Computational Assessment of Botrytis cinerea Lipase for Biofuel Production
Abstract
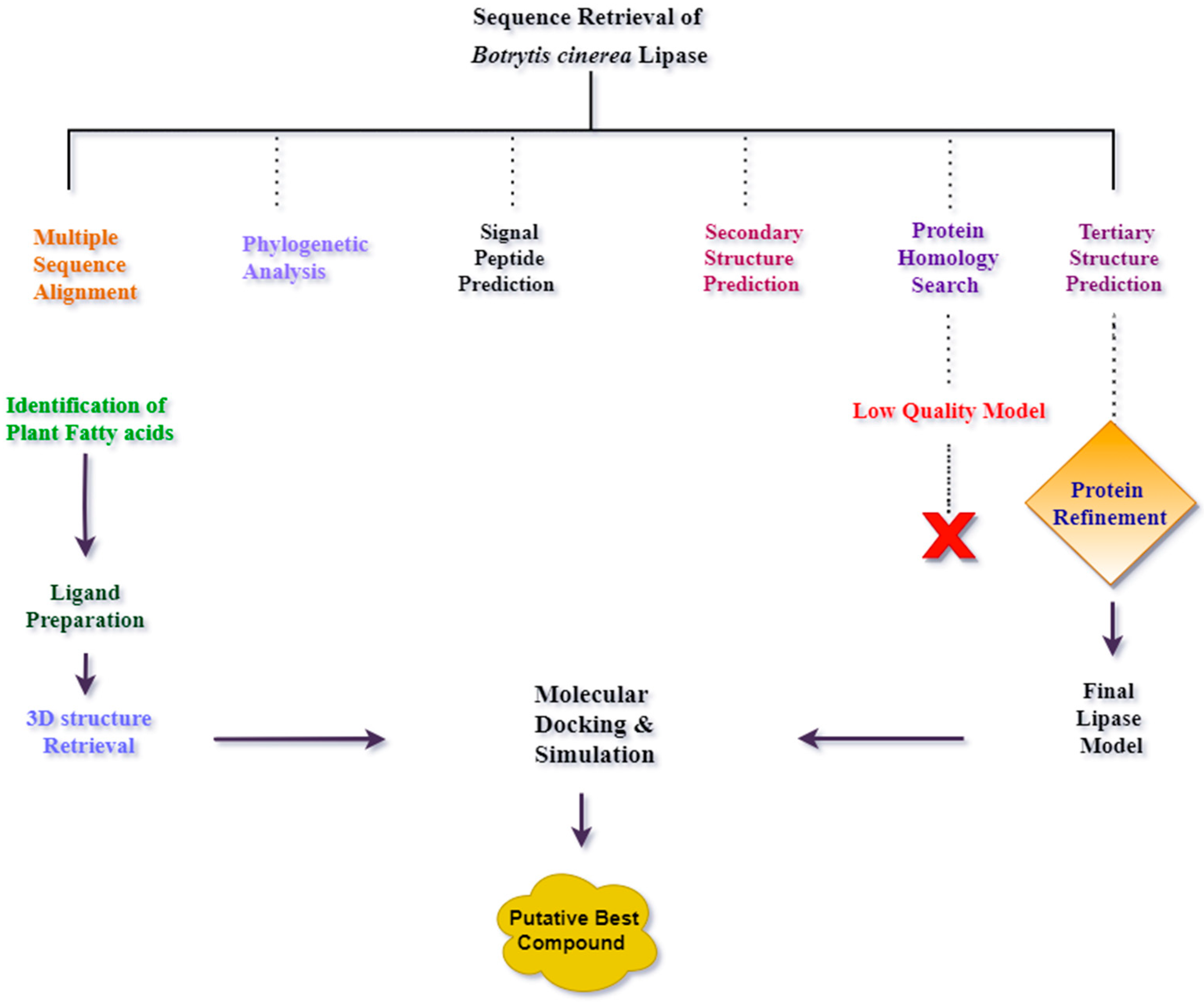
:1. Introduction
2. Results
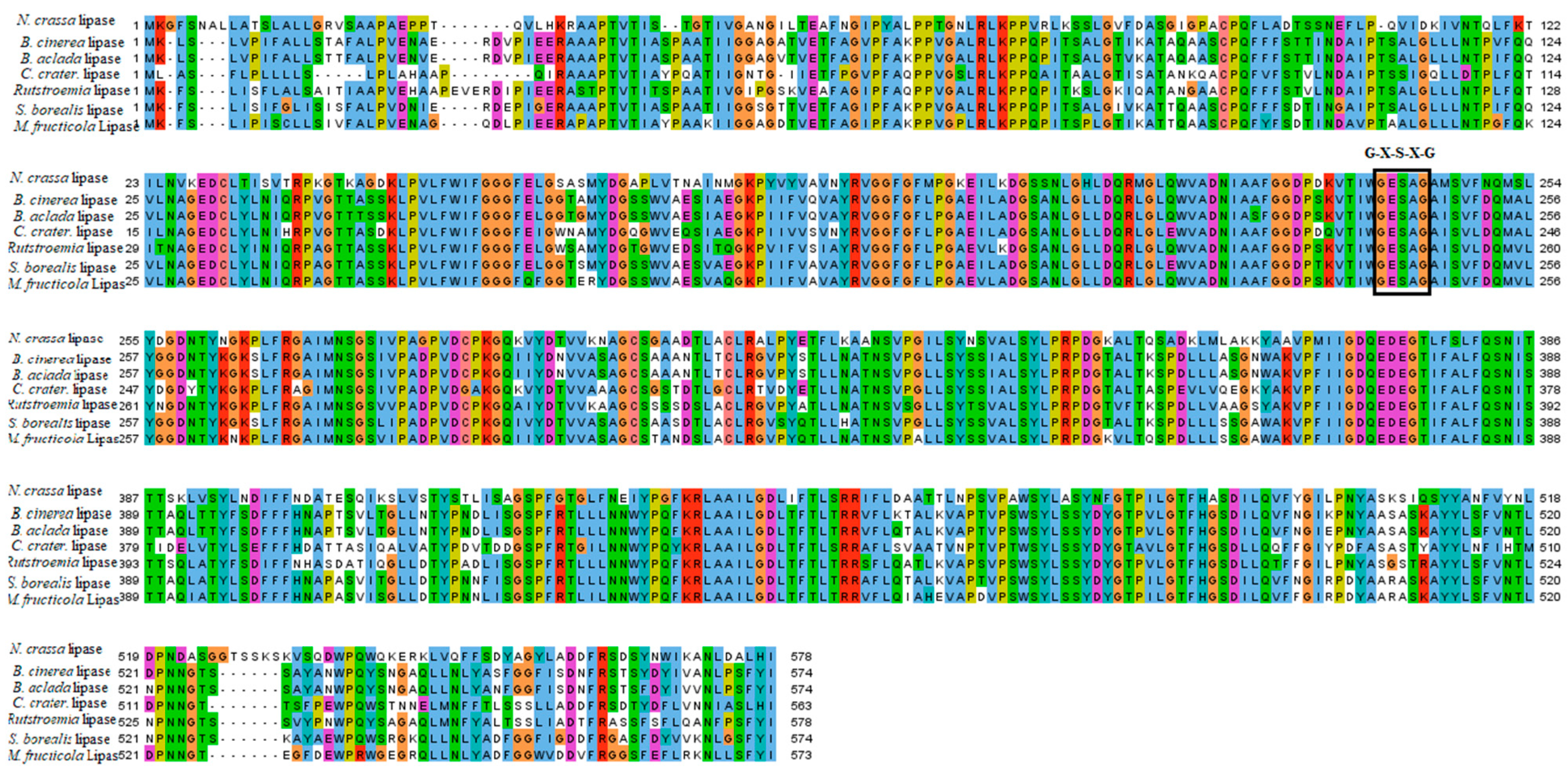
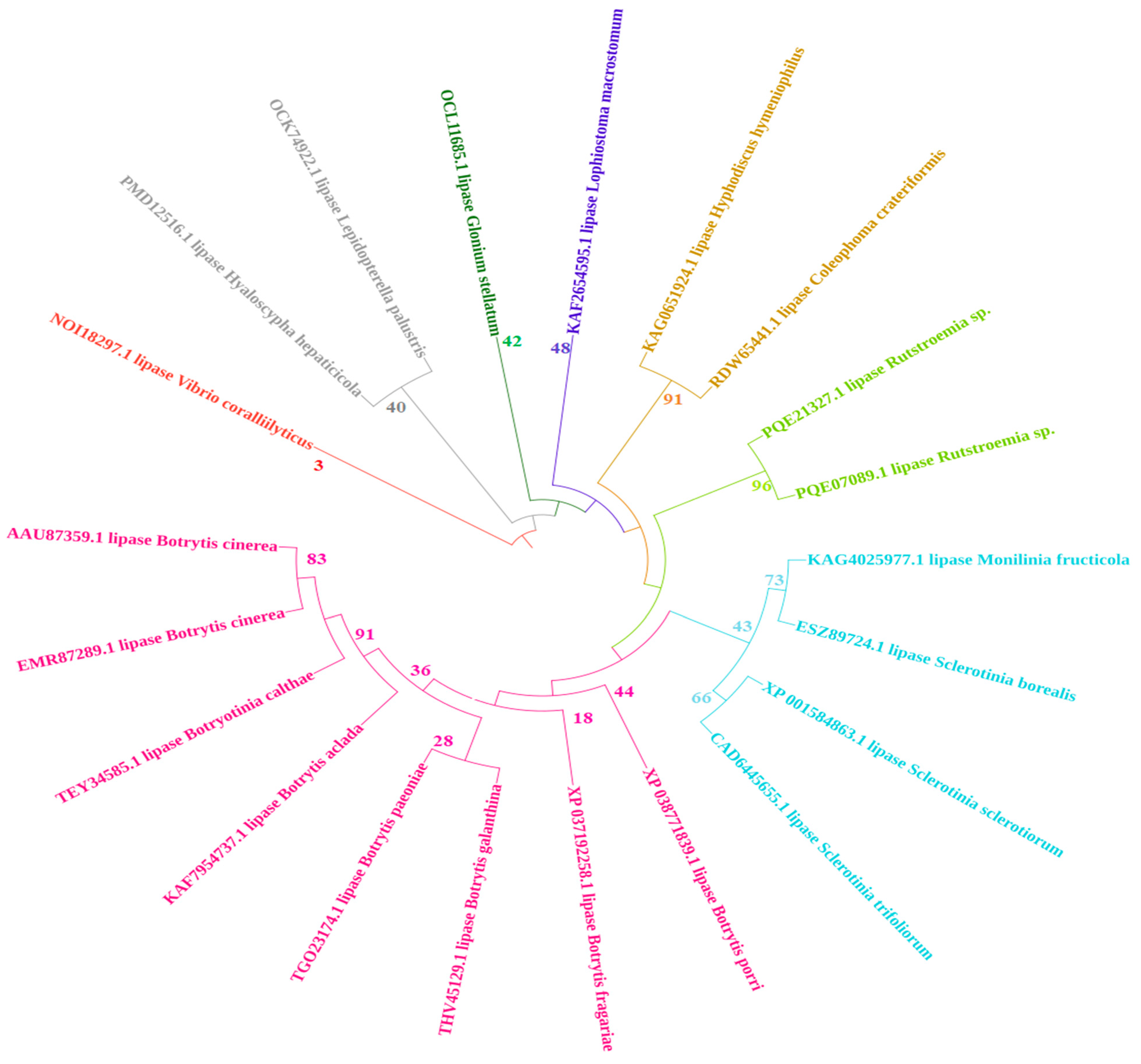
2.1. Multiple Sequence Alignment and Phylogenetic Analysis of B. cinerea Lipase
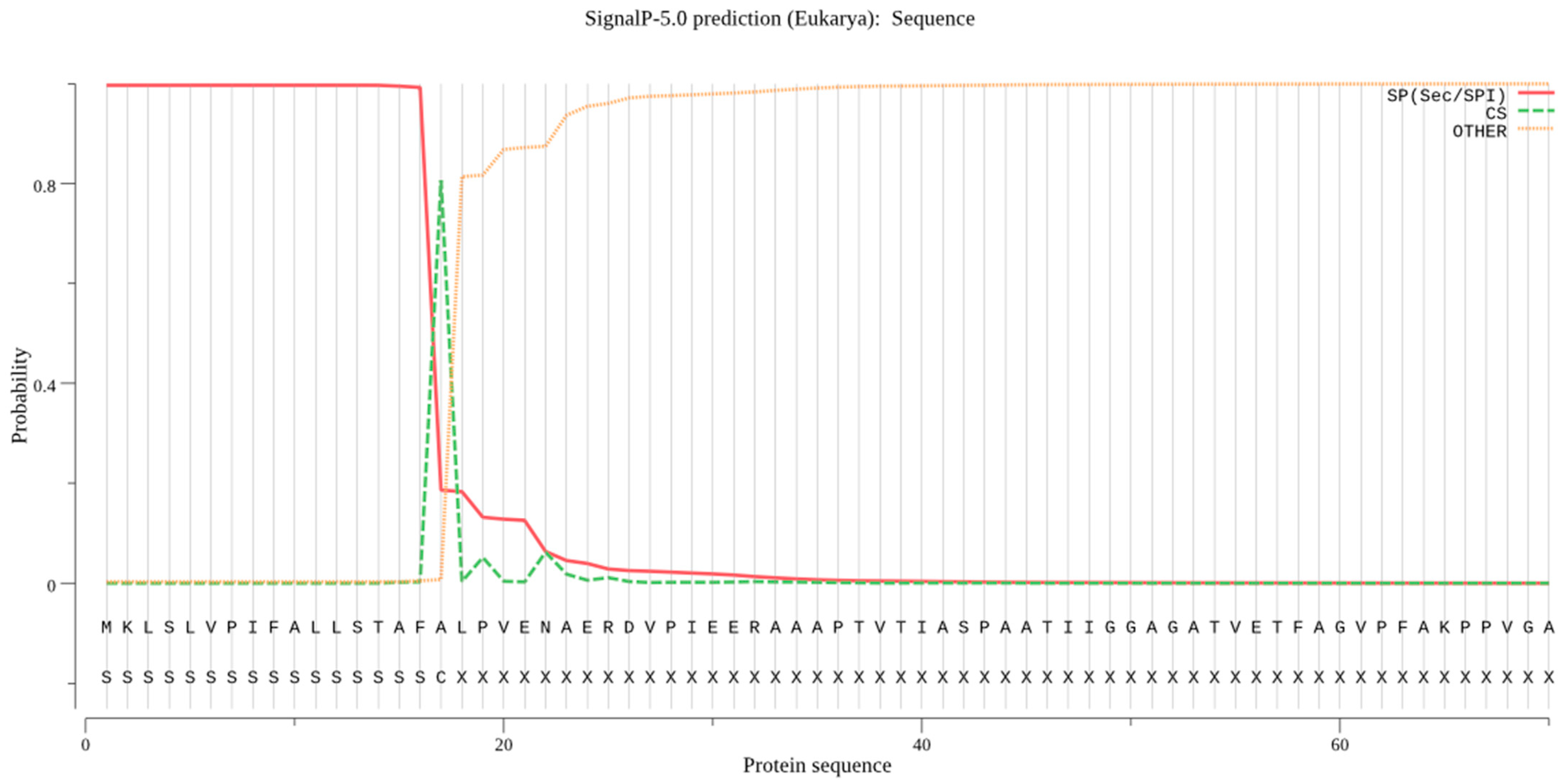
2.2. Signal Peptide Removal
2.3. Prediction of Secondary Structure of B. cinerea Lipase
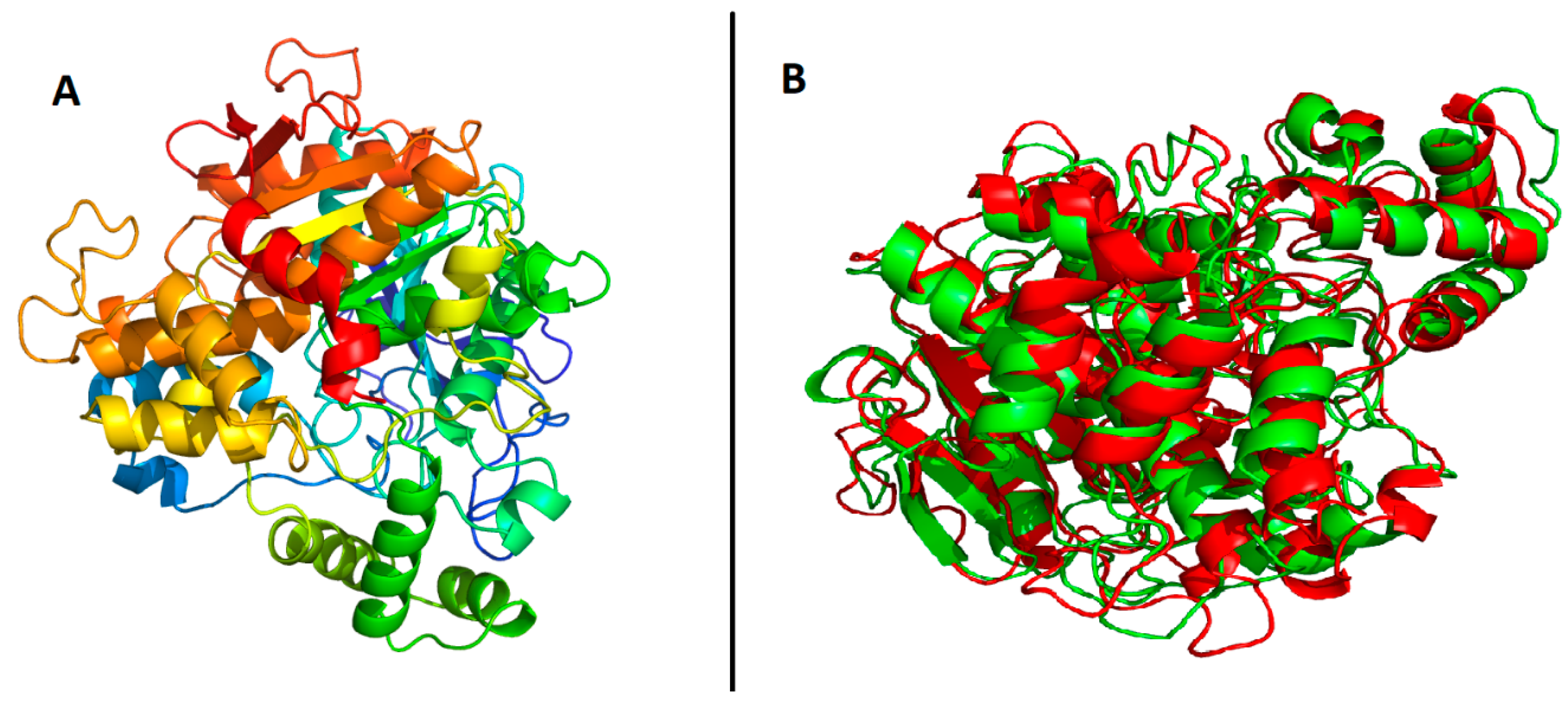
2.4. Prediction of Tertiary Structure of B. cinerea Lipase
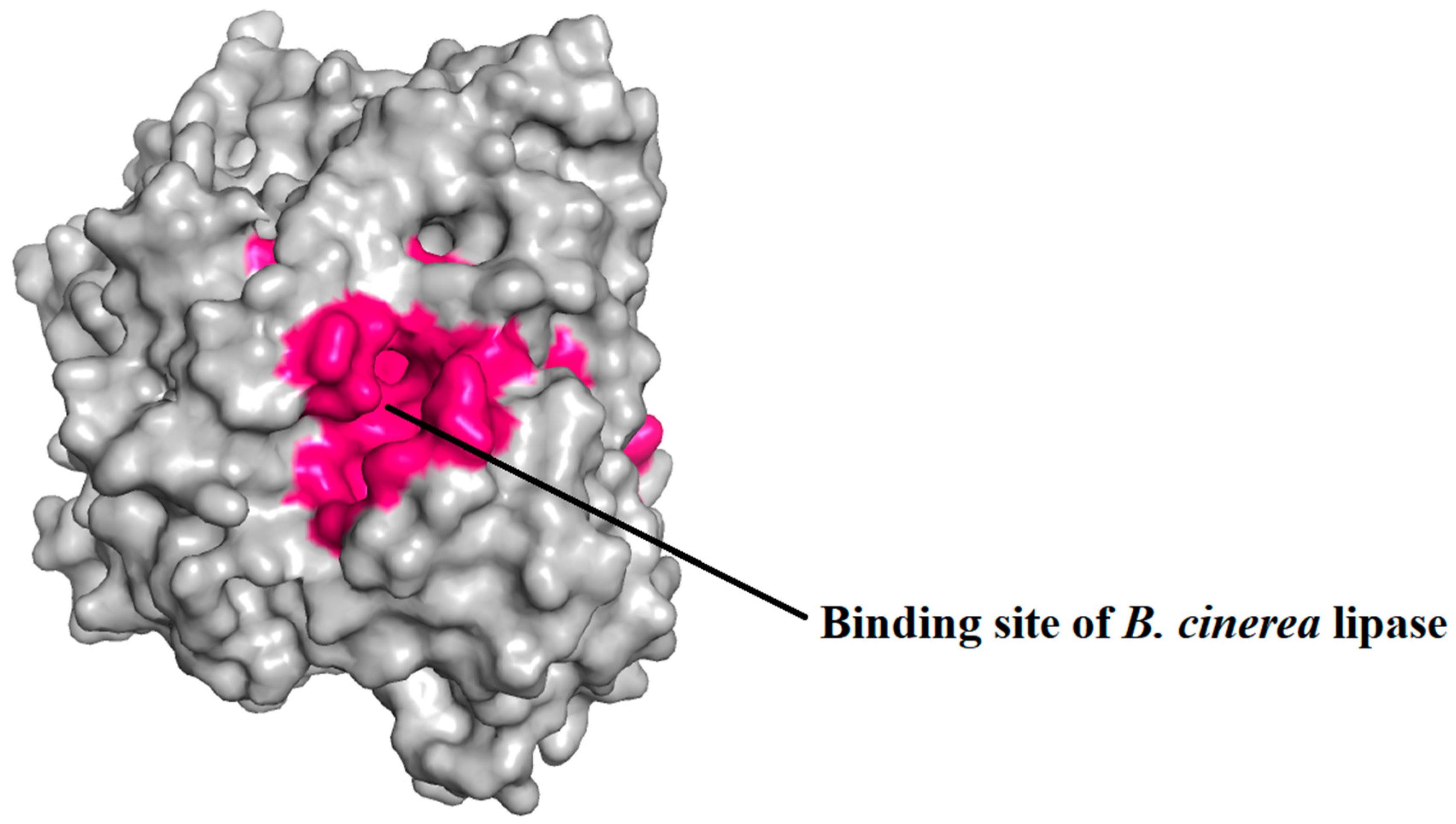
2.5. Prediction of Binding Pocket Site of Protein
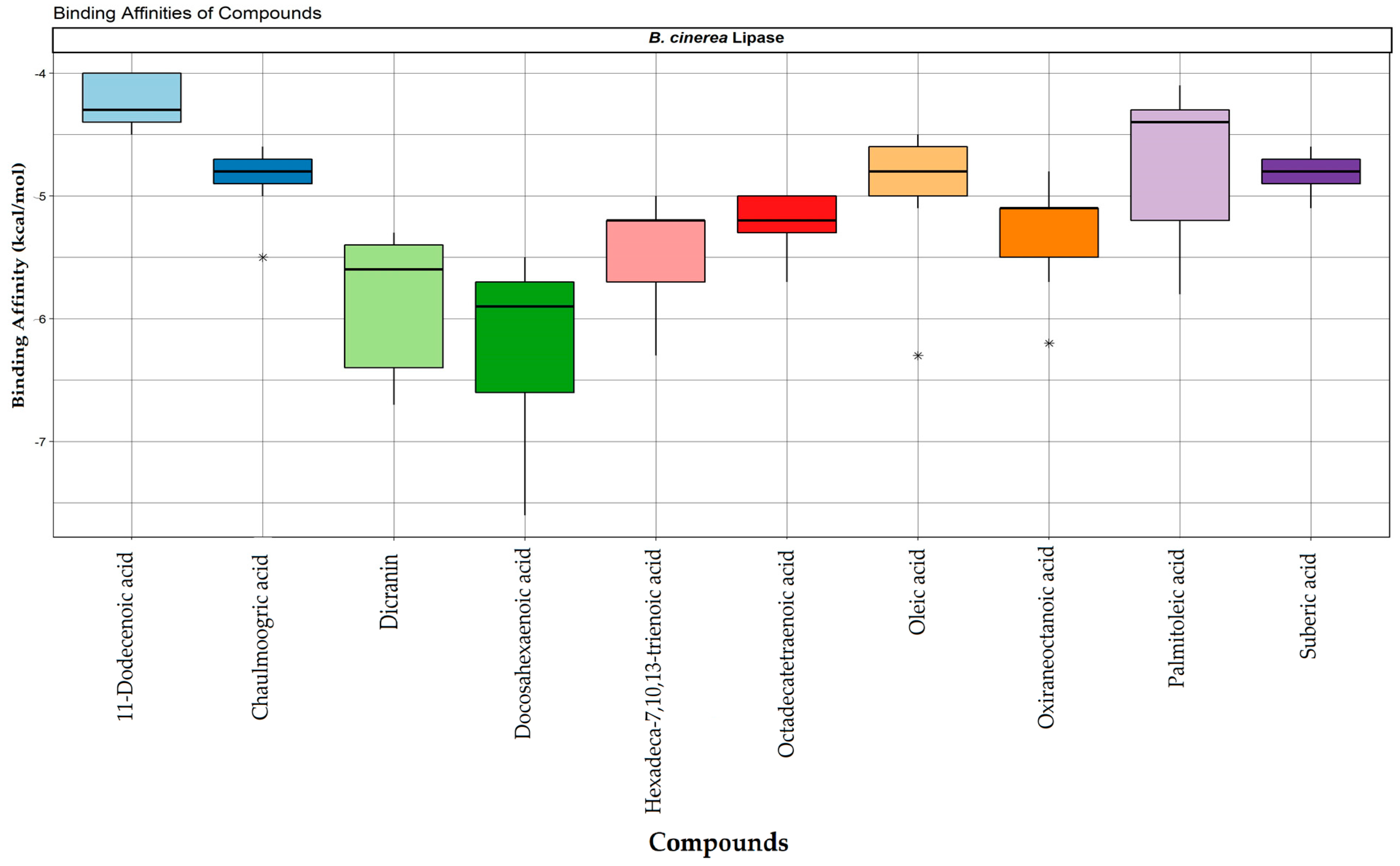
2.6. Molecular Docking of Lipase with Plant Triglycerides
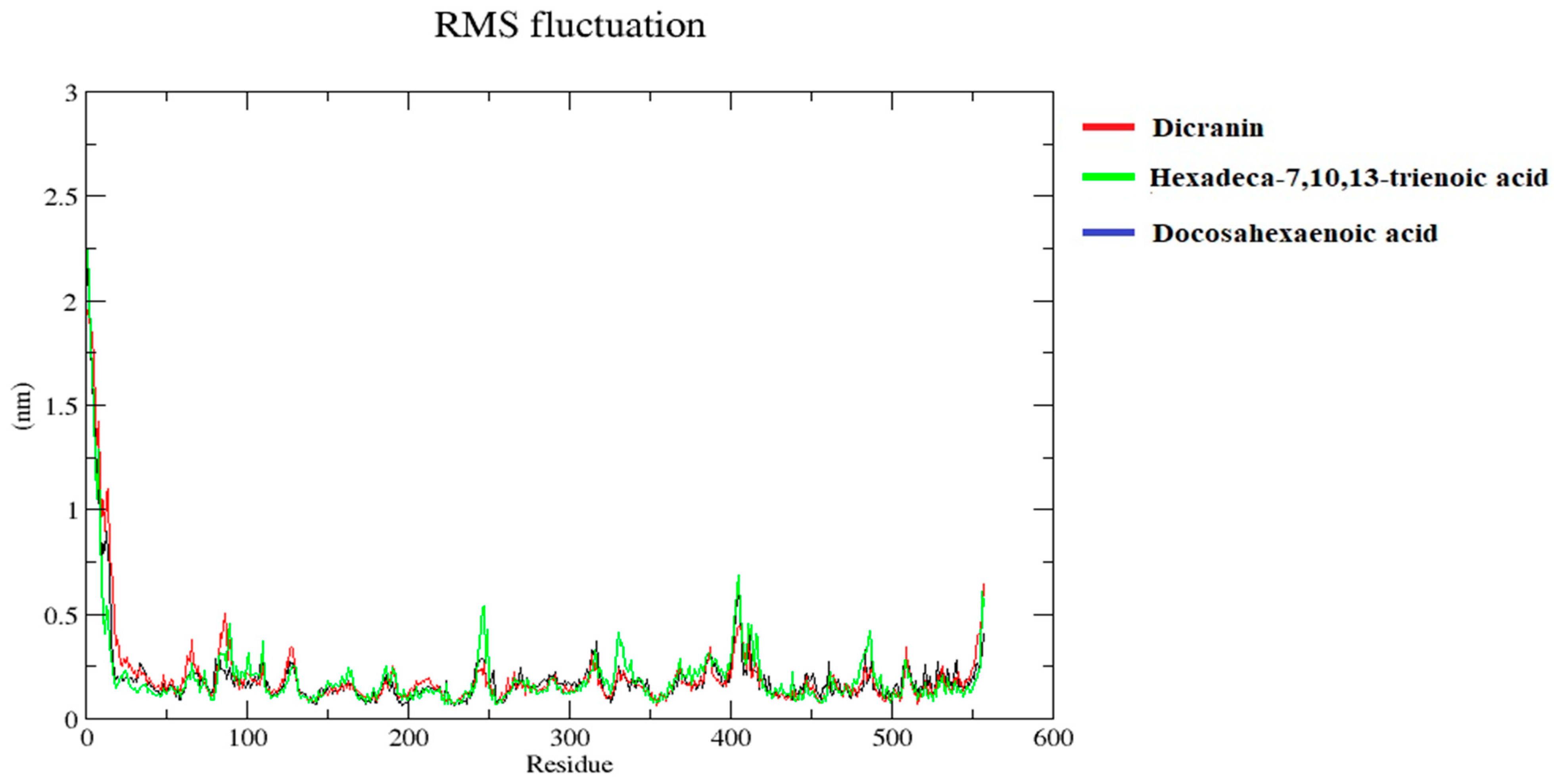
2.7. Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
4.1. Multiple Sequence Alignment and Phylogenetic Analysis of Lipase in Botrytis cinerea
4.2. Signal Peptide Prediction
4.3. Protein Secondary Structure Prediction
4.4. Tertiary Structure Prediction of B. cinerea Lipase
4.5. Protein Model Validation
4.6. Binding Site Prediction
4.7. Ligand Preparation
4.8. Protein Preparation
4.9. Molecular Docking
4.10. Molecular Dynamics Simulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Posarac, D.; Ellis, N. Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol. Chem. Eng. Res. Des. 2011, 89, 2626–2642. [Google Scholar] [CrossRef]
- Aguieiras, E.C.; Cavalcanti-Oliveira, E.D.; Freire, D.M. Current status and new developments of biodiesel production using fungal lipases. Fuel 2015, 159, 52–67. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Department of State Development, Manufacturing, Infrastructure and Planning. Queensland Hydrogen Industry Strategy 2019–2024; Department of State Development, Manufacturing, Infrastructure and Planning: Queensland, Australia, 2019.
- Malani, R.S.; Moholkar, V.S.; Elbashir, N.O.; Choudhury, H.A. Chapter 2: Advancements of Cavitation Technology in Biodiesel Production–from Fundamental Concept to Commercial Scale-Up. In Liquid Biofuels: Fundamentals, Characterization, and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 39–76. [Google Scholar]
- Fleuri, L.F.; de Oliveira, M.C.; Arcuri, M.d.L.C.; Capoville, B.L.; Pereira, M.S.; Delgado, C.H.O.; Novelli, P.K. Production of fungal lipases using wheat bran and soybean bran and incorporation of sugarcane bagasse as a co-substrate in solid-state fermentation. Food Sci. Biotechnol. 2014, 23, 1199–1205. [Google Scholar] [CrossRef]
- Durrett, T.P.; Benning, C.; Ohlrogge, J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008, 54, 593–607. [Google Scholar] [CrossRef]
- No, S.-Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Raghavendra, M.; Nayaka, S.C.; Gupta, V.K. Microbial Enzymes for Conversion of Biomass to Bioenergy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–26. [Google Scholar]
- Li, P.; Makino, H. Liquefied dimethyl ether: An energy-saving, green extraction solvent. In Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; pp. 91–106. [Google Scholar]
- Juangsa, F.B.; Prananto, L.A.; Mufrodi, Z.; Budiman, A.; Oda, T.; Aziz, M. Highly energy-efficient combination of dehydrogenation of methylcyclohexane and hydrogen-based power generation. Appl. Energy 2018, 226, 31–38. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Pang, H.; Yuan, S.; Wang, X.; Hu, Z.; Zhou, Q.; He, Y.; Yan, Y.; Xu, L. Codisplay of Rhizopus oryzae and Candida rugosa Lipases for Biodiesel Production. Catalysts 2021, 11, 421. [Google Scholar] [CrossRef]
- Hama, S.; Tamalampudi, S.; Fukumizu, T.; Miura, K.; Yamaji, H.; Kondo, A.; Fukuda, H. Lipase localization in Rhizopus oryzae cells immobilized within biomass support particles for use as whole-cell biocatalysts in biodiesel-fuel production. J. Biosci. Bioeng. 2006, 101, 328–333. [Google Scholar] [CrossRef]
- Weber, N.; Weitkamp, P.; Mukherjee, K.D. Steryl and stanyl esters of fatty acids by solvent-free esterification and transesterification in vacuo using lipases from Rhizomucor miehei, Candida antarctica, and Carica papaya. J. Agric. Food Chem. 2001, 49, 5210–5216. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, N.; Maeda, A.; Mizuno, T.; Bunya, M.; Sugihara, S.; Sugihara, A. Synthesis of fatty acid sterol esters using cholesterol esterase from Trichoderma sp. AS59. Enzym. Microb. Technol. 2011, 48, 498–504. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Gupta, R.; Kumari, A.; Syal, P.; Singh, Y. Molecular and functional diversity of yeast and fungal lipases: Their role in biotechnology and cellular physiology. Prog. Lipid Res. 2015, 57, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Pleiss, J. The Lipase Engineering Database: A navigation and analysis tool for protein families. Nucleic Acids Res. 2003, 31, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, G.M.; Trono, D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [Green Version]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Plaza, V.; Larrondo, L.F.; Canessa, P.; Science, P. Recent advances in the study of the plant pathogenic fungus Botrytis cinerea and its interaction with the environment. Curr. Protein Pept. Sci. 2017, 18, 976–989. [Google Scholar] [CrossRef]
- Reis, H.; Pfiffi, S.; Hahn, M. Molecular and functional characterization of a secreted lipase from Botrytis cinerea. Mol. Plant Pathol. 2005, 6, 257–267. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.d.P.; Almeida, S.T.d.; Paula, A.S.; Medeiros, V.L.; Vasconcellos, A.d.; Nery, J.G.; Aranda, D.A. Potential new biocatalysts for biofuel production: The fungal lipases of Thermomyces lanuginosus and Rhizomucor miehei immobilized on nanozeolitic supports ion exchanged with lanthanide cations. In Proceedings of the 18. Brazil MRS Meeting, Balneário Camboriú, Brazil, 21–26 September 2019. [Google Scholar]
- Du, W.; Li, W.; Sun, T.; Chen, X.; Liu, D. Biotechnology. Perspectives for biotechnological production of biodiesel and impacts. Appl. Microbiol. Biotechnol. 2008, 79, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Exploitation. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Brännström, H.; Kumar, H.; Alén, R. Current and potential biofuel production from plant oils. BioEnergy Res. 2018, 11, 592–613. [Google Scholar] [CrossRef]
- Juhl, P.B.; Trodler, P.; Tyagi, S.; Pleiss, J. Modelling substrate specificity and enantioselectivity for lipases and esterases by substrate-imprinted docking. BMC Struct. Biol. 2009, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calero-Rueda, O.; Plou, F.J.; Ballesteros, A.; Martinez, A.T.; Martinez, M.J. Production, isolation and characterization of a sterol esterase from Ophiostoma piceae. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2002, 1599, 28–35. [Google Scholar] [CrossRef]
- Barriuso, J.; Vaquero, M.E.; Prieto, A.; Martínez, M.J. Structural traits and catalytic versatility of the lipases from the Candida rugosa-like family: A review. Biotechnol. Adv. 2016, 34, 874–885. [Google Scholar] [CrossRef]
- Kontkanen, H.; Tenkanen, M.; Reinikainen, T. Purification and characterisation of a novel steryl esterase from Melanocarpus albomyces. Enzym. Microb. Technol. 2006, 39, 265–273. [Google Scholar] [CrossRef]
- Moya-Salazar, J.; Vértiz-Osores, J.; Jibaja, S.; Acevedo-Espindola, C.; Rupa, R.; Alarcón-Díaz, M. Fungi lipases homology modeling and molecular docking with fatty acids and tripalmitin of palm oil effluent. Arch. Org. Inorg. Chem. Sci. 2019, 4, 493–500. [Google Scholar]
- Messaoudi, A.; Belguith, H.; Hamida, J. Three-dimensional structure of Arabidopsis thaliana lipase predicted by homology modeling method. Evol. Bioinform. 2011, 7, EBO.S7122. [Google Scholar] [CrossRef]
- Patel, G.B.; Rakholiya, P.; Shindhal, T.; Varjani, S.; Tabhani, N.; Shah, K.R. Lipolytic Nocardiopsis for Reduction of Pollution Load in Textile Industry Effluent and SWISS Model for Structural Study of Lipase. Bioresour. Technol. 2021, 341, 125673. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.A.; Mirza, A.H.; Tahir, R.A.; Mir, A. Quick Guideline for Computational Drug Design; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018. [Google Scholar]
- Tahir, H.M.; Abd Rahman, R.N.Z.R.; Leow, A.T.C.; Ali, M.S.M. Expression, characterisation and homology modelling of a novel hormone-sensitive lipase (HSL)-like esterase from Glaciozyma antarctica. In Novel Enzyme and Whole-Cell Biocatalysts; MDPI: Basel, Switzerland, 2020; p. 85. [Google Scholar]
- Hermoso, J.A.; Sanz-Aparicio, J.; Molina, R.; Juge, N.; Gonzalez, R.; Faulds, C.B. The crystal structure of feruloyl esterase A from Aspergillus niger suggests evolutive functional convergence in feruloyl esterase family. J. Mol. Biol. 2004, 338, 495–506. [Google Scholar] [CrossRef]
- Bahaman, A.H.; Wahab, R.A.; Abdul Hamid, A.A.; Abd Halim, K.B.; Kaya, Y. Molecular docking and molecular dynamics simulations studies on β-glucosidase and xylanase Trichoderma asperellum to predict degradation order of cellulosic components in oil palm leaves for nanocellulose preparation. J. Biomol. Struct. Dyn. 2021, 39, 2628–2641. [Google Scholar] [CrossRef]
- Ezeilo, U.R.; Lee, C.T.; Huyop, F.; Zakaria, I.I.; Wahab, R.A. Raw oil palm frond leaves as cost-effective substrate for cellulase and xylanase productions by Trichoderma asperellum UC1 under solid-state fermentation. J. Environ. Manag. 2019, 243, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Lanvin, B.; Wunsch-Vincent, S. Global Innovation Index 2018: Energizing the World with Innovation; WIPO: Geneva, Switzerland, 2018. [Google Scholar]
- Curto, V.; Einav, L.; Finkelstein, A.; Levin, J.; Bhattacharya, J. Health care spending and utilization in public and private Medicare. Am. Econ. J. Appl. Econ. 2019, 11, 302–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.; Zhu, R.; Li, C.; He, L.; Zeng, X.; Zhang, Q. technology. Gene cloning, expression, purification and characterization of a sn-1, 3 extracellular lipase from Aspergillus niger GZUF36. J. Food Sci. Technol. 2020, 57, 2669–2680. [Google Scholar] [CrossRef]
- Brandao, L.M.d.S.; Barbosa, M.S.; Souza, R.L.; Pereira, M.M.; Lima, A.S.; Soares, C.M.F. Lipase activation by molecular bioimprinting: The role of interactions between fatty acids and enzyme active site. Biotechnol. Prog. 2021, 37, e3064. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.C.; Barbosa, M.S.; de Jesus, F.A.; Santos, R.M.; Fricks, A.T.; Freitas, L.S.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Enzymatic transesterification of coconut oil by using immobilized lipase on biochar: An experimental and molecular docking study. Biotechnol. Appl. Biochem. 2020, 68, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.-J.; Katayama, T.; Maruyama, J.-I.; Kitamoto, K. Comparative genomic analysis identified a mutation related to enhanced heterologous protein production in the filamentous fungus Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2016, 100, 9163–9174. [Google Scholar] [CrossRef]
- Volkamer, A.; Kuhn, D.; Rippmann, F.; Rarey, M. DoGSiteScorer: A web server for automatic binding site prediction, analysis and druggability assessment. Bioinformatics 2012, 28, 2074–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363. [Google Scholar] [CrossRef] [Green Version]
- Hæffner, F.; Norin, T.; Hult, K. Molecular modeling of the enantioselectivity in lipase-catalyzed transesterification reactions. Biophys. J. 1998, 74, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Kumar, A. Computational modelling and protein-ligand interaction studies of SMlipA lipase cloned from forest metagenome. J. Mol. Graph. Model. 2016, 70, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, L.; Jia, Y.; Hu, Y.; Xu, Q.; Xu, X.; Huang, H. Counteraction of trehalose on N, N-dimethylformamide-induced Candida rugosa lipase denaturation: Spectroscopic insight and molecular dynamic simulation. PLoS ONE 2016, 11, e0152275. [Google Scholar] [CrossRef] [PubMed]
- Wedberg, R.; Abildskov, J.; Peters, G.H. Protein dynamics in organic media at varying water activity studied by molecular dynamics simulation. J. Phys. Chem. B 2012, 116, 2575–2585. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Gibson, T.J.; Higgins, D. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2. [Google Scholar] [CrossRef]
- Reynolds, S.M.; Käll, L.; Riffle, M.E.; Bilmes, J.A.; Noble, W.S. Transmembrane topology and signal peptide prediction using dynamic bayesian networks. PLoS Comput. Biol. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Buchan, D.W.; Minneci, F.; Nugent, T.C.; Bryson, K.; Jones, D.T. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2013, 41, W349–W357. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.E.; Chivian, D.; Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004, 32, W526–W531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 571–607. [Google Scholar]
- Ohlrogge, J.; Thrower, N.; Mhaske, V.; Stymne, S.; Baxter, M.; Yang, W.; Liu, J.; Shaw, K.; Shorrosh, B.; Zhang, M.; et al. Plant FA db: A resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 2018, 96, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Release, S.J. LigPrep; Schrödinger, LLC: New York, NY, USA, 2017. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]





| Sr. No | Physiochemical Properties of B. cinerea Lipase | Values |
|---|---|---|
| 1 | Amino acid Residues | 557 |
| 2 | Molecular weight (Da) | 59,090.04 |
| 3 | Theoretical pI | 4.93 |
| 4 | Positively Charged Residue | 30 |
| 5 | Negatively Charged Residue | 38 |
| 6 | Total No. of Atoms | 8312 |
| 7 | Molecular formula | C2703H4129N675O797S8 |
| 8 | Instability index | 36.20 |
| 9 | Aliphatic index (%) | 92.21 |
| 10 | GRAVY | 0.194 |
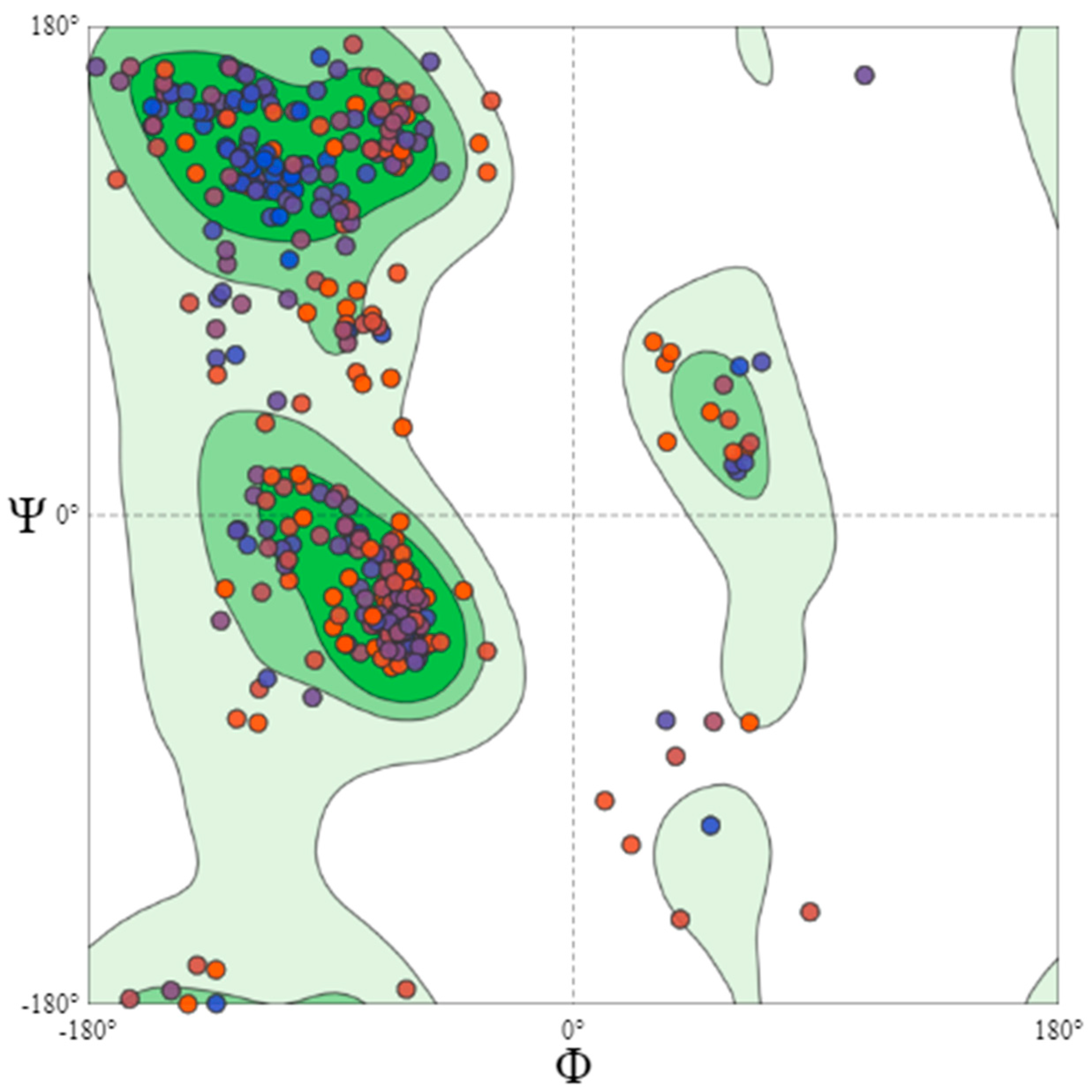
| Protein Type | Method/Tool | Method | ERRAT | Q-Mean | Ramachandran Plot | ||
|---|---|---|---|---|---|---|---|
| Outlier (%) | Allowed Region (%) | Favorable Region (%) | |||||
| B. cinerea Lipase | ITASSAR_1 | Thread-Based | 68.4 | 0.5 | 3.6 | 7.8 | 55.0 |
| PHYRE_2 | Normal | 56.5 | 0.3 | 2.2 | 18.4 | 50.0 | |
| ROBETTA_ Ab | AB-initio | 72.4 | 0.35 | 0.9 | 23.2 | 74.0 | |
| ROBETTA_TR | Thread-Based | 93.2 | 0.68 | 1.5 | 14.4 | 83.0 | |
| Compound | IUPAC Names | PubChem ID | Molecular Formula | Hydrogen Bonds | Hydrophobic Interactions | Binding Affinities |
|---|---|---|---|---|---|---|
| Oxiraneoctanoic acid | 8-(3-oct-2-enyloxiran-2-yl)octanoic acid | 1929 | C18H32O3 | 3 | 10 | −5.7 |
| Docosahexaenoic acid | (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid | 445580 | C22H32O2 | 2 | 14 | −7.6 |
| Hexadeca-7,10,13-trienoic acid | hexadeca-7,10,13-trienoic acid | 2826712 | C16H26O2 | 2 | 9 | −6.3 |
| Suberic acid | octanedioic acid | 10457 | C8H14O4 | 2 | 4 | −5.0 |
| Chaulmoogric acid | 13-cyclopent-2-en-1-yltridecanoic acid | 72853 | C18H32O2 | 1 | 10 | −5.0 |
| 11-Dodecenoic acid | (8Z,10E,12Z)-octadeca-8,10,12-trienoic acid | 125207 | C12H22O2 | 0 | 7 | −4.5 |
| Palmitoleic acid | (Z)-octadec-9-enoic acid | 445638 | C16H30O2 | 1 | 12 | −5.8 |
| Oleic acid | (Z)-octadec-9-enoic acid | 445639 | C16H34O2 | 1 | 13 | −5.1 |
| Dicranin | (9Z,12Z,15Z)-octadeca-9,12,15-trien-6-ynoic acid | 44584408 | C18H26O2 | 3 | 14 | −6.7 |
| Octadecatetraenoic acid | (9Z,11Z,13E,15E)-4-oxooctadeca-9,11,13,15-tetraenoic acid | 5312915 | C18H26O3 | 3 | 10 | −5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatma, T.; Zafar, Z.; Fatima, S.; Paracha, R.Z.; Adnan, F.; Zeshan; Virk, N.; Bhatti, M.F. Computational Assessment of Botrytis cinerea Lipase for Biofuel Production. Catalysts 2021, 11, 1319. https://doi.org/10.3390/catal11111319
Fatma T, Zafar Z, Fatima S, Paracha RZ, Adnan F, Zeshan, Virk N, Bhatti MF. Computational Assessment of Botrytis cinerea Lipase for Biofuel Production. Catalysts. 2021; 11(11):1319. https://doi.org/10.3390/catal11111319
Chicago/Turabian StyleFatma, Tehsin, Zeeshan Zafar, Sidra Fatima, Rehan Zafar Paracha, Fazal Adnan, Zeshan, Nasar Virk, and Muhammad Faraz Bhatti. 2021. "Computational Assessment of Botrytis cinerea Lipase for Biofuel Production" Catalysts 11, no. 11: 1319. https://doi.org/10.3390/catal11111319
APA StyleFatma, T., Zafar, Z., Fatima, S., Paracha, R. Z., Adnan, F., Zeshan, Virk, N., & Bhatti, M. F. (2021). Computational Assessment of Botrytis cinerea Lipase for Biofuel Production. Catalysts, 11(11), 1319. https://doi.org/10.3390/catal11111319








