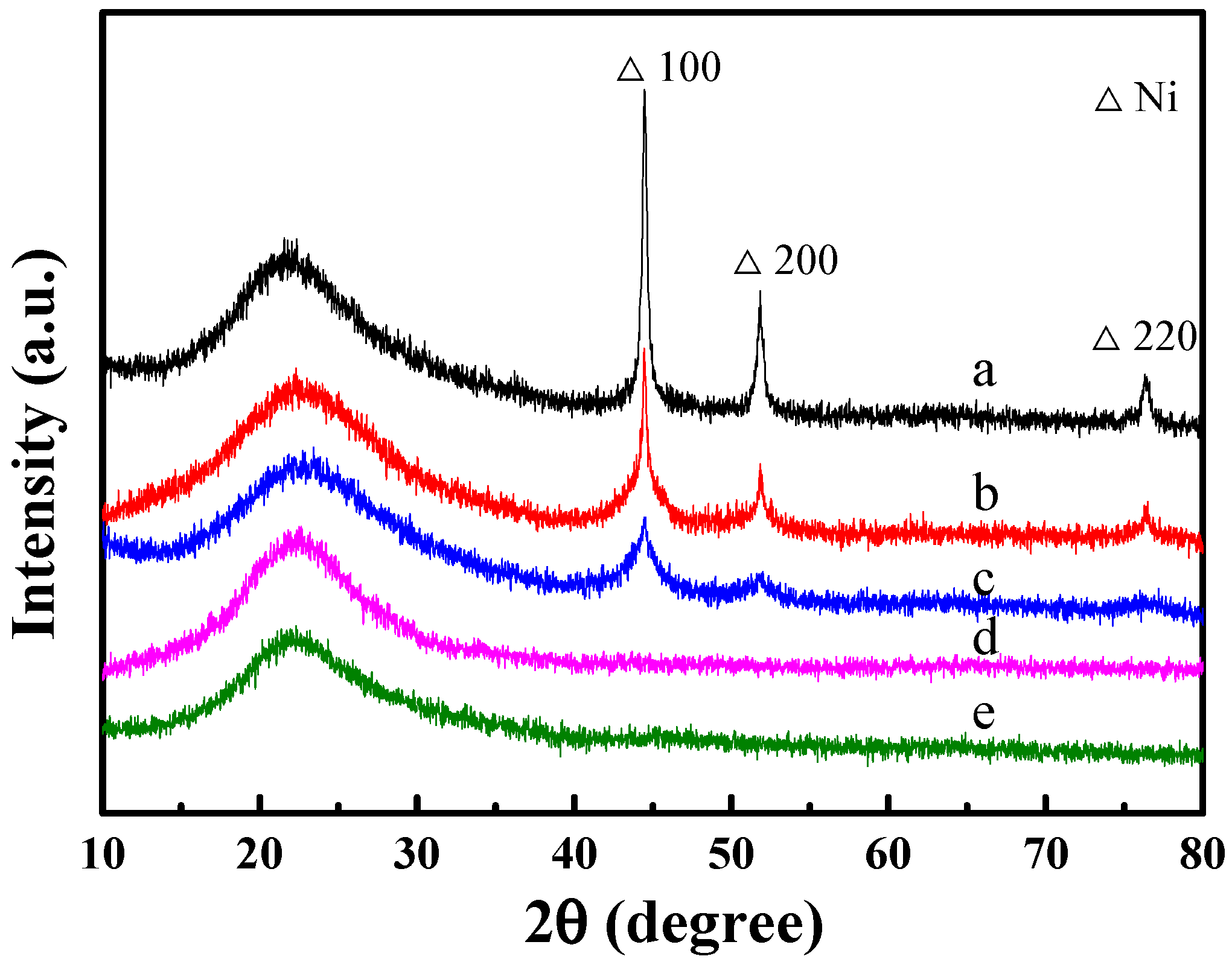
2.5. Characterization of Used Catalysts
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization Techniques
3.3. Activity Evaluation of the Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moura, I.P.; Reis, A.C.; Bresciani, A.E.; Alves, R.M.B. Carbon dioxide abatement by integration of methane bi-reforming process with ammonia and urea synthesis. Renew. Sustain. Energy Rev. 2021, 151, 111619. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Zhu, K.; Sui, Z.; Chen, D.; Zhu, Y.; Zhou, X. High-throughput screening of alloy catalysts for dry methane reforming. ACS Catal. 2021, 11, 8881–8894. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen Production from reforming of biogas: Review of technological advances and an indian perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.; Saw, E.T. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Simonov, M.; Bespalko, Y.; Smal, E.; Valeev, K.; Fedorova, V.; Krieger, T.; Sadykov, V. Nickel-containing ceria-zirconia doped with Ti and Nb. Effect of support composition and preparation method on catalytic activity in methane dry reforming. Nanomaterials 2020, 10, 1281. [Google Scholar] [CrossRef]
- Azancot, L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Effect of potassium loading on basic properties of Ni/MgAl2O4 catalyst for CO2 reforming of methane. J. CO2 Util. 2021, 52, 101681. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Ramantani, T.; Bampos, G.; Vavatsikos, A.; Vatskalis, G.; Kondarides, D.I. Propane steam reforming over catalysts derived from noble metal (Ru, Rh)-substituted LaNiO3 and La0.8Sr0.2NiO3 perovskite precursors. Nanomaterials 2021, 11, 1931. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiu, Y.; Zhang, X.; Zhang, Y.; Sun, N.; Zhao, Y. Geometric design of a Ni@silica nano-capsule catalyst with superb methane dry reforming stability: Enhanced confinement effect over the nickel site anchoring inside a capsule shell with an appropriate inner cavity. Catal. Sci. Technol. 2018, 19, 4877–4890. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, W.; Li, S.; Wu, G.; Ma, X.; Wang, X.; Gong, J. Sintering-resistant Ni-based reforming catalysts obtained via the nanoconfinement effect. Chem. Commun. 2013, 49, 9383–9385. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile route for synthesizing ordered mesoporous Ni-Ce-Al oxide materials and their catalytic performance for methane dry reforming to hydrogen and syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Daoura, O.; Fornasieri, G.; Boutros, M.; Hassan, N.E.; Beaunier, P.; Thomas, C.; Selmane, M.; Miche, A.; Sassoye, C.; Ersen, O.; et al. One-pot prepared mesoporous silica SBA-15-like monoliths with embedded Ni particles as selective and stable catalysts for methane dry reforming. Appl. Catal. B Environ. 2021, 280, 119417. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, M.; Ning, P.; Long, K.; Wang, J.; Tang, T.; Fan, J.; Sun, H.; Yin, L.; Lin, Q. Effect of thermal induction temperature on re-dispersion behavior of Ni nanoparticles over Ni/SBA-15 for dry reforming of methane. Appl. Surf. Sci. 2019, 469, 368–377. [Google Scholar] [CrossRef]
- Danghyan, V.; Kumar, A.; Mukasyan, A.; Wolf, E.E. An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane. Appl. Catal. Environ. 2020, 273, 119056. [Google Scholar] [CrossRef]
- Fang, X.; Peng, C.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W. Methane dry reforming over coke-resistant mesoporous Ni-Al2O3 catalysts prepared by evaporation-induced self-assembly method. ChemCatChem 2015, 7, 3753–3762. [Google Scholar] [CrossRef]
- Dai, Y.; Zou, R.; Ba, T.; Zhang, J.; Liu, C. Highly active and coke resistant Ni/CeZrO2 catalyst prepared by cold plasma decomposition for CO2 reforming of methane. J. CO2 Util. 2021, 51, 101647. [Google Scholar] [CrossRef]
- Zhu, X.; Huo, P.; Zhang, Y.; Cheng, D.; Liu, C. Structure and reactivity of plasma treated Ni/Al2O3 catalyst for CO2 reforming of methane. Appl. Catal. B Environ. 2008, 81, 132–140. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhang, X.; Liu, Y.; Liu, C. Improvement in the activity of Ni/ZrO2 by cold plasma decomposition for dry reforming of methane. Catal. Commun. 2019, 128, 105720. [Google Scholar] [CrossRef]
- Li, B.; Yuan, X.; Li, L.; Li, B.; Wang, X.; Tomishige, K. Lanthanide oxide modified nickel supported on mesoporous silica catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 31608–31622. [Google Scholar] [CrossRef]
- Agueniou, F.; Vidal, H.; Yeste, M.P.; Hernández-Garrido, J.C.; Cauqui, M.A.; Rodríguez-Izquierdo, J.M.; Calvino, J.J.; Gatica, J.M. Ultrathin washcoat and very low loading monolithic catalyst with outstanding activity and stability in dry reforming of methane. Nanomaterials 2020, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Costa, P.D. Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jiang, Q.; Gao, Y.; Liu, J.; Gu, H.; Neal, L.; Li, F. LaNixFe1-xO3-δ as a robust redox catalyst for CO2 splitting and methane partial oxidation. Energy Fuels 2021, 35, 13921–13929. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from LaCoxNi1-x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Han, K.; Wang, S.; Liu, Q.; Wang, F. Optimizing the Ni/Cu ratio in Ni-Cu nanoparticle catalysts for methane dry reforming. ACS Appl. Nano Mater. 2021, 4, 5340–5348. [Google Scholar] [CrossRef]
- Guharoy, U.; Le Saché, E.; Cai, Q.; Reina, T.R.; Gu, S. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane. J. CO2 Util. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Ding, F.; Guo, X.; Wang, G. Syngas production via steam-CO2 dual reforming of methane over La-Ni/ZrO2 catalyst prepared by L-arginine ligand-assisted strategy: Enhanced activity and stability. ACS Sustain. Chem. Eng. 2015, 3, 3461–3476. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, T.; Wang, J.; Sun, M.; Wang, H.; Sun, H.; Ning, P. Facile template-free synthesis of Ni-SiO2 catalyst with excellent sintering-and coking-resistance for dry reforming of methane. Catal. Commun. 2019, 131, 105782. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Beaunier, P.; Che, M.; Train, C. Stabilization of hexagonal close-packed metallic nickel for alumina-supported systems prepared from Ni(II) glycinate. J. Solid State Chem. 2007, 180, 22–30. [Google Scholar] [CrossRef]
- Bian, Z.; Xin, Z.; Meng, X.; Tao, M.; Lv, Y.; Gu, J. Effect of citric acid on the synthesis of CO metalation catalysts with high activity and excellent stability. Ind. Eng. Chem. Res. 2017, 56, 2383–2392. [Google Scholar] [CrossRef]
- Zhang, Q.; Long, K.; Wang, J.; Zhang, T.; Song, Z.; Lin, Q. A novel promoting effect of chelating ligand on the dispersion of Ni species over Ni/SBA-15 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 14103–14114. [Google Scholar] [CrossRef]
- Ren, H.; Hao, Q.; Ding, S.; Zhao, Y.; Zhu, M.; Tian, S.; Ma, Q.; Song, W.; Miao, Z.; Liu, Z. A high-performance Ni/SiO2 prepared by the complexed-impregnation method with citric acid for carbon dioxide reforming of methane. Ind. Eng. Chem. Res. 2018, 57, 16257–16263. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Cai, Y.; Wang, C.; Zhang, Q.; Wang, H. One-step synthesis of metallic Ni-C/Al2O3 directly applied for CO2 reforming of CH4. Int. J. Hydrogen Energy 2014, 44, 21651–21658. [Google Scholar] [CrossRef]
- Ren, H.; Tian, S.; Ding, S.; Huang, G.; Zhu, M.; Ma, Q.; Song, W.; Zhao, Y.; Miao, Z.; Wang, W. Carbon dioxide reforming of methane over Ni supported SiO2: Influence of the preparation method on the resulting structural properties. Catalysts 2020, 10, 795. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Che, M.; Pepe, C. Influence of the morphology and impurities of Ni(OH)2 on the synthesis of neutral Ni(II)-amino acid complexes. J. Solid State Chem. 2007, 180, 3469–3478. [Google Scholar] [CrossRef]
- Gorboletova, G.G.; Metlin, A.A. Standard thermodynamic functions of Co2+ complexation with glycine and L-histidine in aqueous solution. J. Phys. Chem. 2016, 90, 334–338. [Google Scholar] [CrossRef]
- Sun, K.Q.; Marceau, E.; Che, M. Evolution of nickel speciation during preparation of Ni-SiO2 catalysts: Effect of the number of chelating ligands in [Ni(en)x(H2O)6-2x]2+ precursor complexes. Phys. Chem. Chem. Phys. 2006, 8, 1731–1738. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas production from CO2 reforming with methane over core-shell Ni@SiO2 catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]


| Samples | SBET (m2·g−1) a | Pore Volumes (cm3·g−1) b | Pore Diameter (nm) c |
|---|---|---|---|
| SiO2 | 231.3 | 1.28 | 23.6 |
| Ni/SiO2 | 176.9 | 0.91 | 20.8 |
| Ni-G-2/SiO2-C | 188.3 | 0.91 | 20.7 |
| Ni-G-1/SiO2-P | 193.6 | 0.93 | 21.0 |
| Ni-G-2/SiO2-P | 199.5 | 0.89 | 20.7 |
| Ni-G-3/SiO2-P | 201.8 | 0.90 | 20.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-P.; Ding, S.-Y.; Ma, Q.; Song, W.-Q.; Zhao, Y.-Z.; Liu, J.; He, Y.-M.; Tian, S.-P. The Effect of Preparation Method of Ni-Supported SiO2 Catalysts for Carbon Dioxide Reforming of Methane. Catalysts 2021, 11, 1221. https://doi.org/10.3390/catal11101221
Ren H-P, Ding S-Y, Ma Q, Song W-Q, Zhao Y-Z, Liu J, He Y-M, Tian S-P. The Effect of Preparation Method of Ni-Supported SiO2 Catalysts for Carbon Dioxide Reforming of Methane. Catalysts. 2021; 11(10):1221. https://doi.org/10.3390/catal11101221
Chicago/Turabian StyleRen, Hua-Ping, Si-Yi Ding, Qiang Ma, Wen-Qi Song, Yu-Zhen Zhao, Jiao Liu, Ye-Ming He, and Shao-Peng Tian. 2021. "The Effect of Preparation Method of Ni-Supported SiO2 Catalysts for Carbon Dioxide Reforming of Methane" Catalysts 11, no. 10: 1221. https://doi.org/10.3390/catal11101221





