Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions
Abstract
:1. Introduction
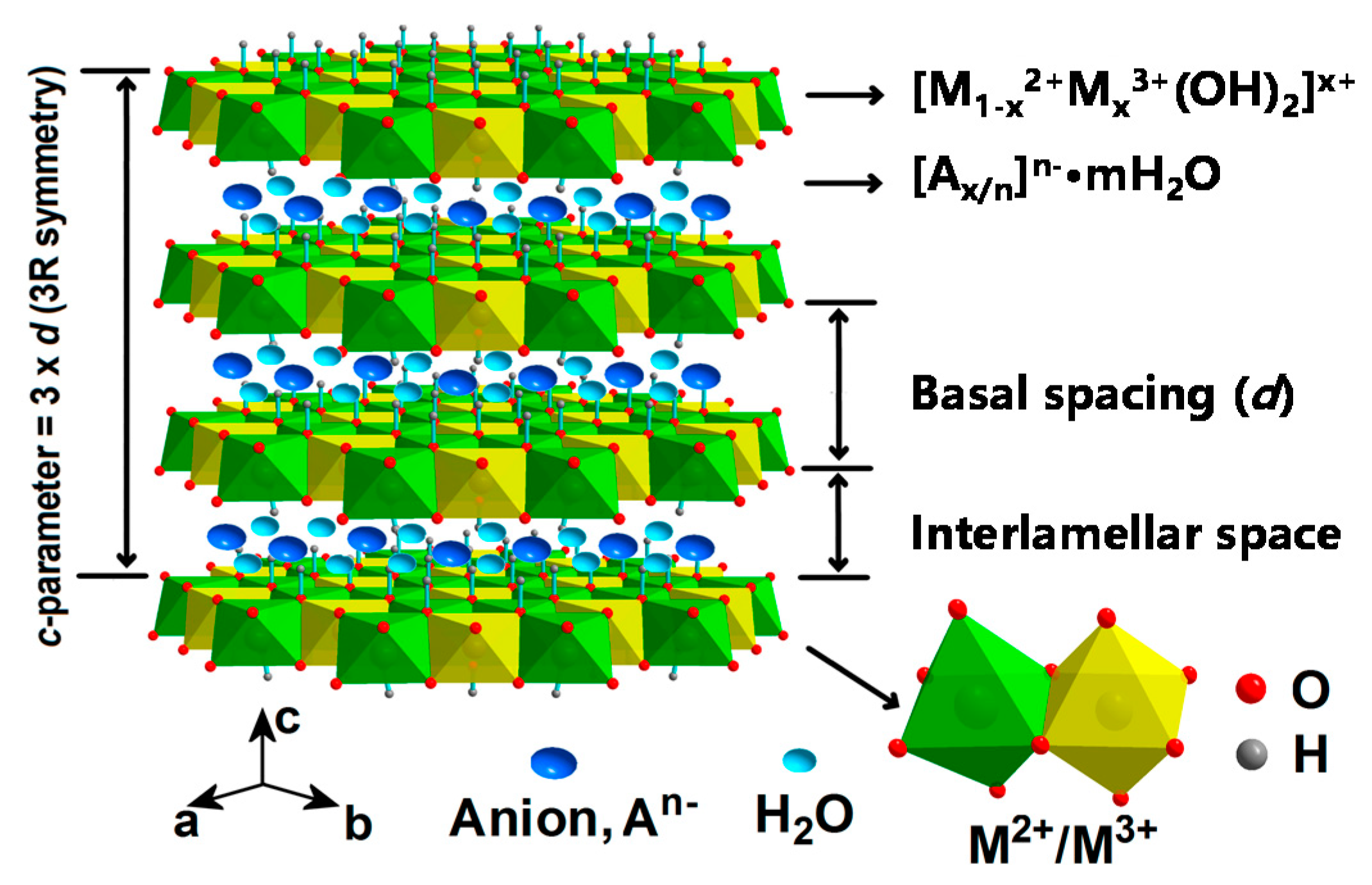
2. Fundamental Characteristics of LDHs
2.1. Binary LDHs
3. Strategies to Functionalize LDHs
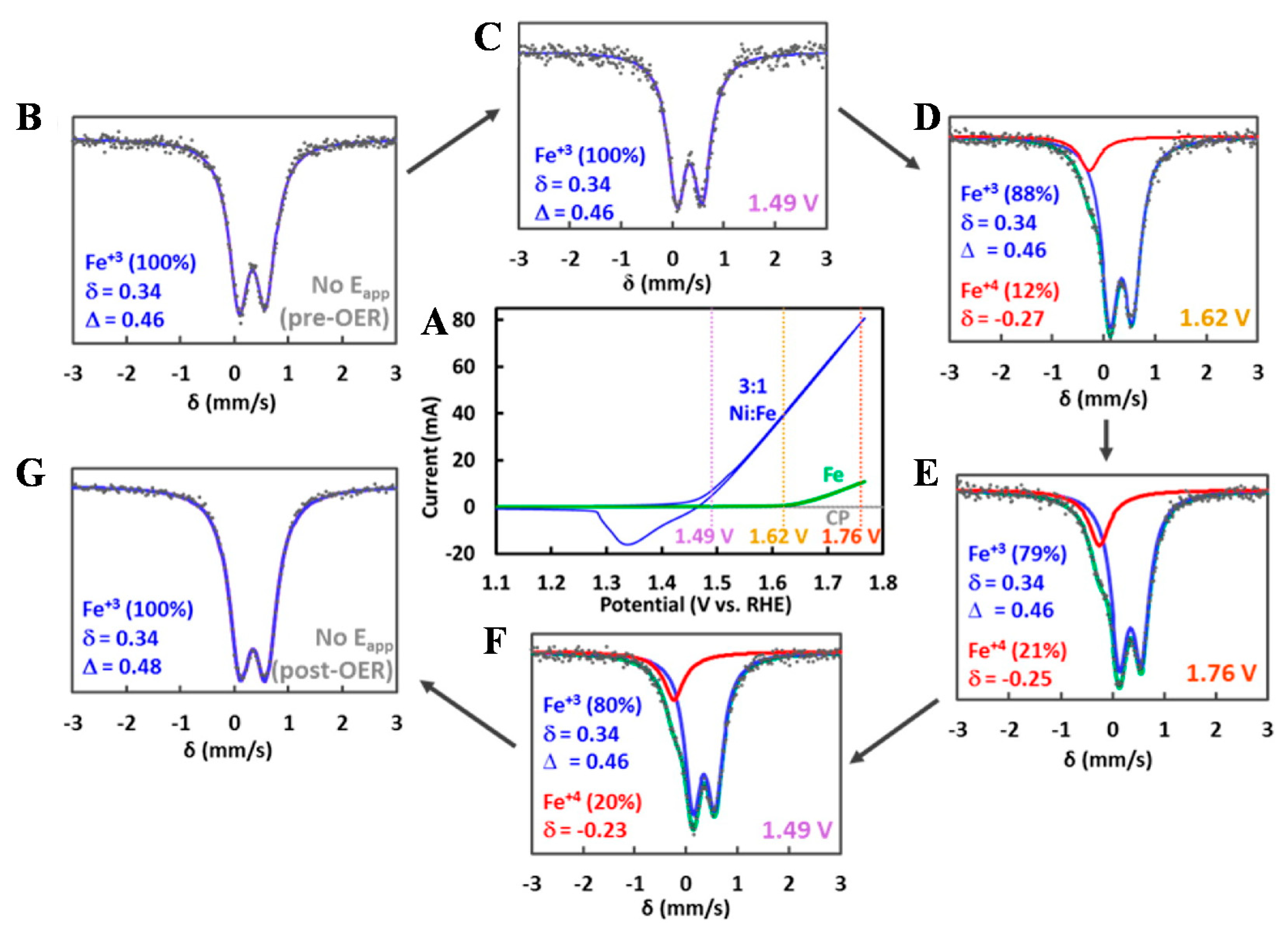
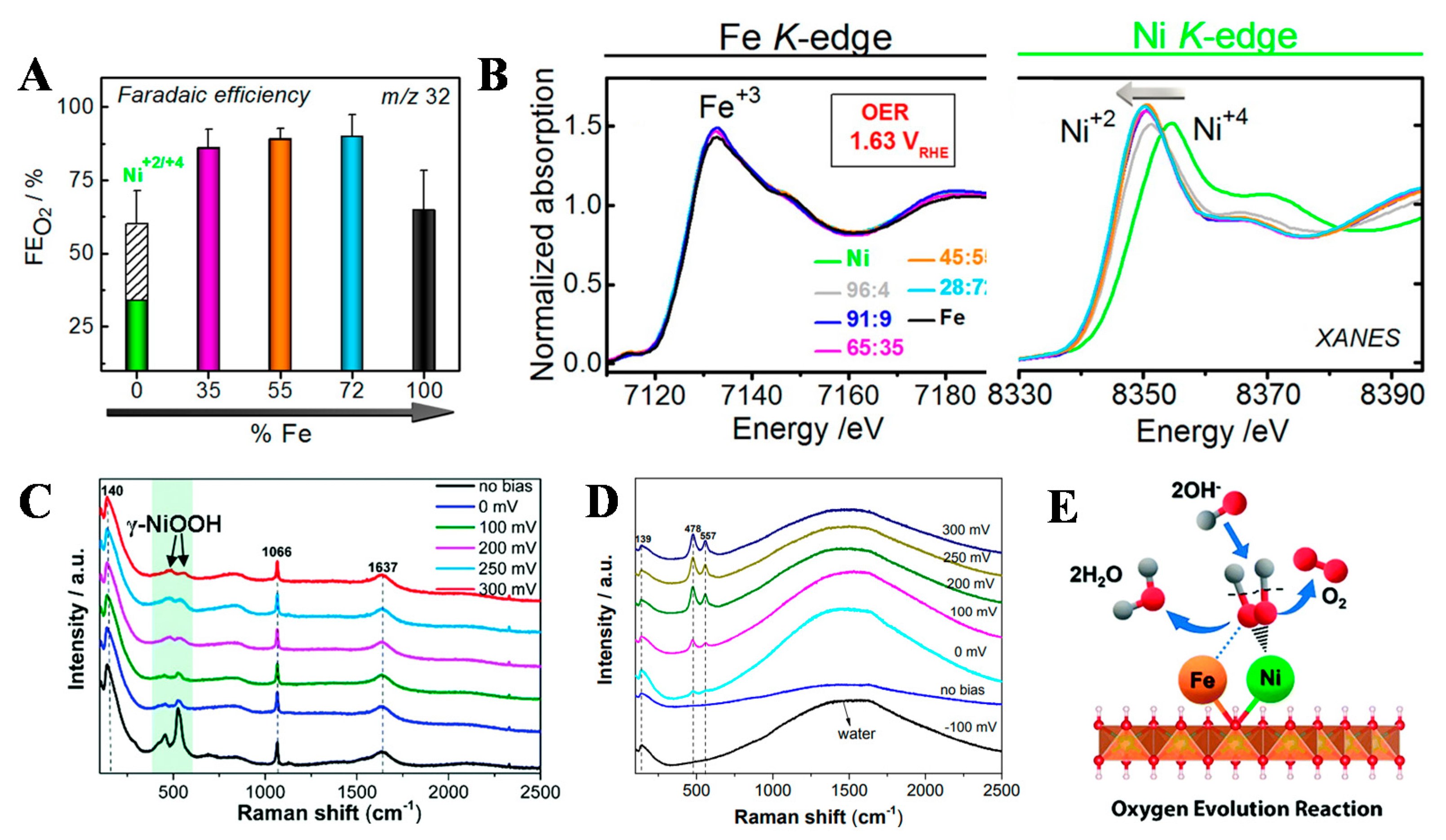
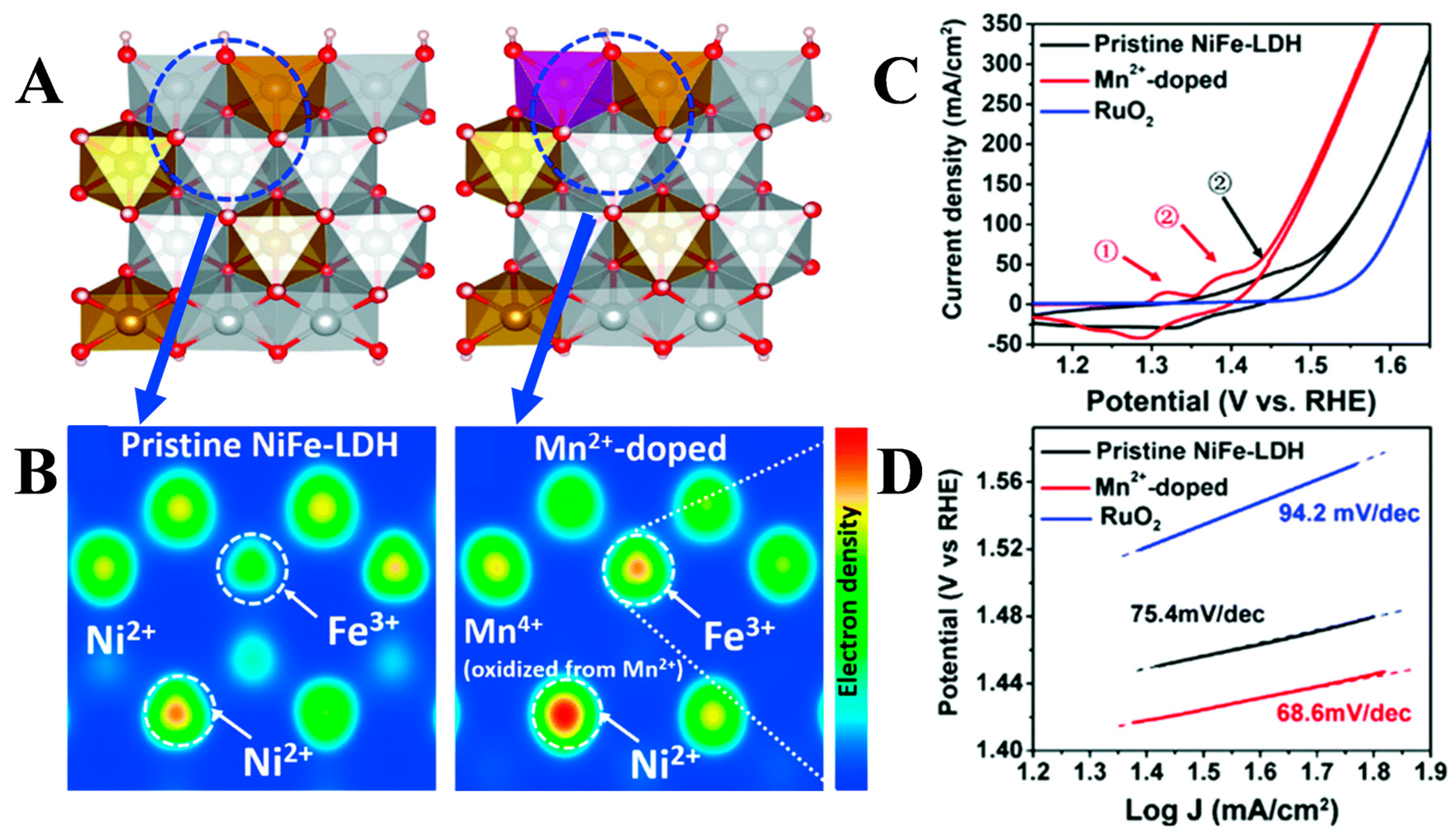
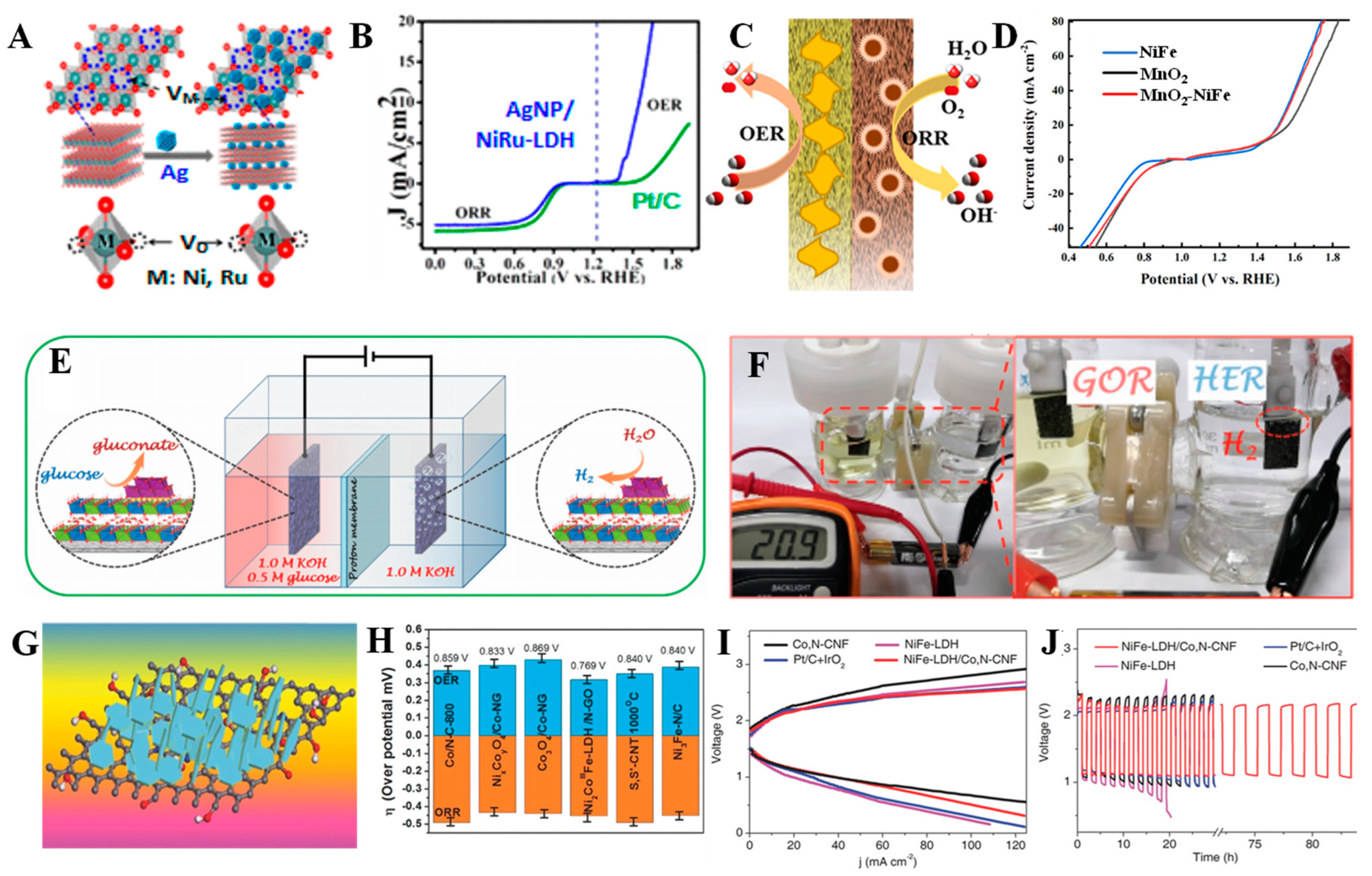
3.1. Regulation of Layer Composition
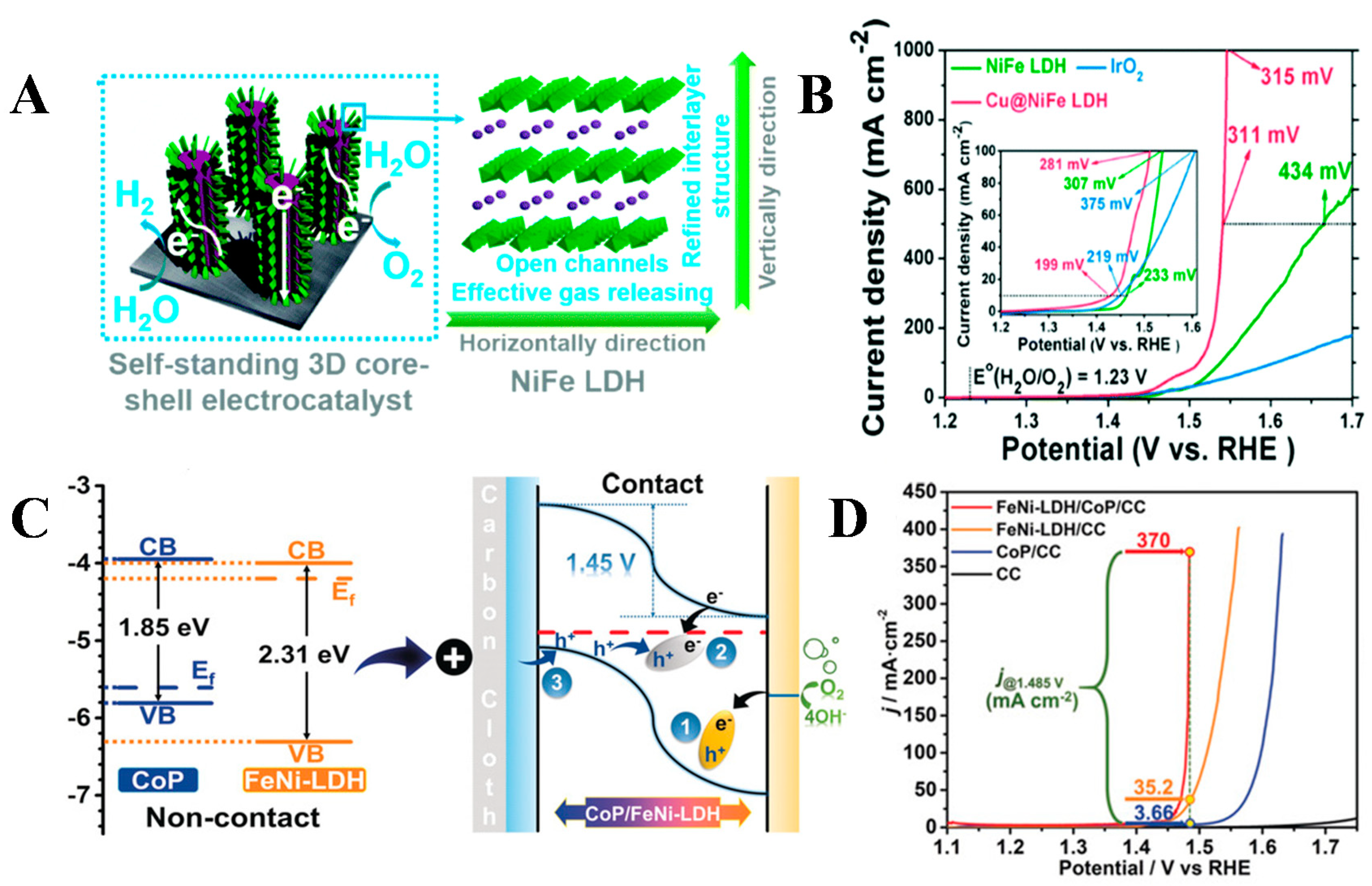
3.2. Intercalation
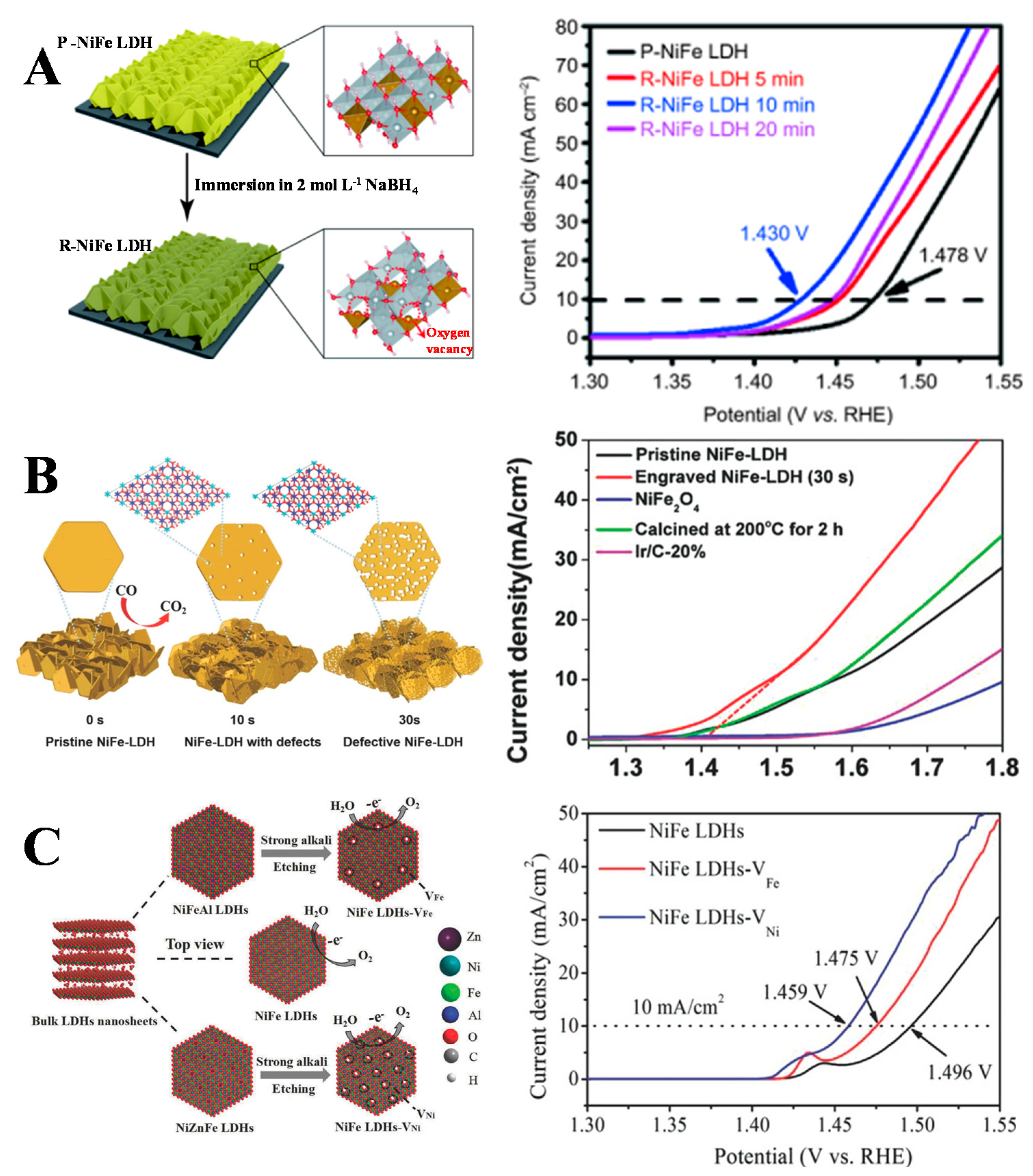
3.3. Vacancy Engineering
3.4. Ultrathin Nanosheets
3.5. Nanostructuring
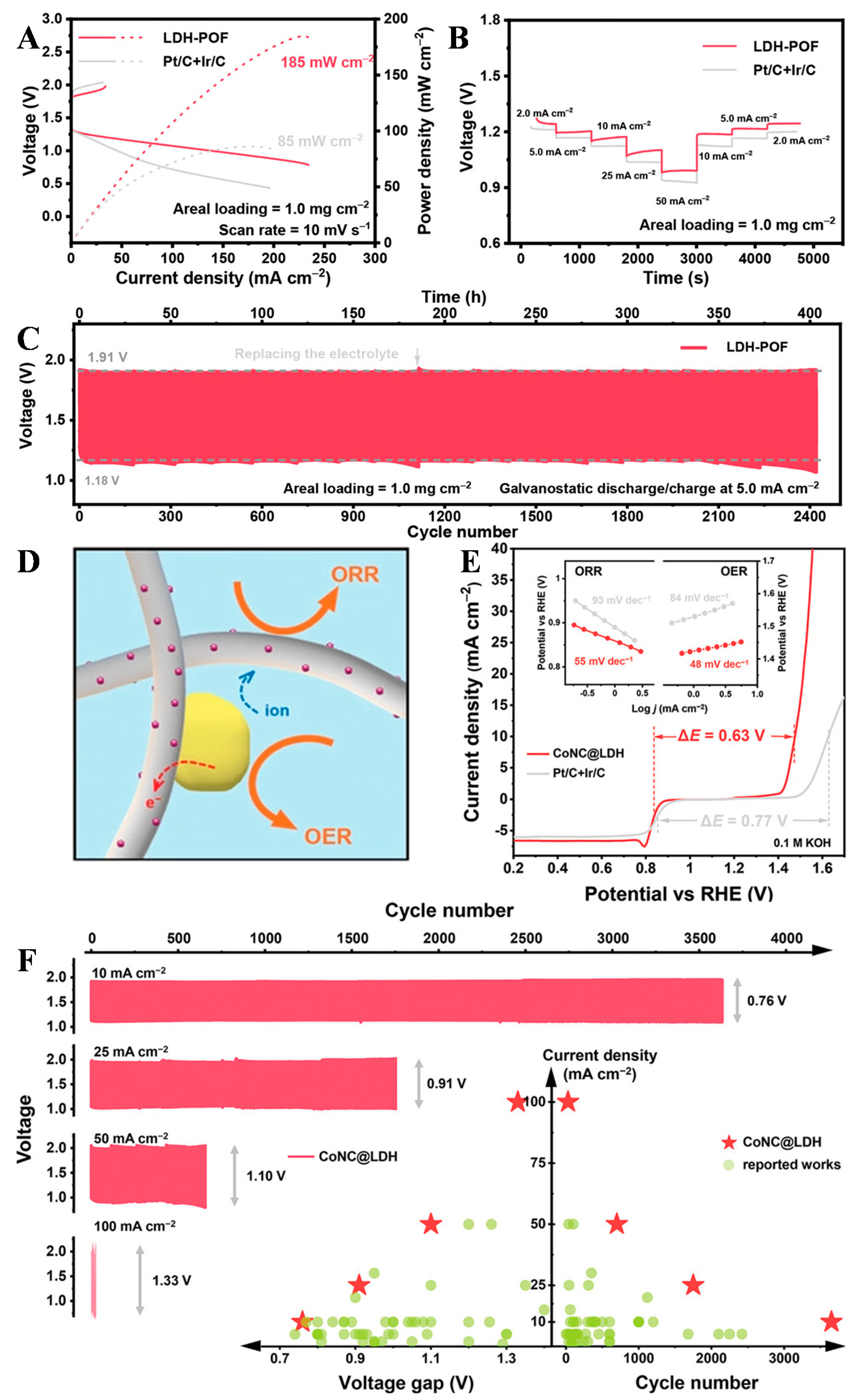
3.6. Hybridization
4. Applications of LDHs-Based Bifunctional Electrocatalysts
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, M.; Li, N.; Bu, X.H. Recent Advances and Perspectives of Metal/Covalent-Organic Frameworks in Metal-air Batteries. J. Energy Chem. 2021. [Google Scholar] [CrossRef]
- She, L.N.; Zhao, G.Q.; Ma, T.Y.; Chen, J.; Sun, W.P.; Pan, H.G. On the Durability of Iridium-based Electrocatalysts toward the Oxygen Evolution Reaction under Acid Environment. Adv. Funct. Mater. 2021. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, Z.W.; Xue, W.J.; Xia, B.Y.; You, B. Emerging Electrocatalysts for Water Oxidation under Near-neutral CO2 Reduction Conditions. Adv. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Abdul Nasir, J.; Munir, A.; Ahmad, N.; Haq, T.u.; Khan, Z.; Rehman, Z. Photocatalytic Z-scheme Overall Water Splitting: Recent Advances in Theory and Experiments. Adv. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.E.G.; Doyle, R.L.; Fernandez, D.; Godwin, I.J.; Browne, M.P.; Rovetta, A. The Mechanism and Kinetics of Electrochemical Water Oxidation at Oxidized Metal and Metal Oxide Electrodes. Part 1. General Considerations: A Mini Review. Electrochem. Commun. 2014, 45, 60–62. [Google Scholar] [CrossRef]
- Tributsch, H. Photovoltaic Hydrogen Generation. Int. J. Hydrogen Energy 2008, 33, 5911–5930. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D.K. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst Approaches and Challenges for Automotive Fuel Cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Peng, P.; Zhou, Z.H.; Guo, J.N.; Xiang, Z.H. Well-defined 2D Covalent Organic Polymers for Energy Electrocatalysis. ACS Energy Lett. 2017, 2, 1308–1314. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and Fuels from Electrochemical Interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, Y.; Li, Y.H.; Cui, X.; Zhang, Y.H.; Xiao, P. NiCo-selenide as a Novel Catalyst for Water Oxidation. J. Mater. Sci. 2016, 51, 3724–3734. [Google Scholar] [CrossRef]
- Song, J.J.; Wei, C.; Huang, Z.F.; Liu, C.T.; Zeng, L.; Wang, X.; Xu, Z.C. A Review on Fundamentals for Designing Oxygen Evolution Electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Zhang, Q. Multiscale Principles to Boost Reactivity in Gas-involving Energy Electrocatalysis. Acc. Chem. Res. 2018, 51, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Audichon, T.; Napporn, T.W.; Canaff, C.; Morais, C.; Comminges, C.; Kokoh, K.B. IrO2 Coated on RuO2 as Efficient and Stable Electroactive Nanocatalysts for Electrochemical Water Splitting. J. Phys. Chem. C 2016, 120, 2562–2573. [Google Scholar] [CrossRef]
- DeSario, P.A.; Chervin, C.N.; Nelson, E.S.; Sassin, M.B.; Rolison, D.R. Competitive Oxygen Evolution in Acid Electrolyte Catalyzed at Technologically Relevant Electrodes Painted with Nanoscale RuO2. ACS Appl. Mater. Interfaces 2017, 9, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and Hydrogen Evolution Reactions on Ru, RuO2, Ir, and IrO2 Thin Film Electrodes in Acidic and Alkaline Electrolytes: A Comparative Study on Activity and Stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Antolini, E. Iridium as Catalyst and Cocatalyst for Oxygen Evolution/Reduction in Acidic Polymer Electrolyte Membrane Electrolyzers and Fuel Cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.W.; Wang, L.; Zou, J.J.; Wang, Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Jung, S.H.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Nanoparticulate Metal Oxide Electrocatalysts for the Alkaline Water Oxidation Reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef] [Green Version]
- Kuai, L.; Geng, J.; Chen, C.Y.; Kan, E.J.; Liu, Y.D.; Wang, Q.; Geng, B.Y. A Reliable Aerosol-spray-assisted Approach to Produce and Optimize Amorphous Metal Oxide Catalysts for Electrochemical Water Splitting. Angew. Chem. Int. Ed. Engl. 2014, 126, 7677–7681. [Google Scholar] [CrossRef]
- Gao, R.q.; Deng, M.; Yan, Q.; Fang, Z.X.; Li, L.C.; Shen, H.Y.; Chen, Z.F. Structural Variations of Metal Oxide-based Electrocatalysts for Oxygen Evolution Reaction. Small Methods 2021. [Google Scholar] [CrossRef]
- Abrham Gebreslase, G.; Victoria Martínez-Huerta, M.; Jesus Lázaro, M. Recent Progress on Bimetallic NiCo and CoFe based Electrocatalysts for Alkaline Oxygen Evolution Reaction: A Review. J. Energy Chem. 2021. [Google Scholar] [CrossRef]
- Wang, H.X.; Zhang, K.H.L.; Hofmann, J.P.; de la Peña O’Shea, V.A.; Oropeza, F.E. The Electronic Structure of Transition Metal Oxides for Oxygen Evolution Reaction. J. Mater. Chem. A 2021, 9, 19465–19488. [Google Scholar] [CrossRef]
- Yu, M.Q.; Budiyanto, E.; Tüysüz, H.R. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zheng, S.S.; Cao, S.; Xue, H.G.; Pang, H. Advances in the Application of Manganese Dioxide and its Composites as Electrocatalysts for the Oxygen Evolution Reaction. J. Mater. Chem. A 2020, 8, 18492–18514. [Google Scholar] [CrossRef]
- Tian, L.; Zhai, X.H.; Wang, X.; Li, J.; Li, Z. Advances in Manganese-based Oxides for Oxygen Evolution Reaction. J. Mater. Chem. A 2020, 8, 14400–14414. [Google Scholar] [CrossRef]
- Burke, M.S.; Enman, L.J.; Batchellor, A.S.; Zou, S.H.; Boettcher, S.W. Oxygen Evolution Reaction Electrocatalysis on Transition Metal Oxides and (Oxy)Hydroxides: Activity Trends and Design Principles. Chem. Mater. 2015, 27, 7549–7558. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Wang, H.S.; Wei, F.; Zhang, Q. Guest-host Modulation of Multi-metallic (Oxy) Hydroxides for Superb Water Oxidation. J. Mater. Chem. A 2016, 4, 3210–3216. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, H.; An, Q.; Song, S.X. Recent Advances in Engineering Cobalt Carbonate Hydroxide for Enhanced Alkaline Water Splitting. J. Alloys Compd. 2021, 887, 161405. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Yu, T.; Wen, H.; Ni, Z.Y.; He, Y.; Guo, R.; You, J.H.; Liu, X.W. The Latest Development of CoOOH Two-dimensional Materials used as OER Catalysts. Chem. Commun. 2020, 56, 15387–15405. [Google Scholar] [CrossRef]
- Zhang, J.; Si, C.H.; Kou, T.Y.; Wang, J.F.; Zhang, Z.H. Recent Progress in Self-supported Two-dimensional Transition Metal Oxides and (Oxy)Hydroxides as Oxygen Evolution Reaction Catalysts. Sustain. Energy Fuels 2020, 4, 2625–2637. [Google Scholar] [CrossRef]
- Lei, L.; Huang, D.L.; Zhou, C.Y.; Chen, S.; Yan, X.L.; Li, Z.H.; Wang, W.J. Demystifying the Active Roles of NiFe-based Oxides/(Oxy)Hydroxides for Electrochemical Water Splitting under Alkaline Conditions. Coord. Chem. Rev. 2020, 408, 213177. [Google Scholar] [CrossRef]
- He, P.L.; Yu, X.Y.; Lou, X.W. Carbon-incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. Engl. 2017, 56, 3897–3900. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.L.; Feng, Y.F.; Nai, J.W.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of Hierarchical Ni-Co-P Hollow Nanobricks with Oriented Nanosheets for Efficient Overall Water Splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, Z.Y. Transition Metal-phosphorus-based Materials for Electrocatalytic Energy Conversion Reactions. Catal. Sci. Technol. 2017, 7, 330–347. [Google Scholar] [CrossRef]
- Zhao, X.D.; Kong, X.L.; Liu, Z.L.; Li, Z.; Xie, Z.W.; Wu, Z.Y.; He, F.; Chang, X.H.; Yang, P.P.; Zheng, J.; et al. The Cutting-edge Phosphorus-rich Metal Phosphides for Energy Storage and Conversion. Nano Today 2021, 40, 101245. [Google Scholar] [CrossRef]
- Li, Z.; Feng, H.; Song, M.; He, C.; Zhuang, W.; Tian, L. Advances in CoP Electrocatalysts for Water Splitting. Mater. Today Energy 2021, 20, 100698. [Google Scholar] [CrossRef]
- Ray, A.; Sultana, S.; Paramanik, L.; Parida, K.M. Recent Advances in Phase, Size, and Morphology-oriented Nanostructured Nickel Phosphide for Overall Water Splitting. J. Mater. Chem. A 2020, 8, 19196–19245. [Google Scholar] [CrossRef]
- Pu, Z.H.; Liu, T.T.; Amiinu, I.S.; Cheng, R.L.; Wang, P.Y.; Zhang, C.T.; Ji, P.X.; Hu, W.H.; Liu, J.; Mu, S.C. Transition-metal Phosphides: Activity Origin, Energy-related Electrocatalysis Applications, and Synthetic Strategies. Adv. Funct. Mater. 2020, 30, 2004009. [Google Scholar] [CrossRef]
- Liu, Y.W.; Xiao, C.; Lyu, M.J.; Lin, Y.; Cai, W.Z.; Huang, P.C.; Tong, W.; Zou, Y.M.; Xie, Y. Ultrathin Co3S4 Nanosheets that Synergistically Engineer Spin States and Exposed Polyhedra that Promote Water Oxidation under Neutral Conditions. Angew. Chem. Int. Ed. Engl. 2015, 54, 11231–11235. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; He, Q.; Jiang, H.L.; Lin, Y.X.; Zhang, Y.K.; Habib, M.; Chen, S.M.; Song, L. Electronic Structure Reconfiguration toward Pyrite NiS2 via Engineered Heteroatom Defect Boosting Overall Water Splitting. ACS Nano 2017, 11, 11574–11583. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, L.Z.; Jia, Y.; Liu, H.L.; Yan, X.C.; Zhang, L.Z.; Yang, D.J.; Zhu, Z.H.; Yao, X.D. Plasma-triggered Synergy of Exfoliation, Phase Transformation, and Surface Engineering in Cobalt Diselenide for Enhanced Water Oxidation. Angew. Chem. Int. Ed. Engl. 2018, 57, 16421–16425. [Google Scholar] [CrossRef]
- Zhao, Y.; You, J.H.; Wang, L.; Bao, W.T.; Yao, R.Y. Recent Advances in Ni3S2-based Electrocatalysts for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 39146–39182. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yin, Z.H.; Cui, M.; Liu, X.; Xiong, J.B.; Chen, S.R.; Ma, T.L. Interface Engineering of Transitional Metal Sulfide-MoS2 Heterostructure Composites as Effective Electrocatalysts for Water-splitting. J. Mater. Chem. A 2021, 9, 2070–2092. [Google Scholar] [CrossRef]
- Ni, Z.Y.; Wen, H.; Zhang, S.Q.; Guo, R.; Su, N.; Liu, X.W.; Liu, C.M. Recent Advances in Layered Tungsten Disulfide as Electrocatalyst for Water Splitting. ChemCatChem 2020, 12, 4962–4999. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Qiao, M.; Li, Y.F.; Wang, S.Y. Tuning Surface Electronic Configuration of NiFe LDHs Nanosheets by Introducing Cation Vacancies (Fe or Ni) as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Small 2018, 14, 1800136. [Google Scholar] [CrossRef]
- Zhou, D.J.; Li, P.S.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.M.; Duan, X. Layered Double Hydroxide-based Electrocatalysts for the Oxygen Evolution Reaction: Identification and Tailoring of Active Sites, and Superaerophobic Nanoarray Electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Gicha, B.B.; Tufa, L.T.; Kang, S.; Goddati, M.; Bekele, E.T.; Lee, J. Transition Metal-based 2D Layered Double Hydroxide Nanosheets: Design Strategies and Applications in Oxygen Evolution Reaction. Nanomaterials 2021, 11, 1388. [Google Scholar] [CrossRef]
- Yu, J.; Yu, F.; Yuen, M.F.; Wang, C.D. Two-dimensional Layered Double Hydroxides as a Platform for Electrocatalytic Oxygen Evolution. J. Mater. Chem. A 2021, 9, 9389–9430. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Zhu, S.; Feng, L.; Xing, W. Ni-based Layered Double Hydroxide Catalysts for Oxygen Evolution Reaction. Mater. Today Phys. 2021, 16, 100292. [Google Scholar] [CrossRef]
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent Advances in Highly Active Nanostructured NiFe LDH Catalyst for Electrochemical Water Splitting. J. Mater. Chem. A 2020, 9, 3180–3208. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Sankar, S.S.; Kumaravel, S.; Madhu, R.; Kundu, S. A Vast Exploration of Improvising Synthetic Strategies for Enhancing the OER Kinetics of LDH Structures: A Review. J. Mater. Chem. A 2020, 9, 1314–1352. [Google Scholar] [CrossRef]
- Goncalves, J.M.; Martins, P.R.; Angnes, L.; Araki, K. Recent Advances in Ternary Layered Double Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. New J. Chem. 2020, 44, 9981–9997. [Google Scholar] [CrossRef]
- Hall, D.E. Ni(OH)2-impregnated Anodes for Alkaline Water Electrolysis. J. Electrochem. Soc. 1983, 130, 317–321. [Google Scholar] [CrossRef]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe Electrolyte Impurities on Ni(OH)2/NiOOH Structure and Oxygen Evolution Activity. J. Phys. Chem. C 2015, 119, 7243–7254. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.H.; Song, M.X.; Liu, P.Z.; Liu, W.W.; Yuan, L.J.; Hao, X.D.; Pei, L.Y.; Xu, B.S.; Guo, J.J.; Sun, Z.Q. Fe-doping Induced Localized Amorphization in Ultrathin α-Ni(OH)2 Nanomesh for Superior Oxygen Evolution Reaction Catalysis. J. Mater. Chem. A 2021, 9, 14372–14380. [Google Scholar] [CrossRef]
- Zhong, H.H.; Cheng, X.K.; Xu, H.T.; Li, L.; Li, D.Q.; Tang, P.G.; Alonso-Vante, N.; Feng, Y.J. Carbon Fiber Paper Supported Interlayer Space Enlarged Ni2Fe-LDHs Improved OER Electrocatalytic Activity. Electrochim. Acta 2017, 258, 554–560. [Google Scholar] [CrossRef]
- Ye, Q.L.; Li, L.F.; Li, H.Y.; Gu, X.Y.; Han, B.M.; Xu, X.T.; Wang, F.; Li, B. Quasi-parallel NiFe Layered Double Hydroxide Nanosheet Arrays for Large-current-density Oxygen Evolution Electrocatalysis. ChemSusChem 2021. [Google Scholar] [CrossRef]
- Wilhelm, M.; Bastos, A.; Neves, C.; Martins, R.; Tedim, J. Ni-Fe Layered Double Hydroxides for Oxygen Evolution Reaction: Impact of Ni/Fe Ratio and Crystallinity. Mater. Des. 2021, 212, 110188. [Google Scholar] [CrossRef]
- Dong, G.F.; Xie, F.Y.; Kou, F.X.; Chen, T.T.; Wang, F.Y.; Zhou, Y.W.; Wu, K.C.; Du, S.W.; Fang, M.; Ho, J.C. NiFe-Layered Double Hydroxides Arrays for Oxygen Evolution Reaction in Fresh Water and Seawater. Mater. Today Energy 2021. [Google Scholar] [CrossRef]
- Chen, W.; Wu, B.B.; Wang, Y.Y.; Zhou, W.; Li, Y.Y.; Liu, T.Y.; Xie, C.; Xu, L.T.; Du, S.Q.; Song, M.L.; et al. Deciphering the Alternating Synergy Between Interlayer Pt Single-atom and NiFe Layered Double Hydroxide for Overall Water Splitting. Energy Environ. Sci. 2021. [Google Scholar] [CrossRef]
- Liu, B.Y.; Zhang, M.; Wang, Y.C.; Chen, Z.; Yan, K. Facile Synthesis of Defect-rich Ultrathin NiCo-LDHs, NiMn-LDHs and NiCoMn-LDHs Nanosheets on Ni Foam for Enhanced Oxygen Evolution Reaction Performance. J. Alloys Compd. 2021, 852, 156949. [Google Scholar] [CrossRef]
- Chen, Z.D.; Zhang, Y.; Yang, P.G.; Xiong, W.; Ren, X.Z.; Li, Y.L.; Wang, L.L.; Ye, S.H.; Liu, J.H.; Zhang, Q.L. Improving Oxygen Evolution Reaction Activity by Constructing Core-shell Structure of Co/N-doped Carbon Polyhedron@NiCo Layered Double Hydroxides. J. Alloys Compd. 2022, 890, 161805. [Google Scholar] [CrossRef]
- Shamloofard, M.; Shahrokhian, S.; Amini, M.K. Mesoporous Nanostructures of NiCo-LDH/ZnCo2O4 as an Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Colloid Interface Sci. 2021, 604, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.X.; Ma, Q.X.; Li, Z.S.; Li, B.L.; Huang, F.R.; Pang, Q.; Chen, Y.B.; Li, B.; Zhang, J.Z. Design and Preparation of Three-dimensional Hetero-electrocatalysts of NiCo-Layered Double Hydroxide Nanosheets Incorporated with Silver Nanoclusters for Enhanced Oxygen Evolution Reactions. Nanoscale 2021, 13, 11150–11160. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Wang, X.; Huang, J.F.; Dong, P.P.; Ji, J.; Li, J.; Cao, L.Y.; Feng, L.L.; Jin, P.; Wang, C.R. Decorating CoNi Layered Double Hydroxides Nanosheet Arrays with Fullerene Quantum Dot Anchored on Ni Foam for Efficient Electrocatalytic Water Splitting and Urea Electrolysis. Chem. Eng. J. 2020, 390, 124525. [Google Scholar] [CrossRef]
- Shi, Y.L.; Li, J.Q.; Zhang, B.Y.; Lv, S.Y.; Wang, T.; Liu, X. Tuning Electronic Structure of CoNi LDHs via Surface Fe Doping for Achieving Effective Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 565, 150506. [Google Scholar] [CrossRef]
- Hu, L.Y.; Li, M.Y.; Wei, X.Q.; Wang, H.J.; Wu, Y.; Wen, J.; Gu, W.L.; Zhu, C.Z. Modulating Interfacial Electronic Structure of CoNi LDH Nanosheets with Ti3C2Tx MXene for Enhancing Water Oxidation Catalysis. Chem. Eng. J. 2020, 398, 125605. [Google Scholar] [CrossRef]
- Li, J.W.; Lian, R.Q.; Wang, J.Y.; He, S.; Jiang, S.P.; Rui, Z.B. Oxygen Vacancy Defects Modulated Electrocatalytic Activity of Iron-Nickel Layered Double Hydroxide on Ni Foam as Highly Active Electrodes for Oxygen Evolution Reaction. Electrochim. Acta 2020, 331, 135395. [Google Scholar] [CrossRef]
- Wang, M.; Wei, Y.S.; Zou, L.L.; Wang, H.F.; Shen, S.H.; Xu, Q. Revealing Active Function of Multicomponent Electrocatalysts from In Situ Nickel Redox for Oxygen Evolution. J. Phys. Chem. Lett. 2021, 125, 16420–16427. [Google Scholar] [CrossRef]
- Zheng, F.Q.; Zhang, W.F.; Zhang, X.X.; Zhang, Y.L.; Chen, W. Sub-2 nm Ultrathin and Robust 2D FeNi Layered Double Hydroxide Nanosheets Packed with 1D FeNi-MOFs for Enhanced Oxygen Evolution Electrocatalysis. Adv. Funct. Mater. 2021, 31, 2103318. [Google Scholar] [CrossRef]
- Han, X.Q.; Lin, Z.H.; He, X.Q.; Cui, L.L.; Lu, D.X. The Construction of Defective FeCo-LDHs by In-situ Polyaniline Curved Strategy as a Desirable Bifunctional Electrocatalyst for OER and HER. Int. J. Hydrogen Energy 2020, 45, 26989–26999. [Google Scholar] [CrossRef]
- Deng, L.Q.; Zhang, K.; Shi, D.; Liu, S.F.; Xu, D.Q.; Shao, Y.L.; Shen, J.X.; Wu, Y.Z.; Hao, X.P. Rational Design of Schottky Heterojunction with Modulating Surface Electron Density for High-performance Overall Water Splitting. Appl. Catal. B Environ. 2021, 299, 120660. [Google Scholar] [CrossRef]
- Hu, L.Y.; Xiao, R.S.; Wang, X.; Wang, X.S.; Wang, C.L.; Wen, J.; Gu, W.L.; Zhu, C.Z. MXene-induced Electronic Optimization of Metal-Organic Framework-derived CoFe LDH Nanosheet Arrays for Efficient Oxygen Evolution. Appl. Catal. B Environ. 2021, 298, 120599. [Google Scholar] [CrossRef]
- Wu, L.B.; Yu, L.; Zhu, Q.C.; McElhenny, B.; Zhang, F.H.; Wu, C.Z.; Xing, X.X.; Bao, J.M.; Chen, S.; Ren, Z.F. Boron-modified Cobalt Iron Layered Double Hydroxides for High Efficiency Seawater Oxidation. Nano Energy 2021, 83, 105838. [Google Scholar] [CrossRef]
- Chen, L.Y.; Guo, Y.T.; Wang, H.F.; Gu, Z.Z.; Zhang, Y.Y.; Li, X.Z.; Wang, H.; Duan, C.Y. Imidazolate-mediated Assembled Structures of Co-LDH Sheets for Efficient Electrocatalytic Oxygen Evolution. J. Mater. Chem. A 2018, 6, 4636–4641. [Google Scholar] [CrossRef]
- Xu, X.; Cao, A.H.; You, W.F.; Tao, Z.J.; Kang, L.T.; Liu, J.J. Assembly of Cobalt Layered Double Hydroxide on Cuprous Phosphide Nanowire with Strong Built-in Potential for Accelerated Overall Water Splitting. Small 2021, 17, 2101725. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhang, Y.Q.; Ye, L.; Guo, B.W.; Gong, Y.Q. Hierarchically Constructed Ag Nanowires Shelled with Ultrathin Co-LDH Nanosheets for Advanced Oxygen Evolution Reaction. Appl. Catal. B Environ. 2021, 298, 120601. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, R.; Liu, H.; Zhou, L.X.; Wang, Y.; Zhang, Y.; Fan, G.Y. Flexible Active-site Engineering of Monometallic Co-Layered Double Hydroxides for Achieving High-performance Bifunctional Electrocatalyst toward Oxygen Evolution and H2O2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 12919–12929. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, Y.F.; Zhou, Y.F.; Yang, F.L. NiFe Layered-double-hydroxide-derived NiO-NiFe2O4/Reduced Graphene Oxide Architectures for Enhanced Electrocatalysis of Alkaline Water Splitting. ChemElectroChem 2016, 3, 1927–1936. [Google Scholar] [CrossRef]
- Zhong, H.Z.; Gao, G.P.; Wang, X.N.; Wu, H.Y.; Shen, S.H.; Zuo, W.B.; Cai, G.X.; Wei, G.; Shi, Y.; Fu, D.J.; et al. Ion Irradiation Inducing Oxygen Vacancy-rich NiO/NiFe2O4 Heterostructure for Enhanced Electrocatalytic Water Splitting. Small 2021, 17, 2103501. [Google Scholar] [CrossRef]
- Fan, X.M.; Ma, Y.X.; Sun, A.K.; Zhang, X.; Tang, L.; Guo, J.X. Engineering Heterogeneous Nickel-Iron Oxide/Iron Phosphate on P, N co-doped Carbon Fibers for Efficient Oxygen Evolution Reaction in Neutral and Alkaline Solutions. Surf. Interfaces 2021, 25, 101193. [Google Scholar] [CrossRef]
- Zhong, H.H.; Tian, R.; Li, D.Q.; Tang, P.G.; Alonso-Vante, N.; Feng, Y.J. Tuning the Adsorption Properties of Layered Double Hydroxides to Tailor Highly Active Oxygen Bifunctional Electrocatalysts. J. Electrochem. Soc. 2017, 164, F491–F498. [Google Scholar] [CrossRef]
- Ortega, K.F.; Anke, S.; Salamon, S.; Ozcan, F.; Heese, J.; Andronescu, C.; Landers, J.; Wende, H.; Schuhmann, W.; Muhler, M.; et al. Topotactic Synthesis of Porous Cobalt Ferrite Platelets from a Layered Double Hydroxide Precursor and Their Application in Oxidation Catalysis. Chem. Eur. J. 2017, 23, 12443–12449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lu, S.Q.; Huang, H.L.; Liu, D.H.; Zhuang, Z.B.; Zhong, C.L. ZIF-67 as Continuous Self-sacrifice Template Derived NiCo2O4/Co,N-CNTs Nanocages as Efficient Bifunctional Electrocatalysts for Rechargeable Zn-air Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10021–10029. [Google Scholar] [CrossRef]
- Yang, M.; Lu, W.; Jin, R.X.; Liu, X.C.; Song, S.Y.; Xing, Y. Superior Oxygen Evolution Reaction Performance of Co3O4/NiCo2O4/Ni Foam Composite with Hierarchical Structure. ACS Sustain. Chem. Eng. 2019, 7, 12214–12221. [Google Scholar] [CrossRef]
- Li, T.T.; Lu, Y.X.; Zhao, S.S.; Gao, Z.D.; Song, Y.Y. Co3O4-doped Co/CoFe Nanoparticles Encapsulated in Carbon Shells as Bifunctional Electrocatalysts for Rechargeable Zn-air Batteries. J. Mater. Chem. A 2018, 6, 3730–3737. [Google Scholar] [CrossRef]
- Peng, D.D.; Zhang, B.W.; Wu, J.S.; Huang, K.; Cao, X.; Lu, Y.; Zhang, Y.; Li, C.J.; Huang, Y.Z. Growth of Lattice Coherent Co9S8/Co3O4 Nano-heterostructure for Maximizing the Catalysis of Co-based Composites. ChemCatChem 2020, 12, 2431–2435. [Google Scholar] [CrossRef]
- He, C.Y.; Han, X.Z.; Kong, X.G.; Jiang, M.H.; Lei, D.Q.; Lei, X.D. Fe-doped Co3O4@C Nanoparticles Derived from Layered Double Hydroxide used as Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Energy Chem. 2019, 32, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Anantharaj, S.; Karthick, K.; Kundu, S. Evolution of Layered Double Hydroxides (LDH) as High Performance Water Oxidation Electrocatalysts: A Review with Insights on Structure, Activity and Mechanism. Mater. Today Energy 2017, 6, 1–26. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Nyamori, V.O. Layered Double Hydroxide- and Graphene-based Hierarchical Nanocomposites: Synthetic Strategies and Promising Applications in Energy Conversion and Conservation. Nano Res. 2016, 9, 3598–3621. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H.J. A Mini Review of NiFe-based Materials as Highly Active Oxygen Evolution Reaction Electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Park, J.T.; Patel, M.; Dash, J.K.; Gowd, E.B.; Karpoormath, R.; Mishra, A.; Kwak, J.; Kim, J.H. Transition-metal-based Layered Double Hydroxides Tailored for Energy Conversion and Storage. J. Mater. Chem. A 2018, 6, 12–29. [Google Scholar] [CrossRef]
- Laipan, M.W.; Yu, J.F.; Zhu, R.L.; Zhu, J.X.; Smith, A.T.; He, H.P.; O’Hare, D.; Sun, L.Y. Functionalized Layered Double Hydroxides for Innovative Applications. Mater. Horiz. 2020, 7, 715–745. [Google Scholar] [CrossRef]
- Yu, J.F.; Wang, Q.; O’Hare, D.; Sun, L.Y. Preparation of Two Dimensional Layered Double Hydroxide Nanosheets and Their Applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Li, C.M.; Wei, M.; Evans, D.G.; Duan, X. Recent Advances for Layered Double Hydroxides (LDHs) Materials as Catalysts Applied in Green Aqueous Media. Catal. Today 2015, 247, 163–169. [Google Scholar] [CrossRef]
- Li, T.F.; Miras, H.N.; Song, Y.F. Polyoxometalate (POM)-Layered Double Hydroxides (LDH) Composite Materials: Design and Catalytic Applications. Catalysts 2017, 7, 260. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gao, Y.S.; Wang, Q.; Lin, W.R. The Synergistic Effect of Layered Double Hydroxides with Other Flame Retardant Additives for Polymer Nanocomposites: A Critical Review. Dalton Trans. 2018, 47, 14827–14840. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.F.; Zhang, F.; Zeng, R.C.; Zeng, J.M. Layered Double Hydroxide Coatings on Magnesium Alloys: A Review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered Double Hydroxides (LDHs) as Functional Materials for the Corrosion Protection of Aluminum Alloys: A Review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent Progress on Layered Double Hydroxide (LDH) Derived Metal-based Catalysts for CO2 Conversion to Valuable Chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered Double Hydroxide (LDH)-based Materials: A Mini-review on Strategies to Improve the Performance for Photocatalytic Water Splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Li, S.D.; Wang, D.D.; Wu, X.F.; Chen, Y.F. Recent Advance on VOCs Oxidation Over Layered Double Hydroxides Derived Mixed Metal Oxides. Chin. J. Catal. 2020, 41, 550–560. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.H.; Liu, H.F.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn-air Batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef]
- Cui, J.Y.; Li, Z.H.; Wang, G.R.; Guo, J.; Shao, M.F. Layered Double Hydroxides and Their Derivatives for Lithium-sulfur Batteries. J. Mater. Chem. A 2020, 8, 23738–23755. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.Q.; Zhang, Y.X. Chemical Modifications of Layered Double Hydroxides in the Supercapacitor. Energy Environ. Mater. 2020, 3, 346–379. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in Activity for the Water Electrolyser Reactions on 3d M(Ni,Co,Fe,Mn) Hydr(oxy)oxide Catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, F.F.; Zhou, S.; Hu, W.; Zhang, H.; Dong, J.C.; Jiang, Z.; Zhao, J.J.; Li, J.F.; Yan, W.S.; et al. Atomic-level Insight into Super-efficient Electrocatalytic Oxygen Evolution on Iron and Vanadium Co-doped Nickel (Oxy)hydroxide. Nat. Commun. 2018, 9, 2885. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.S.; Zhang, H.L.; Chen, L.; Wei, X.F.; Shi, J.L.; He, M.Y. Facile Synthesis of Cu Doped Cobalt Hydroxide (Cu-Co(OH)2) Nano-sheets for Efficient Electrocatalytic Oxygen Evolution. J. Mater. Chem. A 2017, 5, 22568–22575. [Google Scholar] [CrossRef]
- Zou, X.X.; Goswami, A.; Asefa, T. Efficient Noble Metal-Free (Electro)Catalysis of Water and Alcohol Oxidations by Zinc-Cobalt Layered Double Hydroxide. J. Am. Chem. Soc. 2013, 135, 17242–17245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Ji, Y.F.; Nai, J.W.; Niu, X.G.; Luo, Y.; Guo, L.; Yang, S.H. Ultrathin Amorphous Cobalt-Vanadium Hydr(oxy)oxide Catalysts for the Oxygen Evolution Reaction. Energy Environ. Sci. 2018, 11, 1736–1741. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.Y.; Qin, J.Q.; Liu, R.P. Revealing Ni-based Layered Double Hydroxides as High-efficiency Electrocatalysts for the Oxygen Evolution Reaction: A DFT Study. J. Mater. Chem. A 2019, 7, 23091–23097. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.G.; Wang, H.L.; Liang, Y.Y.; Wu, J.Z.; Zhou, J.G.; Wang, J.; Regier, T.; Wei, F.; Dai, H.J. An Advanced Ni-Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-film Ni-Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Morales, O.; Ledezma-Yanez, I.; Koper, M.T.M.; Calle-Vallejo, F. Guidelines for the Rational Design of Ni-based Double Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. ACS Catal. 2015, 5, 5380–5387. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.C.; Dang, L.N.; Liang, H.F.; Bi, W.L.; Gerken, J.B.; Jin, S.; Alp, E.E.; Stahl, S.S. Operando Analysis of NiFe and Fe Oxyhydroxide Electrocatalysts for Water Oxidation: Detection of Fe4+ by Mössbauer Spectroscopy. J. Am. Chem. Soc. 2015, 137, 15090–15093. [Google Scholar] [CrossRef]
- Ahn, H.S.; Bard, A.J. Surface Interrogation Scanning Electrochemical Microscopy of Ni1−xFexOOH (0 < x < 0.27) Oxygen Evolving Catalyst: Kinetics of the “Fast” Iron Sites. J. Am. Chem. Soc. 2016, 138, 313–318. [Google Scholar]
- Gorlin, M.; Chernev, P.; Ferreira de Araujo, J.; Reier, T.; Dresp, S.; Paul, B.; Krahnert, R.; Dau, H.; Strasser, P. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni-Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bediako, D.K.; Hadt, R.G.; Hayes, D.; Kempa, T.J.; von Cube, F.; Bell, D.C.; Chen, L.X.; Nocera, D.G. Influence of Iron Doping on Tetravalent Nickel Content in Catalytic Oxygen Evolving Films. Proc. Natl. Acad. Sci. USA 2017, 114, 1486–1491. [Google Scholar] [CrossRef] [Green Version]
- Hung, S.F.; Hsu, Y.Y.; Chang, C.J.; Hsu, C.S.; Suen, N.T.; Chan, T.S.; Chen, H.M. Unraveling Geometrical Site Confinement in Highly Efficient Iron-doped Electrocatalysts toward Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1701686. [Google Scholar] [CrossRef]
- Smith, R.D.; Prevot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Tai, C.W.; Niklasson, G.A.; Edvinsson, T. Direct Observation of Active Catalyst Surface Phases and the Effect of Dynamic Self-optimization in NiFe-Layered Double Hydroxides for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.J.; Cai, Z.; Jia, Y.; Xiong, X.Y.; Xie, Q.X.; Wang, S.Y.; Zhang, Y.; Liu, W.; Duan, H.H.; Sun, X. Activating Basal Plane in NiFe Layered Double Hydroxide by Mn2+ Doping for Efficient and Durable Oxygen Evolution Reaction. Nanoscale Horiz. 2018, 3, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Qian, L.; Tian, Y.; Li, Y.P.; Sun, X.M.; Duan, X. Ternary NiFeMn Layered Double Hydroxides as Highly-efficient Oxygen Evolution Catalysts. Chem. Commun. 2016, 52, 908–911. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, D.J.; Wang, M.Y.; Bak, S.M.; Wu, Y.S.; Wu, Z.S.; Tian, Y.; Xiong, X.Y.; Li, Y.P.; Liu, W.; et al. Introducing Fe2+ into Nickel-Iron Layered Double Hydroxide: Local Structure Modulated Water Oxidation Activity. Angew. Chem. Int. Ed. Engl. 2018, 57, 9392–9396. [Google Scholar] [CrossRef]
- Thenuwara, A.C.; Attanayake, N.H.; Yu, J.; Perdew, J.P.; Elzinga, E.J.; Yan, Q.M.; Strongin, D.R. Cobalt Intercalated Layered NiFe Double Hydroxides for the Oxygen Evolution Reaction. J. Phys. Chem. B 2018, 122, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.M.; Cai, Z.; Zhou, D.J.; Tian, Y.; Zhang, Q.; Zhang, Q.; Kuang, Y.; Li, Y.P.; Sun, X.M.; Duan, X. Understanding the Incorporating Effect of Co2+/Co3+ in NiFe-Layered Double Hydroxide for Electrocatalytic Oxygen Evolution Reaction. J. Catal. 2018, 358, 100–107. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Zhang, Y.Q.; Li, Z.H.; Shao, M.F. Host Modification of Layered Double Hydroxide Electrocatalyst to Boost the Thermodynamic and Kinetic Activity of Oxygen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2009743. [Google Scholar] [CrossRef]
- Li, P.S.; Duan, X.X.; Kuang, Y.; Li, Y.P.; Zhang, G.X.; Liu, W.; Sun, X.M. Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 1703341. [Google Scholar] [CrossRef]
- Wen, L.L.; Zhang, X.L.; Liu, J.Y.; Li, X.Y.; Xing, C.C.; Lyu, X.J.; Cai, W.P.; Wang, W.C.; Li, Y. Cr-dopant Induced Breaking of Scaling Relations in CoFe Layered Double Hydroxides for Improvement of Oxygen Evolution Reaction. Small 2019, 15, 1902373. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, W.J.; Hu, Y.M.; Guan, M.L.; Xu, L.; Li, H.P.; Bao, J.; Li, H.M. Cr-doped CoFe Layered Double Hydroxides: Highly Efficient and Robust Bifunctional Electrocatalyst for the Oxidation of Water and Urea. Appl. Catal. B Environ. 2020, 272, 118959. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.Y.; Duan, S.; Wang, T.Y.; Li, Q. Tuning the Oxygen Evolution Electrocatalysis on NiFe-Layered Double Hydroxides via Sulfur Doping. Chin. J. Catal. 2020, 41, 847–852. [Google Scholar] [CrossRef]
- Zhang, B.W.; Jiang, K.; Wang, H.T.; Hu, S. Fluoride-induced Dynamic Surface Self-reconstruction Produces Unexpectedly Efficient Oxygen-evolution Catalyst. Nano Lett. 2019, 19, 530–537. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, R.; Qiu, Y.S.; Zheng, L.R.; Hu, Z.B.; Liu, X.F. Tuning Co2+ Coordination in Cobalt Layered Double Hydroxide Nanosheets via Fe3+ Doping for Efficient Oxygen Evolution. Inorg. Chem. 2021, 60, 5252–5263. [Google Scholar] [CrossRef]
- Jeghan, S.M.N.; Kim, J.; Lee, G. Hierarchically Designed CoMo Marigold Flower-like 3D Nano-heterostructure as an Efficient Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Appl. Surf. Sci. 2021, 546, 149072. [Google Scholar] [CrossRef]
- Miyata, S. Anion Exchange Properties of Hydrotalcite-like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Gray, H.B.; Müller, A.M. Effect of Interlayer Anions on [NiFe]-LDH Nanosheet Water Oxidation Activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Cai, Z.; Wang, C.; Bi, Y.M.; Qian, L.; Hao, Y.C.; Li, L.; Kuang, Y.; Li, Y.P.; Lei, X.D.; et al. Phosphorus Oxoanion-intercalated Layered Double Hydroxides for High-performance Oxygen Evolution. Nano Res. 2017, 10, 1732–1739. [Google Scholar] [CrossRef]
- Zhou, D.J.; Cai, Z.; Bi, Y.M.; Tian, W.L.; Luo, M.; Zhang, Q.; Zhang, Q.; Xie, Q.X.; Wang, J.D.; Li, Y.P.; et al. Effects of Redox-active Interlayer Anions on the Oxygen Evolution Reactivity of NiFe-Layered Double Hydroxide Nanosheets. Nano Res. 2018, 11, 1358–1368. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Hao, Y.C.; Zhang, G.X.; Lu, Z.Y.; Han, S.; Li, Y.P.; Sun, X.M. Room-temperature Synthetic NiFe Layered Double Hydroxide with Different Anions Intercalation as an Excellent Oxygen Evolution Catalyst. RSC Adv. 2015, 5, 55131–55135. [Google Scholar] [CrossRef]
- Li, X.M.; Hao, X.G.; Wang, Z.D.; Abudula, A.; Guan, G.Q. In-situ Intercalation of NiFe LDH Materials: An Efficient Approach to Improve Electrocatalytic Activity and Stability for Water Splitting. J. Power Sources 2017, 347, 193–200. [Google Scholar] [CrossRef]
- Dong, Y.; Komarneni, S.; Zhang, F.; Wang, N.; Terrones, M.; Hu, W.C.; Huang, W.Y. “Structural instability” Induced High-performance NiFe Layered Double Hydroxides as Oxygen Evolution Reaction Catalysts for pH-near-neutral Borate Electrolyte: The Role of Intercalates. Appl. Catal. B Environ. 2020, 263, 118343. [Google Scholar] [CrossRef]
- Yang, M.Q.; Wang, J.; Wu, H.; Ho, G.W. Noble Metal-free Nanocatalysts with Vacancies for Electrochemical Water Splitting. Small 2018, 14, 1703323. [Google Scholar] [CrossRef]
- Liu, Y.W.; Cheng, H.; Lyu, M.J.; Fan, S.J.; Liu, Q.H.; Zhang, W.S.; Zhi, Y.D.; Wang, C.M.; Xiao, C.; Wei, S.Q.; et al. Low Overpotential in Vacancy-rich Ultrathin CoSe2 Nanosheets for Water Oxidation. J. Am. Chem. Soc. 2014, 136, 15670–15675. [Google Scholar] [CrossRef]
- Yan, G.B.; Lian, Y.B.; Gu, Y.D.; Yang, C.; Sun, H.; Mu, Q.Q.; Li, Q.; Zhu, W.; Zheng, X.S.; Chen, M.Z.; et al. Phase and Morphology Transformation of MnO2 Induced by Ionic Liquids toward Efficient Water Oxidation. ACS Catal. 2018, 8, 10137–10147. [Google Scholar] [CrossRef]
- Zhou, D.J.; Xiong, X.Y.; Cai, Z.; Han, N.N.; Jia, Y.; Xie, Q.X.; Duan, X.X.; Xie, T.H.; Zheng, X.L.; Sun, X.M.; et al. Flame-engraved Nickel-Iron Layered Double Hydroxide Nanosheets for Boosting Oxygen Evolution Reactivity. Small Methods 2018, 2, 1800083. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D.F.; Gu, Q.F.; Zhu, S.H.; Wang, L.; Peng, H.G.; Zhao, Y. Implanting Cation Vacancies in Ni-Fe LDHs for Efficient Oxygen Evolution Reactions of Lithium-oxygen Batteries. Appl. Catal. B Environ. 2021, 285, 119792. [Google Scholar] [CrossRef]
- Annenkov, M.; Blank, V.; Kulnitskiy, B.; Larionov, K.; Ovsyannikov, D.; Perezhogin, I.; Popov, M.; Sorokin, P. Boron Carbide Nanoparticles for High-hardness Ceramics: Crystal Lattice Defects after Treatment in a Planetary Ball Mill. J. Eur. Ceram. Soc. 2017, 37, 1349–1353. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.Q.; Xi, C.; Cheng, C.Q.; Zou, C.Q.; Zhang, R.; Xie, Y.M.; Guo, Z.L.; Tang, C.C.; Dong, C.K.; et al. A Hydrogen-deficient Nickel-Cobalt Double Hydroxide for Photocatalytic Overall Water Splitting. Angew. Chem. Int. Ed. Engl. 2020, 59, 11510–11515. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Cai, Z.; Zhou, D.J.; Zhang, G.X.; Zhang, Q.; Jia, Y.; Duan, X.X.; Xie, Q.X.; Lai, S.B.; Xie, T.H.; et al. A Highly-efficient Oxygen Evolution Electrode Based on Defective Nickel-Iron Layered Double Hydroxide. Sci. China Mater. 2018, 61, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.S.; Wang, Z.; Ju, M.; Long, X.; Yang, S.H. Dispersing Transition Metal Vacancies in Layered Double Hydroxides by Ionic Reductive Complexation Extraction for Efficient Water Oxidation. Chem. Sci. 2019, 10, 8354–8359. [Google Scholar] [CrossRef] [Green Version]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.F.; Zhao, Y.X.; Shi, R.; Waterhouse, G.I.N.; Zhang, T.R. A Simple Synthetic Strategy toward Defect-rich Porous Monolayer NiFe-Layered Double Hydroxide Nanosheets for Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2019, 9, 1900881. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.Y.; Xie, C.; Chen, C.; Liu, H.W.; Chen, R.; Huo, J.; Wang, S.Y. Acid-etched Layered Double Hydroxides with Rich Defects for Enhancing the Oxygen Evolution Reaction. Chem. Commun. 2017, 53, 11778–11781. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Hu, X. Exfoliation of Layered Double Hydroxides for Enhanced Oxygen Evolution Catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1420–1439. [Google Scholar] [CrossRef] [Green Version]
- Adachi-Pagano, M.; Forano, C.; Besse, J.P. Delamination of Layered Double Hydroxides by use of Surfactants. Chem. Commun. 2000, 91–92. [Google Scholar] [CrossRef]
- Jia, X.D.; Zhang, X.; Zhao, J.Q.; Zhao, Y.F.; Zhao, Y.X.; Waterhouse, G.I.N.; Shi, R.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Ultrafine Monolayer Co-containing Layered Double Hydroxide Nanosheets for Water Oxidation. J. Energy Chem. 2019, 34, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Zhang, Z.; Kim, S.; Lee, S.; Lee, J.; Kim, W.; Yong, K. Ostwald Ripening Driven Exfoliation to Ultrathin Layered Double Hydroxides Nanosheets for Enhanced Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 44518–44526. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, Y.Q.; Liu, Z.J.; Xie, C.; Feng, S.; Liu, D.D.; Shao, M.F.; Wang, S.Y. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. Engl. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Liu, Z.J.; Dong, C.L.; Huang, Y.C.; Cen, J.J.; Yang, H.T.; Chen, X.B.; Tong, X.; Su, D.; Wang, Y.Y.; Wang, S.Y. Modulating the Electronic Structure of Ultrathin Layered Double Hydroxide Nanosheets with Fluorine: An Efficient Electrocatalyst for the Oxygen Evolution Reaction. J. Mater. Chem. A 2019, 7, 14483–14488. [Google Scholar] [CrossRef]
- Hao, J.H.; Yang, W.S.; Peng, Z.; Zhang, C.; Huang, Z.P.; Shi, W.D. A Nitrogen Doping Method for CoS2 Electrocatalysts with Enhanced Water Oxidation Performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D.P. Fast Formation of Single-unit-cell-thick and Defect-rich Layered Double Hydroxide Nanosheets with Highly Enhanced Oxygen Evolution Reaction for Water Splitting. Nano Res. 2018, 11, 1883–1894. [Google Scholar] [CrossRef]
- Zhou, P.; He, J.Y.; Zou, Y.Q.; Wang, Y.Y.; Xie, C.; Chen, R.; Zang, S.Q.; Wang, S.Y. Single-crystalline Layered Double Hydroxides with Rich Defects and Hierarchical Structure by Mild Reduction for Enhancing the Oxygen Evolution Reaction. Sci. China Chem. 2019, 62, 1365–1370. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, X.; Jia, X.D.; Waterhouse, G.I.N.; Shi, R.; Zhang, X.R.; Zhan, F.; Tao, Y.; Wu, L.Z.; Tung, C.H.; et al. Sub-3 nm Ultrafine Monolayer Layered Double Hydroxide Nanosheets for Electrochemical Water Oxidation. Adv. Energy Mater. 2018, 8, 1703585. [Google Scholar] [CrossRef]
- Kuai, C.G.; Zhang, Y.; Wu, D.Y.; Sokaras, D.; Mu, L.Q.; Spence, S.; Nordlund, D.; Lin, F.; Du, X.W. Fully Oxidized Ni-Fe Layered Double Hydroxide with 100% Exposed Active Sites for Catalyzing Oxygen Evolution Reaction. ACS Catal. 2019, 9, 6027–6032. [Google Scholar] [CrossRef]
- Zhong, H.H.; Liu, T.Y.; Zhang, S.W.; Li, D.Q.; Tang, P.G.; Alonso-Vante, N.; Feng, Y.J. Template-free Synthesis of Three-dimensional NiFe-LDH Hollow Microsphere with Enhanced OER Performance in Alkaline Media. J. Energy Chem. 2019, 33, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shao, M.F.; Zhou, L.; Li, Z.H.; Xiao, K.M.; Wei, M. Hierarchical NiFe Layered Double Hydroxide Hollow Microspheres with Highly-efficient Behavior toward Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 33697–33703. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W. Hierarchical Hollow Nanoprisms Based on Ultrathin Ni-Fe Layered Double Hydroxide Nanosheets with Enhanced Electrocatalytic Activity towards Oxygen Evolution. Angew. Chem. Int. Ed. Engl. 2018, 57, 172–176. [Google Scholar] [CrossRef]
- Xu, H.J.; Shan, C.F.; Wu, X.X.; Sun, M.Z.; Huang, B.L.; Tang, Y.; Yan, C.H. Fabrication of Layered Double Hydroxide Microcapsules Mediated by Cerium Doping in Metal-Organic Frameworks for Boosting Water Splitting. Energy Environ. Sci. 2020, 13, 2949–2956. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Chen, J.J.; Wang, W.J.; Liao, J.W.; Dong, J.C.; Chee, M.O.L.; Wang, N.; Dong, P.; Ajayan, P.M.; Gao, S.P.; et al. Etching-doping Sedimentation Equilibrium Strategy: Accelerating Kinetics on Hollow Rh-doped CoFe-Layered Double Hydroxides for Water Splitting. Adv. Funct. Mater. 2020, 30, 2003556. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Zhu, X.L.; Li, B.Q.; Zhang, Q. Advances in Hybrid Electrocatalysts for Oxygen Evolution Reactions: Rational Integration of NiFe Layered Double Hydroxides and Nanocarbon. Part. Part. Syst. Charact. 2016, 33, 473–486. [Google Scholar] [CrossRef]
- Guo, X.L.; Zheng, X.Q.; Hu, X.L.; Zhao, Q.N.; Li, L.; Yu, P.; Jing, C.; Zhang, Y.X.; Huang, G.S.; Jiang, B.; et al. Electrostatic Adsorbing Graphene Quantum Dot into Nickel-based Layered Double Hydroxides: Electron Absorption/donor Effects Enhanced Oxygen Electrocatalytic Activity. Nano Energy 2021, 84, 105932. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Li, Z.Z.; Wang, J.H.; Zhu, L.; Yin, C.; Wang, Y.; Li, X.B.; Liu, Z.F.; Zhang, J.; et al. Superhydrophilic Graphdiyne Accelerates Interfacial Mass/Electron Transportation to Boost Electrocatalytic and Photoelectrocatalytic Water Oxidation Activity. Adv. Funct. Mater. 2019, 29, 1808079. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liu, J.Y.; Xi, L.F.; Yu, Y.F.; Chen, N.; Sun, S.H.; Wang, W.C.; Lange, K.M.; Zhang, B. Single-atom Au/NiFe Layered Double Hydroxide Electrocatalyst: Probing the Origin of Activity for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.Q.; Sun, J.Y.; Qin, F.; Yu, F.; Bao, J.M.; Yu, Y.; Chen, S.; Ren, Z.F. Cu Nanowires Shelled with NiFe Layered Double Hydroxide Nanosheets as Bifunctional Electrocatalysts for Overall Water Splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Wan, L.; Zhao, Z.H.; Chen, X.X.; Liu, P.F.; Wang, P.C.; Xu, Z.; Lin, Y.Q.; Wang, B.G. Controlled Synthesis of Bifunctional NiCo2O4@FeNi LDH Core-shell Nanoarray Air Electrodes for Rechargeable Zinc-air Batteries. ACS Sustain. Chem. Eng. 2020, 8, 11079–11087. [Google Scholar] [CrossRef]
- He, K.; Tadesse Tsega, T.; Liu, X.; Zai, J.T.; Li, X.H.; Liu, X.J.; Li, W.H.; Ali, N.; Qian, X.F. Utilizing the Space-charge Region of the FeNi-LDH/CoP p-n Junction to Promote Performance in Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2019, 58, 11903–11909. [Google Scholar] [CrossRef]
- Gao, Z.W.; Liu, J.Y.; Chen, X.M.; Zheng, X.L.; Mao, J.; Liu, H.; Ma, T.; Li, L.; Wang, W.C.; Du, X.W. Engineering NiO/NiFe LDH Intersection to Bypass Scaling Relationship for Oxygen Evolution Reaction via Dynamic Tridimensional Adsorption of Intermediates. Adv. Mater. 2019, 31, 1804769. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.S.; Zhang, B.; Ruan, Y.J.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J.J. Hierarchical NiCo2S4@NiFe LDH Heterostructures Supported on Nickel Foam for Enhanced Overall-Water-Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 15364–15372. [Google Scholar] [CrossRef]
- Yu, M.Z.; Wang, Z.Y.; Liu, J.S.; Sun, F.; Yang, P.J.; Qiu, J.S. A Hierarchically Porous and Hydrophilic 3D Nickel-Iron/MXene Electrode for Accelerating Oxygen and Hydrogen Evolution at High Current Densities. Nano Energy 2019, 63, 103880. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.N.; Shearer, M.J.; Sheng, H.Y.; Li, W.J.; Chen, J.; Xiao, P.; Zhang, Y.H.; Hamers, R.J.; Jin, S. Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Li, R.S.; Wang, Y.H.; Li, W.; Zhou, S.Y.; Tian, P.F.; Gao, H.W.; Liu, X.Y.; Zang, J.B. Ternary NiFeZr Layered Double Hydroxides: A Highly Efficient Catalyst for the Oxygen Evolution Reaction. Chem. Commun. 2019, 55, 13370–13373. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, Q.; Han, C.; Lu, Q.Q.; Xing, Z.C.; Yang, X.R. Atomic and Electronic Modulation of Self-supported Nickel-Vanadium Layered Double Hydroxide to Accelerate Water Splitting Kinetics. Nat. Commun. 2019, 10, 3899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Xi, C.; Jin, Y.Z.; Wu, D.Y.; Wang, J.Q.; Liu, T.; Wang, H.B.; Dong, C.K.; Liu, H.; Kulinich, S.A.; et al. Ir-O-V Catalytic Group in Ir-doped NiV(OH)2 for Overall Water Splitting. ACS Energy Lett. 2019, 4, 1823–1829. [Google Scholar] [CrossRef]
- Hao, S.Y.; Zheng, G.K.; Gao, S.J.; Qiu, L.S.; Xu, N.; He, Y.; Lei, L.C.; Zhang, X.W. In Situ Synthesis of Ternary NiCoRu-based Layered Double Hydroxide by Chlorine Corrosion toward Electrocatalytic Water Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 14361–14367. [Google Scholar] [CrossRef]
- Hu, Y.M.; Wang, Z.L.; Liu, W.J.; Xu, L.; Guan, M.L.; Huang, Y.P.; Zhao, Y.; Bao, J.; Li, H.M. Novel Cobalt-Iron-Vanadium Layered Double Hydroxide Nanosheet Arrays for Superior Water Oxidation Performance. ACS Sustain. Chem. Eng. 2019, 7, 16828–16834. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.B.; Chen, X.J.; Zhao, C. High Valence Chromium Regulated Cobalt-Iron-hydroxide for Enhanced Water Oxidation. J. Power Sources 2018, 402, 381–387. [Google Scholar] [CrossRef]
- Pi, Y.C.; Shao, Q.; Wang, P.T.; Lv, F.; Guo, S.J.; Guo, J.; Huang, X.Q. Trimetallic Oxyhydroxide Coralloids for Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2017, 56, 4502–4506. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.L.; Xie, J.F.; Xu, L.; Lei, F.C.; Guan, M.L.; Zhao, Y.; Huang, Y.P.; Li, H.M. A Ternary Cobalt-Molybdenum-Vanadium Layered Double Hydroxide Nanosheet Array as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Chem. Commun. 2019, 55, 3521–3524. [Google Scholar] [CrossRef] [PubMed]
- Nejati, K.; Akbari, A.R.; Davari, S.; Asadpour-Zeynali, K.; Rezvani, Z. Zn-Fe-Layered Double Hydroxide Intercalated with Vanadate and Molybdate Anions for Electrocatalytic Water Oxidation. New J. Chem. 2018, 42, 2889–2895. [Google Scholar] [CrossRef]
- Xue, X.Y.; Yu, F.; Peng, B.H.; Wang, G.; Lv, Y.; Chen, L.; Yao, Y.B.; Dai, B.; Shi, Y.L.; Guo, X.H. One-step Synthesis of Nickel-Iron Layered Double Hydroxides with Tungstate Acid Anions via Flash Nano-precipitation for the Oxygen Evolution Reaction. Sustain. Energy Fuels 2019, 3, 237–244. [Google Scholar] [CrossRef]
- Xue, X.Y.; Yu, F.; Li, J.G.; Bai, G.; Yuan, H.F.; Hou, J.; Peng, B.H.; Chen, L.; Yuen, M.F.; Wang, G.; et al. Polyoxometalate Intercalated NiFe Layered Double Hydroxides for Advanced Water Oxidation. Int. J. Hydrogen Energy 2020, 45, 1802–1809. [Google Scholar] [CrossRef]
- Su, L.; Du, H.F.; Tang, C.; Nan, K.K.; Wu, J.G.; Li, C.M. Borate-ion Intercalated NiFe Layered Double Hydroxide to Simultaneously Boost Mass Transport and Charge Transfer for Catalysis of Water Oxidation. J. Colloid Interface Sci. 2018, 528, 36–44. [Google Scholar] [CrossRef]
- Xie, Q.X.; Cai, Z.; Li, P.S.; Zhou, D.J.; Bi, Y.M.; Xiong, X.Y.; Hu, E.Y.; Li, Y.P.; Kuang, Y.; Sun, X.M. Layered Double Hydroxides with Atomic-scale Defects for Superior Electrocatalysis. Nano Res. 2018, 11, 4524–4534. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xie, C.; Zhang, Z.Y.; Liu, D.D.; Chen, R.; Wang, S.Y. In Situ Exfoliated, N-doped, and Edge-rich Ultrathin Layered Double Hydroxides Nanosheets for Oxygen Evolution Reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- Long, X.; Li, J.K.; Xiao, S.; Yan, K.Y.; Wang, Z.L.; Chen, H.N.; Yang, S.H. A Strongly Coupled Graphene and FeNi Double Hydroxide Hybrid as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2014, 53, 7584–7588. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.Z.; Gao, G.P.; Chen, H.; Wang, B.; Zhou, J.Z.; Soo, M.T.; Hong, M.; Yan, X.C.; Qian, G.R.; et al. A Heterostructure Coupling of Exfoliated Ni-Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Wang, B.K.; Shan, C.F.; Xi, P.X.; Liu, W.S.; Tang, Y. Ce-doped NiFe-Layered Double Hydroxide Ultrathin Nanosheets/Nanocarbon Hierarchical Nanocomposite as an Efficient Oxygen Evolution Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 6336–6345. [Google Scholar] [CrossRef]
- Zhang, X.; Marianov, A.N.; Jiang, Y.J.; Cazorla, C.; Chu, D.W. Hierarchically Constructed Silver Nanowire@Nickel-Iron Layered Double Hydroxide Nanostructures for Electrocatalytic Water Splitting. ACS Appl. Nano Mater. 2019, 3, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Li, F.; Chen, W.L.; Ma, Y.L.; Wu, Y.; Gu, X.; Qin, Y.; Tao, P.; Song, C.Y.; Shang, W.; et al. In Situ Vertical Growth of Fe-Ni Layered Double-Hydroxide Arrays on Fe-Ni Alloy Foil: Interfacial Layer Enhanced Electrocatalyst with Small Overpotential for Oxygen Evolution Reaction. ACS Energy Lett. 2018, 3, 2357–2365. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.L.; Jiang, S.S.; Li, M.; Zhang, K.; Yan, Y.; Zhang, H.P.; Xue, J.M. Increasing Gas Bubble Escape Rate for Water Splitting with Nonwoven Stainless Steel Fabrics. ACS Appl. Mater. Interfaces 2017, 9, 40281–40289. [Google Scholar] [CrossRef]
- Chen, J.D.; Zheng, F.; Zhang, S.J.; Fisher, A.; Zhou, Y.; Wang, Z.y.; Li, Y.y.; Xu, B.B.; Li, J.T.; Sun, S.G. Interfacial Interaction between FeOOH and Ni-Fe LDH to Modulate the Local Electronic Structure for Enhanced OER Electrocatalysis. ACS Catal. 2018, 8, 11342–11351. [Google Scholar] [CrossRef]
- Yang, L.T.; Chen, L.; Yang, D.W.; Yu, X.; Xue, H.G.; Feng, L.G. NiMn Layered Double Hydroxide Nanosheets/NiCo2O4 Nanowires with Surface Rich High Valence State Metal Oxide as an Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Power Sources 2018, 392, 23–32. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, P.; Wang, Z.Y.; Wang, P.; Liu, Y.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y.; Zheng, Z.K.; Huang, B.B. Electrodeposition of NiFe Layered Double Hydroxide on Ni3S2 Nanosheets for Efficient Electrocatalytic Water Oxidation. Int. J. Hydrogen Energy 2020, 45, 8659–8666. [Google Scholar] [CrossRef]
- Zou, Y.J.; Xiao, B.; Shi, J.W.; Hao, H.; Ma, D.D.; Lv, Y.X.; Sun, G.T.; Li, J.; Cheng, Y.H. 3D Hierarchical Heterostructure Assembled by NiFe LDH/(NiFe)Sx on Biomass-derived Hollow Carbon Microtubes as Bifunctional Electrocatalysts for Overall Water Splitting. Electrochim. Acta 2020, 348, 136339. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.Q.; Sun, J.Y.; Mishra, I.K.; Luo, D.; Yu, F.; Yu, Y.; Chen, S.; Ren, Z.F. Amorphous NiFe layered double hydroxide nanosheets decorated on 3D nickel phosphide nanoarrays: A hierarchical core-shell electrocatalyst for efficient oxygen evolution. J. Mater. Chem. A 2018, 6, 13619–13623. [Google Scholar] [CrossRef]
- Yu, M.Z.; Zhou, S.; Wang, Z.Y.; Zhao, J.J.; Qiu, J.S. Boosting Electrocatalytic Oxygen Evolution by Synergistically Coupling Layered Double Hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Tian, M.; Jiang, Y.; Tong, H.L.; Xu, Y.M.; Xia, L.X. MXene-supported FeCo-LDHs as Highly Efficient Catalysts for Enhanced Electrocatalytic Oxygen Evolution Reaction. ChemNanoMat 2019, 6, 154–159. [Google Scholar] [CrossRef]
- Chala, S.A.; Tsai, M.C.; Su, W.N.; Ibrahim, K.B.; Thirumalraj, B.; Chan, T.S.; Lee, J.F.; Dai, H.J.; Hwang, B.J. Hierarchical 3D Architectured Ag Nanowires Shelled with NiMn-Layered Double Hydroxide as an Efficient Bifunctional Oxygen Electrocatalyst. ACS Nano 2020, 14, 1770–1782. [Google Scholar] [CrossRef]
- Chala, S.A.; Tsai, M.C.; Su, W.N.; Ibrahim, K.B.; Duma, A.D.; Yeh, M.H.; Wen, C.Y.; Yu, C.H.; Chan, T.S.; Dai, H.J.; et al. Site Activity and Population Engineering of NiRu-Layered Double Hydroxide Nanosheets Decorated with Silver Nanoparticles for Oxygen Evolution and Reduction Reactions. ACS Catal. 2019, 9, 117–129. [Google Scholar] [CrossRef]
- Wang, P.C.; Lin, Y.Q.; Wan, L.; Wang, B.G. Construction of a Janus MnO2-NiFe Electrode via Selective Electrodeposition Strategy as a High-performance Bifunctional Electrocatalyst for Rechargeable Zinc-air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 37701–37707. [Google Scholar] [CrossRef]
- Zhou, D.J.; Cai, Z.; Lei, X.D.; Tian, W.L.; Bi, Y.M.; Jia, Y.; Han, N.N.; Gao, T.F.; Zhang, Q.; Kuang, Y.; et al. NiCoFe-Layered Double Hydroxides/N-doped Graphene Oxide Array Colloid Composite as an Efficient Bifunctional Catalyst for Oxygen Electrocatalytic Reactions. Adv. Energy Mater. 2018, 8, 1701905. [Google Scholar] [CrossRef]
- Sun, F.C.; Zhou, Y.; You, Z.H.; Xia, H.H.; Tuo, Y.X.; Wang, S.T.; Jia, C.P.; Zhang, J. Bi-functional Fe3O4/Au/CoFe-LDH Sandwich-structured Electrocatalyst for Asymmetrical Electrolyzer with Low Operation Voltage. Small 2021. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Zhao, Y.F.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. NiFe Layered Double Hydroxide Nanoparticles on Co,N-codoped Carbon Nanoframes as Efficient Bifunctional Catalysts for Rechargeable Zinc-air Batteries. Adv. Energy Mater. 2017, 7, 1700467. [Google Scholar] [CrossRef]
- Wang, T.T.; Wu, J.H.; Liu, Y.L.; Cui, X.; Ding, P.; Deng, J.; Zha, C.Y.; Coy, E.; Li, Y.G. Scalable Preparation and Stabilization of Atomic-thick CoNi Layered Double Hydroxide Nanosheets for Bifunctional Oxygen Electrocatalysis and Rechargeable Zinc-air Batteries. Energy Storage Mater. 2019, 16, 24–30. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.J.; Zhao, Y.; Song, L.; Zhang, Z.P. FeNi Layered Double Hydroxide Nanosheets on a 3D Carbon Network as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. Part. Part. Syst. Charact. 2016, 33, 158–166. [Google Scholar] [CrossRef]
- Li, Z.S.; Xiao, K.C.; Yu, C.L.; Wang, H.Q.; Li, Q.Y. Three-dimensional Graphene-like Carbon Nanosheets Coupled with MnCo-Layered Double Hydroxides Nanoflowers as Efficient Bifunctional Oxygen Electrocatalyst. Int. J. Hydrogen Energy 2021, 46, 34239–34251. [Google Scholar] [CrossRef]
- Zhan, T.R.; Zhang, Y.M.; Liu, X.L.; Lu, S.S.; Hou, W.G. NiFe Layered Double Hydroxide/Reduced Graphene Oxide Nanohybrid as an Efficient Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions. J. Power Sources 2016, 333, 53–60. [Google Scholar] [CrossRef]
- Zhan, T.R.; Liu, X.L.; Lu, S.S.; Hou, W.G. Nitrogen Doped NiFe Layered Double Hydroxide/Reduced Graphene Oxide Mesoporous Nanosphere as an Effective Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Appl. Catal. B Environ. 2017, 205, 551–558. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Xing, J.Y.; Zhao, Y.Y.; Yang, F.L. Hierarchical N,P Co-doped Graphene Aerogels Framework Assembling Vertically Grown CoMn-LDH Nanosheets as Efficient Bifunctional Electrocatalyst for Rechargeable Zinc-air Battery. J. Colloid Interface Sci. 2021, 590, 476–486. [Google Scholar] [CrossRef]
- Wang, Z.J.; Lu, Y.Z.; Yan, Y.; Larissa, T.Y.P.; Zhang, X.; Wuu, D.; Zhang, H.; Yang, Y.H.; Wang, X. Core-shell Carbon Materials Derived from Metal-Organic Frameworks as an Efficient Oxygen Bifunctional Electrocatalyst. Nano Energy 2016, 30, 368–378. [Google Scholar] [CrossRef]
- Yin, P.Q.; Yao, T.; Wu, Y.E.; Zheng, L.R.; Lin, Y.; Liu, W.; Ju, H.X.; Zhu, J.F.; Hong, X.; Deng, Z.X.; et al. Single Cobalt Atoms with Precise N-coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. Engl. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Shang, L.; Yu, H.J.; Huang, X.; Bian, T.; Shi, R.; Zhao, Y.F.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Well-dispersed ZIF-derived Co,N-co-doped Carbon Nanoframes through Mesoporous-silica-protected Calcination as Efficient Oxygen Reduction Electrocatalysts. Adv. Mater. 2016, 28, 1668–1674. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-lifespan Rechargeable Zinc-air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Wang, J.; Ren, D.; Yu, J.; Chen, X.; Li, B.Q.; Zhang, Q. A ΔE = 0.63 V Bifunctional Oxygen Electrocatalyst Enables High-rate and Long-cycling Zinc-air Batteries. Adv. Mater. 2021, 33, 2008606. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.M.; Xing, Y.Y.; Li, D.; Jiang, D.L.; Chen, M.; Shi, W.D.; Yuan, S.Q. Synergistic Coupling of CoFe-LDH Arrays with NiFe-LDH Nanosheet for Highly Efficient Overall Water Splitting in Alkaline Media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Zhong, H.H.; Tian, R.; Gong, X.M.; Li, D.Q.; Tang, P.G.; Alonso-Vante, N.; Feng, Y.J. Advanced Bifunctional Electrocatalyst Generated through Cobalt Phthalocyanine Tetrasulfonate Intercalated Ni2Fe-Layered Double Hydroxides for a Laminar Flow Unitized Regenerative Micro-cell. J. Power Sources 2017, 361, 21–30. [Google Scholar] [CrossRef]
- Fan, K.; Li, Z.H.; Song, Y.J.; Xie, W.F.; Shao, M.F.; Wei, M. Confinement Synthesis Based on Layered Double Hydroxides: A New Strategy to Construct Single-atom-containing Integrated Electrodes. Adv. Funct. Mater. 2020, 31, 2008064. [Google Scholar] [CrossRef]
- Fu, G.T.; Cui, Z.M.; Chen, Y.F.; Xu, L.; Tang, Y.W.; Goodenough, J.B. Hierarchically Mesoporous Nickel-Iron Nitride as a Cost-efficient and Highly Durable Electrocatalyst for Zn-air Battery. Nano Energy 2017, 39, 77–85. [Google Scholar] [CrossRef]
- Gao, D.Q.; Zhang, J.Y.; Wang, T.T.; Xiao, W.; Tao, K.; Xue, D.S.; Ding, J. Metallic Ni3N Nanosheets with Exposed Active Surface Sites for Efficient Hydrogen Evolution. J. Mater. Chem. A 2016, 4, 17363–17369. [Google Scholar] [CrossRef]
- Sun, Z.M.; Zhang, J.Y.; Xie, J.F.; Zheng, X.J.; Wang, M.; Li, X.M.; Tang, B. High-performance Alkaline Hydrogen Evolution Electrocatalyzed by a Ni3N-CeO2 Nanohybrid. Inorg. Chem. Front. 2018, 5, 3042–3045. [Google Scholar] [CrossRef]
- Wang, B.R.; Jiao, S.H.; Wang, Z.S.; Lu, M.J.; Chen, D.; Kang, Y.T.; Pang, G.S.; Feng, S.H. Rational Design of NiFe LDH@Ni3N Nano/Microsheet Arrays as a Bifunctional Electrocatalyst for Overall Water Splitting. J. Mater. Chem. A 2020, 8, 17202–17211. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. 3D Carbon Nanoframe Scaffold-immobilized Ni3FeN Nanoparticle Electrocatalysts for Rechargeable Zinc-air Batteries’ Cathodes. Nano Energy 2017, 40, 382–389. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Wang, B.; Li, B.Q.; Zhang, Q. Bifunctional Transition Metal Hydroxysulfides: Room-temperature Sulfurization and Their Applications in Zn-air Batteries. Adv. Mater. 2017, 29, 1702327. [Google Scholar] [CrossRef]
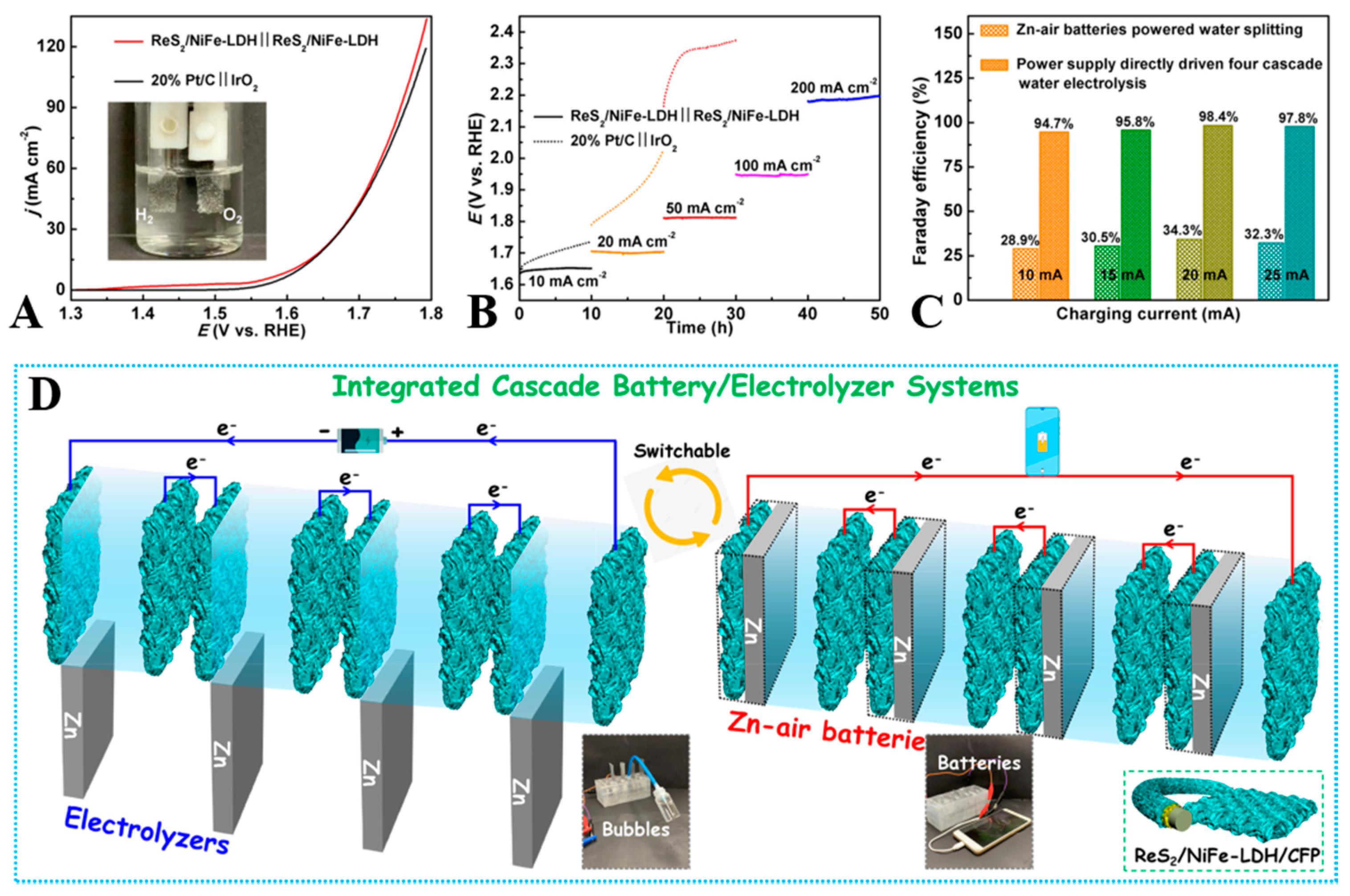
- Han, X.T.; Li, N.N.; Kang, Y.B.; Dou, Q.Y.; Xiong, P.X.; Liu, Q.; Lee, J.Y.; Dai, L.M.; Park, H.S. Unveiling Trifunctional Active Sites of a Heteronanosheet Electrocatalyst for Integrated Cascade Battery/Electrolyzer Systems. ACS Energy Lett. 2021, 6, 2460–2468. [Google Scholar] [CrossRef]
- Qian, L.; Lu, Z.Y.; Xu, T.H.; Wu, X.C.; Tian, Y.; Li, Y.P.; Huo, Z.Y.; Sun, X.M.; Duan, X. Trinary Layered Double Hydroxides as High-performance Bifunctional Materials for Oxygen Electrocatalysis. Adv. Energy Mater. 2015, 5, 1500245. [Google Scholar] [CrossRef]
- Dresp, S.; Luo, F.; Schmack, R.; Kuhl, S.; Gliech, M.; Strasser, P. An Efficient Bifunctional Two-component Catalyst for Oxygen Reduction and Oxygen Evolution in Reversible Fuel Cells, Electrolyzers and Rechargeable Air Electrodes. Energy Environ. Sci. 2016, 9, 2020–2024. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.D.; Gao, S.J.; Liu, T.Y.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Fabrication and Bifunctional Electrocatalytic Performance of Ternary CoNiMn Layered Double Hydroxides/Polypyrrole/Reduced Graphene Oxide Composite for Oxygen Reduction and Evolution Reactions. Electrochim. Acta 2017, 245, 51–60. [Google Scholar] [CrossRef]
- Guo, X.L.; Hu, X.L.; Wu, D.; Jing, C.; Liu, W.; Ren, Z.L.; Zhao, Q.N.; Jiang, X.P.; Xu, C.H.; Zhang, Y.X.; et al. Tuning the Bifunctional Oxygen Electrocatalytic Properties of Core-shell Co3O4@NiFe LDH Catalysts for Zn-air Batteries: Effects of Interfacial Cation Valences. ACS Appl. Mater. Interfaces 2019, 11, 21506–21514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, X.; Xie, Y.; Sun, F.F.; Liang, Z.J.; Wang, L.; Fu, H.G. N-doped Carbon Coating Enhances the Bifunctional Oxygen Reaction Activity of CoFe Nanoparticles for a Highly Stable Zn-air Battery. J. Mater. Chem. A 2020, 8, 21189–21198. [Google Scholar] [CrossRef]
- Li, S.J.; Xie, W.F.; Song, Y.K.; Shao, M.F. Layered Double Hydroxide@Polydopamine Core-shell Nanosheet Arrays-derived Bifunctional Electrocatalyst for Efficient, Flexible, All-solid-state Zinc-air Battery. ACS Sustain. Chem. Eng. 2020, 8, 452–459. [Google Scholar] [CrossRef]







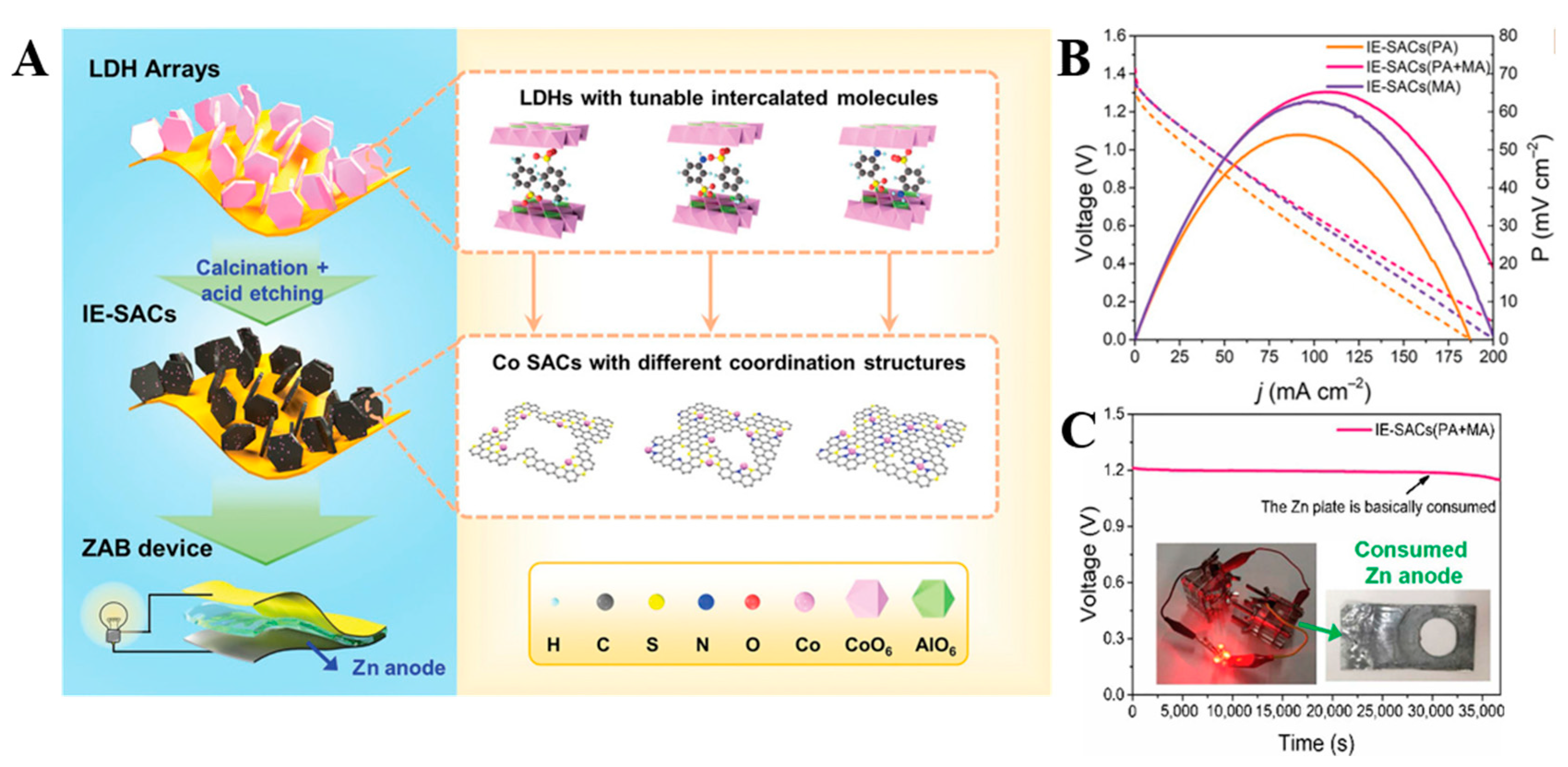
| Catalysts | Modification Method | Overpotential/mV | Tafel Slope/mV dec−1 | Stability | Ref. |
|---|---|---|---|---|---|
| Co2+-doped NiFe-LDHs | Cation-doping | 264@10 mA cm−2 | 58.1 | 10 h | [132] |
| Co3+-doped NiFe-LDHs | - | 249@10 mA cm−2 | 50.5 | 10 h | [132] |
| Ni6Fe2Cr1-LDHs | - | 225@25 mA cm−2 | 69 | At 10 mA cm−2 for 6 h | [187] |
| NiFeZr-LDHs | - | 198@10 mA cm−2 | 53.1 | At 1.47 V for 12 h | [188] |
| Mn2+-doped NiFe-LDHs | - | 190@10 mA cm−2 | 68.6 | At 50 mA cm−2 for 40 h; 750 cycles at 5 mV s−1 | [128] |
| NiFeV-LDHs | - | 195@20 mA cm−2 | 42 | At 1.48 V for 18 h | [134] |
| NiVRu-LDHs | - | 190@10 mA cm−2 | 83 | 2000 cycles; at 50 mA cm−2 for 600 h; at 200 mA cm−2 for 400 h | [189] |
| NiFeMn4+-LDHs | - | 289@20 mA cm−2 | 47 | At η = 310 mV for 15 h; at 10 mA cm−2 for 15 h | [129] |
| NiVIr-LDHs | - | 203@10 mA cm−2 | 55.3 | At 10 mA cm−2 for 15 h | [190] |
| NiCoRu-LDHs | - | 270@100 mA cm−2 | 40 | At 100 mA cm−2 for 55 h | [191] |
| CoFeV-LDHs | - | 242@10 mA cm−2 | 57 | At η = 250 mV for 32 h | [192] |
| CoFeCr-LDHs | - | 270@100 mA cm−2 | 32 | At 1.58 V for 24 h | [193] |
| Cr-CoFe LDHs | - | 238@10 mA cm−2 | 107 | 1000, 3000, and 5000 cycles at 50 mV s−1; at 20 mA cm−2 for 20 h | [135] |
| W0.5Co0.4Fe0.1-LDHs | - | 310@100 mA cm−2 | 32 | 3000 cycles at 5 mV s−1; at 20 mA cm−2 for 50 h | [194] |
| CoMoV-LDHs | - | 270 | 106 | At η = 300 mV for 20 h | [195] |
| ZnFe-VO4-LDHs | Anion- intercalation | 250@10 mA cm−2 | 26 | At 2.4 V for 4000 s; 200 cycles at 200 100 mV s−1 | [196] |
| NiFe-WO4-LDHs | - | 290@10 mA cm−2 | 41.5 | At 10 mA cm−2 for 20 h | [197] |
| Ni2Fe-SDS-LDHs/CFP | - | 289@10 mA cm−2 | 39 | At 50 mV s−1 for 10 h | [60] |
| H2PO2−/NiFe-LDHs | - | 250@10 mA cm−2 | 37.7 | 30,000 s | [143] |
| Formamide intercalated NiFe-LDHs/CR | - | 210@10 mA cm−2 | 39 | At 0.5 V for 18 h | [146] |
| NiFe-LDHs (POM) | - | 287@10 mA cm−2 | 43 | At 50 mA cm−2 for 35 h; at 10 mA cm−2 for 40 h | [198] |
| 0.05-SB-Ni0.8Fe0.2(OH)2 | - | 301@10 mA cm−2 | 42 | 250, 500, 750 and 1000 cycles at 100 mA s−1; at η = 300 mV for 20 h | [199] |
| R-NiFe-LDHs | Defect engineering | 200@10 mA cm−2 | 59.3 | at 1.43 V for 10 h | [155] |
| CoFe-LDHs-Ar | - | 266@10 mA cm−2 | 37.85 | 2000 CV cycles at 5 mV s−1 | [165] |
| PM-LDHs | - | 230@10 mA cm−2 | 47 | At η = 230 mV for 100 h | [158] |
| N-CoFe-LDHs | - | 310@10 mA cm−2 | 74 | 10 h | |
| NiFe-LDHs-VFe | - | 255@10 mA cm−2 | 70 | 2000 cycles at 10 mA cm−2 | [49] |
| NiFe-LDHs-VNi | - | 229@10 mA cm−2 | 62.9 | 2000 CV cycles at 10 mA cm−2 | [49] |
| D-NiFeZn-LDHs | - | 200@20 mA cm−2 | 34.9 | At 30 mA cm−2 for 10 h | [200] |
| ZnCo-UF | ultrathin nanosheets | 360@10 mA cm−2 | 66 | [163] | |
| NiFe-LDHs | - | 254@10 mA cm−2 | 32 | At η = 254 mV for 12 h | [170] |
| E-CoFe-LDHs | - | 302@10 mA cm−2 | 41 | At 10 mA cm−2 for 10 h | [159] |
| DH-CoFe-LDHs | - | 276@10 mA cm−2 | 40.3 | At η = 276 mV for 10 h | [169] |
| N-CoFe–LDHs | - | 233@10 mA cm−2 | 40.03 | 2000 cycles at 5 mV s−1 | [201] |
| Ni3FeAl0.91-LDHs/NF | - | 304@10 mA cm−2 | 50 | 18 h | |
| NiFe-LDHs | - | 300@10 mA cm−2 | 40 | At 10 mA cm−2 for 13 h | [160] |
| FeNi-GO | LDH/carbon composites | 206@10 mA cm−2 | 39 | At 10 mA cm−2 for 8 h | [202] |
| NiFe-LDHs-NS@DG10 | - | 210@10 mA cm−2 | 52 | At 5 mA cm−1 for 10 h; at 10 mA cm−1 for 10 h | [203] |
| NiFe LDHs/GQDs | - | 189@10 mA cm−2 | 52.1 | At 10 mA cm−2 for 15 h | [178] |
| CoFe-LDHs/TEG | - | 301@10 mA cm−2 | 80 | At 1.50 V for 25 h | [179] |
| NiFe-LDHs/CNT | - | 290@10 mA cm−2 | 31 | At 10 and 20 mA cm−2 for 1 h | [116] |
| Ce-NiFe-LDHs/CNT | - | 227@10 mA cm−2 | 33 | At 10 mA cm−2 for 30,000 s | [204] |
| Ag NWs/Ni0.95Fe0.05-LDHs | LDHs/Metal Compounds | 330@10 mA cm−2 | 89 | At 10 mA cm−2 for 12 h | [205] |
| FeNi-LDHs/FeNi foil | - | 130@10 mA cm−2 | 39.8 | At 1.5 V for 10 h | [206] |
| FeNi-LDHs@NWSSF | - | 210@10 mA cm−2 | 56 | At 10 mA cm−2 for 18 h | [207] |
| FeOOH/NiFe-LDHs | - | 174@10 mA cm−2 | 27 | 1000 cycles at 300 mV s−1; at 10 mA cm−2 for 25 h; at 50 mA cm−2 for 15 h | [208] |
| NiO/NiFe-LDHs | - | 205@30 mA cm−2 | 30 | At 20 mA cm−2 for 10 h | [184] |
| NiMn- LDHs/NiCo2O4 | - | 310@10 mA cm−2 | 99 | 1000 cycles at 10 mA cm−2; at 1.65 V for 8 h | [209] |
| Ni3S2/NiFe-LDHs | - | 200@10 mA cm−2 | 29.71 | 1000 cycles at 2 mV s−1; at 10 mA cm−2 for 45,000 s | [210] |
| NiFe-LDHs/(NiFe)Sx/CMT | - | 210@10 mA cm−2 | 31 | 1000 cycles at 10 mA cm−2; at 10 mA cm−2 for 15 h | [211] |
| FeNi-LDHs/CoP | - | 254@350 mA cm−2 | 33.5 | 18.5 h | [183] |
| Ni5P4/NiP2/NiFe-LDHs | - | 197@10 mA cm−2 | 46.6 | At 10, 100, and 200 mA cm−2 for 24 h | [212] |
| FeNi-LDHs/Ti3C2-MXene | - | 240@10 mA cm−2 | 43 | At 10 mA cm−2 for 12 h | [213] |
| FeCo-LDHs/MXene | - | 268@10 mA cm−2 | 85 | At 10 mA cm−2 for 30,000 s | [214] |
| Catalysts | E1/2/V @ORR | Ej=10/V @OER | Specific Capacity/mAh g−1 | Power Density | Open Circuit Potential | Charge/Discharge Voltage Gap | Cycling Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| O-NiCoFe-LDHs | 0.63 | 260 | - | - | - | - | 40 h | [242] |
| Ag NW@NiMn-LDHs | 0.75 | 270 | - | - | - | 0.77 V @ 10 mA cm−2 | - | [215] |
| Ag NP/NiRu-LDHs | 0.8 | 210 | - | - | - | - | 25 h | [216] |
| NiFe-LDHs/NrGO | 0.75 | 250 | - | - | - | - | 9.5 h | [225] |
| CoNi-NS/rGO | 0.85 | 330 | 746 | 300 mW cm−2 | 1.37 V | 0.8 V @ 10 mA cm−2 | 6 h | [221] |
| NiFe-LDHs/Fe-N-C | 0.8 | 300 | - | - | - | - | 24 h | [243] |
| CoNiMn-LDHs/PPy/RGO | 0.77 | 369 | - | - | - | - | 5 h | [244] |
| NiCoIIIFe-LDHs/N-GO | 0.82 | 320 | - | - | - | - | - | [218] |
| LDHs-POF | 0.8 | 250 | 807 | 185 mW cm−2 | - | 0.74 V @ 5 mA cm−2 | 2400 cycles | [230] |
| NiFe-LDHs/Co,N-CNF | 0.79 | 312 | - | - | - | 1 V @ 25 mA cm−2 | 80 h | [220] |
| CoNC@LDHs | 0.84 | 240 | 743 | 173 mW cm−2 | 1.22 V | 0.67 V @ 10 mA cm−2 | 3600 cycles | [231] |
| Co3O4@NiFe-LDHs | - | 226 | 667.5 | 798 Wh kg−1 | 1.38 V | 0.8 V @ 15 mA cm−2 | 2000 h | [245] |
| MnO2-NiFe/Ni | 0.81 | 226 | - | 94 mW cm−2 | - | 0.98 V @ 50 mA cm−2 | 25 h | [217] |
| CoFe@NC/CC | 0.75 | 254 | - | 154 mW cm−2 | 1.469 V | 0.77 V @ 10 mA cm−2 | 42 h | [246] |
| IE-SAC(PA+MA) | 0.851 | 349 | 690.3 | 963 Wh kg−1 | 1.38 V | 0.57 V @ 1 mA cm−2 | 12 h | [234] |
| Ni3FeN | 0.78 | 355 | - | - | 1.547 V | 0.7 V @ 10 mA cm−2 | 100 h | [235] |
| Ni3FeN/Co,N-CNF | 0.81 | 270 | - | 200 mW cm−2 | 1.43 V | 0.79 V @ 6 mA cm−2 | 540 h | [239] |
| Co3FeS1.5(OH)6 | 0.721 | 358 | 898 | 113 mW cm−2 | - | 0.86 V @ 20 mA cm−2 | 36 h | [240] |
| Co3O4-doped Co/CoFe | 0.82 | 370 | 757 | 942 Wh kg−1 | 1.43 V | 0.65 V @ 5 mA cm−2 | 65 h | [90] |
| Co-CoOx/N-C | 0.75 | 278 | - | 21 mW cm−2 | 1.32 V | - | 11 h | [247] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Kong, H.; Zhong, H.; Jiang, Y.; Guo, F.; Alonso-Vante, N.; Feng, Y. Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions. Catalysts 2021, 11, 1394. https://doi.org/10.3390/catal11111394
Wang J, Kong H, Zhong H, Jiang Y, Guo F, Alonso-Vante N, Feng Y. Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions. Catalysts. 2021; 11(11):1394. https://doi.org/10.3390/catal11111394
Chicago/Turabian StyleWang, Jing, Heng Kong, Haihong Zhong, Yu Jiang, Fei Guo, Nicolas Alonso-Vante, and Yongjun Feng. 2021. "Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions" Catalysts 11, no. 11: 1394. https://doi.org/10.3390/catal11111394
APA StyleWang, J., Kong, H., Zhong, H., Jiang, Y., Guo, F., Alonso-Vante, N., & Feng, Y. (2021). Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions. Catalysts, 11(11), 1394. https://doi.org/10.3390/catal11111394








