Effect of Adding Chelating Ligands on the Catalytic Performance of Rh-Promoted MoS2 in the Hydrodesulfurization of Dibenzothiophene
Abstract
1. Introduction
2. Results and Discussion
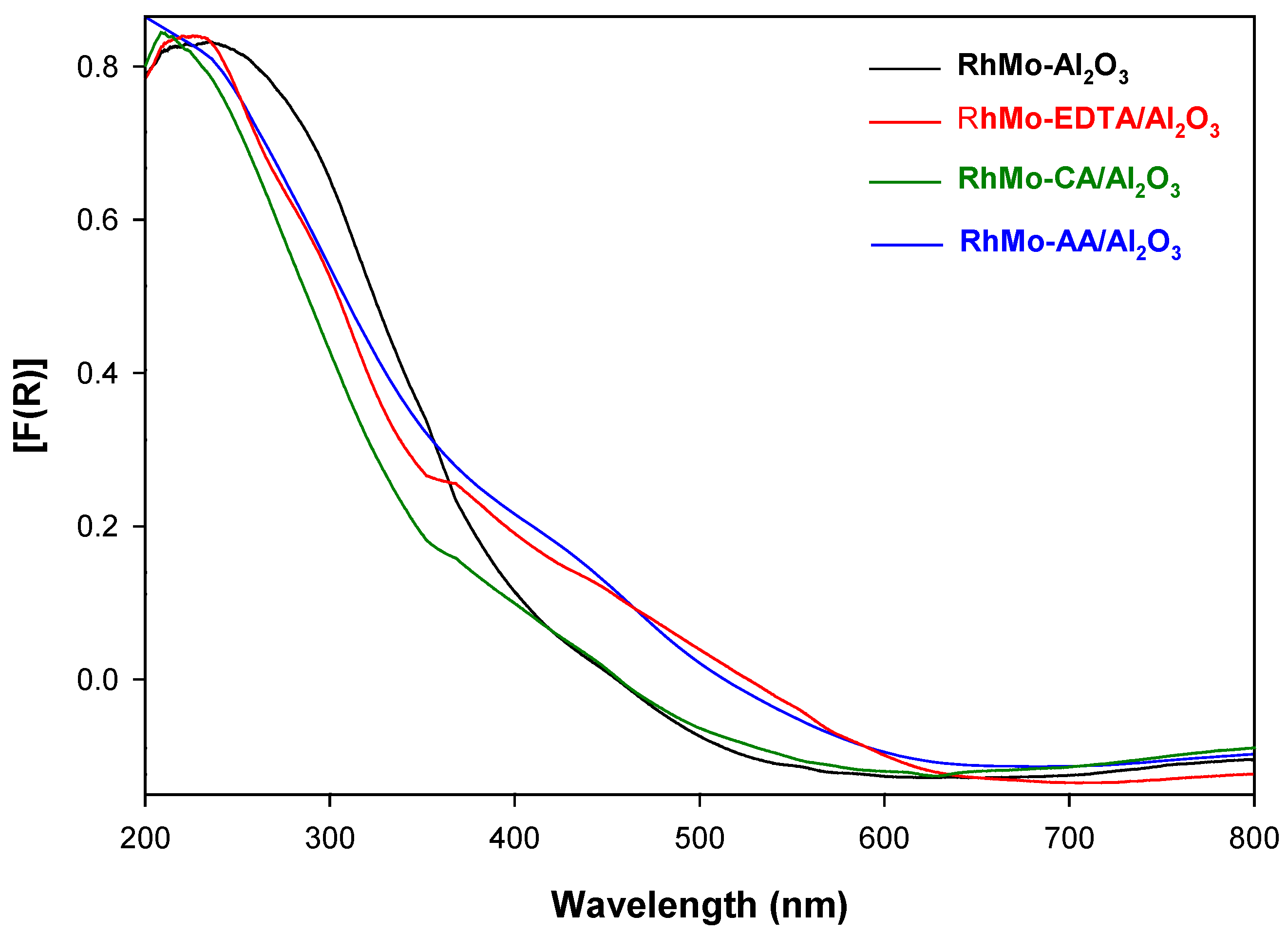
2.1. UV-Vis Spectroscopy
2.2. Band Gaps of RhMo Catalysts
2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Energy Dispersion Spectroscopy (EDX)
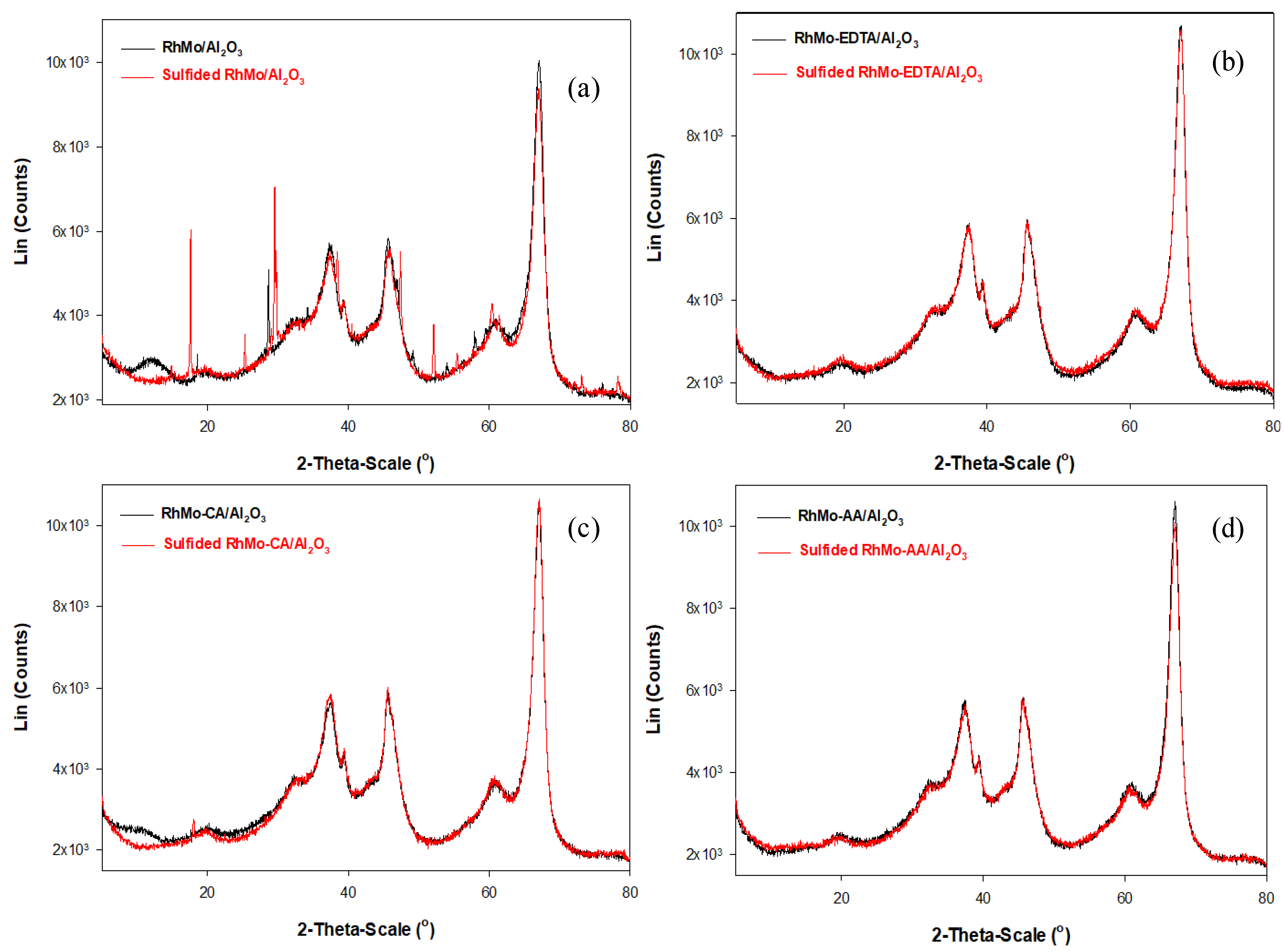
2.5. X-ray Diffraction (XRD)
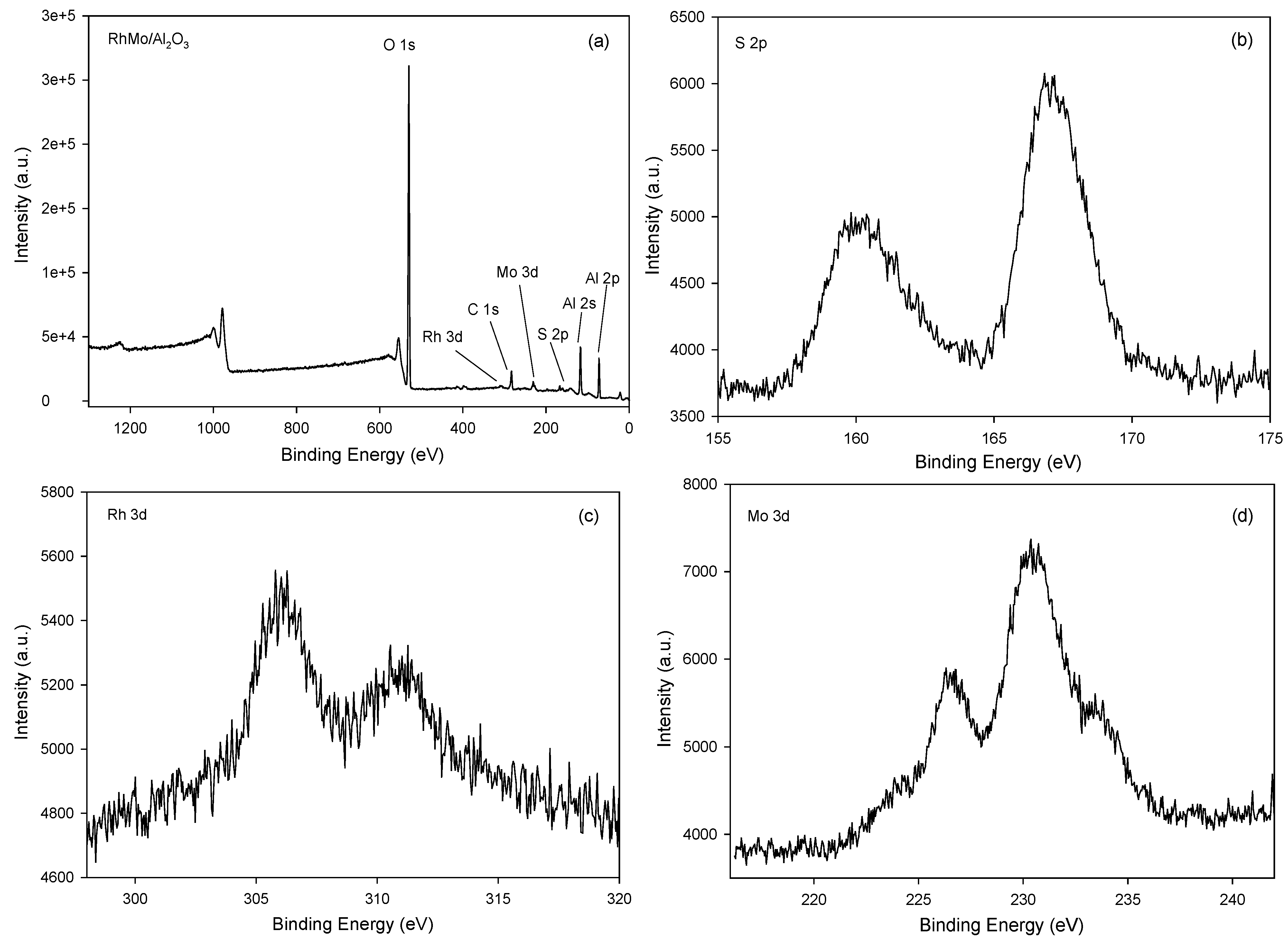
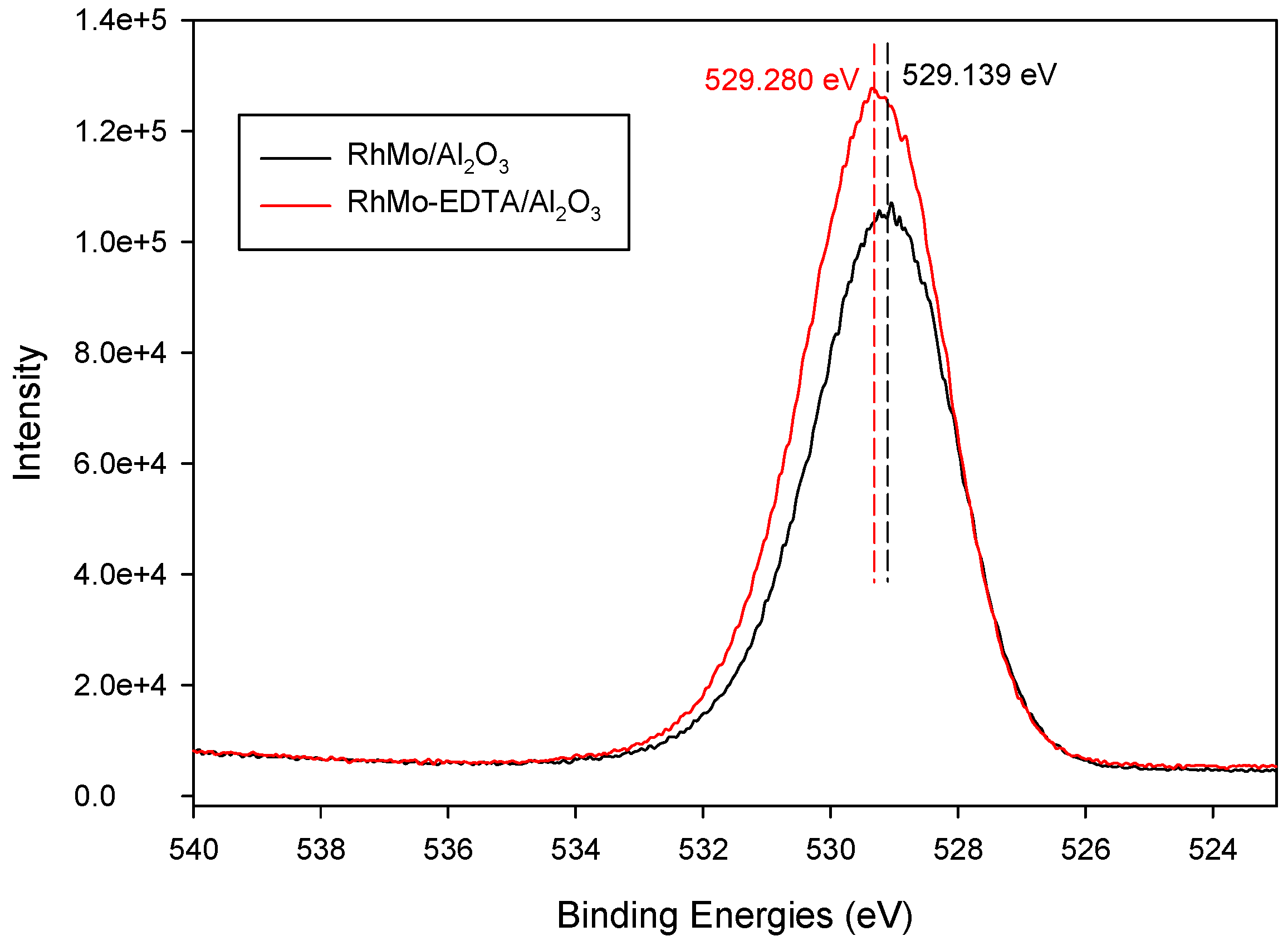
2.6. XPS Analysis
2.7. Transmission Electron Microscopy (TEM)
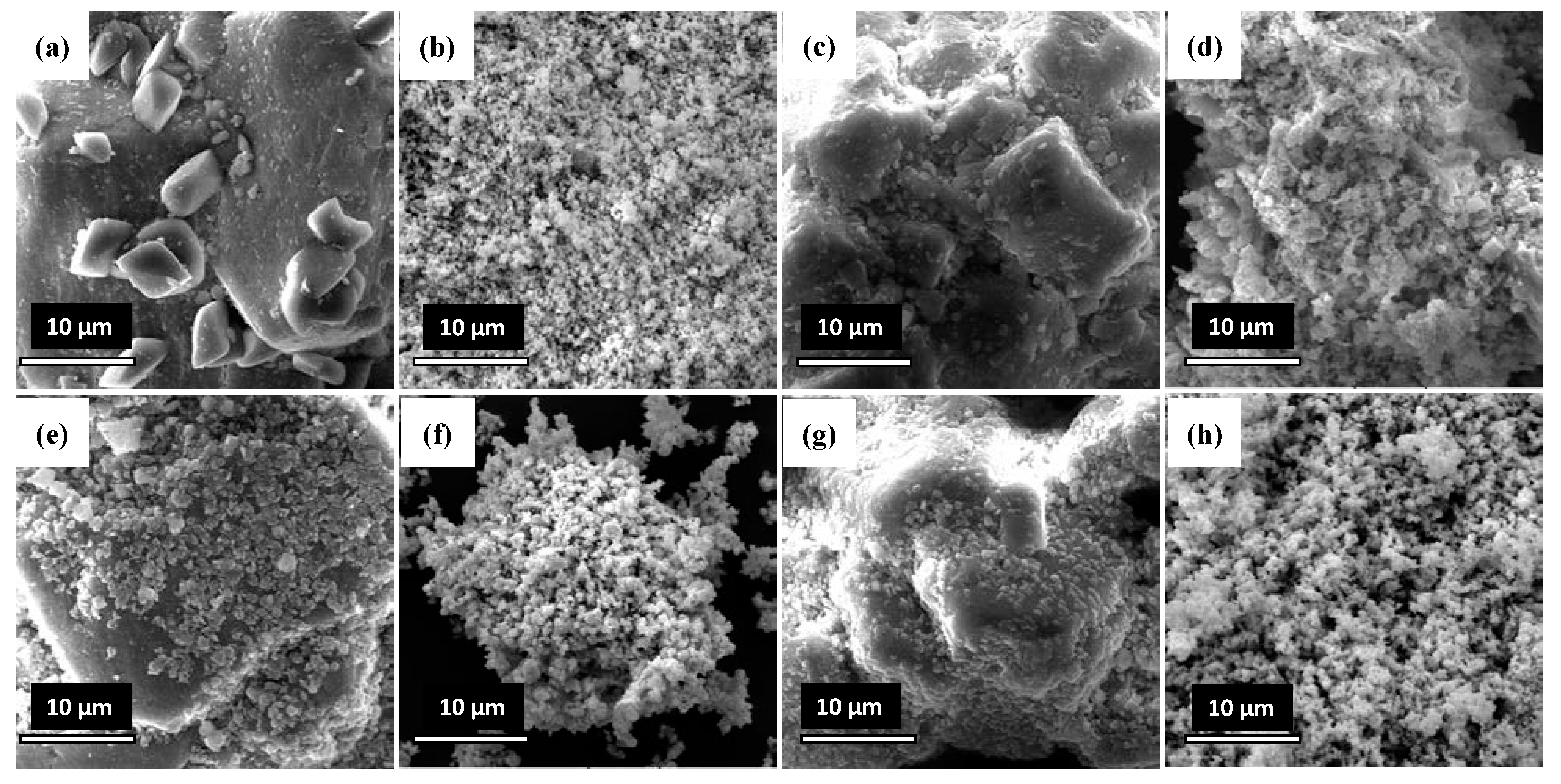
2.8. Scanning Electron Microscopy (SEM)
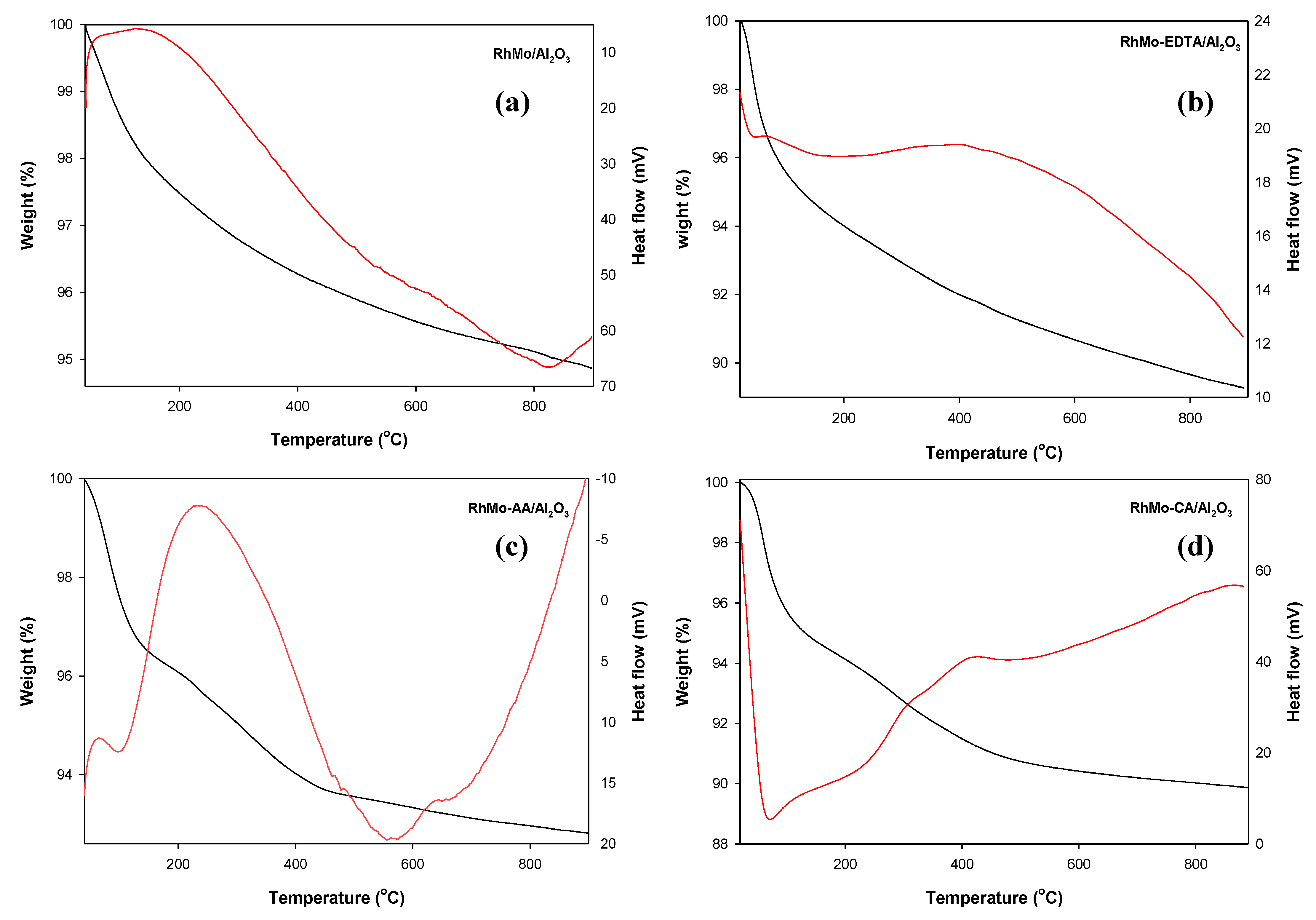
2.9. Stability of Catalysts—TGA and DSC Thermal Analyses
2.10. Catalytic Activity
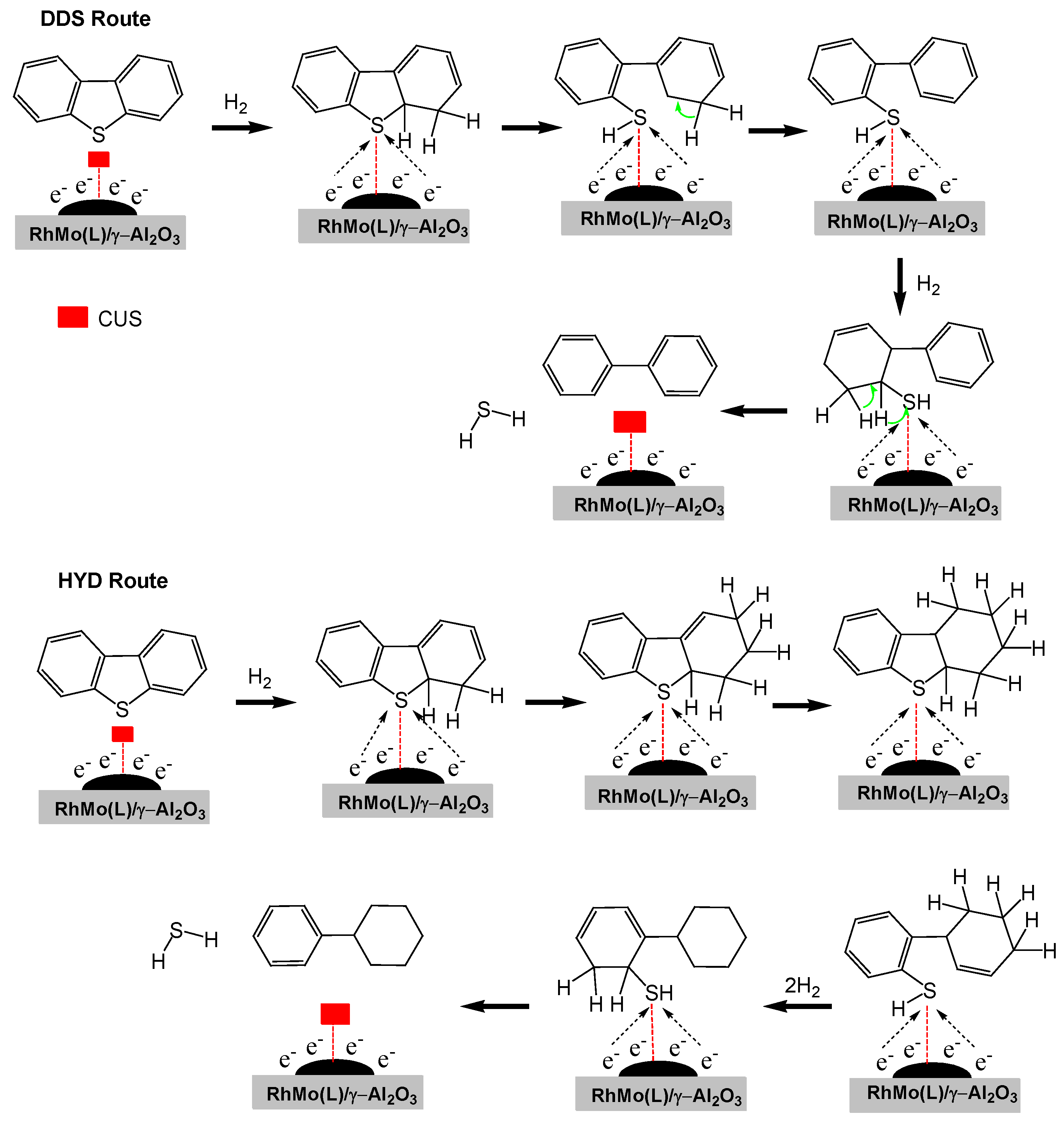
2.11. Proposed Mechanism
3. Experimental Section
3.1. Materials
3.2. Synthesis of RhMo Catalysts Prepared with Ethylenediaminetetraacetic Acid (EDTA), Citric Acid (CA) and Acetic Acid (AA)
3.3. Catalyst Characterization
3.4. Catalyst Sulfidation and Hydrodesulfurization Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dembaremba, T.O.; Ogunlaja, A.S.; Tshentu, Z.R. Coordination polymers and polymer nanofibers for effective adsorptive desulfurization. In Nanocomposites Desulfurization Fuels; Saleh, T., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 168–234. Available online: https://www.igi-global.com/chapter/coordination-polymers-and-polymer-nanofibers-for-effective-adsorptive-desulfurization/246161 (accessed on 4 March 2021). [CrossRef]
- Tahira, S.; Qazib, U.Y.; Naseema, Z.; Tahira, N.; Zahida, M.; Javaidc, R.; Shahid, I. Deep eutectic solvents as alternative green solvents for the efficient desulfurization of liquid fuel: A comprehensive review. Fuel 2021, 305, 121502. [Google Scholar] [CrossRef]
- Nascimento, I.G.; Locatel, W.R.; Magalhães, B.C.; Travalloni, L.; Zotin, J.L.; da Silva, M.A.P. Kinetics of dibenzothiophene hydrodesulfurization reactions using CoMoP/Al2O3 and NiMoP/Al2O3. Catal. Today 2021, 381, 200–208. [Google Scholar] [CrossRef]
- Mundotiya, S.; Singh, R.; Saha, S.; Kakkar, R.; Pal, S.; Kunzru, D.; Pala, R.G.S.; Sivakumar, S. Effect of Sodium on Ni-Promoted MoS2 Catalyst for Hydrodesulfurization Reaction: Combined Experimental and Simulation Study. Energy Fuels 2021, 35, 2368–2378. [Google Scholar] [CrossRef]
- Huang, T.; Peng, Q.; Gai, H.; Fan, Y. Surfactant-Confined Synthesis of CoMoS Catalysts Using Polyoxometalate Precursors for Superior Fuel Hydrodesulfurization. Energy Fuels 2021, 35, 2402–2415. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Han, Z.; Wang, K.; Zhang, Z.; Guan, G.; Abudula, A.; Xu, G. Catalyst Ni-Mo/Al2O3 promoted with infrared heating calcination for hydrodesulfurization of shale oil. Fuel 2021, 305, 121537. [Google Scholar] [CrossRef]
- Vinogradov, N.A.; Glotov, A.P.; Savinov, A.A.; Vutolkina, A.V.; Vinokurov, V.A.; Pimerzin, A.A. The mesoporous silicate-alumina composites application as supports for bifunctional sulfide catalysts for n-hexadecane hydroconversion. J. Porous Mater. 2021, 28, 1449–1458. [Google Scholar] [CrossRef]
- Ghosh, S.; Courthéoux, L.; Brunet, S.; Lacroix-Desmazes, P.; Pradel, A.; Girard, E.; Uzio, D. Hybrid CoMoS—Polyaniline nanowires catalysts for hydrodesulfurisation applications. Appl. Catal. A Gen. 2021, 623, 118264. [Google Scholar] [CrossRef]
- Soltanali, S.; Mashayekhi, M.; Mohaddecy, S.R.S. Comprehensive investigation of the effect of adding phosphorus and/or boron to NiMo/γ-Al2O3 catalyst in diesel fuel hydrotreating. Process Saf. Environ. Prot. 2020, 137, 273–281. [Google Scholar] [CrossRef]
- Hamidi, R.; Khoshbin, R.; Karimzadeh, R. Facile fabrication, characterization and catalytic activity of a NiMo/Al2O3 nanocatalyst via a solution combustion method used in a low temperature hydrodesulfurization process: The effect of fuel to oxidant ratio. RSC Adv. 2020, 10, 12439–12450. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Yang, W.; Hussain, W. Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: A technical review. Catal. Rev. 2020, 1–86. [Google Scholar] [CrossRef]
- Vutolkina, A.V.; Glotov, A.P.; Zanina, A.V.; Mahmutov, D.F.; Maksimov, A.L.; Karakhanov, E.A. Mesoporous Al-HMS and Al-MCM-41 supported Ni-Mo sulfide catalysts for HYD and HDS via in situ hydrogen generation through a WGSR. Catal. Today 2019, 329, 156–166. [Google Scholar] [CrossRef]
- Rinaldi, N.; Kubota, T.; Okamoto, Y. Effect of citric acid addition on the hydrodesulfurization activity of MoO3/Al2O3 catalysts. Appl. Catal. A: Gen. 2010, 374, 228–236. [Google Scholar] [CrossRef]
- Ninh, T.K.T.; Massin, L.; Laurenti, D.; Vrinat, M. A new approach in the evaluation of the support effect for NiMo hydrodesulfurization catalysts. Appl. Catal. A: Gen. 2011, 407, 29–39. [Google Scholar] [CrossRef]
- Sun, M.; Nicosia, D.; Prins, R. The effects of fluorine, phosphate and chelating agents on hydrotreating catalysts and catalysis. Catal. Today 2003, 86, 173–189. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Yin, X.; Li, G.; Li, L.; Guo, R.; Liu, X.; Guo, Q.; Shen, B. Migration and rearrangement of Fe–Zn species in the preparation of Fe–Zn bimetallic catalysts and their effects on the hydrodesulfurization reactivity. Energy Fuels 2021, 35, 16768–16777. [Google Scholar] [CrossRef]
- Ramírez, J.; Romualdo-Escobar, D.; Castillo-Villalón, P.; Gutiérrez-Alejandre, A. Improved NiMoSA catalysts: Analysis of EDTA post-treatment in the HDS of 4,6-DMDBT. Catal. Today 2020, 349, 168–177. [Google Scholar] [CrossRef]
- Hussain, M.; Song, S.-K.; Lee, J.-H.; Ihm, S.-K. Characteristics of CoMo catalysts supported on modified MCM-41 and MCM-48 materials for thiophene hydrodesulfurization. Ind. Eng. Chem. Res. 2006, 45, 536–543. [Google Scholar] [CrossRef]
- Hussain, M.; Ihm, S.K. Synthesis, characterization, and hydrodesulfurization activity of new mesoporous carbon supported transition metal sulfide catalysts. Ind. Eng. Chem. Res. 2009, 48, 698–707. [Google Scholar] [CrossRef]
- Hussain, M.; Yun, J.S.; Ihm, S.-K.; Russo, N.; Geobaldo, F. Synthesis, characterization, and thiophene hydrodesulfurization activity of novel macroporous and mesomacroporous carbon. Ind. Eng. Chem. Res. 2011, 50, 2530–2535. [Google Scholar] [CrossRef]
- Aray, Y.; Rodriguez, J.; Vega, D.; Rodriguez-Arias, E.N. Correlation of the topology of the electron density of pyrite-type transition metal sulfides with their catalytic activity in hydrodesulfurization. Angew. Chem. Int. Ed. 2000, 39, 3810–3813. [Google Scholar] [CrossRef]
- Vissers, J.P.R.; Groot, C.K.; Van Oers, E.M.; Beer, D.; Prins, R. Carbon-supported transition metal sulfides. Bull. Des Sociétés Chim. Belg. 1984, 93, 813–822. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Michaux, O.; Agostini, G.; Panissod, P. The influence of sulfide structures on the hydrodesulfurization activity of carbon-supported catalysts. J. Catal. 1986, 102, 275–288. [Google Scholar] [CrossRef]
- Raybaud, P.; Hafner, J.; Kresse, G.; Toulhoat, H. Ab initio density functional studies of transition-metal sulphides: II. Electronic structure. J. Phys. Condens. Matter 1997, 9, 11107. [Google Scholar] [CrossRef]
- Toulhoat, H.; Raybaud, P.; Kasztelan, S.; Kresse, G.; Hafner, J. Transition metals to sulfur binding energies relationship to catalytic activities in HDS: Back to Sabatier with first principle calculations. Catal. Today 1999, 50, 629–636. [Google Scholar] [CrossRef]
- Bataille, F.; Lemberton, J.L.; Michaud, P.; Pérot, G.; Vrinat, M.; Lemaire, M.; Kasztelan, S. Alkyldibenzothiophenes hydrodesulfurization-promoter effect, reactivity, and reaction mechanism. J. Catal. 2000, 191, 409–422. [Google Scholar] [CrossRef]
- Lee, J.; Ishihara, A.; Dumeignil, F.; Miyazaki, K.; Oomori, Y.; Qian, E.W.; Kabe, T. Novel hydrodesulfurization catalysts derived from a rhodium carbonyl complex. J. Mol. Catal. A Chem. 2004, 209, 155–162. [Google Scholar] [CrossRef]
- Giraldo, S.A.; Pinzón, M.H.; Centeno, A. Behavior of catalysts with rhodium in simultaneous hydrodesulfurization and hydrogenation reactions. Catal. Today 2008, 133, 239–243. [Google Scholar] [CrossRef]
- Rana, M.S.; Ramírez, J.; Gutiérrez-Alejandre, A.; Ancheyta, J.; Cedeño, L.; Maity, S.K. Support effects in CoMo hydrodesulfurization catalysts prepared with EDTA as a chelating agent. J. Catal. 2007, 246, 100–108. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra-low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Ríos-Caloch, G.; Santes, V.; Escobar, J.; Valle-Orta, M.; Barrera, M.C.; Hernández-Barrera, M. Effect of chitosan addition on NiMo/Al2O3 catalysts for Dibenzothiophene Hydrodesulfurization. Int. J. Chem. Reactor Eng. 2012, 10, 1–24. [Google Scholar] [CrossRef]
- Bui, N.Q.; Geantet, C.; Berhault, G. Activation of regenerated CoMo/Al2O3 hydrotreating catalysts by organic additives–The particular case of maleic acid. Appl. Catal. A Gen. 2019, 572, 185–196. [Google Scholar] [CrossRef]
- Mendes, F.M.; Schmal, M. The cyclohexanol dehydrogenation on Rh-CuAl2O3 catalysts Part 1. Characterization of the catalyst. Appl. Catal. A Gen. 1997, 151, 393–408. [Google Scholar] [CrossRef]
- Bergwerff, J.A.; Visser, T.; Weckhuysen, B.M. On the interaction between Co-and Mo-complexes in impregnation solutions used for the preparation of Al2O3-supported HDS catalysts: A combined Raman/UV–vis–NIR spectroscopy study. Catal. Today 2008, 130, 117–125. [Google Scholar] [CrossRef]
- Vatutina, Y.V.; Klimov, O.V.; Stolyarova, E.A.; Nadeina, K.A.; Danilova, I.G.; Chesalov, Y.A.; Noskov, A.S. Influence of the phosphorus addition ways on properties of CoMo-catalysts of hydrotreating. Catal. Today 2019, 329, 13–23. [Google Scholar] [CrossRef]
- Matralis, H.; Papadopoulou, C.; Lycourghiotis, A. Fluorinated hydrotreatment catalysts effect of the deposition order of F− ions on F-CoMo/γ-Al2O3 catalysts. Appl. Catal. A Gen. 1994, 116, 221–236. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Vakros, J.; Matralis, H.K.; Voyiatzis, G.A.; Kordulis, C. Preparation, characterization, and catalytic activity of CoMo/γ-Al2O3 catalysts prepared by equilibrium deposition filtration and conventional impregnation techniques. J. Colloid Interface Sci. 2004, 274, 159–166. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Zepeda, T.A.; Rivera-Muñoz, E.M.; Nava, R.; Loricera, C.V.; Pawelec, B. Characterization and HDS performance of sulfided CoMoW catalysts supported on mesoporous Al-SBA-16 substrates. Fuel 2015, 149, 149–161. [Google Scholar] [CrossRef]
- Rinaldi, N.; Al-Dalama, K.; Kubota, T.; Okamoto, Y. Preparation of Co–Mo/B2O3/Al2O3 catalysts for hydrodesulfurization: Effect of citric acid addition. Appl. Catal. A Gen. 2009, 360, 130–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, W.; Long, X.; Nie, H. Redispersion effects of citric acid on CoMo/γ-Al2O3 hydrodesulfurization catalysts. Catal. Commun. 2016, 82, 20–23. [Google Scholar] [CrossRef]
- Weber, R. Effect of local structure on the UV-Visible absorption edges of molybdenum oxide clusters and supported molybdenum oxides. J. Catal. 1995, 151, 470–474. [Google Scholar] [CrossRef]
- Tian, H.; Roberts, C.A.; Wachs, I.E. Molecular structural determination of molybdena in different environments: Aqueous solutions, bulk mixed oxides, and supported MoO3 catalysts. J. Phys. Chem. C 2010, 114, 14110–14120. [Google Scholar] [CrossRef]
- Xu, J.; Huang, T.; Fan, Y. Highly efficient NiMo/SiO2-Al2O3 hydrodesulfurization catalyst prepared from gemini surfactant-dispersed Mo precursor. Appl. Catal. B Environ. 2017, 203, 839–850. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Gutiérrez, A.W.; Terrazas, J.E. Benzothiophene hydrodesulfurization over NiMo/alumina catalysts modified by citric acid. Effect of addition stage of organic modifier. Fuel Process. Technol. 2017, 156, 33–42. [Google Scholar] [CrossRef]
- Cabello, C.I.; Cabrerizo, F.M.; Alvarez, A.; Thomas, H.J. Decamolybdodicobaltate (III) heteropolyanion: Structural, spectroscopical, thermal and hydrotreating catalytic properties. J. Mol. Catal. A Chem. 2002, 186, 89–100. [Google Scholar] [CrossRef]
- Valencia, D.; Klimova, T. Citric acid loading for MoS2-based catalysts supported on SBA-15. New catalytic materials with high hydrogenolysis ability in hydrodesulfurization. Appl. Catal. B Environ. 2013, 129, 137–145. [Google Scholar] [CrossRef]
- Yurum, A.; Karakas, G. Synthesis of Na-, Fe-, and Co-promoted TiO2/multiwalled carbon nanotube composites and their use as a photocatalyst. Turk. J. Chem. 2017, 41, 440–454. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Seentrakoon, B. Hydrodesulfurization activity of MoS2 and bimetallic catalysts prepared by in situ decomposition of thiosalt. Ind. Eng. Chem. Res. 2007, 46, 1874–1882. [Google Scholar] [CrossRef]
- Alonso, G.; Petranovskii, V.; Del Valle, M.; Cruz-Reyes, J.; Licea-Claverie, A.; Fuentes, S. Preparation of WS2 catalysts by in situ decomposition of tetraalkylammonium thiotungstates. Appl. Catal. A Gen. 2000, 197, 87–97. [Google Scholar] [CrossRef]
- Badoga, S.; Mouli, K.C.; Soni, K.K.; Dalai, A.K.; Adjaye, J. Beneficial influence of EDTA on the structure and catalytic properties of sulfided NiMo/SBA-15 catalysts for hydrotreating of light gas oil. Appl. Catal. B Environ. 2012, 125, 67–84. [Google Scholar] [CrossRef]
- Calderón-Magdaleno, M.Á.; Mendoza-Nieto, J.A.; Klimova, T.E. Effect of the amount of citric acid used in the preparation of NiMo/SBA-15 catalysts on their performance in HDS of dibenzothiophene-type compounds. Catal. Today. 2014, 220, 78–88. [Google Scholar] [CrossRef]
- Chen, S.; Luo, L.; Cheng, X. Influence of preparation method on the performance of Pd/ZrO2-Al2O3 catalysts for HDS. Indian J. Chem. Technol. 2009, 16, 272–277. [Google Scholar]
- Gutiérrez, O.Y.; Pérez, F.; Fuentes, G.A.; Bokhimi, X.; Klimova, T. Deep HDS over NiMo/Zr-SBA-15 catalysts with varying MoO3 loading. Catal. Today 2008, 130, 292–301. [Google Scholar] [CrossRef]
- Hara, K.; Iwahashi, K.; Takakusagi, S.; Uosaki, K.; Sawamura, M. Construction of self-assembled monolayer terminated with N-heterocyclic carbene–rhodium(I) complex moiety. Surf. Sci. 2007, 601, 5127–5132. [Google Scholar] [CrossRef]
- Larichev, Y.V.; Netskina, O.V.; Komova, O.V.; Simagina, V.I. Comparative XPS study of Rh/Al2O3 and Rh/TiO2 as catalysts for NaBH4 hydrolysis. Int. J. Hydrog. Energy 2010, 35, 6501–6507. [Google Scholar] [CrossRef]
- Masud, J.; Van Nguyen, T.; Singh, N.; McFarland, E.; Ikenberry, M.; Hohn, K.; Hwang, B.J. A RhxSy/C catalyst for the hydrogen oxidation and hydrogen evolution reactions in HBr. J. Electrochem. Soc. 2015, 162, F455. [Google Scholar] [CrossRef][Green Version]
- Galtayries, A.; Wisniewski, S.; Grimblot, J. Formation of thin oxide and sulphide films on polycrystalline molybdenum foils: Characterization by XPS and surface potential variations. J. Electron Spectrosc. Relat. Phenom. 1997, 87, 31–44. [Google Scholar] [CrossRef]
- Venezia, A.M. X-ray photoelectron spectroscopy (XPS) for catalysts characterization. Catal. Today 2003, 77, 359–370. [Google Scholar] [CrossRef]
- Budukva, S.V.; Klimov, O.V.; Uvarkina, D.D.; Chesalov, Y.A.; Prosvirin, I.P.; Larina, T.V.; Noskov, A.S. Effect of citric acid and triethylene glycol addition on the reactivation of CoMo/γ-Al2O3 hydrotreating catalysts. Catal. Today 2019, 329, 35–43. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Huang, T.; Fan, Y. Hexamethonium bromide-assisted synthesis of CoMo/graphene catalysts for selective hydrodesulfurization. Appl. Catal. B Environ. 2019, 244, 385–395. [Google Scholar] [CrossRef]
- Huang, T.; Xu, J.; Fan, Y. Effects of concentration and microstructure of active phases on the selective hydrodesulfurization performance of sulfided CoMo/Al2O3 catalysts. Appl. Catal. B Environ. 2018, 220, 42–56. [Google Scholar] [CrossRef]
- Matsumoto, K.; Matsumoto, T.; Kawano, M.; Ohnuki, H.; Shichi, Y.; Nishide, T.; Sato, T. Syntheses and crystal structures of disulfide-bridged binuclear ruthenium compounds: The First UV—Vis, Raman, ESR, and XPS Spectroscopic Characterization of a Valence-Averaged Mixed-Valent RuIIISSRuII Core. J. Am. Chem. Soc. 1996, 118, 3597–3609. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, L.; Du, M.; Lyu, Z.; Wen, X.D.; Li, X.; Ge, H. A novel reactive adsorption desulfurization Ni/MnO adsorbent and its hydrodesulfurization ability compared with Ni/ZnO. Catal. Commun. 2015, 61, 37–40. [Google Scholar] [CrossRef]
- Liu, B.; Chai, Y.; Li, Y.; Wang, A.; Liu, Y.; Liu, C. Effect of sulfidation atmosphere on the performance of the CoMo/γ-Al2O3 catalysts in hydrodesulfurization of FCC gasoline. Appl. Catal. A Gen. 2014, 471, 70–79. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Smith, K.J. Understanding selectivity changes during hydrodesulfurization of dibenzothiophene on Mo2C/carbon catalysts. J. Catal. 2019, 369, 427–439. [Google Scholar] [CrossRef]
- Rashidi, F.; Sasaki, T.; Rashidi, A.M.; Kharat, A.N.; Jozani, K.J. Ultradeep hydrodesulfurization of diesel fuels using highly efficient nanoalumina-supported catalysts: Impact of support, phosphorus, and/or boron on the structure and catalytic activity. J. Catal. 2013, 299, 321–335. [Google Scholar] [CrossRef]
- Wang, X.; Du, P.; Chi, K.; Duan, A.; Xu, C.; Zhao, Z.; Zhang, H. Synthesis of NiMo catalysts supported on mesoporous silica FDU-12 with different morphologies and their catalytic performance of DBT HDS. Catal. Today 2017, 291, 146–152. [Google Scholar] [CrossRef]
- Nikulshina, M.; Kokliukhin, A.; Mozhaev, A.; Nikulshin, P. CoMo/Al2O3 hydrotreating catalysts prepared from single Co2Mo10-heteropolyacid at extremely high metal loading. Catal. Commun. 2019, 127, 51–57. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rahman, A.U.; Rashid, H.U.; Sahibzada, M.; Subhan, S.; Tong, Z. Hydrodesulfurization of dibenzothiophene using Pd-promoted Co–Mo/Al₂O₃ and Ni–Mo/Al₂O₃ catalysts coupled with ionic liquids at ambient operating conditions. RSC Adv. 2019, 9, 10371–10385. [Google Scholar] [CrossRef]
- Pereyma, V.Y.; Klimov, O.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Yashnik, S.A.; Noskov, A.S. Effect of thermal treatment on morphology and catalytic performance of NiW/Al2O3 catalysts prepared using citric acid as chelating agent. Catal. Today 2018, 305, 162–170. [Google Scholar] [CrossRef]
- Zhang, M.H.; Fan, J.Y.; Chi, K.; Duan, A.J.; Zhao, Z.; Meng, X.L.; Zhang, H.L. Synthesis, characterization, and catalytic performance of NiMo catalysts supported on different crystal alumina materials in the hydrodesulfurization of diesel. Fuel Process. Technol. 2017, 156, 446–453. [Google Scholar] [CrossRef]
- Shimizu, T.; Hiroshima, K.; Honma, T.; Mochizuki, T.; Yamada, M. Highly active hydrotreatment catalysts prepared with chelating agents. Catal. Today 1998, 45, 271–276. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ochiai, K.; Kawano, M.; Kubota, T. Evaluation of the maximum potential activity of Co–Mo/Al2O3 catalysts for hydrodesulfurization. J. Catal. 2004, 222, 143–151. [Google Scholar] [CrossRef]
- Yoosuk, B.; Kim, J.H.; Song, C.; Ngamcharussrivichai, C.; Prasassarakich, P. Highly active MoS2, CoMoS2 and NiMoS2 unsupported catalysts prepared by hydrothermal synthesis for hydrodesulfurization of 4, 6-dimethyldibenzothiophene. Catal. Today 2008, 130, 14–23. [Google Scholar] [CrossRef]
- Escobar, M.C.; Barrera, J.A.; De los Reyes, J.A.; Toledo, V.; Santes, J.A. Colín, Effect of chelating ligands on Ni-Mo impregnation over wide-pore ZrO2-TiO2. J. Mol. Catal. A Chem. 2008, 278, 33–40. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Xiao, T.C.; Costa, P.M.; Fontal, B.; Green, M.L. Urea–organic matrix method: An alternative approach to prepare Co-MoS2/γ-Al2O3 HDS catalyst. Appl. Catal. A Gen. 2004, 270, 209–222. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Xiao, T.C.; Rodulfo-Baechler, S.M.; Green, M.L. Impact of the urea–matrix combustion method on the HDS performance of Ni-MoS2/γ-Al2O3 catalysts. J. Mol. Catal. A Chem. 2005, 240, 214–225. [Google Scholar]
- Bin, L.I.U.; Lei, L.I.U.; Chai, Y.M.; Zhao, J.C.; Liu, C.G. Essential role of promoter Co on the MoS2 catalyst in selective hydrodesulfurization of FCC gasoline. J. Fuel Chem. Technol. 2018, 46, 441–450. [Google Scholar]
- Li, X.; Bai, J.; Wang, A.; Prins, R.; Wang, Y. Hydrodesulfurization of dibenzothiophene and its hydrogenated intermediates over bulk Ni2P. Top. Catal. 2011, 54, 290–298. [Google Scholar] [CrossRef]
- García-Martínez, J.C.; Dutta, A.; Chávez, G.; De los Reyes, J.A.; Castillo-Araiza, C.O. Hydrodesulfurization of dibenzothiophene in a micro trickle bed catalytic reactor under operating conditions from reactive distillation. Int. J. Chem. React. Eng. 2015, 14, 769–783. [Google Scholar] [CrossRef]
- Torres-García, N.L.; Huirache-Acuña, R.; Zepeda-Partida, T.A.; Pawelec, B.; Fierro, J.L.G.; Vázquez-Salas, P.J.; Maya-Yescas, R.; Rivera-Garnica, J.M. Trimetallic RuxMoNi catalysts supported on SBA-15 for the hydrodesulfurization of dibenzothiophene. Int. J. Chem. React. Eng. 2019, 17, 2194–5748. [Google Scholar] [CrossRef]
- Li, M.; Song, J.; Yue, F.; Pan, F.; Yan, W.; Hua, Z.; Li, L.; Yang, Z.; Li, L.; Wen, G.; et al. Complete Hydrodesulfurization of Dibenzothiophene via Direct Desulfurization Pathway over Mesoporous TiO2-Supported NiMo Catalyst Incorporated with Potassium. Catalysts 2019, 9, 448. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wang, Y.; Chen, Y.; Liu, Y.; Hu, Y. Hydrodesulfurization of dibenzothiophene over Ni-Mo sulfides supported by proton-exchanged siliceous MCM-41. Catal. Lett. 2002, 84, 107–113. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Li, L.; Guo, R.; Zhang, X.; Ren, S.; Guo, Q.; Wen, X.-D.; Shen, B. Hydrodesulfurization of dibenzothiophene on TiO2−x-modified Fe-based catalysts: Electron transfer behavior between TiO2−x and Fe species. ACS Catal. 2020, 10, 9019–9033. [Google Scholar] [CrossRef]



| Catalyst | Eg Values | NMo-O-Mo |
|---|---|---|
| RhMo/γ-Al2O3 | 3.779 | 1.975 |
| RhMo-AA/γ-Al2O3 | 4.341 | 0.5134 |
| RhMo-EDTA/γ-Al2O3 | 4.394 | 0.3756 |
| RhMo-CA/γ-Al2O3 | 4.478 | 0.1572 |
| Catalysts | Atomic Percentage (wt. %) | S/Mo | C/Mo | |||||
|---|---|---|---|---|---|---|---|---|
| C K | O K | Al K | S K | Rh L | Mo L | |||
| RhMo/ɣ-Al2O3 | 7.97 | 63.24 | 26.47 | 0.68 | 0.17 | 1.46 | 0.47 | 5.46 |
| RhMo-EDTA/ɣ-Al2O3 | 10.20 | 57.52 | 30.03 | 1.48 | 0.44 | 0.32 | 4.63 | 31.88 |
| RhMo-AA/ɣ-Al2O3 | 9.38 | 62.61 | 25.63 | 1.35 | 0.19 | 0.73 | 1.85 | 12.85 |
| RhMo-CA/ɣ-Al2O3 | 12.07 | 57.56 | 26.88 | 1.60 | 0.94 | 0.90 | 1.78 | 13.41 |
| Elements (eV) | RhMo/ɣ-Al2O3 | RhMo-EDTA/ɣ-Al2O3 |
|---|---|---|
| C 1s | 289.5 | 285.5 |
| O 1s | 530.0 | 529.0 |
| Mo 3d | 226.2; 230.0; 233.2 | 226.0; 230.0 |
| Rh 3d | 306.5; 310.6 | 305.0; 310.1 |
| S 2p | 160.1; 167.0 | 160.5; 166.1 |
| Al 2p | 72.4 | 72.0 |
| Al 2s | 117.0 | 117.0 |
| Catalysts | Average Diameter ± SD (nm) |
|---|---|
| RhMo/ɣ-Al2O3 | 4.4 (±1.38) |
| RhMo-EDTA/ɣ-Al2O3 | 4.1 (±1.220) |
| RhMo-AA/ɣ-Al2O3 | 3.3 (±0.757) |
| RhMo-CA/ɣ-Al2O3 | 1.6 (±0.860) |
| Catalysts | Crystallite Sizes (nm) | Eg Values | HDS (%) | BP(%) | PhCh(%) | HYD/DDS Ratio | TOF (h−1) a |
|---|---|---|---|---|---|---|---|
| RhMo/ɣ-Al2O3 | 5.903 | 3.779 | 88 | 65 | 13 | 0.20 | 51 |
| RhMo-EDTA/ɣ-Al2O3 | 5.770 | 4.394 | 68 | 16 | 1 | 0.06 | 60 |
| RhMo-AA/ɣ-Al2O3 | 5.750 | 4.341 | 73 | 65 | 3 | 0.05 | 79 |
| RhMo-CA/ɣ-Al2O3 | 5.809 | 4.478 | 72 | 36 | 2 | 0.06 | 223 |
| Catalysts | Model Compound | Reaction Temperature (°C) | HDS (%) | Reaction Pressure (Bar) | Reference |
|---|---|---|---|---|---|
| RhMo/ɣ-Al2O3 | DBT | 300 | 88 | 40 | This work |
| RhMo-EDTA/ɣ-Al2O3 | DBT | 300 | 68 | 40 | This work |
| RhMo-AA/ɣ-Al2O3 | DBT | 300 | 73 | 40 | This work |
| RhMo-CA/ɣ-Al2O3 | DBT | 300 | 72 | 40 | This work |
| Ni2P | DBT | 340 | 35 | 40 | [79] |
| Ni2P | TH-DBT | 340 | 50 | 40 | [79] |
| NiMoP/γ-Al2O3 | DBT | <320 | 22–90 | <25 | [80] |
| RuxMoNi | DBT | 320 | 24–92 | 54.5 | [81] |
| NiMo | DBT | 320 | 62 | 54.5 | [81] |
| NiMo/TiO2-6 | DBT | 300 | 90 | 20 | [82] |
| NiMo/MCM-41-Na | DBT | 300 | >95 | 50 | [83] |
| Fe-Zn/TiO2-Al2O3 | DBT | 380 | >98 | 40 | [84] |
| RhMo/ɣ-Al2O3 | DBT | 310 | 84 | 50 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majodina, S.; Tshentu, Z.R.; Ogunlaja, A.S. Effect of Adding Chelating Ligands on the Catalytic Performance of Rh-Promoted MoS2 in the Hydrodesulfurization of Dibenzothiophene. Catalysts 2021, 11, 1398. https://doi.org/10.3390/catal11111398
Majodina S, Tshentu ZR, Ogunlaja AS. Effect of Adding Chelating Ligands on the Catalytic Performance of Rh-Promoted MoS2 in the Hydrodesulfurization of Dibenzothiophene. Catalysts. 2021; 11(11):1398. https://doi.org/10.3390/catal11111398
Chicago/Turabian StyleMajodina, Siphumelele, Zenixole R. Tshentu, and Adeniyi S. Ogunlaja. 2021. "Effect of Adding Chelating Ligands on the Catalytic Performance of Rh-Promoted MoS2 in the Hydrodesulfurization of Dibenzothiophene" Catalysts 11, no. 11: 1398. https://doi.org/10.3390/catal11111398
APA StyleMajodina, S., Tshentu, Z. R., & Ogunlaja, A. S. (2021). Effect of Adding Chelating Ligands on the Catalytic Performance of Rh-Promoted MoS2 in the Hydrodesulfurization of Dibenzothiophene. Catalysts, 11(11), 1398. https://doi.org/10.3390/catal11111398








