Understanding the Surface Characteristics of Biochar and Its Catalytic Activity for the Hydrodeoxygenation of Guaiacol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of BCR
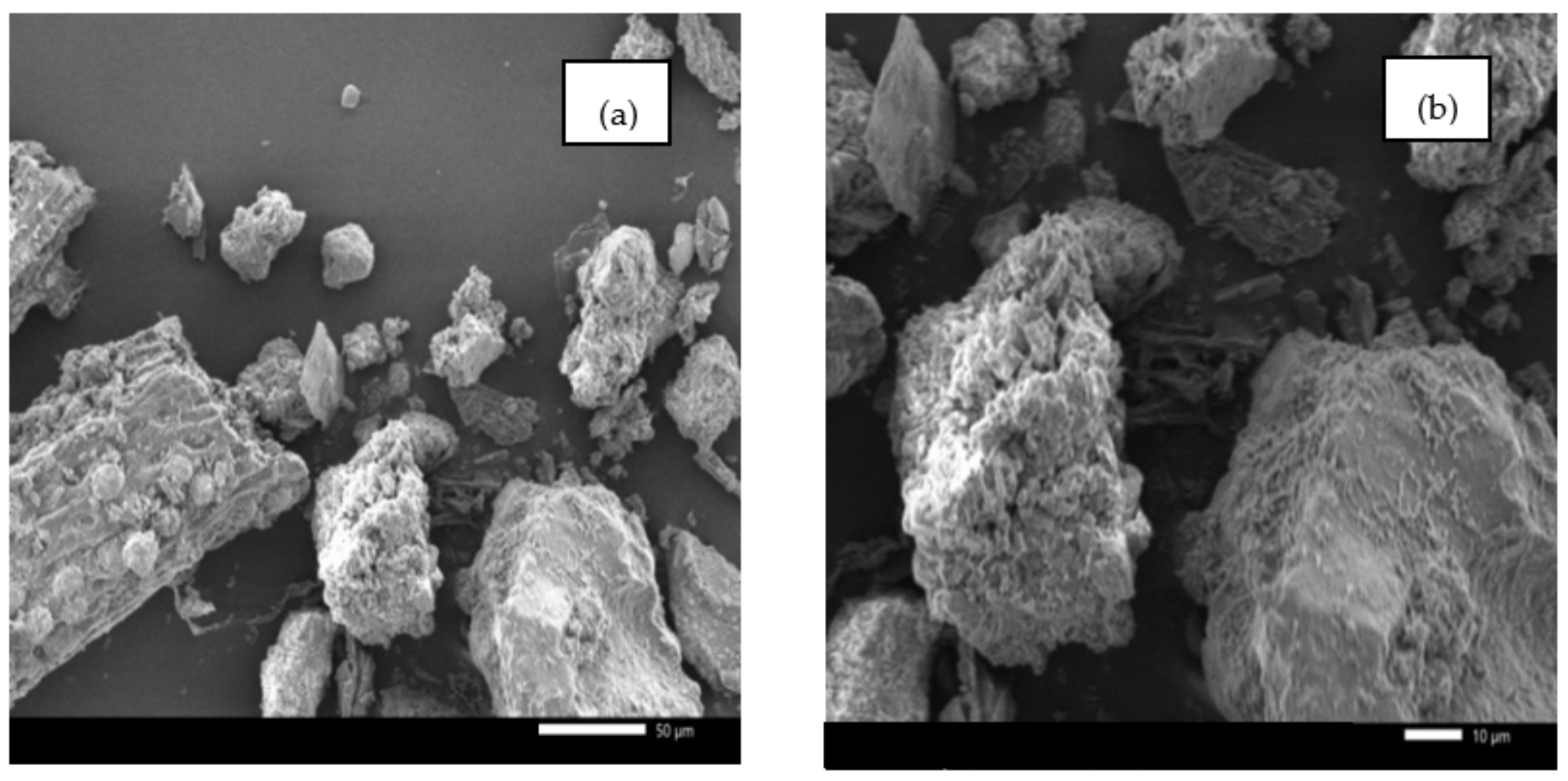
2.1.1. Morphology and Elemental Analyses
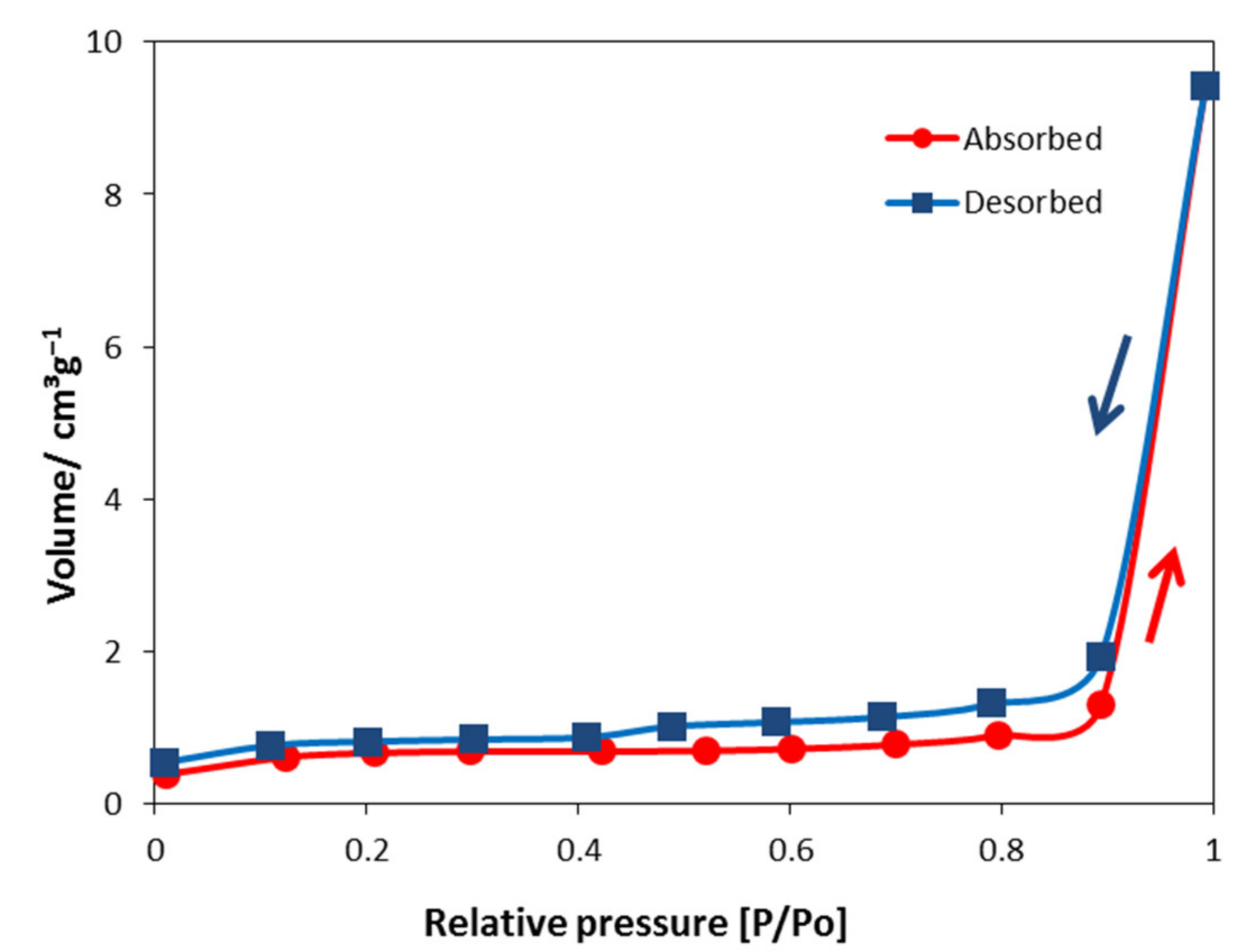
2.1.2. BET Surface Area, Pore Size, and Total Acidity–Basicity
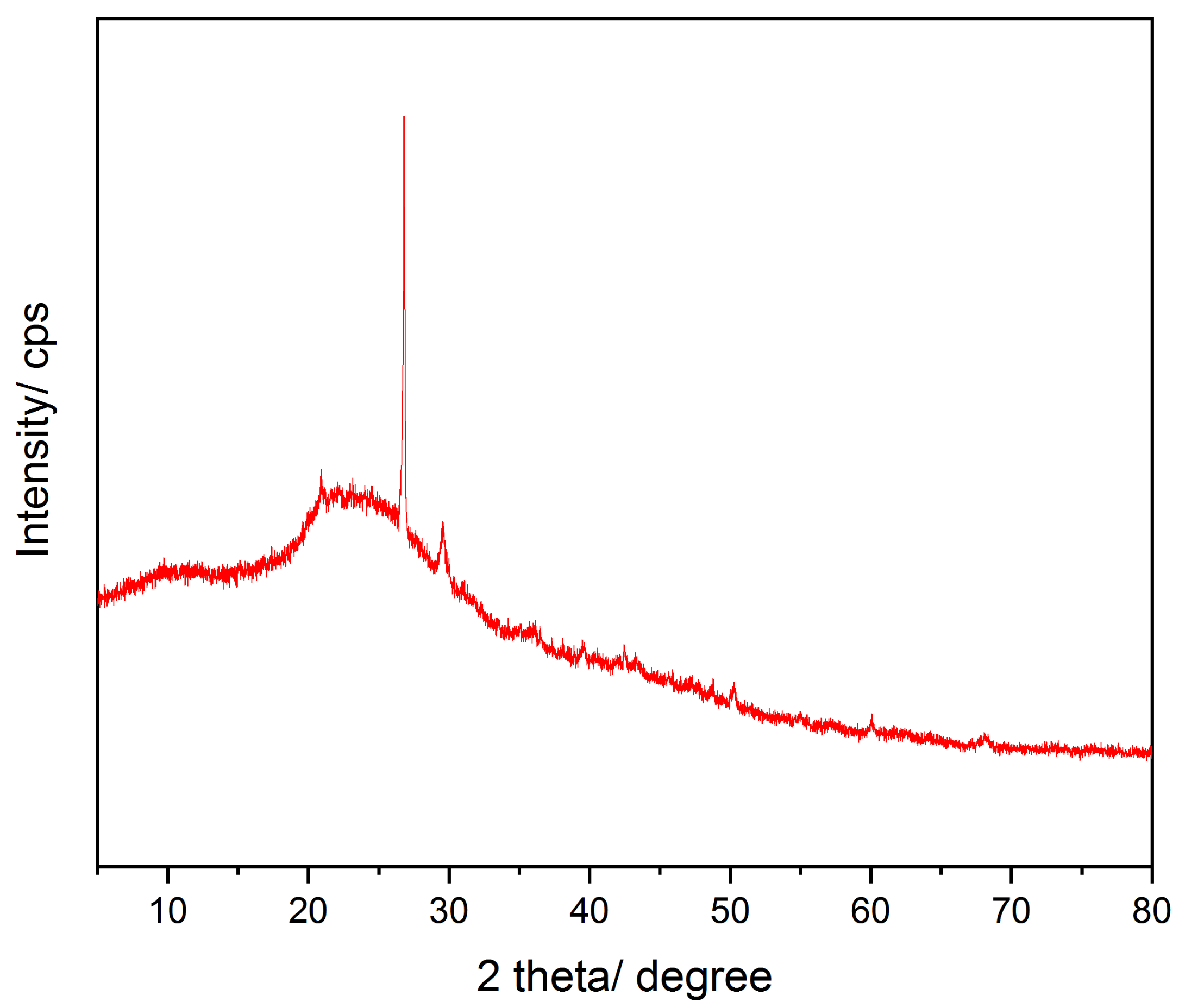
2.1.3. Powder X-ray Diffraction Analysis
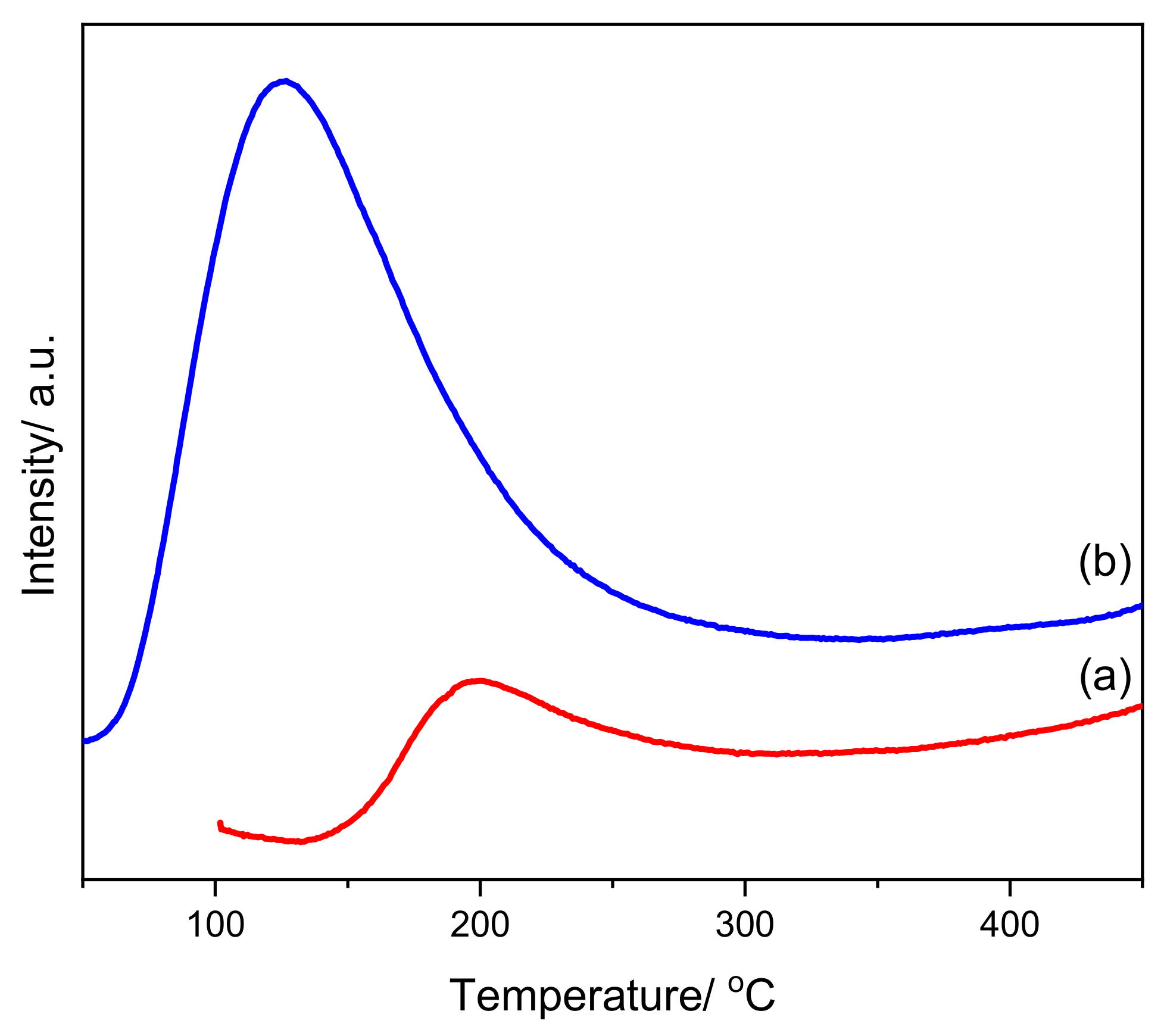
2.2. Vibrational Studies
2.3. Catalytic HDO of GUA
3. Materials and Methods
3.1. Materials
3.2. Characterization of BCR
3.3. Catalytic Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Prananta, W.; Kubiszewski, I. Assessment of Indonesia’s future renewable energy plan: A meta-analysis of biofuel energy return on investment (eroi). Energies 2021, 14, 2803. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Rulli, M.C.; Bellomi, D.; Cazzoli, A.; De Carolis, G.; D’Odorico, P. The water-land-food nexus of first-generation biofuels. Sci. Rep. 2016, 6, 22521. [Google Scholar] [CrossRef] [Green Version]
- Sudiyani, Y.; Styarini, D.; Triwahyuni, E.; Sudiyarmanto; Sembiring, K.C.; Aristiawan, Y.; Abimanyu, H.; Han, M.H. Utilization of biomass waste empty fruit bunch fiber of palm oil for bioethanol production using pilot-Scale unit. Energy Procedia 2013, 32, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Ruddy, D.A.; Schaidle, J.A.; Ferrell, J.R.; Wang, J.; Moens, L.; Hensley, J.E. Recent advances in heterogeneous catalysts for bio-oil upgrading via “ex situ catalytic fast pyrolysis”: Catalyst development through the study of model compounds. Green Chem. 2014, 16, 454–490. [Google Scholar] [CrossRef]
- Mora-Vergara, I.D.; Hernández Moscoso, L.; Gaigneaux, E.M.; Giraldo, S.A.; Baldovino-Medrano, V.G. Hydrodeoxygenation of guaiacol using NiMo and CoMo catalysts supported on alumina modified with potassium. Catal. Today 2018, 302, 125–135. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, C.L.; Wan, H.P.; Lee, H.T.; Liu, C.F. Catalytic hydrodeoxygenation of guaiacol on Rh-based and sulfided CoMo and NiMo catalysts. Energy Fuels 2011, 25, 890–896. [Google Scholar] [CrossRef]
- Adilina, I.B.; Rinaldi, N.; Simanungkalit, S.P.; Aulia, F.; Oemry, F.; Stenning, G.B.G.; Silverwood, I.P.; Parker, S.F. Hydrodeoxygenation of Guaiacol as a Bio-Oil Model Compound over Pillared Clay-Supported Nickel-Molybdenum Catalysts. J. Phys. Chem. C 2019, 123, 21429–21439. [Google Scholar] [CrossRef] [Green Version]
- Duchet, J.C.; van Oers, E.M.; de Beer, V.H.J.; Prins, R. Carbon-supported sulfide catalysts. J. Catal. 1983, 80, 386–402. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Galarneau, A.; Abid, Z.; Said, B.; Didi, Y.; Szymanska, K.; Jarzebski, A.; Tancret, F.; Hamaizi, H.; Bengueddach, A.; Di Renzo, F.; et al. Synthesis and textural characterization of mesoporous and meso-/macroporous silica monoliths obtained by spinodal decomposition. Inorganics 2016, 4, 9. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Shariff, A.; Aziz, N.S.M.; Abdullah, N. Slow Pyrolysis of Oil Palm Empty Fruit Bunches for Biochar Production and Characterisation. J. Phys. Sci. 2014, 25, 97–112. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- De Mendonça, F.G.; da Cunha, I.T.; Soares, R.R.; Tristão, J.C.; Lago, R.M. Tuning the surface properties of biochar by thermal treatment. Bioresour. Technol. 2017, 246, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourhosseini, S.E.M.; Norouzi, O.; Naderi, H.R. Study of micro/macro ordered porous carbon with olive-shaped structure derived from Cladophora glomerata macroalgae as efficient working electrodes of supercapacitors. Biomass Bioenergy 2017, 107, 287–298. [Google Scholar] [CrossRef]
- Cui, L.; Fan, Q.; Sun, J.; Quan, G.; Yan, J.; Hina, K.; Wang, H.; Zhang, Z.; Hussain, Q. Changes in surface characteristics and adsorption properties of 2,4,6-trichlorophenol following Fenton-like aging of biochar. Sci. Rep. 2021, 11, 4293. [Google Scholar] [CrossRef] [PubMed]
- Balajii, M.; Niju, S. Biochar-derived heterogeneous catalysts for biodiesel production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- Fraga, M.A.; Jordão, E.; Mendes, M.J.; Freitas, M.M.A.; Faria, J.L.; Figueiredo, J.L. Properties of carbon-supported platinum catalysts: Role of carbon surface sites. J. Catal. 2002, 209, 355–364. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Zhang, L.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Hu, G.; Hu, X. Co-presence of hydrophilic and hydrophobic sites in Ni/biochar catalyst for enhancing the hydrogenation activity. Fuel 2021, 293, 120426. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Liu, W.J.; Zeng, F.X.; Jiang, H.; Zhang, X.S. Preparation of high adsorption capacity bio-chars from waste biomass. Bioresour. Technol. 2011, 102, 8247–8252. [Google Scholar] [CrossRef]
- Popov, A.; Kondratieva, E.; Goupil, J.M.; Mariey, L.; Bazin, P.; Gilson, J.P.; Travert, A.; Maugé, F. Bio-oils hydrodeoxygenation: Adsorption of phenolic molecules on oxidic catalyst supports. J. Phys. Chem. C 2010, 114, 15661–15670. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Do, H.N.; Do, H.N.; Long, Q.N. One-step preparation of rice husk-based magnetic biochar and its catalytic activity for p-nitrophenol degradation. Chem. Eng. Trans. 2020, 78, 379–384. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ling, L.L.; Jiang, H. Selective hydrogenation of lignin to produce chemical commodities by using a biochar supported Ni-Mo2C catalyst obtained from biomass. Green Chem. 2016, 18, 4032–4041. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Nunes, P.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; García, A.M.; Díaz-Díez, M.A. Production of activated carbons from coffee endocarp by CO2 and steam activation. Fuel Process. Technol. 2008, 89, 262–268. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kołtowski, M.; Charmas, B.; Skubiszewska-Zięba, J.; Oleszczuk, P. Effect of biochar activation by different methods on toxicity of soil contaminated by industrial activity. Ecotoxicol. Environ. Saf. 2017, 136, 119–125. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Shao, Q.; Wang, G. Physicochemical properties evolution of chars from palm kernel shell pyrolysis. J. Therm. Anal. Calorim. 2018, 133, 1271–1280. [Google Scholar] [CrossRef]
- Bakhtiar, M.H.A.B.M.; Sari, N.B.A.; Yaacob, A.B.; Yunus, M.F.B.M.; Ismail, K.B. Characterization of oil palm Empty Fruit Bunch (EFB) biochar activated with potassium hydroxide under different pyrolysis temperature. J. Eng. Sci. Technol. 2019, 14, 2792–2807. [Google Scholar]
- Zhong, Y.; Deng, Q.; Yao, Q.; Lu, C.; Zhang, P.; Li, H.; Wang, J.; Zeng, Z.; Zou, J.J.; Zou, J.J.; et al. Functionalized Biochar with Superacidity and Hydrophobicity as a Highly Efficient Catalyst in the Synthesis of Renewable High-Density Fuels. ACS Sustain. Chem. Eng. 2020, 8, 7785–7794. [Google Scholar] [CrossRef]
- Cui, X.; Shao, H.; Song, Y.; Yang, S.; Wang, F.; Liu, H. Preparation of highly interconnected porous polymer microbeads: Via suspension polymerization of high internal phase emulsions for fast removal of oil spillage from aqueous environments. RSC Adv. 2019, 9, 25730–25738. [Google Scholar] [CrossRef] [Green Version]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Fan, X.D.; Wu, Y.J.; Tu, R.; Sun, Y.; Jiang, E.C.; Xu, X.W. Hydrodeoxygenation of guaiacol via rice husk char supported Ni based catalysts: The influence of char supports. Renew. Energy 2020, 157, 1035–1045. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- He, S.; Zhong, L.; Duan, J.; Feng, Y.; Yang, B.; Yang, L. Bioremediation of wastewater by iron Oxide-Biochar nanocomposites loaded with photosynthetic bacteria. Front. Microbiol. 2017, 8, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Silverwood, I.P.; Hamilton, N.G.; Laycock, C.J.; Staniforth, J.Z.; Ormerod, R.M.; Frost, C.D.; Parker, S.F.; Lennon, D. Quantification of surface species present on a nickel/alumina methane reforming catalyst. Phys. Chem. Chem. Phys. 2010, 12, 3102–3107. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, T.; Bourdéron, C.; Sandorfy, C. Model Calculations on the Influence of Mechanical and Electrical Anharmonicity on Infrared Intensities: Relation to Hydrogen Bonding. Can. J. Chem. 1972, 50, 3161–3166. [Google Scholar] [CrossRef] [Green Version]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Albers, P.W.; Weber, W.; Möbus, K.; Wieland, S.D.; Parker, S.F. Neutron scattering study of the terminating protons in the basic structural units of non-graphitising and graphitising carbons. Carbon 2016, 109, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Angoni, K. Remarks on the structure of carbon materials on the basis of Raman spectra. Carbon 1993, 31, 537–547. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Saito, R. Characterizing graphene, graphite, and carbon nanotubes by Raman spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Albers, P.W.; Pietsch, J.; Krauter, J.; Parker, S.F. Investigations of activated carbon catalyst supports from different natural sources. Phys. Chem. Chem. Phys. 2003, 5, 1941–1949. [Google Scholar] [CrossRef]
- Spencer, E.C.; Ross, N.L.; Parker, S.F.; Olsen, R.E.; Woodfield, B.F. Inelastic neutron scattering studies of hydrated CuO, ZnO and CeO2 nanoparticles. Chem. Phys. 2013, 427, 66–70. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Bifunctional transalkylation and hydrodeoxygenation of anisole over a Pt/HBeta catalyst. J. Catal. 2011, 281, 21–29. [Google Scholar] [CrossRef]
- Davidson, A.L.; Webb, P.B.; Parker, S.F.; Lennon, D. Hydrogen Partitioning as a Function of Time-on-Stream for an Unpromoted Iron-Based Fischer-Tropsch Synthesis Catalyst Applied to CO Hydrogenation. Ind. Eng. Chem. Res. 2020, 59, 52–60. [Google Scholar] [CrossRef]
- Olcese, R.N.; Bettahar, M.; Petitjean, D.; Malaman, B.; Giovanella, F.; Dufour, A. Gas-phase hydrodeoxygenation of guaiacol over Fe/SiO2 catalyst. Appl. Catal. B Environ. 2012, 115–116, 63–73. [Google Scholar] [CrossRef]
- Li, C.; Nakagawa, Y.; Tamura, M.; Nakayama, A.; Tomishige, K. Hydrodeoxygenation of guaiacol to phenol over ceria-supported iron catalysts. ACS Catal. 2020, 10, 14624–14639. [Google Scholar] [CrossRef]
- Tieuli, S.; Mäki-Arvela, P.; Peurla, M.; Eränen, K.; Wärnå, J.; Cruciani, G.; Menegazzo, F.; Murzin, D.Y.; Signoretto, M. Hydrodeoxygenation of isoeugenol over Ni-SBA-15: Kinetics and modelling. Appl. Catal. A Gen. 2019, 580, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Luo, Z.; Wang, W.; Cen, J. A study of the mechanisms of guaiacol pyrolysis based on free radicals detection technology. Catalysts 2020, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Centeno, A.; Laurent, E.; Delmon, B. Influence of the support of CoMo Sulfide Catalysts and of the Addition of Potassium and Platinum on the Catalytic Performances for the hydrodeoxygenation of Carbonyl, Carboxyl, and Guaiacol-Type Molecules. J. Catal. 1995, 154, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Sulman, A.; Mäki-Arvela, P.; Bomont, L.; Alda-Onggar, M.; Fedorov, V.; Russo, V.; Eränen, K.; Peurla, M.; Akhmetzyanova, U.; Skuhrovcová, L.; et al. Kinetic and Thermodynamic Analysis of Guaiacol Hydrodeoxygenation. Catal. Lett. 2019, 149, 2453–2467. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.L.; Mäki-Arvela, P.; Wärnå, J.; Monzón, A.; Centeno, M.A.; Murzin, D.Y. Hydrodeoxygenation of vanillin over noble metal catalyst supported on biochars: Part II: Catalytic behaviour. Appl. Catal. B Environ. 2020, 268. [Google Scholar] [CrossRef]
- Tran, C.C.; Mohan, O.; Banerjee, A.; Mushrif, S.H.; Kaliaguine, S. A combined experimental and DFT investigation of selective hydrodeoxygenation of guaiacol over bimetallic carbides. Energy Fuels 2020, 34, 16265–16273. [Google Scholar] [CrossRef]
- ISIS Neutron and Muon Source. Available online: https://www.isis.stfc.ac.uk/Pages/home.aspx (accessed on 1 October 2021).
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Škoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2018, 896, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Parker, S.F.; Lennon, D.; Albers, P.W. Vibrational spectroscopy with neutrons: A review of new directions. Appl. Spectrosc. 2011, 65, 1325–1341. [Google Scholar] [CrossRef]
- Ewings, R.A.; Stewart, J.R.; Perring, T.G.; Bewley, R.I.; Le, M.D.; Raspino, D.; Pooley, D.E.; Škoro, G.; Waller, S.P.; Zacek, D.; et al. Upgrade to the MAPS neutron time-of-flight chopper spectrometer. Rev. Sci. Instrum. 2019, 90, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
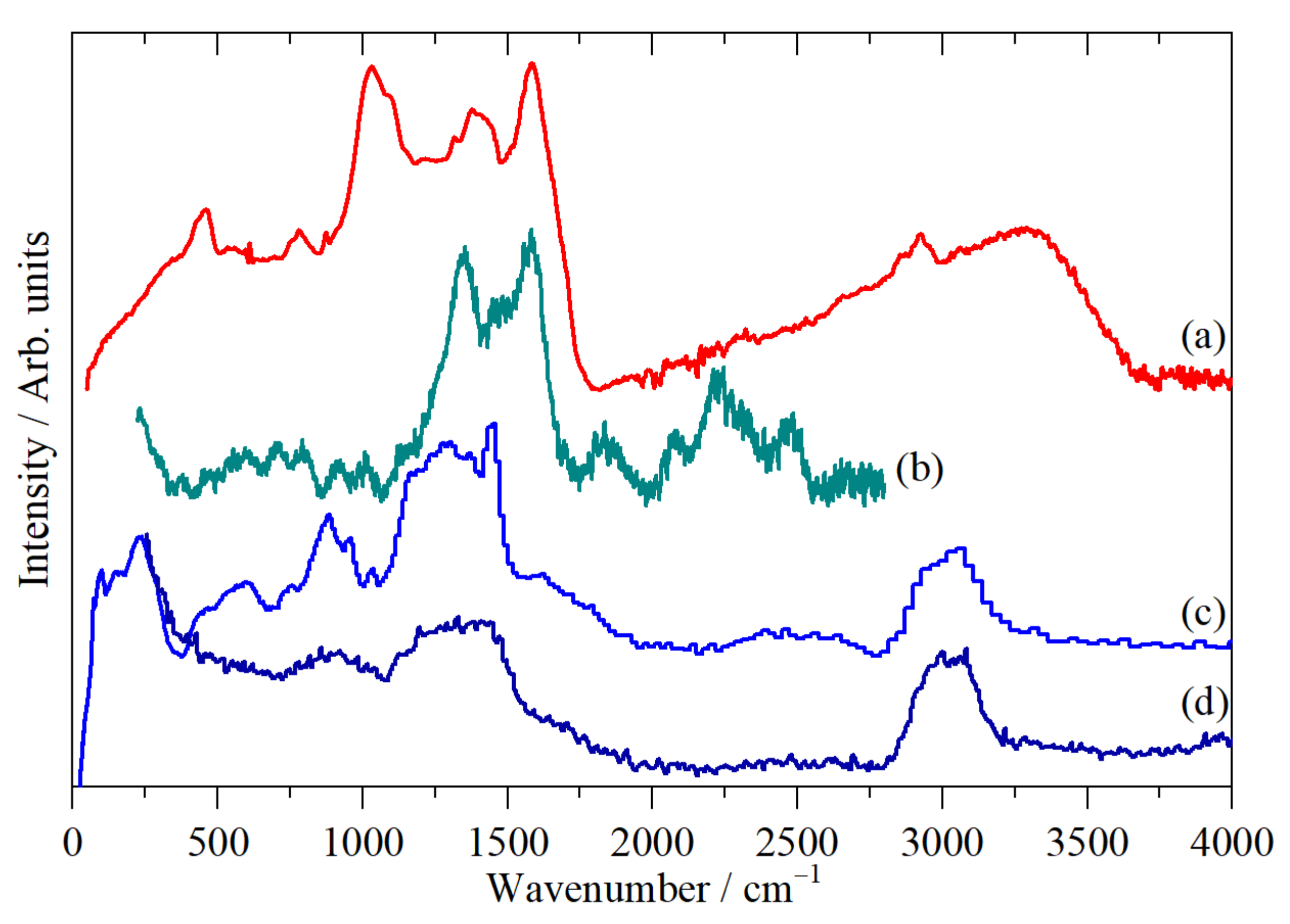
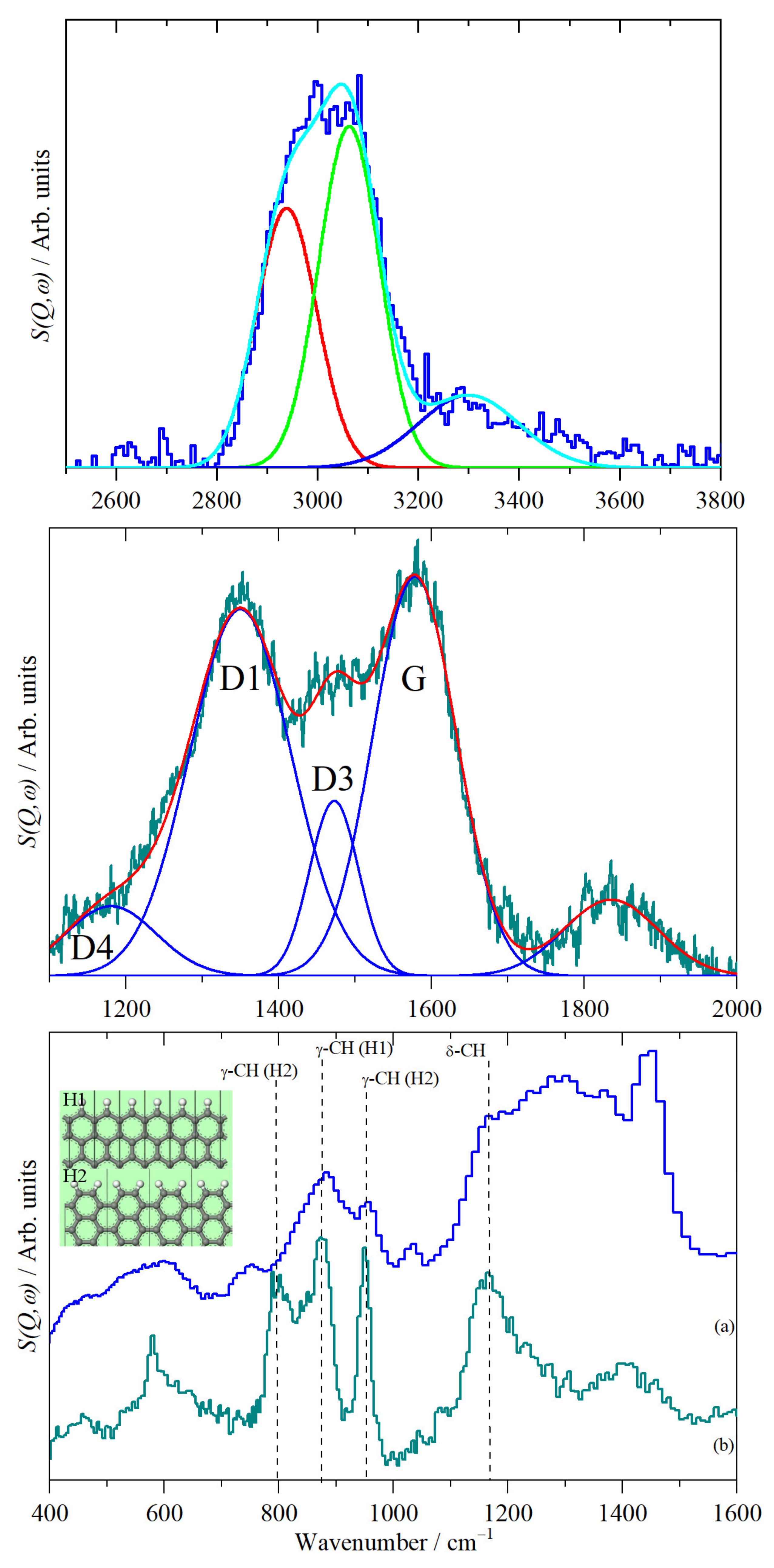
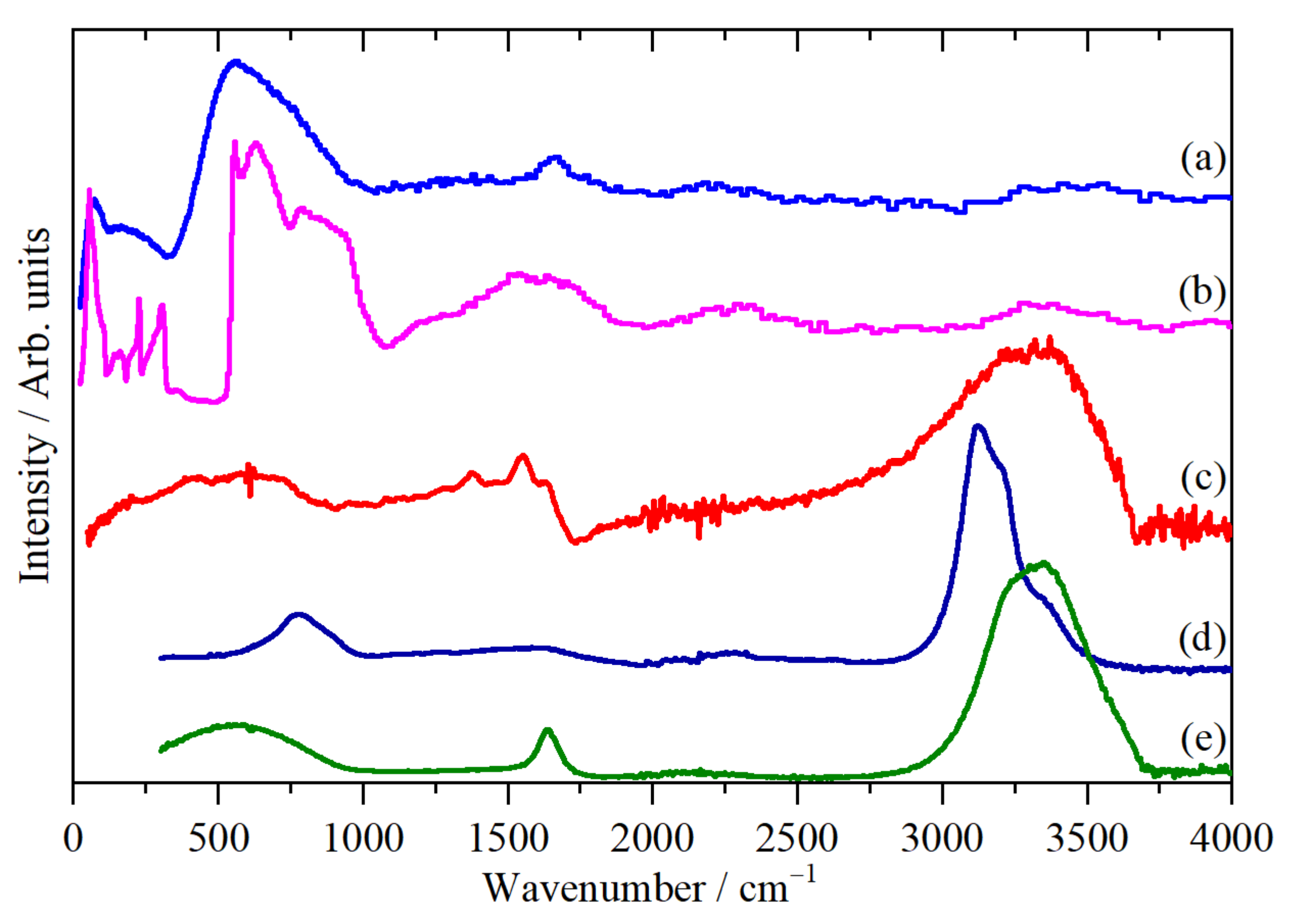

| Element | CHN/wt % | SEM-EDS/wt % | XRF/wt % |
|---|---|---|---|
| C | 40.59 | 66.71 | - |
| H | 2.72 | - | - |
| N | 3.53 | - | - |
| O | 53.16 a | 30.45 | - |
| Mg | - | - | 4.98 |
| Al | - | - | 3.76 |
| Si | - | 1.18 | 37.63 |
| P | - | - | 4.62 |
| S | - | - | 3.88 |
| Cl | - | - | 1.27 |
| K | - | 0.52 | 14.16 |
| Ca | - | 0.56 | 18.45 |
| Ti | - | - | 0.48 |
| Mn | - | - | 0.56 |
| Fe | - | - | 9.51 |
| H/C molar ratio | 0.80 | - | - |
| O/C molar ratio | - | 0.34 | - |
| Properties | Value |
|---|---|
| BET surface area/m2 g−1 | 2.14 |
| Pore size/nm | 27.31 |
| Pore volume/cm3 g−1 | 0.01 |
| Total acidity/mmol g−1 | 0.15 |
| Total basicity/mmol g−1 | 0.50 |
| FTIR | Raman | INS | Assignment 1 |
|---|---|---|---|
| 3300 | - | 3300 | ν OH |
| - | - | 3060 | ν sp2-CH |
| 2924 | - | 2940 | ν sp3-CH2 |
| 2855 | - | ||
| - | 2475 | - | G + 915 |
| - | 2230 | - | D1 + 915 |
| - | 2070 | - | D1 + 790 |
| - | 1835 | - | 2 × 915 |
| 1585 | 1578 | - | aromatic ring stretch (G-band) |
| 1450 | - | 1450 | CH2 scissors |
| - | 1472 | - | aromatic ring stretch (D3-band) |
| 1378 | - | 1370 | CH2 wag |
| - | 1350 | 1300 | aromatic ring stretch (D1-band) |
| - | 1180 | - | aromatic ring stretch (D4-band) |
| - | - | 1160 | δ sp2-CH |
| 1099 | - | - | ν C–O–C |
| 1028 | - | 1032 | |
| - | - | 955 | γ CH |
| - | 920 | - | - |
| 874 | - | 882 | γ CH (H1) |
| - | 790 | - | - |
| 781 | - | - | γ CH |
| - | - | 752 | γ CH |
| - | 712 | - | - |
| - | 604 | 605 | - |
| - | - | 525 | τ CC |
| 465 | 470 | 457 | |
| 423 | - | 431 | |
| - | - | 232 | |
| - | - | 148 | |
| - | - | 99 |

| Catalyst | Temperature /K | Pressure/ bar | Time/ h | Conversion /% | Product Distribution/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | Others | |||||
| BCR | 523 | 20 | 6 | 100 | 60 | 10 | 19 | 0 | 0 | 11 |
| PILC [9] | 623 | 20 | 6 | 78 | 42 | 10 | 5 | 6 | 5 | 32 |
| No catalyst | 523 | 20 | 6 | 22 | 0 | 0 | 0 | 0 | 60 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adilina, I.B.; Widjaya, R.R.; Hidayati, L.N.; Supriadi, E.; Safaat, M.; Oemry, F.; Restiawaty, E.; Bindar, Y.; Parker, S.F. Understanding the Surface Characteristics of Biochar and Its Catalytic Activity for the Hydrodeoxygenation of Guaiacol. Catalysts 2021, 11, 1434. https://doi.org/10.3390/catal11121434
Adilina IB, Widjaya RR, Hidayati LN, Supriadi E, Safaat M, Oemry F, Restiawaty E, Bindar Y, Parker SF. Understanding the Surface Characteristics of Biochar and Its Catalytic Activity for the Hydrodeoxygenation of Guaiacol. Catalysts. 2021; 11(12):1434. https://doi.org/10.3390/catal11121434
Chicago/Turabian StyleAdilina, Indri Badria, Robert Ronal Widjaya, Luthfiana Nurul Hidayati, Edi Supriadi, Muhammad Safaat, Ferensa Oemry, Elvi Restiawaty, Yazid Bindar, and Stewart F. Parker. 2021. "Understanding the Surface Characteristics of Biochar and Its Catalytic Activity for the Hydrodeoxygenation of Guaiacol" Catalysts 11, no. 12: 1434. https://doi.org/10.3390/catal11121434
APA StyleAdilina, I. B., Widjaya, R. R., Hidayati, L. N., Supriadi, E., Safaat, M., Oemry, F., Restiawaty, E., Bindar, Y., & Parker, S. F. (2021). Understanding the Surface Characteristics of Biochar and Its Catalytic Activity for the Hydrodeoxygenation of Guaiacol. Catalysts, 11(12), 1434. https://doi.org/10.3390/catal11121434






