Immobilization of Potassium-Based Heterogeneous Catalyst over Alumina Beads and Powder Support in the Transesterification of Waste Cooking Oil
Abstract
:1. Introduction
2. Results and Discussion
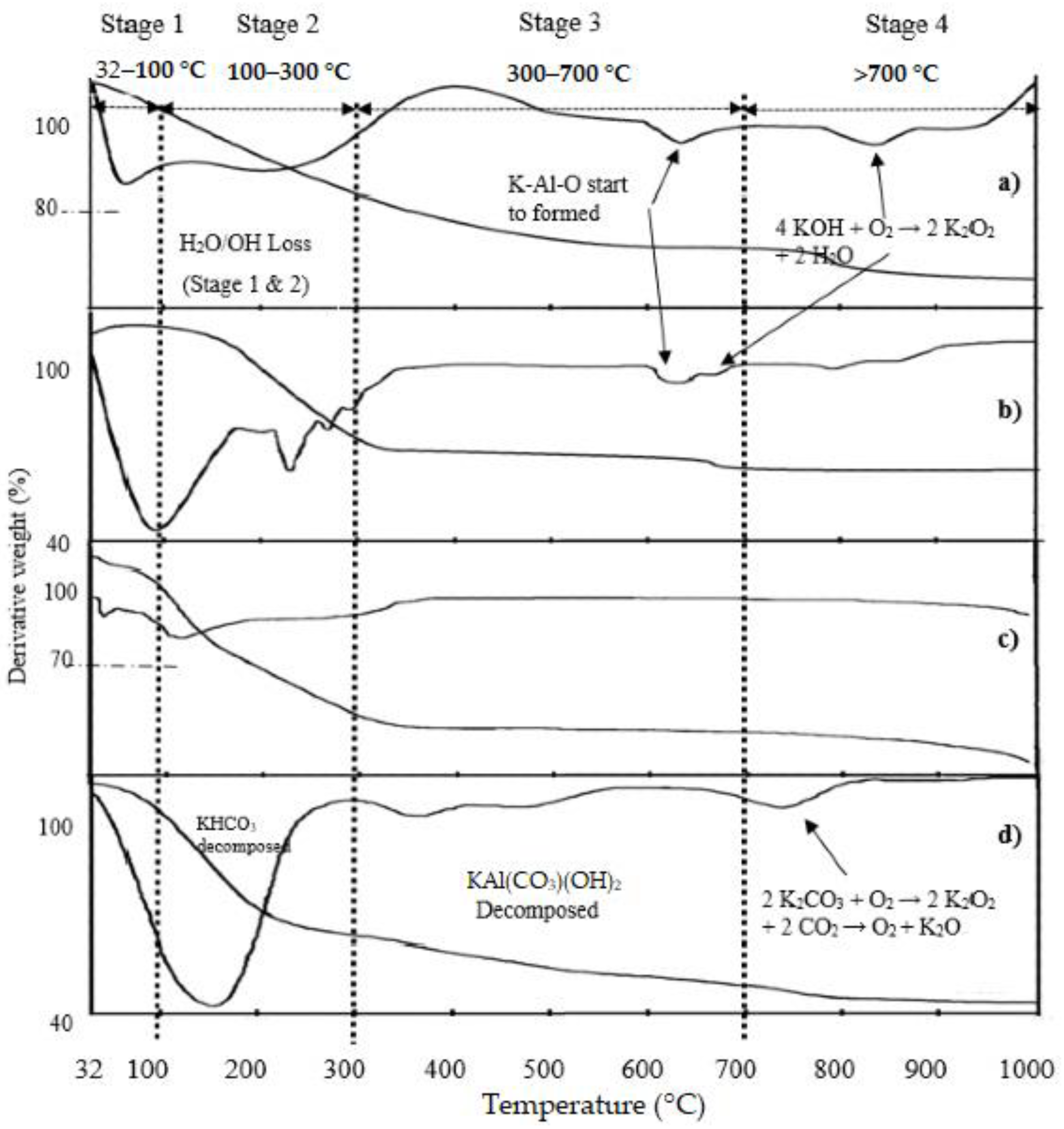
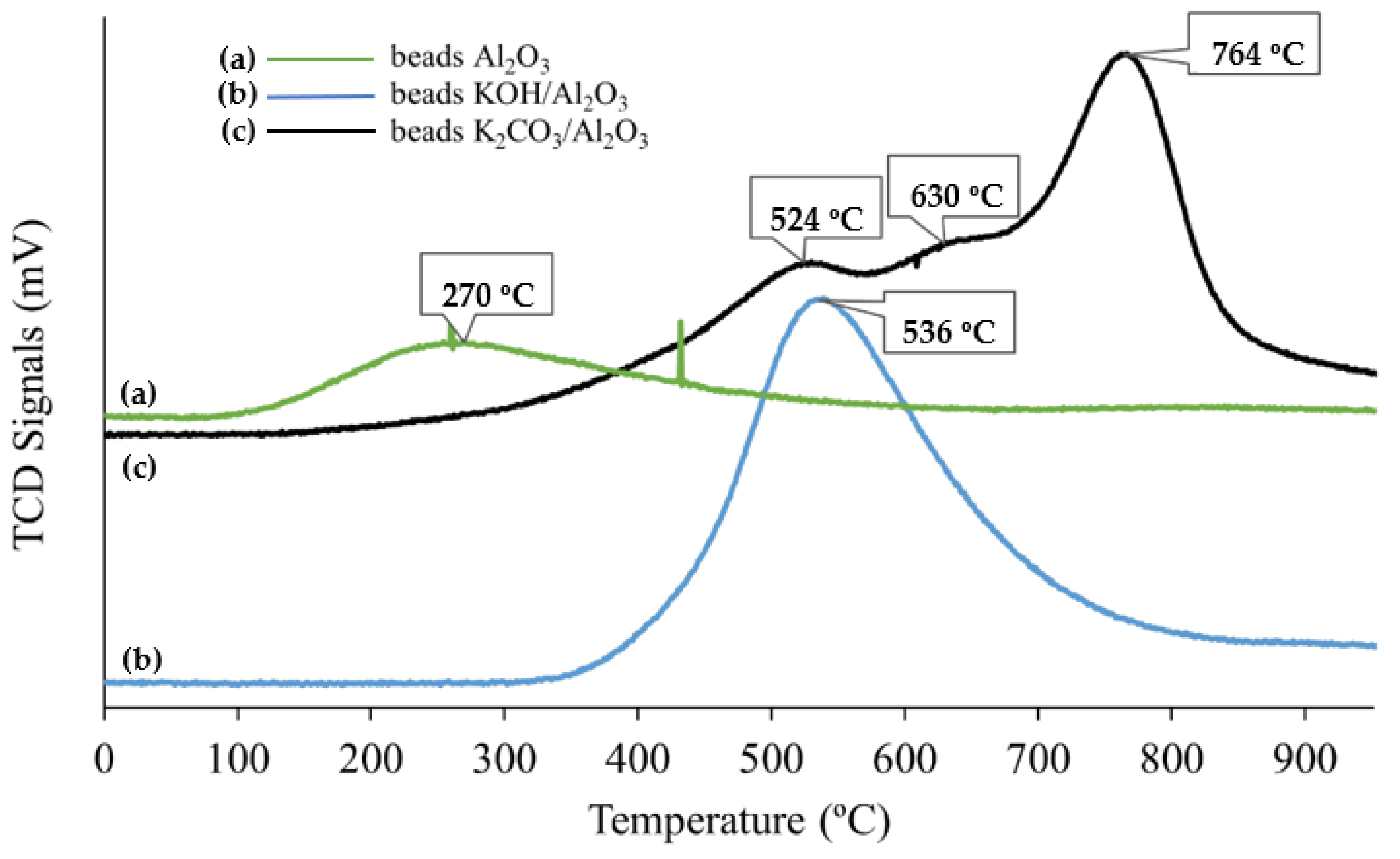
2.1. Thermogravimetric Analysis
2.2. XRD Analysis of K2O2/Al2O3 and K2O/Al2O3 Catalysts
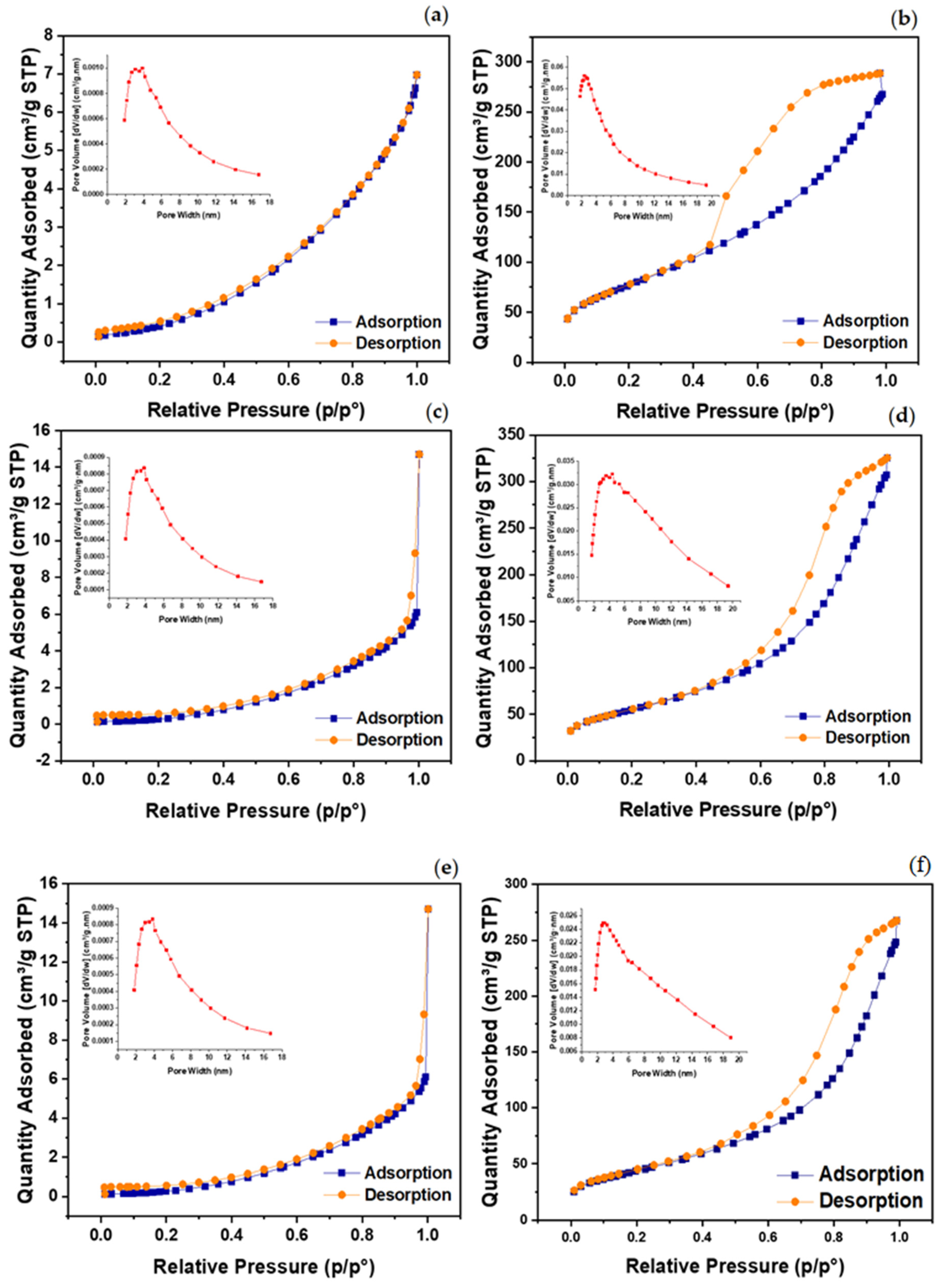
2.3. Catalyst Surface Characteristics
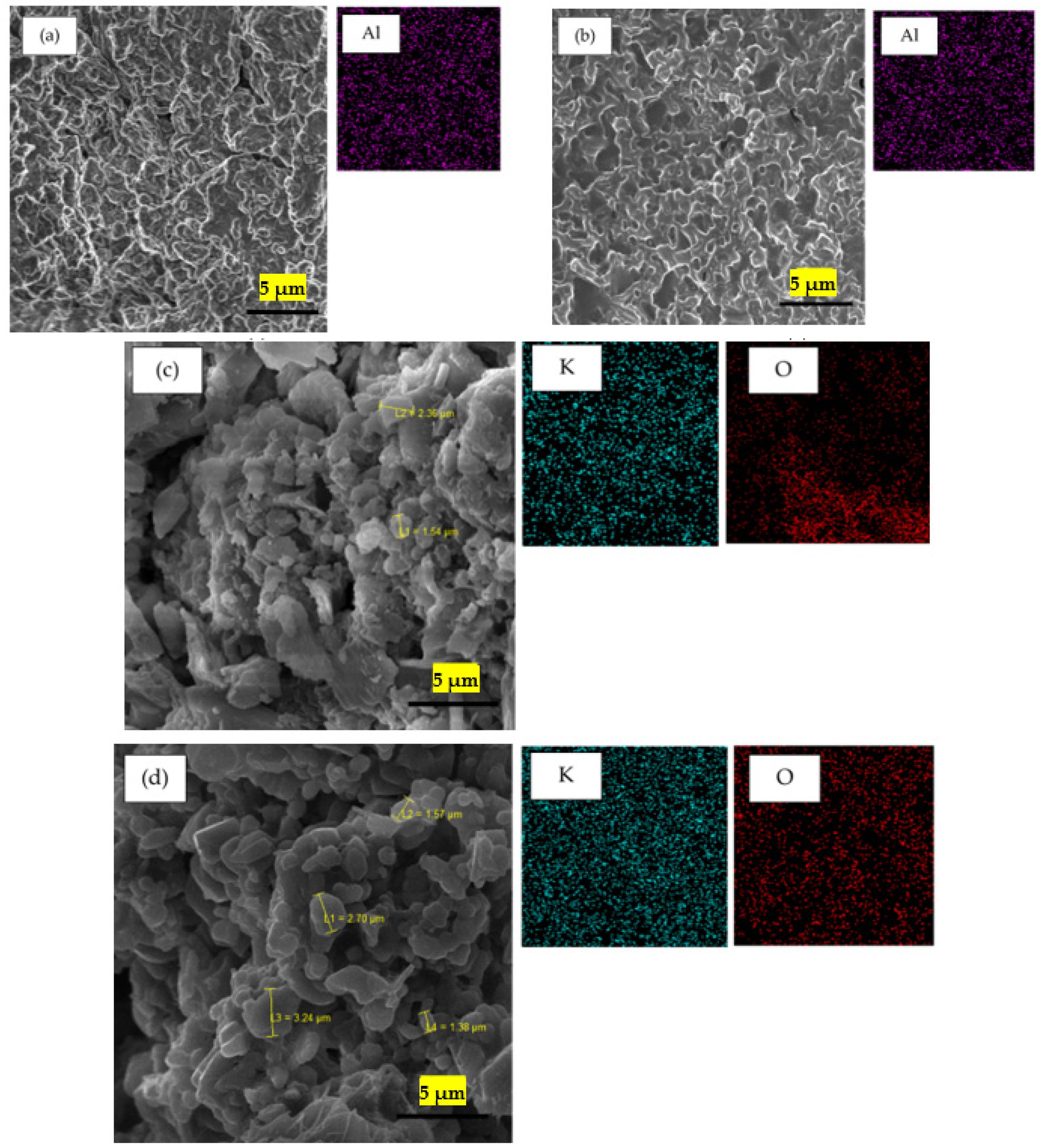
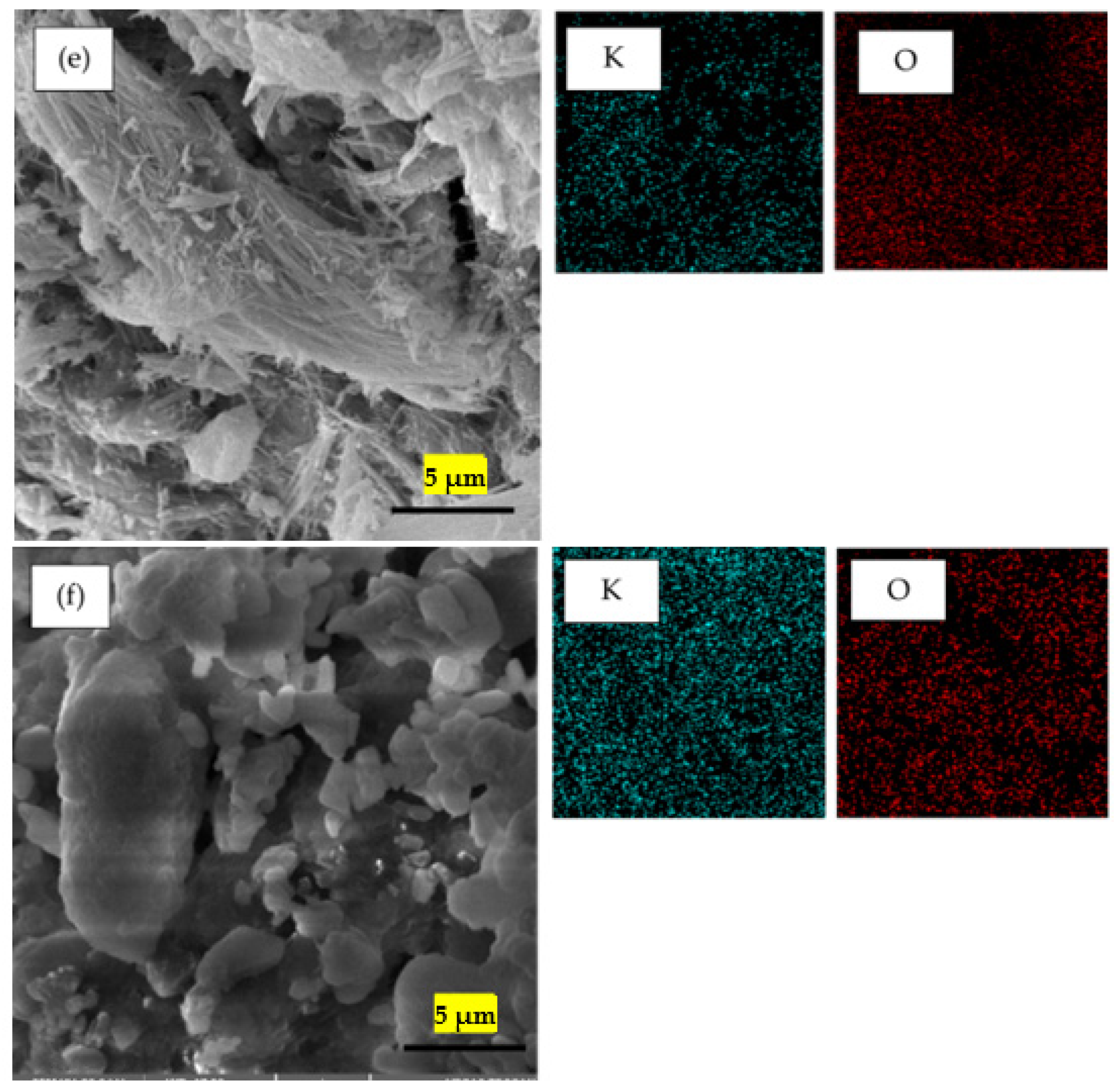
2.4. Scanning Electron Microscope—Energy Dispersive X-ray Analysis
2.5. Temperature Programmed Desorption-Carbon Dioxide Analysis
2.6. Transesterification Reaction
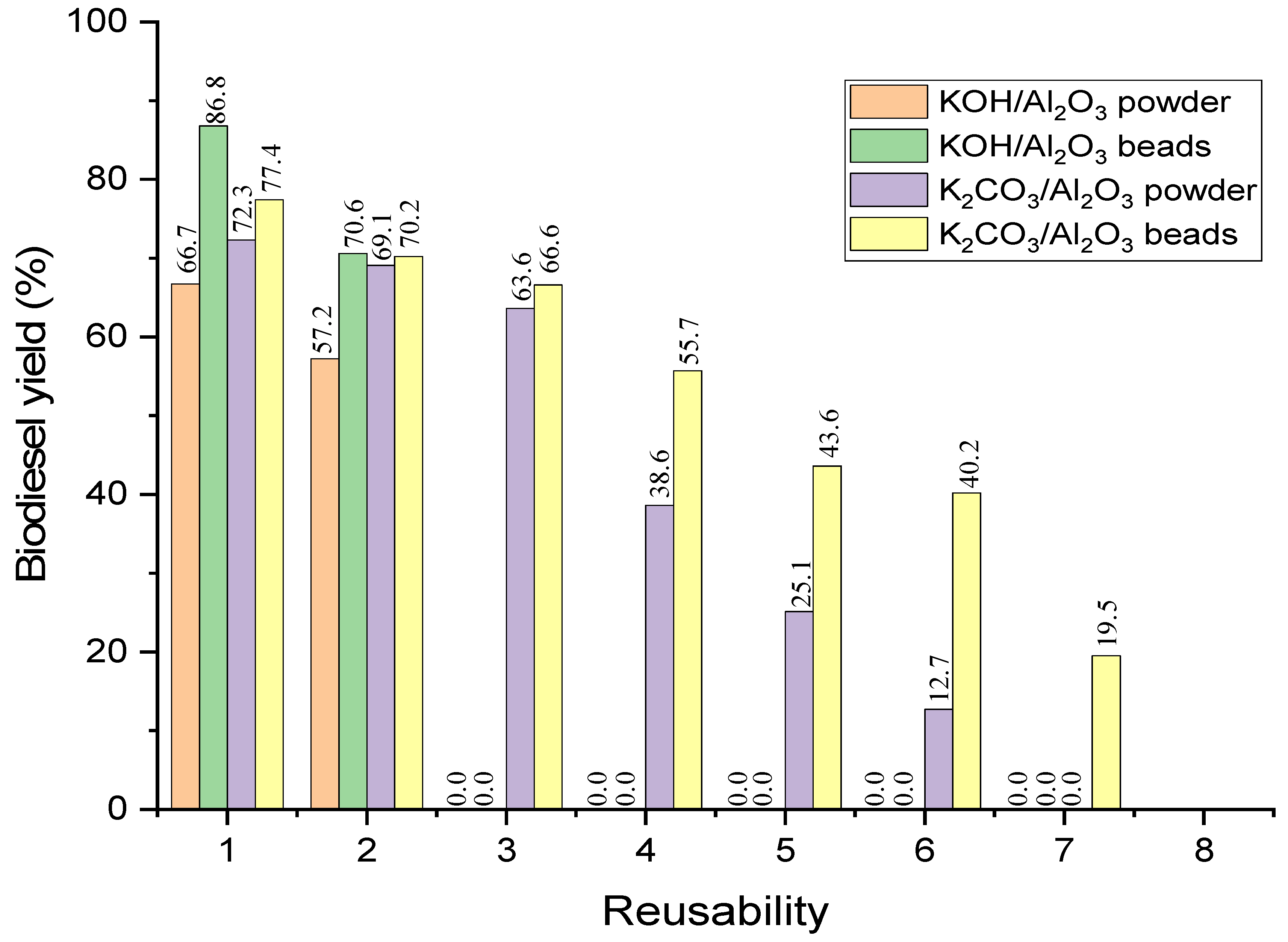
2.7. Reusability Testing for Beads KOH/Al2O3 and K2CO3/Al2O3
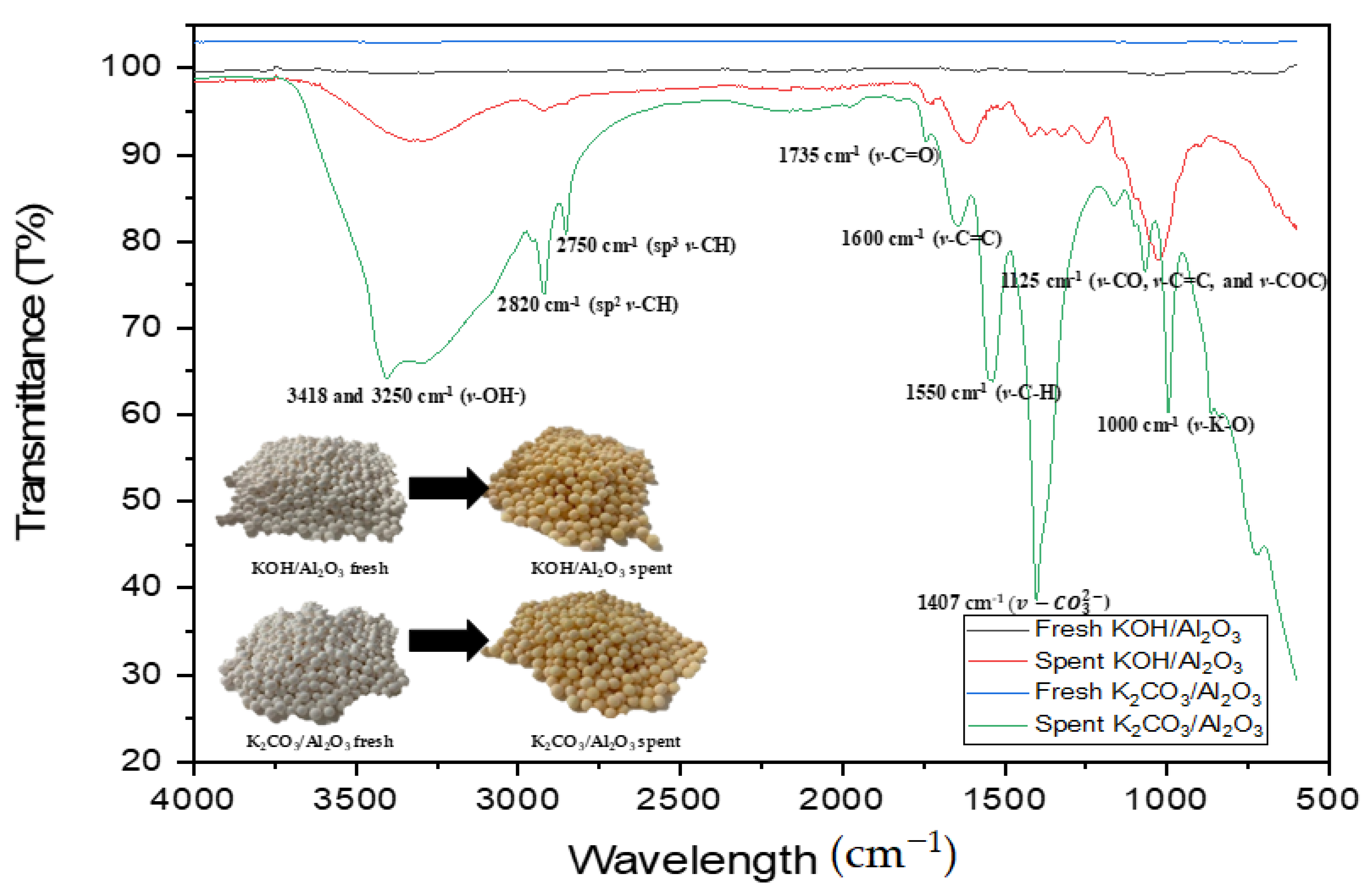
2.8. Identification of Post-Reaction Compounds on the Catalyst Surface Using Fourier-Transform Infrared Spectroscopy
2.9. XRF and ICP-AES Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of K2CO3 and KOH/Al2O3 Catalysts
3.3. Catalyst Characterization of K2CO3/Al2O3 and KOH/Al2O3 Catalysts
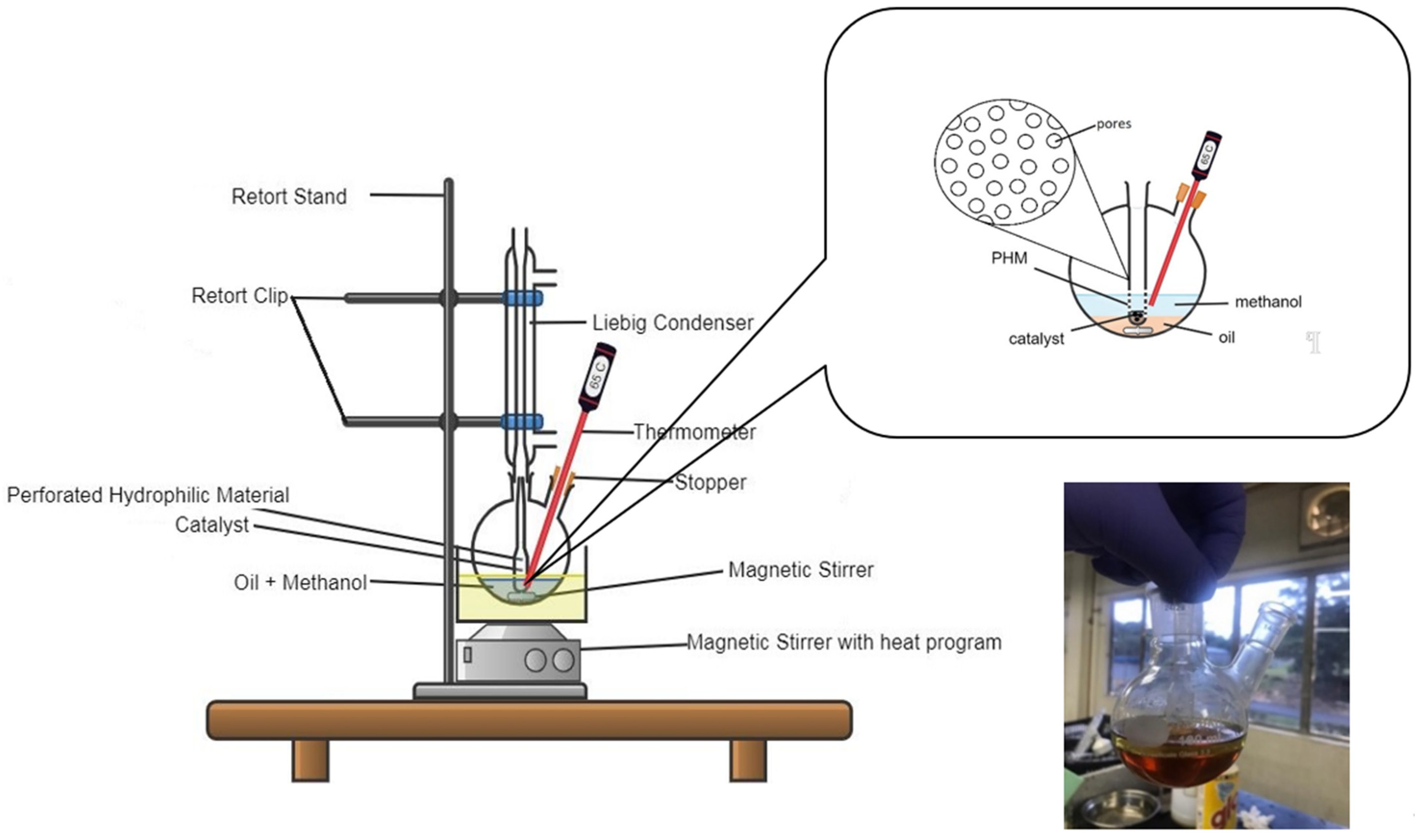
3.4. Transesterification Reaction of WCO
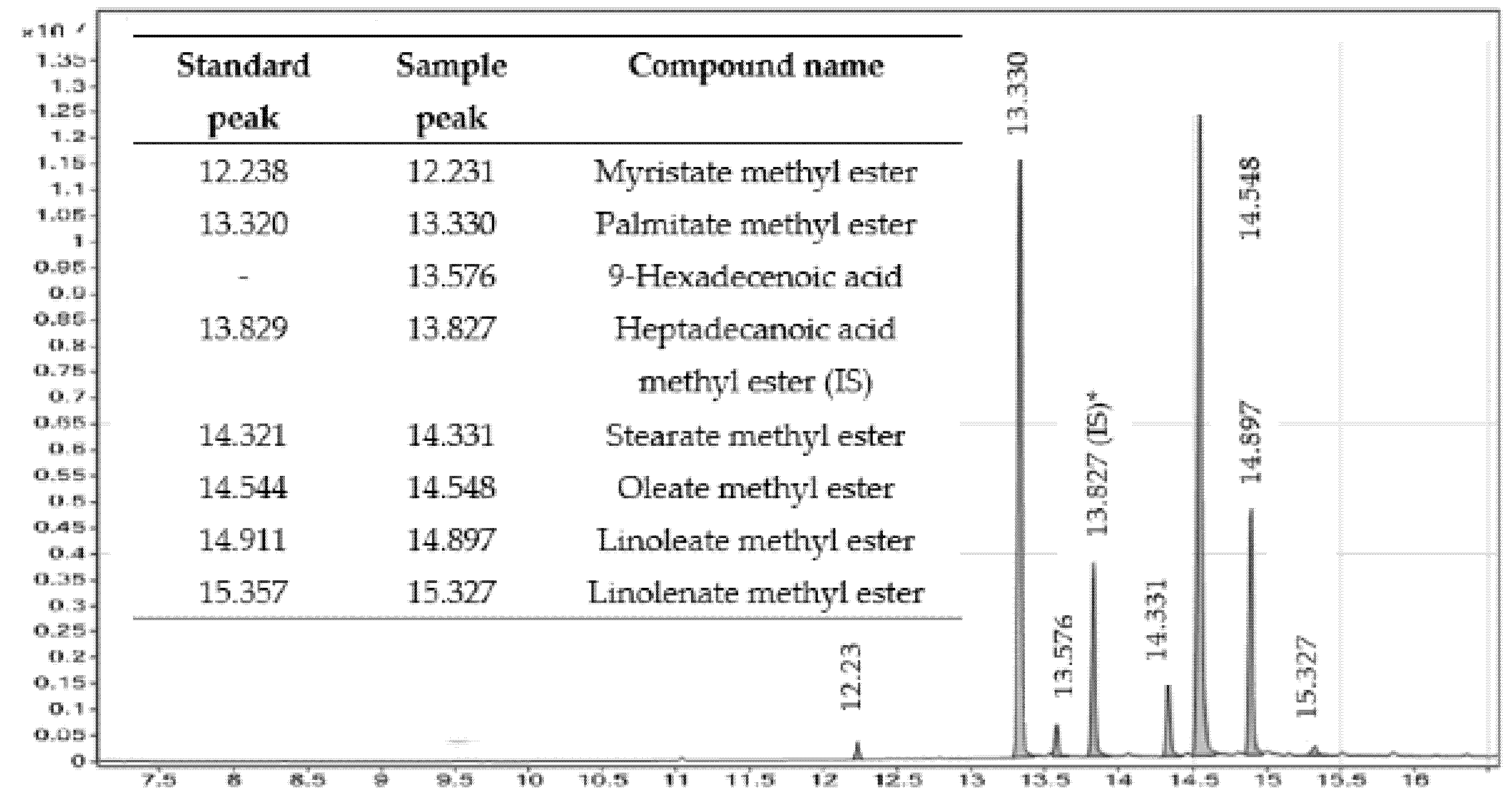
3.5. Determination of Ester Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Trejda, M.; Nurwita, A.; Kryszak, D. Synthesis of solid acid catalysts for esterification with the assistance of elevated pressure. Microporous Mesoporous Mater. 2019, 278, 115–120. [Google Scholar] [CrossRef]
- Ganesan, S.; Arunkumar, T.; Munuswamy, D.; Appavu, P.; Devarajan, Y. Effect of egr & nanoparticles on performance and emission characteristics of a diesel engine fuelled with palm biodiesel and diesel blends. J. Oil Palm. Res. 2019, 31, 130–137. [Google Scholar] [CrossRef]
- Mohd Shohaimi, N.A.; Wan Abu Bakar, W.A.; Jaafar, J. The catalytic deacidification of acidic crude oil using Cu-doped alkaline earth metal oxide catalysts. Pet. Sci. Technol. 2017, 35, 1097–1103. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. The use of KOH/Clinoptilolite catalyst in pilot of microreactor for biodiesel production from waste cooking oil. Fuel 2019, 116659. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Liu, F.; Ma, X.; Li, H.; Wang, Y.; Cui, P.; Guo, M.; Yaxin, H.; Lu, W.; Zhou, S.; Yu, M. Dilute sulfonic acid post functionalized metal organic framework as a heterogeneous acid catalyst for esterification to produce biodiesel. Fuel 2020, 266, 117149. [Google Scholar] [CrossRef]
- Khodamorady, M.; Sohrabnezhad, S.; Bahrami, K. Efficient one-pot synthetic methods for the preparation of 3,4-dihydropyrimidinones and 1,4-dihydropyridine derivatives using BNPs@SiO2(CH2)3NHSO3H as a ligand and metal free acidic heterogeneous nano-catalyst. Polyhedron 2020, 178, 114340. [Google Scholar] [CrossRef]
- Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew. Energy 2009, 34, 1145–1150. [Google Scholar] [CrossRef]
- Shohaimi, N.A.M.; Wan Abu Bakar, W.A.; Jaafar, J. Catalytic neutralization method for naphthenic acid removal in crude oil by alumina supported Ca and Ba catalysts. Pet. Sci. Technol. 2014, 32, 2365–2375. [Google Scholar] [CrossRef]
- Du, L.; Li, Z.; Ding, S.; Chen, C.; Qu, S.; Yi, W.; Lu, J.; Ding, J. Synthesis and characterization of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 2019, 258, 116122. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Hassan, M.A.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Hasan, M.Y.; Ali, A.A.M.; Othman, M.R.; Shirai, Y. Kinetic and thermodynamic of heterogeneously K3PO4/AC-catalysed transesterification via pseudo-first order mechanism and Eyring-Polanyi equation. Fuel 2018, 232, 653–658. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Taufiq-yap, Y.H.; Ibrahim, M.L. Supermagnetic nano-bifunctional catalyst from rice husk: Synthesis, characterization and application for conversion of used cooking oil to biodiesel. Catalysts 2020, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Nayebzadeh, H.; Saghatoleslami, N.; Tabasizadeh, M. Optimization of the activity of KOH/calcium aluminate nanocatalyst for biodiesel production using response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 68, 379–386. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Tangy, A.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Continuous flow through a microwave oven for the large-scale production of biodiesel from waste cooking oil. Bioresour. Technol. 2017, 224, 333–341. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y. A review of microwave-assisted reactions for biodiesel production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Silveira Junior, E.G.; Perez, V.H.; Reyero, I.; Serrano-Lotina, A.; Justo, O.R. Biodiesel production from heterogeneous catalysts based K2CO3 supported on extruded Γ-Al2O3. Fuel 2019, 241, 311–318. [Google Scholar] [CrossRef]
- Da Costa Evangelista, J.P.; Gondim, A.D.; Souza, L.D.; Araujo, A.S. Alumina-supported potassium compounds as heterogeneous catalysts for biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 59, 887–894. [Google Scholar] [CrossRef]
- Zhu, M.; Li, B.; Jehng, J.M.; Sharma, L.; Taborda, J.; Zhang, L.; Stach, E.; Wachs, I.E.; Wu, Z.; Baltrusaitis, J. Molecular structure and sour gas surface chemistry of supported K2O/WO3/Al2O3 catalysts. Appl. Catal. B Environ. 2018, 232, 146–154. [Google Scholar] [CrossRef]
- Ilgen, O.; Akin, A.N. Development of alumina supported alkaline catalysts used for biodiesel production. Turkish J. Chem. 2009, 33, 281–287. [Google Scholar] [CrossRef]
- Ma, G.; Hu, W.; Pei, H.; Jiang, L.; Ji, Y.; Mu, R. Study of KOH/Al2O3 as heterogeneous catalyst for biodiesel production via in situ transesterification from microalgae. Environ. Technol. 2015, 36, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Verdooren, A.; Caram, H.S.; Sircar, S. Chemisorption of carbon dioxide on potassium-carbonate-promoted hydrotalcite. J. Colloid Interface Sci. 2007, 308, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.; Fierro, V.; Szczurek, A.; Izquierdo, M.T.; Celzard, A. Physisorption, chemisorption and spill-over contributions to hydrogen storage. Int. J. Hydrogen Energy 2016, 41, 17442–17452. [Google Scholar] [CrossRef]
- Abdullah, A.; Rahmawati Sianipar, R.N.; Ariyani, D.; Nata, I.F. Conversion of palm oil sludge to biodiesel using alum and KOH as catalysts. Sustain. Environ. Res. 2017, 27, 291–295. [Google Scholar] [CrossRef]
- Boonprasop, S.; Chalermsinsuwan, B.; Piumsomboon, P. Effect of operating parameters of potassium carbonate supported on gamma alumina (K2CO3/γ-Al2O3) on CO2 capture capacity using depressurized regeneration. J. Taiwan Inst. Chem. Eng. 2018, 88, 215–225. [Google Scholar] [CrossRef]
- Postole, G.; Nguyen, T.S.; Aouine, M.; Gélin, P.; Cardenas, L.; Piccolo, L. Efficient hydrogen production from methane over iridium-doped ceria catalysts synthesized by solution combustion. Appl. Catal. B Environ. 2015, 166–167, 580–591. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Derevschikov, V.S.; Kardash, T.Y.; Stonkus, O.A.; Trubitsina, T.A.; Okunev, A.G. Direct CO2 capture from ambient air using K2CO3/Al2O3 composite sorbent. Int. J. Greenh. Gas. Control. 2013, 17, 332–340. [Google Scholar] [CrossRef]
- Silveira, E.G.; Barcelos, L.F.T.; Perez, V.H.; Justo, O.R.; Ramirez, L.C.; Rêgo Filho, L.; de Castro, M.P.P. Biodiesel production from non-edible forage turnip oil by extruded catalyst. Ind. Crops Prod. 2019, 139, 111503. [Google Scholar] [CrossRef]
- Sharikh, A.M.; Sulaiman, S.; Azmi, A.S.; Sulaiman, S.Z. Potassium carbonate from pineapple and orange peels as catalyst for biodiesel production. AIP Conf. Proc. 2018, 2030, 020290. [Google Scholar] [CrossRef]
- Malins, K. The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production. Fuel Process. Technol. 2018, 179, 302–312. [Google Scholar] [CrossRef]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Wan Salleh, W.N.; Jaafar, J.; Rosmi, M.S.; Zul, Z.A.; Abd Mutalib, M.; Ismail, A.F.; Tanemura, M. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO 2: Bio-Template assisted sol-gel synthesis and photocatalytic activity. Appl. Surf. Sci. 2017, 393, 46–59. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Ravindra, P.; Teo, S.H.; Sivasangar, S.; Chan, E.S. Biodiesel synthesis over millimetric γ-Al2O3/KI catalyst. Energy 2015, 89, 965–973. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Ramaswamy, A.V.; Viswanathan, B. Surface area, pore size, and particle size engineering of titania with seeding technique and phosphate modification. J. Phys. Chem. C 2009, 113, 13750–13757. [Google Scholar] [CrossRef]
- Mohd Hir, Z.A.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Visible light-active hybrid film photocatalyst of polyethersulfone–reduced TiO2: Photocatalytic response and radical trapping investigation. J. Mater. Sci. 2018, 53, 13264–13279. [Google Scholar] [CrossRef]
- Shan, R.; Shi, J.; Yan, B.; Chen, G.; Yao, J.; Liu, C. Transesterification of palm oil to fatty acids methyl ester using K2CO3/palygorskite catalyst. Energy Convers. Manag. 2016, 116, 142–149. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Nik Abdul Khalil, N.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Sinar Mashuri, S.I.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 112445. [Google Scholar] [CrossRef]
- Smyrnioti, M.; Tampaxis, C.; Steriotis, T.; Ioannides, T. Study of CO2 adsorption on a commercial CuO/ZnO/Al2O3 catalyst. Catal. Today 2020, 357, 495–502. [Google Scholar] [CrossRef]
- Rahmani Vahid, B.; Haghighi, M.; Alaei, S.; Toghiani, J. Reusability enhancement of combustion synthesized MgO/MgAl2O4 nanocatalyst in biodiesel production by glow discharge plasma treatment. Energy Convers. Manag. 2017, 143, 23–32. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Hassan, M.A.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Othman, M.R.; Ali, A.A.M.; Shirai, Y. Production of methyl esters from waste cooking oil using a heterogeneous biomass-based catalyst. Renew. Energy 2017, 114, 638–643. [Google Scholar] [CrossRef]
- Lokman NolHakim, M.A.H.; Mohd Shohaimi, N.A.; Ibrahim, M.L.; Wan Mokhtar, W.N.A.; Abdul Halim, A.Z. Transesterification of waste cooking oil utilizing heterogeneous K2CO3/Al2O3 and KOH/Al2O3 catalysts. Malays. Inst. Chem. 2021, 23, 74–83. [Google Scholar]
- Hong, Y.; Wu, G. Heterogeneous and efficient transesterification of Jatropha curcas L. seed oil to produce biodiesel catalysed by nano-sized SO42−/TiO2. R. Soc. Open Sci. 2018, 5, 181331. [Google Scholar]
- Alonso, D.M.; Mariscal, R.; Moreno-Tost, R.; Poves, M.D.Z.; Granados, M.L. Potassium leaching during triglyceride transesterification using K/γ-Al2O3 catalysts. Catal. Commun. 2007, 8, 2074–2080. [Google Scholar] [CrossRef]
- Astuti, W.; Prilitasari, N.M.; Iskandar, Y.; Bratakusuma, D.; Petrus, H.T.B.M. Leaching behavior of lanthanum, nickel and iron from spent catalyst using inorganic acids. IOP Conf. Ser. Mater. Sci. Eng. 2018, 285, 012007. [Google Scholar] [CrossRef]
- Saba, T.; Estephane, J.; El Khoury, B.; El Khoury, M.; Khazma, M.; El Zakhem, H.; Aouad, S. Biodiesel production from refined sunflower vegetable oil over KOH/ZSM5 catalysts. Renew. Energy 2016, 90, 301–306. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Wiwatnimit, W.; Wangnoi, S. Modified dolomites as catalysts for palm kernel oil transesterification. J. Mol. Catal. A Chem. 2007, 276, 24–33. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Pasupulety, N.; Gunda, K.; Liu, Y.; Rempel, G.L.; Ng, F.T.T. Production of biodiesel from soybean oil on CaO/Al2O3 solid base catalysts. Appl. Catal. A Gen. 2013, 452, 189–202. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Saikia, R.A.; Deka, D. Biodiesel production from waste cooking oil catalyzed by in-situ decorated TiO2 on reduced graphene oxide nanocomposite. Energy 2018, 158, 881–889. [Google Scholar] [CrossRef]
- Fadzilah, R.; Rashid, U.; Lokman, M.; Hazmi, B.; Alharthi, A.; Arbi, I. Bifunctional nano-catalyst produced from palm kernel shell via hydrothermal-assisted carbonization for biodiesel production from waste cooking oil. Renew. Sustain. Energy Rev. 2021, 137, 110638. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, R.F.; Rashid, U.; Tau, H. Synthesis of bifunctional nanocatalyst from waste palm kernel shell and its application for biodiesel production. RSC Adv. 2020, 4, 27183–27193. [Google Scholar] [CrossRef]
- Mohd Hir, Z.A.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Photoactive hybrid photocatalyst of polyethersulfone-zno for the degredation of methyl orange dye: Kinetic study and operational parameters. Catalyst 2017, 17, 313. [Google Scholar] [CrossRef] [Green Version]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Transesterification of canola oil to biodiesel using calcium bentonite functionalized with K compounds. Appl. Catal. B Environ. 2013, 138, 236–242. [Google Scholar] [CrossRef]
- Rajkhowa, T.; Marin, G.B.; Thybaut, J.W. Quantifying the dominant factors in Cu catalyst deactivation during glycerol hydrogenolysis. J. Ind. Eng. Chem. 2017, 54, 270–277. [Google Scholar] [CrossRef]
- Ning, X.; Zhan, L.; Wang, H.; Yu, H.; Peng, F. Deactivation and regeneration of: In situ formed bismuth-promoted platinum catalyst for the selective oxidation of glycerol to dihydroxyacetone. New J. Chem. 2018, 42, 18837–18843. [Google Scholar] [CrossRef]
- Yori, J.C.; D’Ippolito, S.A.; Pieck, C.L.; Vera, C.R. Deglycerolization of biodiesel streams by adsorption over silica beds. Energy Fuels 2007, 21, 347–353. [Google Scholar] [CrossRef]
- Delmon, B.; Haber, J.; Block, J.H. Manual of methods and procedures for catalyst characterization (technical report). Pure Appl. Chem. 1995, 67, 1257–1306. [Google Scholar] [CrossRef] [Green Version]
- Yaşar, F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: Its effects on some critical fuel properties and comparison with CaO. Fuel 2019, 255, 115828. [Google Scholar] [CrossRef]
- Vargas, E.M.; Neves, M.C.; Tarelho, L.A.C.; Nunes, M.I. Solid catalysts obtained from wastes for FAME production using mixtures of refined palm oil and waste cooking oils. Renew. Energy 2019, 136, 873–883. [Google Scholar] [CrossRef]
- Labib, T.M.; Hawash, S.I.; El-Khatib, K.M.; Sharaky, A.M.; El Diwani, G.I.; Abdel Kader, E. Kinetic study and techno-economic indicators for base catalyzed transesterification of Jatropha oil. Egypt. J. Pet. 2013, 22, 9–16. [Google Scholar] [CrossRef] [Green Version]


| Catalyst | Physical Properties | Mass of Element (%) | Density of Active Site (mmol/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) 1 | Pore Volume (cm3/g) 2 | Pore Size (nm) 2 | K | O | Al | Weak (<160 °C) | Moderate (160–400 °C) | Strong (>400 °C) | |
| powder Al2O3 | 2.50 | 0.01 | 5.03 | 0.00 | 0.00 | 99.98 | 2.36 | - | - |
| beads Al2O3 | 282.10 | 0.44 | 6.24 | 0.00 | 0.00 | 99.98 | 0.34 | 0.20 | 0.45 |
| powder KOH/Al2O3 | 2.40 | 0.01 | 5.16 | 22.50 | 45.26 | 32.24 | N/A | N/A | N/A |
| beads KOH/Al2O3 | 133.40 | 0.35 | 7.09 | 25.37 | 41.48 | 33.15 | 0.15 | 0.08 | 2.38 |
| powder K2CO3/Al2O3 | 1.506 | 0.0069 | 5.437 | 18.96 | 43.27 | 37.77 | N/A | N/A | N/A |
| beads K2CO3/Al2O3 | 199.94 | 0.474 | 6.512 | 28.41 | 45.44 | 26.15 | 0.00 | 0.17 | 3.94 |
| Catalyst | Reusability | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | |
| K2CO3/Al2O3 (PHM) | 77.4 | 70.2 | 66.7 | 55.7 | 43.6 | 40.2 | 19.5 | 0 |
| K2CO3/Al2O3 | 72.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KOH/Al2O3 (PHM) | 86.8 | 70.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| KOH/Al2O3 | 73.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Element Analyzed | Fresh (%) | 1st Cycle (%) | 4th Cycle (%) | 8th Cycle (%) |
|---|---|---|---|---|
| Potassium, (K) | 0.21 | 0.97 | 4.3 | 6.3 |
| Aluminum, (Al) | 0.00 | 0.02 | 0.05 | 2.4 |
| Others | 0.03 | 1.03 | 1.52 | 2.0 |
| Element Analyzed | 1st Cycle (%) | 4th Cycle (%) | 8th Cycle (%) |
|---|---|---|---|
| Potassium, (K) | 1.0 | 3.3 | 6.4 |
| Aluminum, (Al) | 0.0 | 0.1 | 1.5 |
| Formula | Equation | Nr. |
|---|---|---|
| Conversion (%) * | (1) | |
| Ester content (%) From EN 14103 | (2) | |
| Mass of pure biodiesel from biodiesel conversion (g) | (3) | |
| Pure biodiesel yield (%) | (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokman NolHakim, M.A.H.; Shohaimi, N.A.M.; Mokhtar, W.N.A.W.; Ibrahim, M.L.; Abdullah, R.F. Immobilization of Potassium-Based Heterogeneous Catalyst over Alumina Beads and Powder Support in the Transesterification of Waste Cooking Oil. Catalysts 2021, 11, 976. https://doi.org/10.3390/catal11080976
Lokman NolHakim MAH, Shohaimi NAM, Mokhtar WNAW, Ibrahim ML, Abdullah RF. Immobilization of Potassium-Based Heterogeneous Catalyst over Alumina Beads and Powder Support in the Transesterification of Waste Cooking Oil. Catalysts. 2021; 11(8):976. https://doi.org/10.3390/catal11080976
Chicago/Turabian StyleLokman NolHakim, Muhammad Amirrul Hakim, Norshahidatul Akmar Mohd Shohaimi, Wan Nur Aini Wan Mokhtar, Mohd Lokman Ibrahim, and Rose Fadzilah Abdullah. 2021. "Immobilization of Potassium-Based Heterogeneous Catalyst over Alumina Beads and Powder Support in the Transesterification of Waste Cooking Oil" Catalysts 11, no. 8: 976. https://doi.org/10.3390/catal11080976
APA StyleLokman NolHakim, M. A. H., Shohaimi, N. A. M., Mokhtar, W. N. A. W., Ibrahim, M. L., & Abdullah, R. F. (2021). Immobilization of Potassium-Based Heterogeneous Catalyst over Alumina Beads and Powder Support in the Transesterification of Waste Cooking Oil. Catalysts, 11(8), 976. https://doi.org/10.3390/catal11080976








