New Insight into the Interplay of Method of Deposition, Chemical State of Pd, Oxygen Storage Capability and Catalytic Activity of Pd-Containing Perovskite Catalysts for Combustion of Methane
Abstract
:1. Introduction
2. Results and Discussions
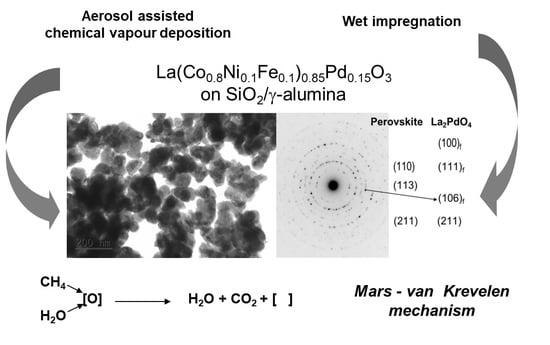
2.1. Structure and Texture of Pd-Containing Perovskite Supported on SiO2-Modified/γ-Alumina
2.2. Catalytic Activity of Supported Pd-Containing Perovskite over SiO2-Modified Alumina
2.2.1. Pre-Treatment Tests
2.2.2. Reaction Kinetics
2.3. Ex-Situ XPS Analysis
2.4. Preliminary Data on Possible Practical Application
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federal Register/Vol. 78, No. 63/Tuesday, April 2, 2013/Proposed Rules, Environmental Protection Agency, 40 CFR Part 98, 2013 Revisions to the Greenhouse Gas Reporting Rule and Proposed Confidentiality Determinations for New or Substantially Revised Data Elements. Available online: https://www.govinfo.gov/content/pkg/FR-2013-04-02/pdf/2013-06093.pdf (accessed on 2 April 2013).
- European Commission Global Methane Reduction Actions, Ref. Ares (2013) 2843722-06/08/2013. Available online: https://www.globalmethane.org/documents/EC_GMI_reduction_actions.pdf (accessed on 6 August 2013).
- Bordelanne, O.; Montero, M.; Bravin, F.; Prieur-Vernat, A.; Oliveti-Selmi, O.; Pierre, H.; Papadopoulo, M.; Muller, T. Biomethane CNG hybrid: A reduction by more than 80% of the greenhouse gases emissions compared to gasoline. J. Nat. Gas Sci. Eng. 2011, 3, 617–624. [Google Scholar] [CrossRef]
- Machin, N.E.; Cakırca, E.E.; Ates, A. Catalytic combustion of methane. In Proceedings of the 6th International Advanced Technologies Symposium (IATS’11), Elazığ, Turkey, 16–18 May 2011; pp. 253–256. [Google Scholar]
- Hurtado, P.; Ordycez, S.; Sastre, H.; Diez, F.V. Combustion of methane over palladium catalyst in the presence of inorganic compounds: Inhibition and deactivation phenomena. Appl. Catal. B Environ. 2004, 47, 85–93. [Google Scholar] [CrossRef]
- Ersson, A. Materials for High-Temperature Catalytic Combustion. Ph.D. Thesis, Kungliga Tekniska Hцgskolan Department of Chemical Engineering and Technology Chemical Technology, Stockholm, Sweden, 2003. [Google Scholar]
- Cimino, S.; Lisi, L.; Pirone, R.; Russo, G.; Turco, M. Methane combustion on perovskites-based structured catalysts. Catal. Today 2000, 59, 19–31. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Sarli, V.; Landi, G.; Di Benedetto, A. High pressure methane catalytic combustion over novel partially coated LaMnO3-based monoliths. Chem. Eng. J. 2015, 259, 381–390. [Google Scholar] [CrossRef]
- Di Sarli, V.; Barbato, P.S.; Benedetto, A.D.; Landi, G. Start-up behavior of a LaMnO3 partially coated monolithic combustorat high pressure. Catal. Today 2015, 242, 200–210. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Wang, Y.; Zhang, F.; Liu, X.; Guo, Y.; Lu, G. Nanocasted Synthesis of Mesoporous LaCoO3 Perovskite with Extremely High Surface Area and Excellent Activity in Methane Combustion. J. Phys. Chem. C 2008, 112, 15293–15298. [Google Scholar] [CrossRef]
- Ivanova, S.; Senyshyn, A.; Zhecheva, E.; Tenchev, K.; Stoyanova, R.; Fuess, H. Crystal structure, microstructure and reducibility of LaNixCo1−xO3 and LaFexCo1−xO3 Perovskites (0 < x ≤ 0.5). J. Solid State Chem. 2010, 183, 940–950. [Google Scholar] [CrossRef]
- Stanchovska, S.; Zhecheva, E.; Stoyanova, R.; Naydenov, A. Preparation and characterization of palladium containing nickel-iron-cobalt perovskite catalysts for complete oxidation of propane. Bulg. Chem. Commun. 2017, 49, 107–113. [Google Scholar]
- Stanchovska, S.; Markov, P.; Tenchev, K.; Stoyanova, R.; Zhecheva, E.; Naydenov, A. Preparation and characterization of palladium containing nickel–iron–cobalt perovskite catalysts for the complete oxidation of C1–C6 alkanes. React. Kinet. Mech. Catal. 2017, 122, 931–942. [Google Scholar] [CrossRef]
- Eyssler, A.; Winkler, A.; Safonova, O.; Nachtegal, M.; Matam, S.K.; Hug, P.; Weidenkaff, A.; Ferri, D. On the State of Pd in Perovskite-Type Oxidation Catalysts of Composition A(B,Pd)O3±δ (A = La, Y.; B = Mn, Fe, Co). Chem. Mater. 2012, 24, 1864–1875. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164. [Google Scholar] [CrossRef]
- Uenishi, M.; Taniguchi, M.; Tanaka, H.; Kimura, M.; Nishihata, Y.; Mizuki, J.; Kobayashi, T. Redox behavior of palladium at start-up in the Perovskite-type LaFePdOx automotive catalysts showing a self-regenerative function. Appl. Catal. B 2005, 57, 267–273. [Google Scholar] [CrossRef]
- Uenishi, M.; Tanaka, H.; Taniguchi, M.; Tan, I.; Sakamoto, Y.; Matsunaga, S.; Yokota, K.; Kobayashi, T. The reducing capability of palladium segregated from perovskite-type LaFePdOx automotive catalysts. Appl. Catal. A 2005, 296, 114–119. [Google Scholar] [CrossRef]
- Tanaka, H.; Taniguchi, M.; Kajita, N.; Uenishi, M.; Tan, I.; Sato, N.; Narita, K.; Kimura, M. Design of the intelligent catalyst for Japan ULEV standard. Top. Catal. 2004, 30, 389–396. [Google Scholar] [CrossRef]
- Tanaka, H.; Taniguchi, M.; Uenishi, M.; Kajita, N.; Tan, I.; Nishihata, Y.; Mizuki, J.; Narita, K.; Kimura, M.; Kaneko, K. Self-Regenerating Rh- and Pt-Based Perovskite Catalysts for Automotive-Emissions Control. Angew. Chem. Int. Ed. 2006, 45, 5998–6002. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Cabiй, M.; Henry, C.R.; Lancelot, C.; Dujardin, C.; Raouf, S.R.; Granger, P. Structural changes of nano-Pt particles during thermal ageing: Support-induced effect and related impact on the catalytic performances. J. Catal. 2010, 270, 299–309. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Lancelot, C.; Dujardin, C.; Cordier-Robert, C.; Granger, P. Support-Induced Effects of LaFeO3 Perovskite on the Catalytic Performances of Supported Pt Catalysts in DeNOx Applications. J. Phys. Chem. C 2011, 115, 1911–1921. [Google Scholar] [CrossRef]
- Wenge, L.; Deyong, G.; Xin, X. Research Progress of Palladium Catalysts for Methane Combustion. China Pet. Proc. Petrochem. Techn. Rev. 2012, 14, 1–9. [Google Scholar]
- Zasada, F.; Janas, J.; Piskorz, W.; Gorczynska, M.; Sojka, Z. Total Oxidation of Lean Methane over Cobalt Spinel Nanocubes Controlled by the Self-Adjusted Redox State of the Catalyst: Experimental and Theoretical Account for Interplay between the Langmuir–Hinshelwood and Mars–Van Krevelen Mechanisms. ACS Catal. 2017, 7, 2853–2867. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Keav, S.; Matam, S.K.; Ferri, D.; Weidenkaff, A. Structured Perovskite-Based Catalysts and Their Application as Three-Way Catalytic Converters—A Review. Catalysts 2014, 4, 226–255. [Google Scholar] [CrossRef] [Green Version]
- Eyssler, A.; Mandaliev, P.; Winkler, A.; Hug, P.; Safonova, O.; Figi, R.; Weidenkaff, A.; Ferri, D. The Effect of the State of Pd on Methane Combustion in Pd-Doped LaFeO3. J. Phys. Chem. C 2010, 114, 4584–4594. [Google Scholar] [CrossRef]
- Zhou, R.; Zhao, B.; Yue, B. Effects of CeO2-ZrO2 present in Pd/Al2O3 catalysts on the redox behavior of PdOx and their combustion activity. Appl. Surface Sci. 2008, 254, 4701–4707. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.H.; Kim, D.H. Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl. Catal. A 2015, 505, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Books, C.S. The kinetics of hydrogen and carbon monoxide oxidation over a manganese oxide. J. Catal. 1967, 8, 272–282. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Уrfгo, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Baylet, A.; Marйcot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd° oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Liu, W.; Gao, X.; Gao, D.; Wang, M.; Wang, S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl. Catal. A Gen. 2016, 525, 94–102. [Google Scholar] [CrossRef]
- Kucharczyk, B. Activity of monolithic Pd/Al2O3 catalysts in the combustion of mine ventilation air methane. Pol. J. Chem. Technol. 2011, 13, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, R.; Huang, G.; Istratescu, G.M.; Wu, L.; Hayes, R.E. Methane oxidation over Pt, Pt:Pd, and Pd based catalysts: Effects of pre-treatment. Can. J. Chem. Eng. 2015, 93, 1474–1482. [Google Scholar] [CrossRef]
- Duprat, F. Light-off curve of catalytic reaction and kinetics. Chem. Eng. Sci. 2002, 57, 901–911. [Google Scholar] [CrossRef]
- Todorova, S.; Naydenov, A.; Kolev, H.; Holgado, J.P.; Ivanov, G.; Kadinov, G.; Caballero, A. Mechanism of complete n-hexane oxidation on silica supported cobalt and manganese catalysts. Appl. Catal. A 2012, 413–414, 43–51. [Google Scholar] [CrossRef]
- Markova-Velichkova, M.; Lazarova, T.; Tumbalev, V.; Ivanov, G.; Kovacheva, D.; Stefanov, P.; Naydenov, A. Complete oxidation of hydrocarbons on YFeO3 and LaFeO3 catalysts. Chem. Eng. J. 2013, 231, 236–244. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd Catalysts by Water during Low Temperature Methane Oxidation Relevant to Natural Gas Vehicle Converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and Reactivity of PdOx/ZrO2 Catalysts for Methane Oxidation at Low Temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.; Iglesia, E. Elementary Steps, the Role of Chemisorbed Oxygen, and the Effects of Cluster Size in Catalytic CH4–O2 Reactions on Palladium. J. Phys. Chem. C 2011, 115, 17845–17855. [Google Scholar] [CrossRef]
- Ciuparu, D.; Pfefferle, L. Contributions of lattice oxygen to the overall oxygen balance during methane combustion over PdO-based catalysts. Catal. Today 2002, 77, 167–179. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Catalytic Deactivation and Role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Support Interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Vannice, M.A. Kinetics of Catalytic Reactions; Springer Science-Business Media, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Boudart, M. Two-step catalytic reactions. AIChE J. 1972, 18, 465–478. [Google Scholar] [CrossRef]
- Vannice, M.A.; Hyun, S.H.; Kalpakci, B.; Liauh, W.C. Entropies of adsorption in heterogeneous catalytic reactions. J. Catal. 1979, 56, 358–362. [Google Scholar] [CrossRef]
- Toops, T.J.; Walters, A.B.; Vannice, M.A. Methane combustion over La2O3-based catalysts and γ-Al2O3. Appl. Catal. A: Gen. 2002, 233, 125–140. [Google Scholar] [CrossRef]
- Ciuparu, D.; Perkins, E.; Pfefferle, L. In situ DR-FTIR investigation of surface hydroxyls on γ-Al2O3 supported PdO catalysts during methane combustion. Appl. Catal. A Gen. 2004, 263, 145–153. [Google Scholar] [CrossRef]
- Łojewska, J.; Kołodziej, A.; Żak, J.; Stoch, J. Pd/Pt promoted Co3O4 catalysts for VOCs combustion: Preparation of active catalyst on metallic carrier. Catal. Today 2005, 105, 655–661. [Google Scholar] [CrossRef]
- Belfiore, L.A. Transport Phenomena for Chemical Reactor Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Marchand, P.; Hassan, I.A.; Parkin, I.P.; Carmalt, C.J. Aerosol-assisted delivery of precursors for chemical vapour deposition: Expanding the scope of CVD for materials fabrication. Dalton Trans. 2013, 42, 9406–9422. [Google Scholar] [CrossRef]
- Powell, M.J.; Potter, D.B.; Wilson, R.L.; Darr, J.A.; Parkin, I.P.; Carmalt, C.J. Scaling aerosol assisted chemical vapour deposition: Exploring the relationship between growth rate and film properties. Mater. Des. 2017, 129, 116–124. [Google Scholar] [CrossRef]
- Mazhar, M.; Hussain, S.M.; Rabbani, F.; Kociok-Kцhn, G.; Molloy, K.C. Synthesis, Thermal Decomposition Pattern and Single Crystal X-Ray Studiesof Dimeric [Cu(dmae)(OCOCH3)(H2O)]2: A Precursor for the Aerosol Assisted Chemical Vapour Deposition of Copper Metal Thin Films. Bull. Korean Chem. Soc. 2006, 27, 1572–1576. [Google Scholar] [CrossRef] [Green Version]










| Samples | Signal 1 | Signal 2 | IHE/ILE | ||
|---|---|---|---|---|---|
| Pd 3d5/2, eV | Line Width, eV | Pd 3d5/2, eV | Line Width, eV | ||
| Pd-LMO/Imp—fresh | 336.4 | 1.40 | 337.5 | 2.43 | 0.76 |
| Pd-LMO/Imp—after catalytic test | 336.3 | 1.28 | 338.1 | 2.5 | 0.36 |
| Pd-LMO/U-AACVD—fresh | 335.9 | 1.45 | 337.2 | 2.2 | 1.67 |
| Pd-LMO/U-AACVD—after catalytic test | 336.3 | 1.69 | - | - | 0 |
| Sample | S, m2/g | Vt, cm3/g | D, nm |
|---|---|---|---|
| γ-Al2O3 | 219 | 0.41 | 7.4 |
| SiO2 modified γ-Al2O3 | 204 | 0.39 | 7.8 |
| Pd-LMO/U-AACVD | 181 | 0.37 | 8.1 |
| Pd-LMO/Imp | 172 | 0.36 | 8.7 |
| LMO/U-AACVD | 164 | 0.38 | 9.2 |
| LMO/Imp | 153 | 0.35 | 9.1 |
| Fresh Catalyst | After Working Cycle | |||
|---|---|---|---|---|
| Reacted Oxygen (Uptake) | mg O2 to CO | mg O2 to CO2 | mg O2 to CO | mg O2 to CO2 |
| Pd-LMO/U-AACVD | 1.0 | 0.4 | 2.7 | 0.7 |
| Pd-LMO/Imp | 2.6 | 0.8 | 2.3 | 1.0 |
| Model: PWL | Catalyst | ko | Ea | m (CH4) | n (O2) | p (H2O) | RSS | R2 |
|---|---|---|---|---|---|---|---|---|
| Pd-LMO/U-AACVD | 68.3 | 1.36 × 102 | 0.84 | 0.18 | −0.10 | 5.4 | 0.977 | |
| Pd-LMO/Imp | 69.2 | 4.14 × 102 | 0.90 | 0.21 | −0.11 | 4.0 | 0.986 |
| Model: MVK-1 | Catalyst | ko,ox | Ea,ox | ko,red | Ea,red | ko,water | −ΔHwater | RSS | R2 |
|---|---|---|---|---|---|---|---|---|---|
| water adsorbs on oxidized sites | Pd-LMO/U-AACVD | 1.29 × 104 | 85.2 | 1.13 × 102 | 56.5 | 4.02 × 103 | 58.0 | 2.7 | 0.988 |
= 2 | Pd-LMO/Imp | 2.90 × 104 | 91.2 | 2.18 × 102 | 56.5 | 6.60 × 103 | 54.4 | 3.4 | 0.980 |
| Model: MVK-2 | |||||||||
| water adsorbs on reduced sites | Pd-LMO/U-AACVD | 8.39 × 102 | 16.2 | 3.16 × 102 | 62.3 | 5.84 ×1 03 | 82.0 | 4.4 | 0.980 |
= 2 | Pd-LMO/Imp | 2.95 × 102 | 15.3 | 1.99 × 103 | 68.4 | 6.61 × 103 | 72.8 | 4.8 | 0.983 |
| Model | Catalyst | ko | Ea | ko,ox | −ΔHox | ko,water | −ΔHwater | RSS | R2 |
|---|---|---|---|---|---|---|---|---|---|
| ER | Pd-LMO/U-AACVD | 7.42 × 102 | 65.9 | 6.32 × 103 | 65.5 | 2.17 × 103 | 88.4 | 5.2 | 0.971 |
| Pd-LMO/Imp | 1.22 × 104 | 76.9 | 9.15 × 105 | 78.0 | 6.39 × 103 | 72.5 | 5.5 | 0.978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanchovska, S.; Ivanov, G.; Harizanova, S.; Tenchev, K.; Zhecheva, E.; Naydenov, A.; Stoyanova, R. New Insight into the Interplay of Method of Deposition, Chemical State of Pd, Oxygen Storage Capability and Catalytic Activity of Pd-Containing Perovskite Catalysts for Combustion of Methane. Catalysts 2021, 11, 1399. https://doi.org/10.3390/catal11111399
Stanchovska S, Ivanov G, Harizanova S, Tenchev K, Zhecheva E, Naydenov A, Stoyanova R. New Insight into the Interplay of Method of Deposition, Chemical State of Pd, Oxygen Storage Capability and Catalytic Activity of Pd-Containing Perovskite Catalysts for Combustion of Methane. Catalysts. 2021; 11(11):1399. https://doi.org/10.3390/catal11111399
Chicago/Turabian StyleStanchovska, Silva, Georgy Ivanov, Sonya Harizanova, Krasimir Tenchev, Ekaterina Zhecheva, Anton Naydenov, and Radostina Stoyanova. 2021. "New Insight into the Interplay of Method of Deposition, Chemical State of Pd, Oxygen Storage Capability and Catalytic Activity of Pd-Containing Perovskite Catalysts for Combustion of Methane" Catalysts 11, no. 11: 1399. https://doi.org/10.3390/catal11111399
APA StyleStanchovska, S., Ivanov, G., Harizanova, S., Tenchev, K., Zhecheva, E., Naydenov, A., & Stoyanova, R. (2021). New Insight into the Interplay of Method of Deposition, Chemical State of Pd, Oxygen Storage Capability and Catalytic Activity of Pd-Containing Perovskite Catalysts for Combustion of Methane. Catalysts, 11(11), 1399. https://doi.org/10.3390/catal11111399