The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review
Abstract
:1. Introduction
2. Raw Material—The Vegetable Oils and Animal Fats
| Oil/Fat | The Content of Major Fatty Acid, % | ||||
|---|---|---|---|---|---|
| Palmitic (16:0) * | Stearic (18:0) | Oleic (18:1) | Linoleic (18:2) | Linolenic (18:3) | |
| Palm | 45 | 8 | 38 | 10 | 0.5 |
| Soybean | 10 | 4 | 21 | 56 | 8 |
| Rapeseed | 4.5 | 1.5 | 56 | 21 | 10 |
| Sunflower | 6.5 | 5 | 24 | 63 | 0.3 |
| Olive | 11.5 | 2.5 | 74 | 9.5 | 1.5 |
| Jatropha oil | 15.4 | 5 | 37 | 42.2 | 0.3 |
| Camelina sativa | 8.3 | 0.5 | 19.5 | 46.5 | 25.1 |
| Lard | 24 | 14 | 44 | 10.7 | 0.4 |
| Beef tallow | 26 | 20 | 40 | 4.5 | 0.5 |
| Mutton tallow | 27 | 32 | 32 | 1.6 | 0.2 |
3. The Properties and Comparison of Biodiesel and Green Diesel
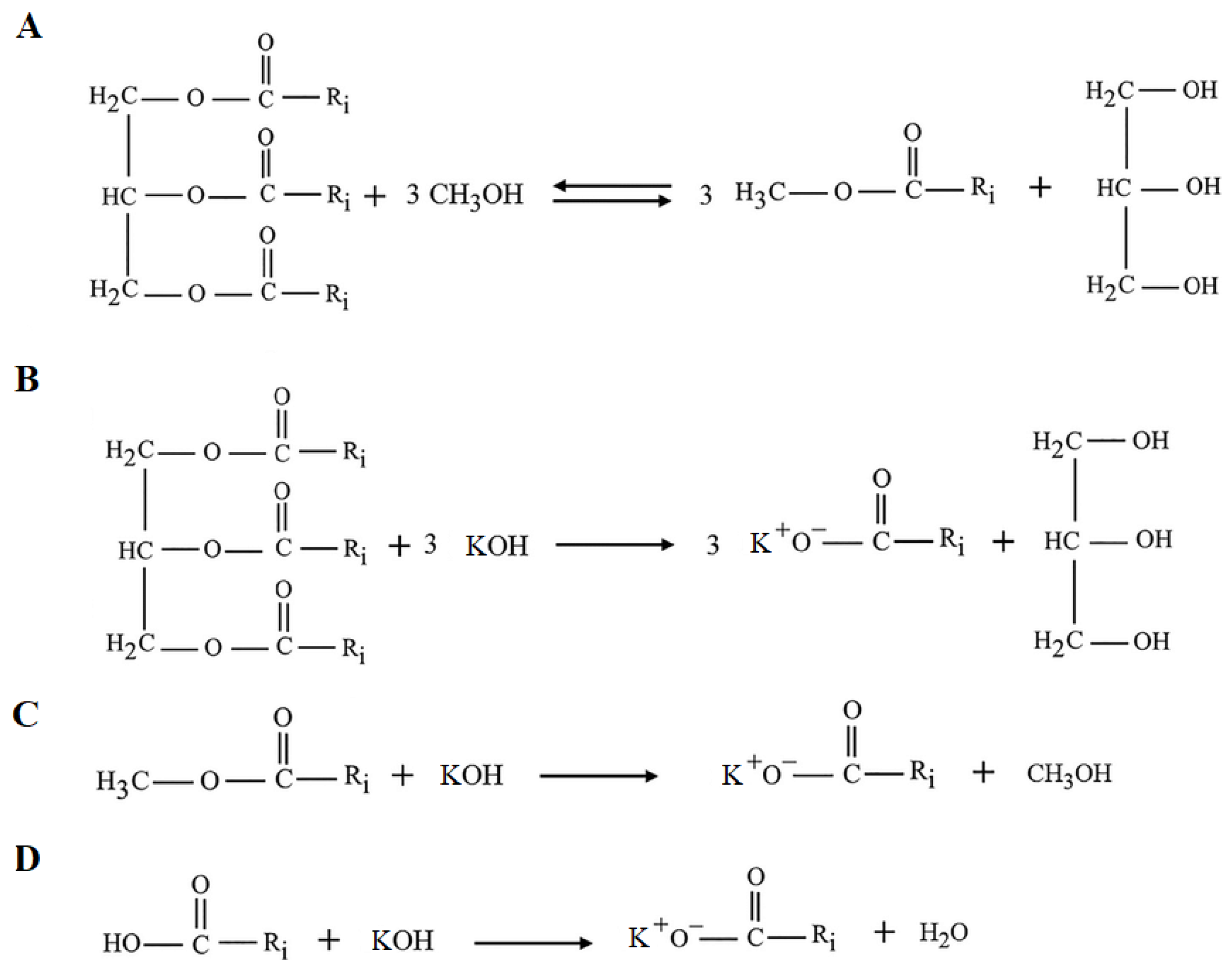
4. The Ester Production—Transesterification
4.1. Alcohols
4.2. The Types of Catalysis
4.2.1. Homogeneous Catalysis
4.2.2. Heterogeneous Catalysis
| Type of Catalyst | Feedstock (Oil/Alcohol) | Operating Conditions (Temp./Amount of Catalyst/Molar Ratio Alcohol to Oil) | Yield, % | Ref. |
|---|---|---|---|---|
| La-Ca/halloysite | palm oil/MeOH | 150 °C/7 wt%/18:1 | 97.5 | [103] |
| quicklime | mustard seed oil/MeOH | 60 °C/9.8 wt%/6.1:1 | 96.5 | [104] |
| kettle limescale | UCO/MeOH | 60 °C/14 wt%/2.15:5 (v/v) | 97.2 | [105] |
| GO5.0Al(HSP) | sunflower oil/MeOH | 120 °C/1 wt%/30:1 | 97 | [106] |
| Fe/Ba/Al2O3 | UCO/MeOH | 65 °C/6 wt%/18:1 (wt/wt) | 84.2 | [107] |
| Ce/dolomite | palm oil/MeOH | 65 °C/0.05 wt%/15 | 97.2 | [108] |
| CaO/wollastonite | palm oil/MeOH | 65 °C/8 wt%/15:1 | 97.6 | [109] |
| Mg/clinoptilolite | UCO/MeOH | 70 °C/4 wt%/16:1 | 98.7 | [110] |
| K/kaolinite | UCO/MeOH | 70 °C/15 wt%/14:1 | 94.8 | [111] |
| Zn/CaO | UCO/MeOH | 65 °C/5 wt%/20:1 | 96.7 | [112] |
| Al2O3/Fe3O4 | UCO/MeOH | 100 °C/5 wt%/30:1 | 77 | [113] |
| S-La2O3/NaY | castor oil/EtOH | 70 °C/10 wt%/15.1:1 | 84.6 | [114] |
| TiO2-CaO | palm oil/MeOH | 65 °C/0.5 wt%/5:3 | 95 | [115] |
| MgO-Al2O3 | castor oil/BuOH | 80 °C/5 wt%/6:1 | 97 | [116] |
| Cu/Zn/Al2O3 | UCO/MeOH | 65 °C/10 wt%/20:1 | 89.5 | [117] |
| sulfonated active carbon | soybean oil/EtOH | 75 °C/20 wt%/6:1 | 88.7 | [118] |
| modified graphene | RSO/MeOH | 130 °C/1 wt%/12:1 | 80 | [119] |
| Ca-Al mixed oxide | RSO/MeOH | 65 °C/4 wt%/24:1 | 90 | [92] |
| Mg-Fe mixed oxide | RSO/MeOH | 117 °C/4 wt%/24:1 | 70 | [120] |
| Mg-Al mixed oxide | RSO/MeOH | 117 °C/4 wt%/24:1 | 75 | [121] |
4.3. The Separation and Purification
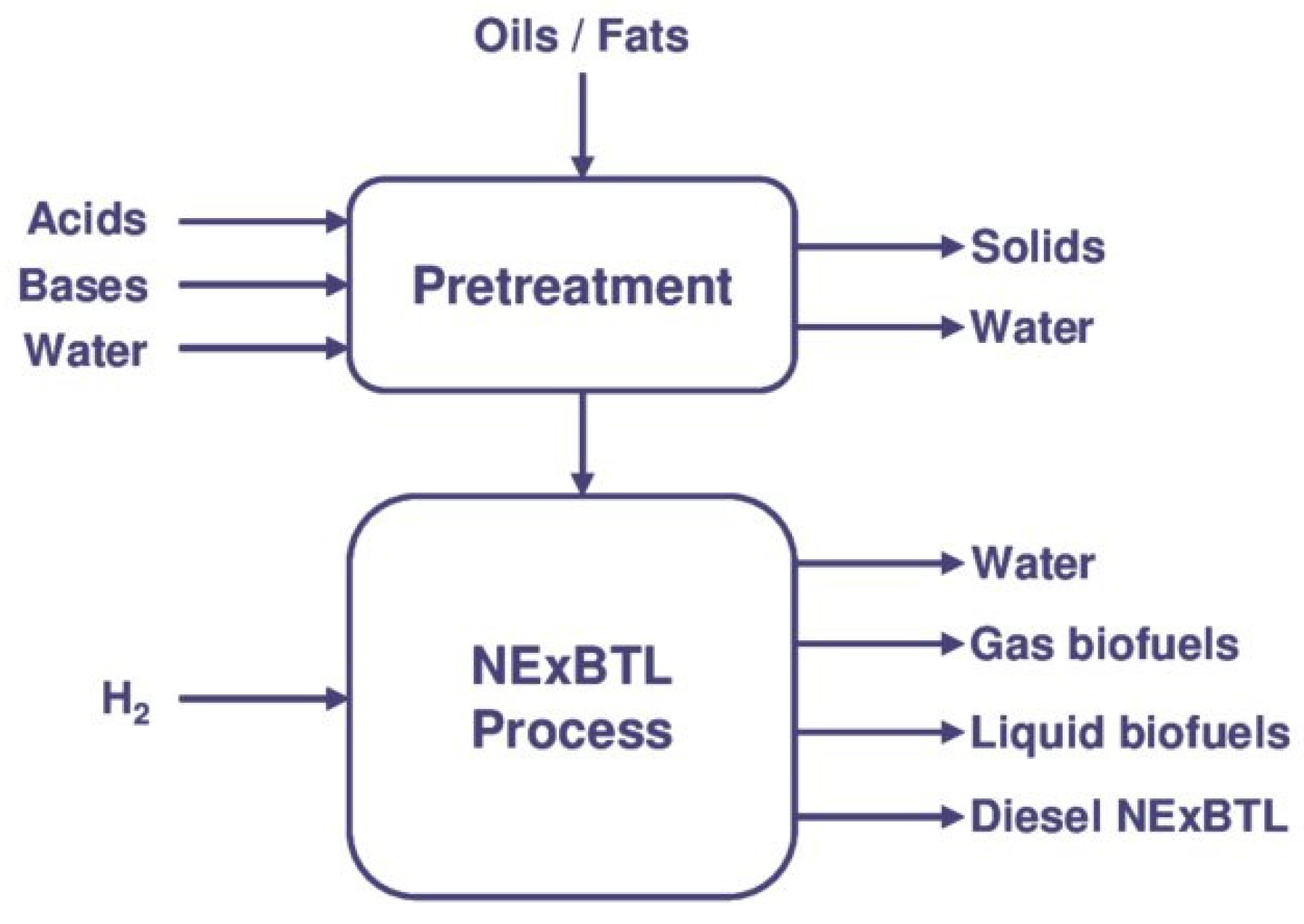
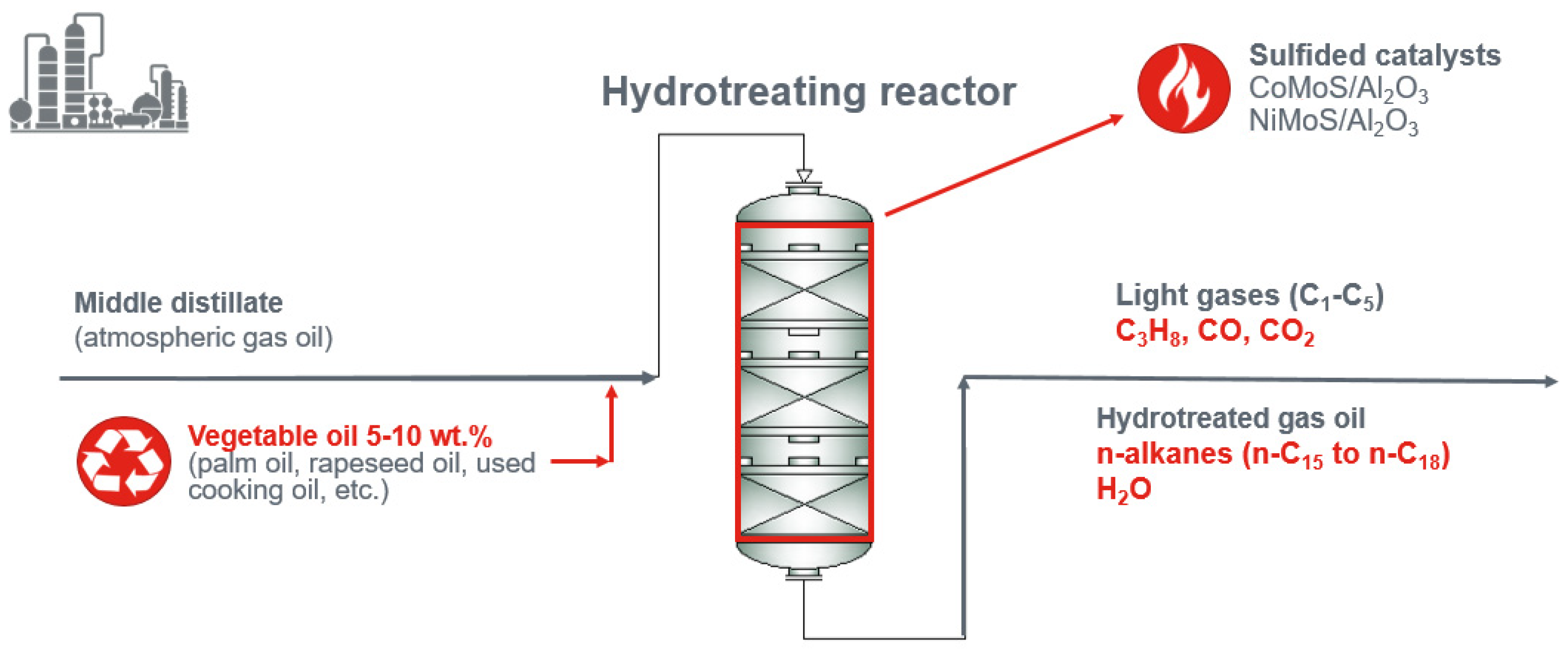
5. The Green Diesel Production–Co-Processing
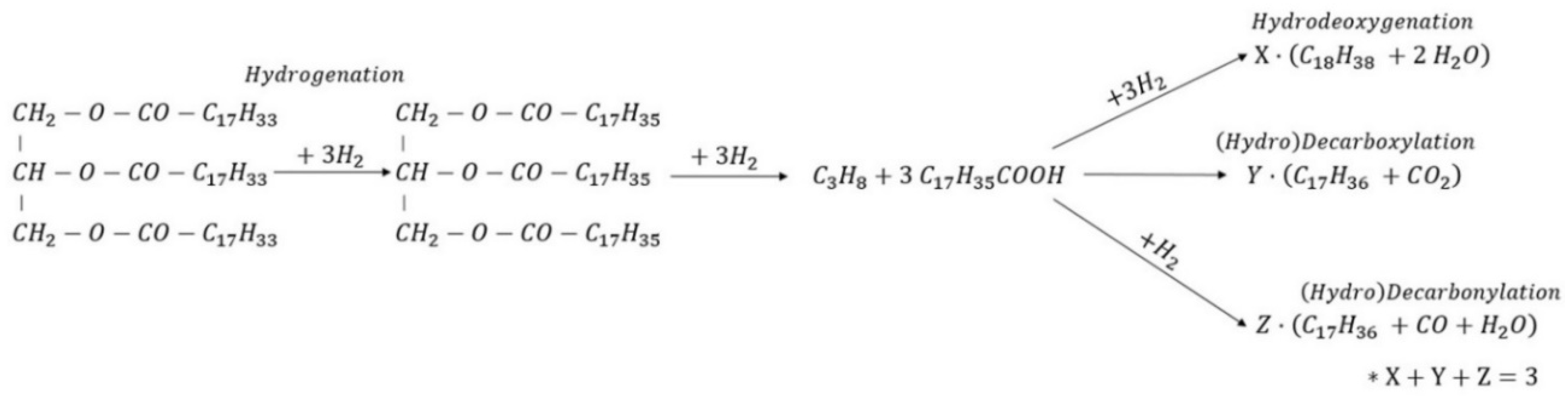
5.1. Reaction Pathways of Triacylglycerols Hydrotreating
5.2. Hydrogen Consumption Due to Triacylglycerols Co-Processing
5.3. Suitable Catalysts for Triacylglycerols Co-Processing
| Catalysts | Feedstock | Operating Conditions (Temperature, °C/Pressure, MPa/L/W-HSV, h−1) | Reference |
|---|---|---|---|
| NiMo/Al2O3 (commercial) | Heavy gas oil/UCO: 90/10, 70/30 | 310, 330, 350/8.4/LHSV = 1.0 | [150] |
| NiMo/Al2O3 CoMo/Al2O3 (both commercial) | Heavy atmospheric gas oil/UCO: 90/10, 70/30 | 330, 350, 370/5.6/LHSV = 1.0 | [136] |
| NiMo/Al2O3 (commercial) | AGO/Used frying oil: 80/20, 50/50 | 320, 350/5.5/WHSV = 2.0 | [141] |
| NiMo/Al2O3 (commercial) | SRGO/UCO: 80/20 SRGO/Animal fat: 80/20 SRGO/Palm oil: 80/20 | 350/5.5/LHSV = 2.0 | [151] |
| NiMo/Al2O3 (commercial) | Light gas oil/RSO: 90/10, 80/20 | 320, 350, 380/3.0, 5.0/LHSV = 2.0 | [36] |
| CoMo/Al2O3 (commercial) NiMo/Al2O3 (synthesized) | SRGO/Jatropha oil: 90/10, 80/20, 70/30, 60/40, 50/50 | 300/5.0 | [152] |
| NiW/SiO2-Al2O3 NiMo/Al2O3 | Gas oil/UCO: 75/25 | 340–380/5.0/LHSV = 2.0, 4.0 | [153] |
| NiMo/Al2O3 (commercial) | Heavy vacuum gas oil/canola oil: 95/5, 90/10, 80/20 | 360–395/8.0–10.0/LHSV = 1.0–2.5 | [154] |
| CoMo/γ-Al2O3 (commercial) | Gas oil/Palm oil: 95/5, 90/10 | 330, 350, 365/3.3/WHSV = 0.85, 1.0, 1.4 | [155] |
| NiW/(Pseudoboehmite + SBA-15 | LCO/WCO 50/50 | 365/9.5/75 min | [156] |
| Mo/Al2O3 + NiMo/Al2O3-SAPO-11) | SRGO/RSO 70/30 | 350, 380/4.0–7.0/LHSV = 1.0–1.5 | [157] |
| CoMo/Al2O3 (commercial) | Gas oil/cottonseed oil: 90/10 | 305–345/3.0/WHSV = 5–25 | [158] |
5.4. Sulphur-Free Catalysts
6. The Used Oil as Bio-Lubricants
7. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGO | Atmospheric gasoil |
| DG | Diacylglycerols(s) |
| E | Ester(s) |
| EP | The ester phase |
| EH | Ethylhexyl |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid(s) |
| HDS | Hydrodesulfurization |
| HDN | Hydrodenitrogenation |
| HDO | Hydrodeoxygenation |
| HVO | Hydrotreated vegetable oil |
| G | Glycerol |
| GP | The glycerol phase |
| ME | Methyl esters |
| MG | Monoacylglycerols(s) |
| RSO | Rapeseed oil |
| SRGO | Straight run gas oil |
| TG | Triacylglycerol(s) |
| TOFA | Tall oil fatty acids |
| UCO | Used cooking oil |
References
- Rutter, P.; Keirstead, J. A brief history and the possible future of urban energy systems. Energ Policy 2012, 50, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Rodrigue, J.P. The Geography of Transport. Systems; Taylor & Francis: New York, NY, USA, 2020. [Google Scholar]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Aron, N.S.M.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels—A review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef]
- Carriquiry, M.A.; Du, X.D.; Timilsina, G.R. Second generation biofuels: Economics and policies. Energy Policy 2011, 39, 4222–4234. [Google Scholar] [CrossRef] [Green Version]
- Peralta, Y.; Sanchez, E.; Kafarov, V. Exergy Analysis for Third Generation Biofuel Production from Microalgae Biomass. Chem. Eng. Trans. 2010, 21, 1363–1368. [Google Scholar]
- Plata, V.; Kafarov, V.; Moreno, N. Optimization of Third Generation Biofuels Production: Biodiesel from Microalgae Oil by Homogeneous Transesterification. Chem. Eng. 2010, 21, 1201–1206. [Google Scholar]
- European Parliament. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources; European Parliament: Brussels, Belgium, 2018.
- Weaver, J. Biodiesel Industry Overview & Technical Update; National Biodiesel Board: Jefferson City, MO, USA, 2020. [Google Scholar]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2021, 285, 119110. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Aitlaalim, A.; Ouanji, F.; Benzaouak, A.; El Mahi, M.; Lotfi, E.; Kacimi, M.; Liotta, L.F. Utilization of Waste Grooved Razor Shell (GRS) as a Catalyst in Biodiesel Production from Refined and Waste Cooking Oils. Catalysts 2020, 10, 703. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Lee, D.S.; Noh, B.S.; Bae, S.Y.; Kim, K. Characterization of fatty acids composition in vegetable oils by gas chromatography and chemometrics. Anal. Chim. Acta 1998, 358, 163–175. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Environ. Sci. Technol. 2009, 6, 677–694. [Google Scholar] [CrossRef] [Green Version]
- Smidrkal, J.; Filip, V.; Belohlav, Z.; Zamostny, P.; Honig, V. Current State and Prospects of Utilization of Vegetable Oils. Chem. Listy 2008, 102, 984–991. [Google Scholar]
- Kocik, J.; Samikannu, A.; Bourajoini, H.; Pham, T.N.; Mikkola, J.P.; Hajek, M.; Capek, L. Screening of active solid catalysts for esterification of tall oil fatty acids with methanol. J. Clean. Prod. 2017, 155, 34–38. [Google Scholar] [CrossRef]
- Ramos, M.; Dias, A.P.S.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel Production Processes and Sustainable Raw Materials. Energies 2019, 12, 4408. [Google Scholar] [CrossRef] [Green Version]
- Savoire, R.; Lanoiselle, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Bowden, S.A.; Monaghan, P.B.; Wilson, R.; Parnell, J.; Cooper, J.M. The liquid-liquid diffusive extraction of hydrocarbons from a North Sea oil using a microfluidic format. Lab Chip 2006, 6, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Widmann, B. Production of vegetable oils in decentral plants and aspects of quality management—Investigations on plants in practice to optimise the process. Biomass Energy Ind. 1998, 124–127. [Google Scholar]
- Šmidrkal, J.; Filip, V.; Bělohlav, Z.; Zámostný, P.; Hönig, V. Současný stav a perspektivity využití rostlinného oleje. Chem. Pap. 2008, 102, 984–991. [Google Scholar]
- Santori, G.; Di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Demirbas, A. Relationships derived from physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]
- Huo, H.; Wang, M.; Bloyd, C.; Putsche, V. Life-Cycle Assessment of Energy Use and Greenhouse Gas Emissions of Soybean-Derived Biodiesel and Renewable Fuels. Environ. Sci. Technol. 2009, 43, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [Green Version]
- Geller, D.P.; Goodrum, J.W. Effects of specific fatty acid methyl esters on diesel fuel lubricity. Fuel 2004, 83, 2351–2356. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Sastry, N.V.; Patel, M.C. Densities, excess molar volumes, viscosities, speeds of sound, excess isentropic compressibilities, and relative permittivities for alkyl (methyl, ethyl, butyl, and isoamyl) acetates plus glycols at different temperatures. J. Chem. Eng. Data 2003, 48, 1019–1027. [Google Scholar] [CrossRef]
- Walendziewski, J. Hydroprocessing of Light Cracking Catalytic Oil, Vacuum Gas Oil and Their Blends. Erdol. Kohle Erdgas Petrochem. 1991, 44, 475–479. [Google Scholar]
- Simacek, P.; Kubicka, D.; Kubickova, I.; Homola, F.; Pospisil, M.; Chudoba, J. Premium quality renewable diesel fuel by hydroprocessing of sunflower oil. Fuel 2011, 90, 2473–2479. [Google Scholar] [CrossRef]
- Geller, D.P.; Goodrum, J.W. Rheology of vegetable oil analogs and triglycerides. J. Am. Oil Chem. Soc. 2000, 77, 111–114. [Google Scholar] [CrossRef]
- Liang, X.Z.; Gao, S.; Wu, H.H.; Yang, J.G. Highly efficient procedure for the synthesis of biodiesel from soybean oil. Fuel Process. Technol. 2009, 90, 701–704. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.M.R.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Kumar, N.; Varun; Chauhan, S.R. Performance and emission characteristics of biodiesel from different origins: A review. Renew. Sustain. Energy Rev. 2013, 21, 633–658. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Liaquat, A.M.; Masjuki, H.H.; Kalam, M.A.; Mofijur, M. Ignition delay, combustion and emission characteristics of diesel engine fueled with biodiesel. Renew. Sustain. Energy Rev. 2013, 21, 623–632. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Robbins, C. Review of the effects of biodiesel on NOx emissions. Fuel Process. Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M. Influence of fuel properties and composition on NOx emissions from biodiesel powered diesel engines: A review. Renew. Sustain. Energy Rev. 2012, 16, 3702–3710. [Google Scholar] [CrossRef]
- Altin, R.; Cetinkaya, S.; Yucesu, H.S. The potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers. Manag. 2001, 42, 529–538. [Google Scholar] [CrossRef]
- Miao, X.L.; Wu, Q.Y. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Firoz, S. A review: Advantages and Disadvantages of Biodiesel. Int. Res. J. Eng. Technol. 2017, 4, 530–535. [Google Scholar]
- Hollebone, B.; Yang, Z. Biofuels in the environment: A review of behaviours, fates, effects and possible remediation techniques. In Proceedings of the 32. AMOP Technical Seminar on Environmental Contamination and Response, Ottawa, ON, Canada, 9–11 June 2009. [Google Scholar]
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Hajek, M.; Skopal, F.; Kwiecien, J. Biodiesel preparation in a batch emulsification reactor. Eur. J. Lipid Sci. Technol. 2009, 111, 979–984. [Google Scholar] [CrossRef] [Green Version]
- Hajek, M.; Vavra, A.; Mach, P.; Strakova, A. The use of cosolvents in heterogeneously and homogeneously catalysed methanolysis of oil. J. Environ. Manag. 2020, 262, 110295. [Google Scholar] [CrossRef]
- Cernoch, M.; Hajek, M.; Skopal, F. Study of effects of some reaction conditions on ethanolysis of rapeseed oil with dispergation. Bioresour. Technol. 2010, 101, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Cernoch, M.; Hajek, M.; Skopal, F. Relationships among flash point, carbon residue, viscosity and some impurities in biodiesel after ethanolysis of rapeseed oil. Bioresour. Technol. 2010, 101, 7397–7401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajek, M.; Skopal, F.; Capek, L.; Cernoch, M.; Kutalek, P. Ethanolysis of rapeseed oil by KOH as homogeneous and as heterogeneous catalyst supported on alumina and CaO. Energy 2012, 48, 392–397. [Google Scholar] [CrossRef]
- Hajek, M.; Skopal, F.; Vavra, A.; Kocik, J. Transesterification of rapeseed oil by butanol and separation of butyl ester. J. Clean. Prod. 2017, 155, 28–33. [Google Scholar]
- Nimcevic, D.; Puntigam, R.; Worgetter, M.; Gapes, J.R. Preparation of rapeseed oil esters of lower aliphatic alcohols. J. Am. Oil Chem. Soc. 2000, 77, 275–280. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Hajek, M.; Vavra, A.; Muck, J. Butanol as a co-solvent for transesterification of rapeseed oil by methanol under homogeneous and heterogeneous catalyst. Fuel 2020, 277, 118239. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Zeng, D.; Yang, L.; Fang, T. Process optimization, kinetic and thermodynamic studies on biodiesel production by supercritical methanol transesterification with CH3ONa catalyst. Fuel 2017, 203, 739–748. [Google Scholar] [CrossRef]
- Andreo-Martinez, P.; Garcia-Martinez, N.; Duran-del-Amor, M.D.; Quesada-Medina, J. Advances on kinetics and thermodynamics of non-catalytic supercritical methanol transesterification of some vegetable oils to biodiesel. Energy Convers. Manag. 2018, 173, 187–196. [Google Scholar] [CrossRef]
- Santos, S.; Puna, J.; Gomes, J. A Review on Bio-Based Catalysts (Immobilized Enzymes) Used for Biodiesel Production. Energies 2020, 13, 3013. [Google Scholar] [CrossRef]
- Lopez-Fernandez, J.; Benaiges, M.D.; Valero, F. Rhizopus oryzae Lipase, a Promising Industrial Enzyme: Biochemical Characteristics, Production and Biocatalytic Applications. Catalysts 2020, 10, 1277. [Google Scholar] [CrossRef]
- Aarthy, M.; Saravanan, P.; Gowthaman, M.K.; Rose, C.; Kamini, N.R. Enzymatic transesterification for production of biodiesel using yeast lipases: An overview. Chem. Eng. Res. Des. 2014, 92, 1591–1601. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Veljković, V.B.; Avramović, J.M.; Stamenković, O.S. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2012, 16, 1193–1209. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Kwiecien, J.; Hajek, M.; Skopal, F. The effect of the acidity of rapeseed oil on its transesterification. Bioresour. Technol. 2009, 100, 5555–5559. [Google Scholar] [CrossRef] [Green Version]
- Kwiecien, J.; Hajek, M.; Skopal, F. Combined effect of water and KOH on rapeseed oil methanolysis. Bioresour. Technol. 2010, 101, 3121–3125. [Google Scholar] [CrossRef] [Green Version]
- Pullen, J.; Saeed, K. Investigation of the factors affecting the progress of base-catalyzed transesterification of rapeseed oil to biodiesel FAME. Fuel Process. Technol. 2015, 130, 127–135. [Google Scholar] [CrossRef]
- Hajek, M.; Kwiecien, J.; Skopal, F. Biodiesel: The influence of dealcoholization on reaction mixture composition after neutralization of catalyst by carbon dioxide. Fuel 2012, 96, 85–89. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; Skopal, F.; Straková, A.; Douda, M. The description of catalyst behaviour during transesterification of rapeseed oil—Formation of micellar emulsion. Renew. Energy 2020, 159, 938–943. [Google Scholar] [CrossRef]
- Hajek, M.; Skopal, F.; Machek, J. Simplification of separation of the reaction mixture after transesterification of vegetable oil. Eur. J. Lipid Sci. Technol. 2008, 110, 347–350. [Google Scholar] [CrossRef] [Green Version]
- Hajek, M.; Skopal, F. Factors affecting the separation of the reaction mixture after transesterification of rapeseed oil. Eur. J. Lipid Sci. Technol. 2008, 110, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Vavra, A.; Hajek, M.; Skopal, F. The removal of free fatty acids from methyl ester. Renew. Energy 2017, 103, 695–700. [Google Scholar] [CrossRef]
- Vavra, A.; Hajek, M.; Skopal, F. Acceleration and simplification of separation by addition of inorganic acid in biodiesel production. J. Clean. Prod. 2018, 192, 390–395. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Guerreiro, L.; Castanheiro, J.E.; Fonseca, I.M.; Martin-Aranda, R.M.; Ramos, A.M.; Vital, J. Transesterification of soybean oil over sulfonic acid functionalised polymeric membranes. Catal. Today 2006, 118, 166–171. [Google Scholar] [CrossRef]
- Goff, M.J.; Bauer, N.S.; Lopes, S.; Sutterlin, W.R.; Suppes, G.J. Acid-catalyzed alcoholysis of soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 415–420. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenergy 2008, 32, 892–895. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification Kinetics of Soybean Oil. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Das, S.; Thakur, A.J.; Deka, D. Two-Stage Conversion of High Free Fatty Acid Jatropha curcas Oil to Biodiesel Using Bronsted Acidic Ionic Liquid and KOH as Catalysts. Sci. World J. 2014, 2014, 180983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canakci, M.; Van Gerpen, J. Biodiesel production via acid catalysis. ASABE Trans. 1999, 42, 1203–1210. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Korstad, J. Latest developments on application of heterogenous basic catalysts for an efficient and eco friendly synthesis of biodiesel: A review. Fuel 2011, 90, 1309–1324. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production? A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Q.; Zhao, X.Q.; Wang, Y.J.; Zhang, J.Y. Synthesis of diphenyl carbonate by transesterification over lead and zinc double oxide catalyst. Appl. Catal. Gen. 2004, 260, 19–24. [Google Scholar]
- Kocik, J.; Hajek, M.; Troppova, I. The factors influencing stability of Ca-Al mixed oxides as a possible catalyst for biodiesel production. Fuel Process. Technol. 2015, 134, 297–302. [Google Scholar] [CrossRef]
- Hajek, M.; Kocik, J.; Frolich, K.; Vavra, A. Mg-Fe mixed oxides and their rehydrated mixed oxides as catalysts for transesterification. J. Clean. Prod. 2017, 161, 1423–1431. [Google Scholar] [CrossRef]
- Perveen, S.; Hanif, M.A.; Nadeem, R.; Rashid, U.; Azeem, M.W.; Zubair, M.; Nisar, N.; Alharthi, F.A.; Moser, B.R. A Novel Route of Mixed Catalysis for Production of Fatty Acid Methyl Esters from Potential Seed Oil Sources. Catalysts 2021, 11, 811. [Google Scholar] [CrossRef]
- Gutierrez-Ortega, N.; Ramos-Ramirez, E.; Serafin-Munoz, A.; Zamorategui-Molina, A.; Monjaraz-Vallejo, J. Use of Co/Fe-Mixed Oxides as Heterogeneous Catalysts in Obtaining Biodiesel. Catalysts 2019, 9, 403. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, M.C.G.; Jimenez-Urbistondo, I.; Santamaria-Gonzalez, J.; Merida-Robles, J.M.; Moreno-Tost, R.; Rodriguez-Castellon, E.; Jimenez-Lopez, A.; Azevedo, D.C.S.; Cavalcante, C.L.; Maireles-Torres, P. CaO supported on mesoporous silicas as basic catalysts for transesterification reactions. Appl. Catal. Gen. 2008, 334, 35–43. [Google Scholar] [CrossRef]
- Hussain, F.; Alshahrani, S.; Abbas, M.M.; Khan, H.M.; Jamil, A.; Yaqoob, H.; Soudagar, M.E.M.; Imran, M.; Ahmad, M.; Munir, M. Waste Animal Bones as Catalysts for Biodiesel Production; A Mini Review. Catalysts 2021, 11, 630. [Google Scholar] [CrossRef]
- Catarino, M.; Ramos, M.; Dias, A.P.S.; Santos, M.T.; Puna, J.F.; Gomes, J.F. Calcium Rich Food Wastes Based Catalysts for Biodiesel Production. Waste Biomass Valoriz. 2017, 8, 1699–1707. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Kant Bhatia, R.; Jeon, J.-M.; Pugazhendhi, A.; Kumar Awasthi, M.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An overview on advancements in biobased transesterification methods for biodiesel production: Oil resources, extraction, biocatalysts, and process intensification technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Frolich, K.; Vavra, A.; Kocik, J.; Hajek, M.; Jilkova, A. The long-term catalytic performance of mixed oxides in fixed-bed reactors in transesterification. Renew. Energy 2019, 143, 1259–1267. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous catalysts for biodiesel production. Energy Fuel 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, S.; Niu, S.; Lyu, Z.; Han, K.; Hu, X. Halloysite nanotube functionalized with La-Ca bimetallic oxides as novel transesterification catalyst for biodiesel production with molecular simulation. Energy Convers. Manag. 2020, 220, 113138. [Google Scholar] [CrossRef]
- Kostić, M.D.; Djalović, I.G.; Stamenković, O.S.; Mitrović, P.M.; Adamović, D.S.; Kulina, M.K.; Veljković, V.B. Kinetic modeling and optimization of biodiesel production from white mustard (Sinapis alba L.) seed oil by quicklime-catalyzed transesterification. Fuel 2018, 223, 125–139. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Study of the transesterification of waste cooking oil for the production of biodiesel in a microreactor pilot: The effect of acetone as the co-solvent. Fuel 2020, 273, 117736. [Google Scholar] [CrossRef]
- AbdelDayem, H.M.; Salib, B.G.; El-Hosiny, F.I. Facile synthesis of hydrothermal stable hierarchically macro-mesoporous hollow microspheres γ-Al2O3-graphene oxide composite: As a new efficient acid-base catalyst for transesterification reaction for biodiesel production. Fuel 2020, 277, 118106. [Google Scholar] [CrossRef]
- Sulaiman, N.F.; Hashim, A.N.N.; Toemen, S.; Rosid, S.J.M.; Mokhtar, W.N.A.W.; Nadarajan, R.; Abu Bakar, W.A.W. Biodiesel production from refined used cooking oil using co-metal oxide catalyzed transesterification. Renew. Energy 2020, 153, 1–11. [Google Scholar]
- Niu, S.L.; Zhang, X.Y.; Ning, Y.L.; Zhang, Y.J.; Qu, T.X.; Hu, X.; Gong, Z.Q.; Lu, C.M. Dolomite incorporated with cerium to enhance the stability in catalyzing transesterification for biodiesel production. Renew. Energy 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Gong, Z.; Han, K.; Wang, Y.; Lu, C. Wollastonite decorated with calcium oxide as heterogeneous transesterification catalyst for biodiesel production: Optimized by response surface methodology. Renew. Energy 2020, 159, 873–884. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Basyouny, M.G.; El Sherbeeny, A.M.; El Meligy, M.A.; Abb Elgawad, A.E.E. Transesterification of commercial waste cooking oil into biodiesel over innovative alkali trapped zeolite nanocomposite as green and environmental catalysts. Sustain. Chem. Pharm. 2020, 17, 100289. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Sayed, M.A. K+ trapped kaolinite (Kaol/K+) as low cost and eco-friendly basic heterogeneous catalyst in the transesterification of commercial waste cooking oil into biodiesel. Energy Convers. Manag. 2018, 177, 468–476. [Google Scholar] [CrossRef]
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Bayat, A.; Baghdadi, M.; Bidhendi, G.N. Tailored magnetic nano-alumina as an efficient catalyst for transesterification of waste cooking oil: Optimization of biodiesel production using response surface methodology. Energy Convers. Manag. 2018, 177, 395–405. [Google Scholar] [CrossRef]
- Du, L.X.; Ding, S.X.; Li, Z.; Lv, E.M.; Lu, J.; Ding, J.C. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar]
- Mohamad, M.; Ngadi, N.; Wong, S.L.; Jusoh, M.; Yahya, N.Y. Prediction of biodiesel yield during transesterification process using response surface methodology. Fuel 2017, 190, 104–112. [Google Scholar] [CrossRef]
- Navas, M.B.; Lick, I.D.; Bolla, P.A.; Casella, M.L.; Ruggera, J.F. Transesterification of soybean and castor oil with methanol and butanol using heterogeneous basic catalysts to obtain biodiesel. Chem. Eng. Sci. 2018, 187, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, N.F.; Lee, S.L.; Toemen, S.; Bakar, W.Z.W. Physicochemical characteristics of Cu/Zn/γ-Al2O3 catalyst and its mechanistic study in transesterification for biodiesel production. Renew. Energy 2020, 156, 142–157. [Google Scholar] [CrossRef]
- Rocha, P.D.; Oliveira, L.S.; Franca, A.S. Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renew. Energy 2019, 143, 1710–1716. [Google Scholar] [CrossRef]
- Gaidukevic, J.; Barkauskas, J.; Malaika, A.; Rechnia-Goracy, P.; Mozdzynska, A.; Jasulaitiene, V.; Kozlowski, M. Modified graphene-based materials as effective catalysts for transesterification of rapeseed oil to biodiesel fuel. Chin. J. Catal. 2018, 39, 1633–1645. [Google Scholar] [CrossRef]
- Hajek, M.; Tomasova, A.; Kocik, J.; Podzemna, V. Statistical evaluation of the mutual relations of properties of Mg/Fe hydrotalcites and mixed oxides as transesterification catalysts. Appl. Clay. Sci. 2018, 154, 28–35. [Google Scholar] [CrossRef]
- Hajek, M.; Kutalek, P.; Smolakova, L.; Troppova, I.; Capek, L.; Kubicka, D.; Kocik, J.; Thanh, D.N. Transesterification of rapeseed oil by Mg-Al mixed oxides with various Mg/Al molar ratio. Chem. Eng. J. 2015, 263, 160–167. [Google Scholar] [CrossRef]
- Vavra, A.; Hajek, M.; Kocian, D. The influence of vegetable oils composition on separation of transesterification products, especially quality of glycerol. Renew. Energy 2021, 176, 262–268. [Google Scholar] [CrossRef]
- Hajek, M.; Skopal, F.; Kwiecien, J.; Cernoch, M. Determination of esters in glycerol phase after transesterification of vegetable oil. Talanta 2010, 82, 283–285. [Google Scholar] [CrossRef]
- Hajek, M.; Skopal, F.; Cernoch, M. Effect of phase separation temperature on ester yields from ethanolysis of rapeseed oil in the presence of NaOH and KOH as catalysts. Bioresour. Technol. 2012, 110, 288–291. [Google Scholar] [CrossRef]
- Hajek, M.; Vavra, A.; Skopal, F.; Mekotova, M.; Musil, M. Biodiesel: The study of methyl esters loss in the glycerol phase at various conditions. J. Clean. Prod. 2018, 197, 1573–1578. [Google Scholar] [CrossRef]
- Cernoch, M.; Hajek, M.; Skopal, F. Ethanolysis of rapeseed oil—Distribution of ethyl esters, glycerides and glycerol between ester and glycerol phases. Bioresour. Technol. 2010, 101, 2071–2075. [Google Scholar] [CrossRef] [Green Version]
- Hajek, M.; Skopal, F. Treatment of glycerol phase formed by biodiesel production. Bioresour. Technol. 2010, 101, 3242–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; Cesar, A.D. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Fontaras, G.; Karavalakis, G.; Kousoulidou, M.; Tzamkiozis, T.; Ntziachristos, L.; Bakeas, E.; Stournas, S.; Samaras, Z. Effects of biodiesel on passenger car fuel consumption, regulated and non-regulated pollutant emissions over legislated and real-world driving cycles. Fuel 2009, 88, 1608–1617. [Google Scholar] [CrossRef]
- Katajisto, M. Neste Annual Report 2020; Neste: Helsinki, Finland, 2021. [Google Scholar]
- Bezergianni, S. Catalytic Hydroprocessing of Liquid Biomass for Biofuels Production; INTECH Open: London, UK, 2013. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kikhtyanin, O.; Kubicka, D. Refinery co-processing of renewable feeds. Prog. Energy Combust. Sci. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Cardenas, J.; Orjuela, A.; Sanchez, D.L.; Narvaez, P.C.; Katryniok, B.; Clark, J. Pre-treatment of used cooking oils for the production of green chemicals: A review. J. Clean. Prod. 2021, 289, 125129. [Google Scholar] [CrossRef]
- Douvartzides, S.L.; Charisiou, N.D.; Papageridis, K.N.; Goula, M.A. Green Diesel: Biomass Feedstocks, Production Technologies, Catalytic Research, Fuel Properties and Performance in Compression Ignition Internal Combustion Engines. Energies 2019, 12, 809. [Google Scholar] [CrossRef] [Green Version]
- Bezergianni, S.; Dimitriadis, A.; Meletidis, G. Effectiveness of CoMo and NiMo catalysts on co-hydroprocessing of heavy atmospheric gas oil-waste cooking oil mixtures. Fuel 2014, 125, 129–136. [Google Scholar] [CrossRef]
- Vonortas, A.; Kubicka, D.; Papayannakos, N. Catalytic co-hydroprocessing of gasoil-palm oil/AVO mixtures over a NiMo/gamma-Al2O3 catalyst. Fuel 2014, 116, 49–55. [Google Scholar] [CrossRef]
- El-Sawy, M.S.; Hanafi, S.A.; Ashour, F.; Aboul-Fotouh, T.M. Co-hydroprocessing and hydrocracking of alternative feed mixture (vacuum gas oil/waste lubricating oil/waste cooking oil) with the aim of producing high quality fuels. Fuel 2020, 269, 117437. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.W.; van Haveren, J.; de Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction Pathways for the Deoxygenation of Vegetable Oils and Related Model Compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef]
- Kubickova, I.; Kubicka, D. Utilization of Triglycerides and Related Feedstocks for Production of Clean Hydrocarbon Fuels and Petrochemicals: A Review. Waste Biomass Valoriz. 2010, 1, 293–308. [Google Scholar] [CrossRef]
- Carmona, H.D.; Horacek, J.; Alayon, A.B.; Hernandez, J.J.M. Suitability of used frying oil for co-processing with atmospheric gas oil. Fuel 2018, 214, 165–173. [Google Scholar] [CrossRef]
- Walendziewski, J.; Stolarski, M.; Luzny, R.; Klimek, B. Hydroprocesssing of light gas oil—Rape oil mixtures. Fuel Process. Technol. 2009, 90, 686–691. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; Chen, J.W.; Ng, S. Fluid Catalytic Cracking of Biomass-Derived Oils and Their Blends with Petroleum Feedstocks: A Review. Energy Fuel 2012, 26, 5355–5372. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dagonikou, V. Effect of CO2 on catalytic hydrotreatment of gas-oil. Can. J. Chem. Eng. 2015, 93, 1017–1023. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dagonikou, V.; Sklari, S. The suspending role of H2O and CO on catalytic hydrotreatment of gas-oil; myth or reality? Fuel Process. Technol. 2016, 144, 20–26. [Google Scholar] [CrossRef]
- Egeberg, R.; Skyum, L.; Zeuthen, P. Hydrotreating in the production of green diesel. Pet. Technol. Q. 2010, 15, 101–113. [Google Scholar]
- Imai, H.; Kimura, T.; Terasaka, K.; Li, X.H.; Sakashita, K.; Asaoka, S.; Al-Khattaf, S.S. Hydroconversion of fatty acid derivative over supported Ni-Mo catalysts under low hydrogen pressure. Catal. Today 2018, 303, 185–190. [Google Scholar] [CrossRef]
- Kubicka, D.; Horacek, J. Deactivation of HDS catalysts in deoxygenation of vegetable oils. Appl. Catal. Gen. 2011, 394, 9–17. [Google Scholar] [CrossRef]
- Veriansyah, B.; Han, J.Y.; Kim, S.K.; Hong, S.A.; Kim, Y.J.; Lim, J.S.; Shu, Y.W.; Oh, S.G.; Kim, J. Production of renewable diesel by hydroprocessing of soybean oil: Effect of catalysts. Fuel 2012, 94, 578–585. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Temperature effect on co-hydroprocessing of heavy gas oil-waste cooking oil mixtures for hybrid diesel production. Fuel 2013, 103, 579–584. [Google Scholar] [CrossRef]
- Carmona, H.D.; Alfaro, O.D.; Alayon, A.B.; Vazquez, M.A.R.; Hernandez, J.J.M. Co-processing of straight run gas oil with used cooking oil and animal fats. Fuel 2019, 254, 115583. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Chiranjeevi, T.; Gokak, D.T.; Viswanathan, P.S. Studies on co-processing of jatropha oil with diesel fraction in hydrodesulfurization. Fuel Process. Technol. 2014, 118, 180–186. [Google Scholar] [CrossRef]
- Rana, B.S.; Kumar, R.; Tiwari, R.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Transportation fuels from co-processing of waste vegetable oil and gas oil mixtures. Biomass Bioenergy 2013, 56, 43–52. [Google Scholar] [CrossRef]
- Chen, J.W.; Farooqi, H.; Fairbridge, C. Experimental Study on Co-hydroprocessing Canola Oil and Heavy Vacuum Gas Oil Blends. Energy Fuel 2013, 27, 3306–3315. [Google Scholar] [CrossRef]
- Vonortas, A.; Templis, C.; Papayannakos, N. Effect of Palm Oil Content on Deep Hydrodesulfurization of Gas Oil-Palm Oil Mixtures. Energy Fuel 2012, 26, 3856–3863. [Google Scholar] [CrossRef]
- Herrador, J.M.H.; Psenicka, M.; Horacek, J.; Tisler, Z.; Vrablik, A.; Cerny, R.; Murat, M. Co-processing of Waste Cooking Oil and Light Cycle Oil with NiW/(Pseudoboehmite + SBA-15) Catalyst. Chem. Eng. Technol. 2019, 42, 512–517. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Porsin, A.A.; Aleksandrov, P.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Co-hydroprocessing of straight-run gasoil—Rapeseed oil mixture over stacked bed Mo/Al2O3 + NiMo/Al2O3-SAPO-11 catalysts. Fuel 2021, 285, 115583. [Google Scholar] [CrossRef]
- Sebos, I.; Matsoukas, A.; Apostolopoulos, V.; Papayannakos, N. Catalytic hydroprocessing of cottonseed oil in petroleum diesel mixtures for production of renewable diesel. Fuel 2009, 88, 145–149. [Google Scholar] [CrossRef]
- Furimsky, E. Metal carbides and nitrides as potential catalysts for hydroprocessing. Appl. Catal. Gen. 2003, 240, 1–28. [Google Scholar] [CrossRef]
- Sousa, L.A.; Zotin, J.L.; da Silva, V.T. Hydrotreatment of sunflower oil using supported molybdenum carbide. Appl. Catal. Gen. 2012, 449, 105–111. [Google Scholar] [CrossRef]
- Carmona, H.D.; Horacek, J.; Tisler, Z.; Akhmetzyanova, U. Sulfur free supported MoCx and MoNx catalysts for the hydrotreatment of atmospheric gasoil and its blends with rapeseed oil. Fuel 2019, 254, 115582. [Google Scholar] [CrossRef]
- Carmona, H.D.; Akhmetzyanova, U.; Tisler, Z.; Vondrova, P. Hydrotreating atmospheric gasoil and co-processing with rapeseed oil using supported Ni-Mo and Co-Mo carbide catalysts. Fuel 2020, 268, 117363. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.M.; Jiang, J.C.; Liu, P.; Li, F.L.; Ye, J.; Zhou, M.H. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Nickel sulfide, nickel phosphide and nickel carbide catalysts for bio-hydrotreated fuel production. Energy Convers. Manag. 2017, 151, 324–333. [Google Scholar] [CrossRef]
- Horacek, J.; Akhmetzyanova, U.; Skuhrovcova, L.; Tisler, Z.; Carmona, H.D. Alumina-supported MoNx, MoCx and MoPx catalysts for the hydrotreatment of rapeseed oil. Appl. Catal. B-Environ. 2020, 263, 118328. [Google Scholar] [CrossRef]
- Tran, C.C.; Akmach, D.; Kaliaguine, S. Hydrodeoxygenation of vegetable oils over biochar supported bimetallic carbides for producing renewable diesel under mild conditions. Green Chem. 2020, 22, 6424–6436. [Google Scholar] [CrossRef]
- Wang, H.L.; Yan, S.L.; Salley, S.O.; Ng, K.Y.S. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Carmona, H.D.; Svobodova, E.; Tisler, Z.; Akhmetzyanova, U.; Strejcova, K. Hydrotreating of Atmospheric Gas Oil and Co-Processing with Rapeseed Oil Using Sulfur-Free PMoCx/Al2O3 Catalysts. ACS Omega 2021, 6, 7680–7692. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B.; Sawhill, S.J.; Bale, D.H.; Main, R.; Phillips, D.C.; Korlann, S.; Self, R.; Bussell, M.E. Hydrodesulfurization over supported monometallic, bimetallic and promoted carbide and nitride catalysts. Catal. Today 2003, 86, 191–209. [Google Scholar] [CrossRef]
- Jin, G.Z.; Zhu, J.H.; Fan, X.J.; Sun, G.D.; Gao, J.B. Effect of Ni promoter on dibenzothiophene hydrodesulfurization performance of molybdenum carbide catalyst. Chin. J. Catal 2006, 27, 899–903. [Google Scholar] [CrossRef]
- Masudi, A.; Muraza, O. Vegetable Oil to Biolubricants: Review on Advanced Porous Catalysts. Energy Fuel 2018, 32, 10295–10310. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Mohamad, E.N.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The prospects of biolubricants as alternatives in automotive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
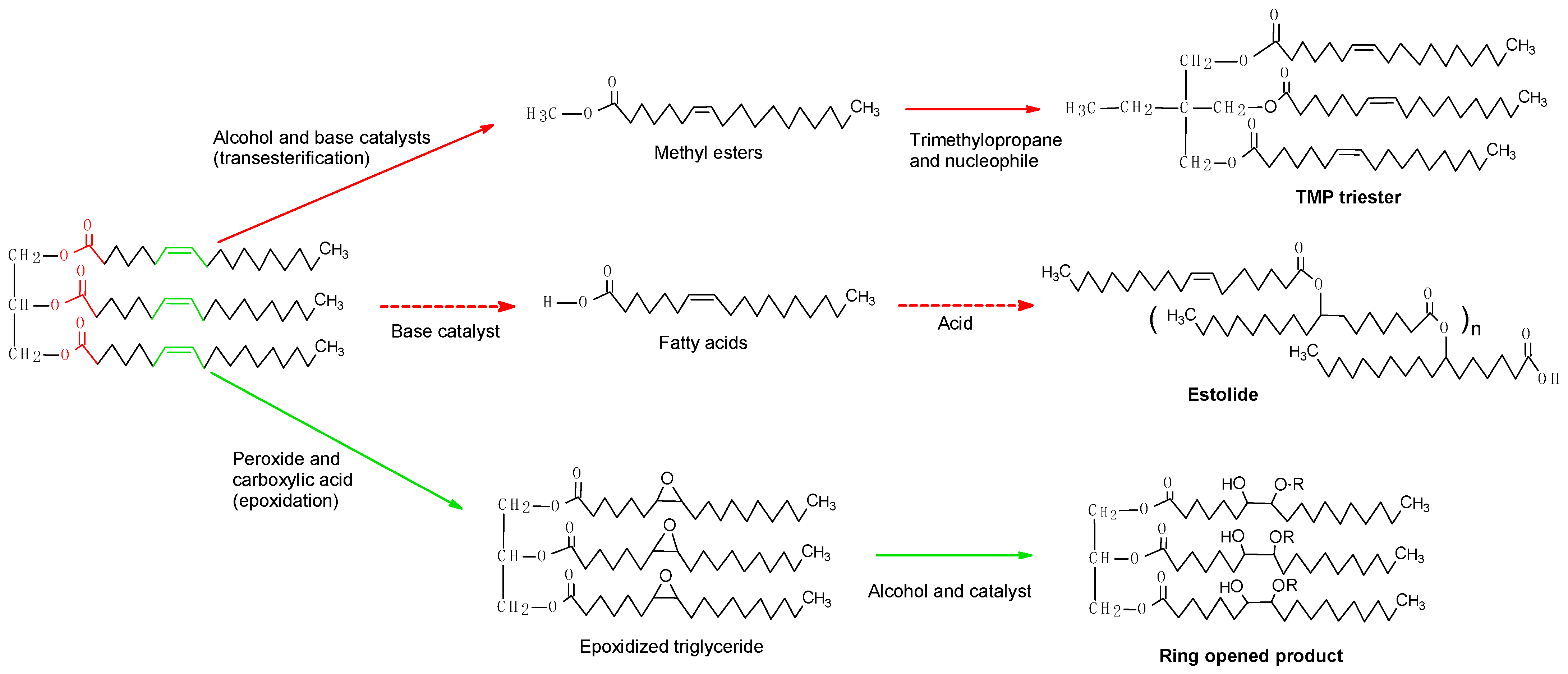
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical modification of vegetable oils for the production of biolubricants using trimethylolpropane: A review. Egypt. J. Pet. 2020, 29, 75–82. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Ghazi, T.I.M.; Resul, M.F.M.G.; Idris, A. Bioenergy II: Production of Biodegradable Lubricant from Jatropha curcas and Trimethylolpropane. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Koh, M.Y.; Ghazi, T.I.M.; Idris, A. Synthesis of palm based biolubricant in an oscillatory flow reactor (OFR). Ind. Crops Prod. 2014, 52, 567–574. [Google Scholar] [CrossRef]
- Masood, H.; Yunus, R.; Choong, T.S.Y.; Rashid, U.; Yap, Y.H.T. Synthesis and characterization of calcium methoxide as heterogeneous catalyst for trimethylolpropane esters conversion reaction. Appl. Catal. Gen. 2012, 425, 184–190. [Google Scholar] [CrossRef]
- Sripada, P.K.; Sharma, R.V.; Dalai, A.K. Comparative study of tribological properties of trimethylolpropane-based biolubricants derived from methyl oleate and canola biodiesel. Ind. Crops Prod. 2013, 50, 95–103. [Google Scholar] [CrossRef]
- Da Silva, J.A.C.; Habert, A.C.; Freire, D.M.G. A potential biodegradable lubricant from castor biodiesel esters. Lubr. Sci. 2013, 25, 53–61. [Google Scholar] [CrossRef]
- Wang, E.P.; Ma, X.; Tang, S.Z.; Yan, R.; Wang, Y.; Riley, W.W.; Reaney, M.J.T. Synthesis and oxidative stability of trimethylolpropane fatty acid triester as a biolubricant base oil from waste cooking oil. Biomass Bioenergy 2014, 66, 371–378. [Google Scholar] [CrossRef]
- Sreeprasanth, P.S.; Srivastava, R.; Srinivas, D.; Ratnasamy, P. Hydrophobic, solid acid catalysts for production of biofuels and lubricants. Appl. Catal. Gen. 2006, 314, 148–159. [Google Scholar] [CrossRef]
- Kamalakar, K.; Rajak, A.K.; Prasad, R.B.N.; Karuna, M.S.L. Rubber seed oil-based biolubricant base stocks: A potential source for hydraulic oils. Ind. Crops Prod. 2013, 51, 249–257. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Muszynski, M.; Nowicki, J. Enzymatic synthesis of rapeseed oil-based lubricants. Ind. Crops Prod. 2013, 45, 25–29. [Google Scholar] [CrossRef]
- Kamalakar, K.; Manoj, G.N.V.T.S.; Prasad, R.B.N.; Karuna, M.S.L. Thumba (Citrullus colocynthis L.) seed oil: A potential bio-lubricant base-stock. Grasas Aceites 2015, 66, e055. [Google Scholar]
- Resul, M.F.M.G.; Ghazi, T.I.M.; Idris, A. Temperature Dependence on the Synthesis of Jatropha Biolubricant. In Proceedings of the Conference on Advanced Materials and Nanotechnology (Caman 2009), Wellington, New Zealand, 7–11 February 2011; Volume 17. [Google Scholar]
- Menkiti, M.; Anaehobi, H.; Oyoh, K.; Nnaji, P. Process optimization and kinetics of bio-lubricants synthesis from fluted pumpkin seed. Eur. Sci. J. 2015, 11, 1857–7881. [Google Scholar]
- Ishak, A.A.; Salimon, J. Synthesis of Rubber Seed Oil and Trimethylolpropane Based Biolubricant Base Stocks. Malays. J. Anal. Sci. 2013, 17, 414–421. [Google Scholar]
- Heikal, E.K.; Elmelawy, M.S.; Khalil, S.A.; Elbasuny, N.M. Manufacturing of environment friendly biolubricants from vegetable oils. Egypt. J. Pet. 2017, 26, 53–59. [Google Scholar]
- Musa, U.; Mohammed, I.A.; Sadiq, M.M.; Aberuagba, F.; Olurinde, A.O.; Obamina, R. Synthesis and Characterization of Trimethylolpropane-Based Biolubricants from Castor Oil. In Proceedings of the Annual Conference of NSChE, Warri, Nigeria, 7 November 2015. [Google Scholar]
- Bethala, L.A.P.D. Ionic Liquids in Lipid Processing and Analysis—Opportunities and Challenges; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cermak, S.C.; Durham, A.L.; Isbell, T.A.; Evangelista, R.L.; Murray, R.E. Synthesis and physical properties of pennycress estolides and esters. Ind. Crops Prod. 2015, 67, 179–184. [Google Scholar] [CrossRef]
- Garcia-Zapateiro, L.A.; Franco, J.M.; Valencia, C.; Delgado, M.A.; Gallegos, C.; Ruiz-Mendez, M.V. Chemical, thermal and viscous characterization of high-oleic sunflower and olive pomace acid oils and derived estolides. Grasas Aceites 2013, 64, 497–508. [Google Scholar]
- Garcia-Zapateiro, L.A.; Franco, J.M.; Valencia, C.; Delgado, M.A.; Gallegos, C. Viscous, thermal and tribological characterization of oleic and ricinoleic acids-derived estolides and their blends with vegetable oils. J. Ind. Eng. Chem. 2013, 19, 1289–1298. [Google Scholar] [CrossRef]
- Biresaw, G.; Bantchev, G.B.; Cermak, S.C. Tribological Properties of Vegetable Oils Modified by Reaction with Butanethiol. Tribol. Lett. 2011, 43, 17–32. [Google Scholar] [CrossRef]
- Cermak, S.C.; Brandon, K.B.; Isbell, T.A. Synthesis and physical properties of estolides from lesquerella and castor fatty acid esters. Ind. Crops Prod. 2006, 23, 54–64. [Google Scholar] [CrossRef]
- Cermak, S.C.; Bredsguard, J.W.; John, B.L.; McCalvin, J.S.; Thompson, T.; Isbell, K.N.; Feken, K.A.; Isbell, T.A.; Murray, R.E. Synthesis and physical properties of new estolide esters. Ind. Crops Prod. 2013, 46, 386–391. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A.; Evangelista, R.L.; Johnson, B.L. Synthesis and physical properties of petroselinic based estolide esters. Ind. Crops Prod. 2011, 33, 132–139. [Google Scholar] [CrossRef]
- Salih, N.; Salimon, J.; Yousif, E. The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind. Crops Prod. 2011, 34, 1089–1096. [Google Scholar] [CrossRef]
- Silva, M.S.; Foletto, E.L.; Alves, S.M.; Dantas, T.N.D.C.; Neto, A.A.D. New hydraulic biolubricants based on passion fruit and moringa oils and their epoxy. Ind. Crops Prod. 2015, 69, 362–370. [Google Scholar] [CrossRef]
- Sammaiah, A.; Padmaja, K.V.; Prasad, R.B.N. Synthesis of Epoxy Jatropha Oil and its Evaluation for Lubricant Properties. J. Oleo Sci. 2014, 63, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somidi, A.K.R.; Sharma, R.V.; Dalai, A.K. Synthesis of Epoxidized Canola Oil Using a Sulfated-SnO2 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18668–18677. [Google Scholar] [CrossRef]
- Sharma, B.K.; Doll, K.M.; Erhan, S.Z. Oxidation, friction reducing, and low temperature properties of epoxy fatty acid methyl esters. Green Chem. 2007, 9, 469–474. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Deshpande, P.S.; Mahajan, S.U.; Mahulikar, P.P. Epoxidation of mustard oil and ring opening with 2-ethylhexanol for biolubricants with enhanced thermo-oxidative and cold flow characteristics. Ind. Crops Prod. 2013, 49, 586–592. [Google Scholar] [CrossRef]
- Sharma, R.V.; Dalai, A.K. Synthesis of bio-lubricant from epoxy canola oil using sulfated Ti-SBA-15 catalyst. Appl. Catal. B-Environ. 2013, 142, 604–614. [Google Scholar] [CrossRef]
- Hwang, H.S.; Erhan, S.Z. Synthetic lubricant basestocks from epoxidized soybean oil and Guerbet alcohols. Ind. Crops Prod. 2006, 23, 311–317. [Google Scholar] [CrossRef]
- Li, W.M.; Wang, X.B. Bio-lubricants Derived from Waste Cooking Oil with Improved Oxidation Stability and Low-temperature Properties. J. Oleo Sci. 2015, 64, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Kleinova, A.; Fodran, P.; Brncalova, L.; Cvengros, J. Substituted esters of stearic acid as potential lubricants. Biomass Bioenergy 2008, 32, 366–371. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Abdullah, B. Improvement of Physicochemical Characteristics of Monoepoxide Linoleic Acid Ring Opening for Biolubricant Base Oil. J. Biomed. Biotechnol. 2011, 2011, 196565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madankar, C.S.; Dalai, A.K.; Naik, S.N. Green synthesis of biolubricant base stock from canola oil. Ind. Crops Prod. 2013, 44, 139–144. [Google Scholar] [CrossRef]
- Hashem, A.I.; Abou Elmagd, W.S.I.; Salem, A.E.; El-Kasaby, M.; El-Nahas, A.M. Conversion of Some Vegetable Oils into Synthetic Lubricants via Two Successive Transesterifications. Energy Source Part A 2013, 35, 909–912. [Google Scholar] [CrossRef]
| Properties | Biodiesel | HVO | Fossil Diesel | Standard Min. | Standard Max. |
|---|---|---|---|---|---|
| Chemical composition | ester | hydrocarbon | hydrocarbon | - | - |
| Oxygen, wt% | 11 | 0 | 0 | - | - |
| Density (15 °C), g cm−3 | 0.86–0.90 | 0.77–0.83 | 0.85 | 0.80 | 0.845 |
| Kinematic viscosity (40 °C), mm2 s−1 | 4.0–5.5 | 2.5–3.5 | 2.7–5.5 | 2.0 | 4.5 |
| Cloud point, °C | −13 to 10 | >20 | −6 | −5 | +6 |
| Sulphur content, mg.kg−1 | <0.01 | <10 | 10 | - | 10 |
| Caloric value, MJ kg−1 | 37.5–38 | 44 | 44.7–46.7 | - | - |
| Flash point, °C | 96–188 | 68–120 | 52–136 | 60 | 170 |
| Pour point, °C | −15 to 16 | −3 to 29 | −21 | −13 | 10 |
| Cetane number | 50–65 | 50–105 | 52–136 | 60 | 170 |
| Alcohols | Number of Carbons | Boiling Point, °C | Miscible with Oil | Reactive | Hygroscopic | Bio-Source | Price |
|---|---|---|---|---|---|---|---|
| methanol | 1 | 65 | no | ++ | + | − | ++ |
| ethanol | 2 | 78 | no | + | − | ++ | + |
| butanol | 4 | 118 | yes | − | − | + | − |
| Type of Catalyst | Advantages | Disadvantages |
|---|---|---|
| Homogeneous alkali |
|
|
| Heterogeneous alkali |
|
|
| Homogeneous acid |
|
|
| Heterogeneous acid |
|
|
| Enzyme |
|
|
| Catalysts | Feedstock | Operating Conditions (Temperature, °C/Pressure, MPa/L/W-HSV, h−1) | Reference |
|---|---|---|---|
| NiMoCx/Al2O3 CoMoCx/Al2O3 NiMoCx/TiO2 CoMoCx/TiO2 | AGO/RSO: 95/5, 90/10, 75/25 | 330, 340, 350/5.5/WHSV = 1.0, 2.0 | [162] |
| Mo2C/Activated carbon | Rapeseed oil and Soya oil | 360, 380/2.0–3.0/2 h (batch reactor) | [163] |
| NiS, NiPx, NiCx | Spent coffee oil | 375, 425/2.0–4.0/3 h (batch reactor) | [164] |
| MoNx, MoCx, MoPx | Rapeseed oil | 350, 370, 390/5.5/WHSV = 1.0, 2.0, 4.0 | [165] |
| Mo2C/C and Ru/Al2O3 | Canola oil | 300/WHSV = 5–25 h−1 | [166] |
| NiMoCx | Soybean oil | 400/4.5/LHSV = 1.0 | [167] |
| MoCx/Al2O3 MoCx/TiO2 MoCx/ZrO2 MoNx/Al2O3 MoNx/TiO2 MoNx/ZrO2 | AGO/RSO: 95/5, 90/10, 75/25 | 330, 340, 350/5.5/WHSV = 1.0, 2.0 | [161] |
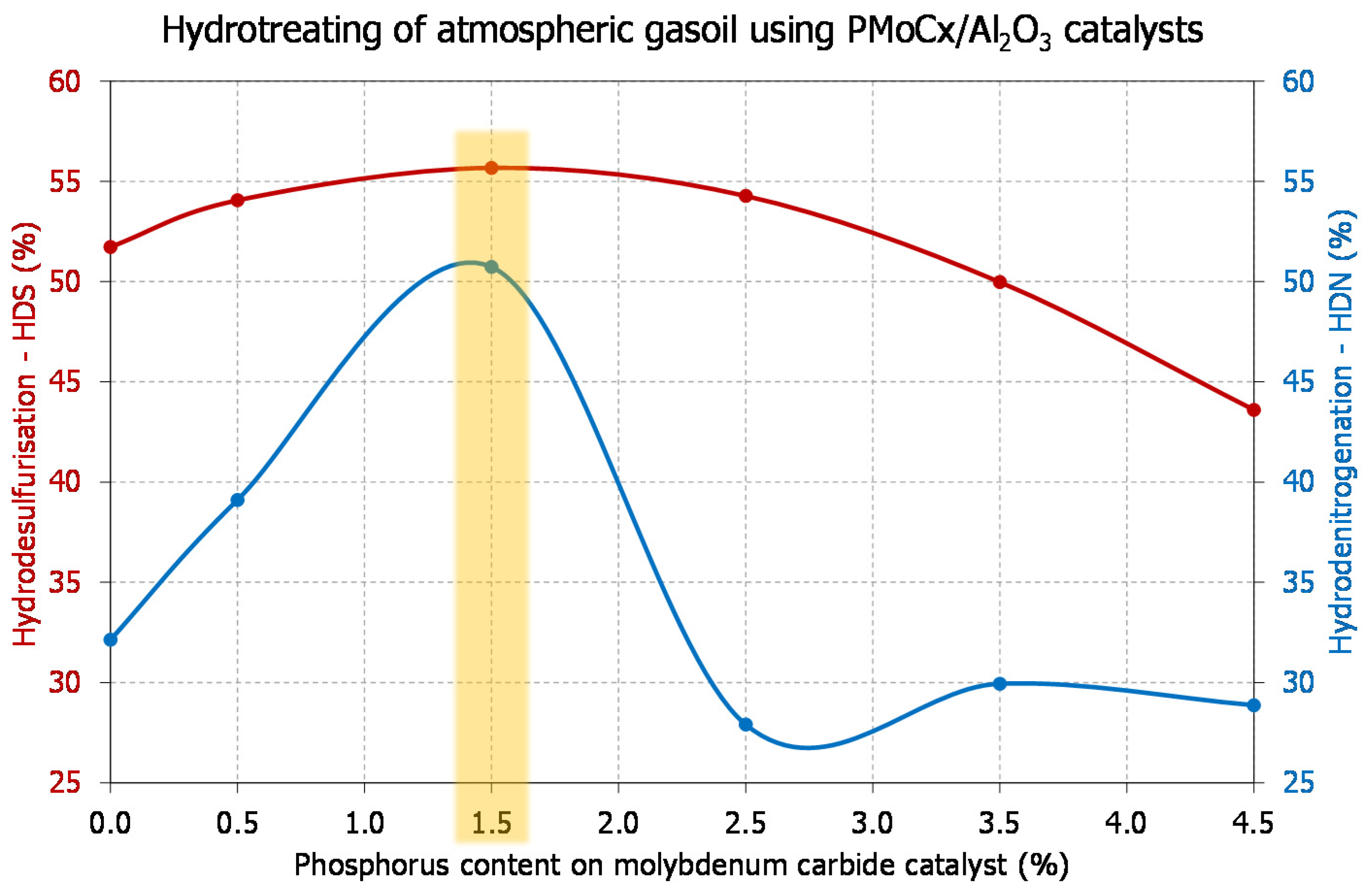
| PMoCx/Al2O3 (P content 0.0–4.5) | AGO/RSO: 95/5, 90/10, 75/25 | 330, 340, 350/5.5/WHSV = 1.0, 2.0 | [168] |
| Catalysts | Feedstock | Operating Conditions (Temp./Pressure/Time/Other) | Yield, % | Ref. |
|---|---|---|---|---|
| Sodium Methoxide | Jatropha oil, TMP | 150 °C/10 mbar/3 h | >80 | [178] |
| Sodium Methoxide | Palm ME, TMP | 140 °C/25 mbar/25 min/oscillatory flow reactor at 1.5 Hz | 94.6 | [179] |
| Calcium Methoxide | Palm ME, TMP | 180 °C/50 mbar/8 h | 92.4 | [180] |
| Sodium Methoxide | Canolabiodiesel, ME, TMP | 110 °C/1 mbar/5 h | 90.9 | [181] |
| Dibutyltin dilaurate | Castor biodiesel, TMP | 170 °C/0.01 bar | 89.7 | [182] |
| KOH | UCO ME, TMP | 128 °C/200 Pa/1.5 h | 85.7 | [183] |
| Fe-Zn double-metal cyanide | Sunflower oil, octanol | 170 °C/8 h | 98 | [184] |
| p-Toluensulphonic acid | Rubber ME, NPG/TMP/PE | 135–140 °C until theoretical reaction complete | 94.5–96.5 | [185] |
| C Antarctica lipase | Rapeseed ME, NPG/TMP/PE | 200 °C/50 h | 98 | [186] |
| p-Toluensulphonic acid | Thumba ME, xylene, NPG/TMP/PE | 135–140 °C until complete | 89–95 | [187] |
| 1% NaOCH3 | Jatropha seed, TMP | 150 °C/10 mbar/3 h | 47 | [188] |
| Ca(OH)2 | Fluted Pumpkin, TMP | 160 °C/6 h | 81.4 | [189] |
| 2% H2SO4 | Rubber seed, TMP | 150 °C/5 h | 79 | [190] |
| 0.9% NaOCH3 | Palm oil, TMP | 130 °C/10 mmHg/4 h | 97.8 | [191] |
| 0.8% o-phosphoric acid | Castor seed, TMP | 120 °C/1 h | 96.6 | [192] |
| Catalysts | Feedstock | Operating Conditions (Temp./Pressure/Time/Other) | Yield, % | Ref. |
|---|---|---|---|---|
| H2SO4, HClO4, or p-Toluensulphonic acid | Sunflower oil | 50–100 (depending on catalyst)/3–24 | - | [195] |
| H2SO4 | Olive oil | 100/3–24 h | - | [195] |
| H2SO4, HClO4, or p-Toluensulphonic acid | Ricinoleic acid | 50–100 °C (depending on catalyst) | - | [196] |
| Tin (II) 2-ethylhexanoate | Castor 2-EH ester, lauric acid | 130 °C/12–18 Pa/24 h | 73 | [197] |
| - | Castor 2-EH ester, estolide, butanethiol | −28 to −18 °C/3 h/photochemical reactor | 91 | [197] |
| - | Saturated Castor FA ester, capping FA | 200 °C/20 Pa/24 h | - | [198] |
| - | Unsaturated Castor FA ester, capping FA | 200 °C/20 Pa/24 h | - | [198] |
| BF3 | Oleic acid estolide, linear alcohols | 60–80 °C until 99% complete | - | [199] |
| HClO4 | Coriander FA, 2-EH, capped with various FA | Estolide: 60 °C/7.5–10.9 kPa/24 h Ester: additional 3–4 h after 2-EH added | 65–76 | [200] |
| - | Castor 2-EH ester estolide, butanethiol | −28 to −18 °C/3 h/photochemical reactor | 96 | [197] |
| Catalysts | Feedstock | Operating Conditions (Temper./Time/Other) | Yield, % | Ref. |
|---|---|---|---|---|
| - | Oleic acid, formic acid, H2O2 | 4 °C/2 h | - | [201] |
| - | Passion fruit oil, formic acid, H2O2 | 10 °C then adding H2O2 and heating to 60 °C, 7 h | - | [202] |
| H2SO4 | Jatropha oil, formic acid, H2O2 | 10 °C for 2 h while H2O2 added then 60 °C until complete | 96 | [203] |
| Sulfated-SnO2 Catalyst | Canola oil, acetic acid, H2O2 | 70 °C/6.5 h | - | [204] |
| - | Methyl oleate, formic acid, H2O2 | - | 97 | [205] |
| H2SO4, H3NSO3, or CH4O3S | Epoxidized mustard oil, 2-EH | 120 °C until complete | 92–95 | [206] |
| Sulfated Ti-SBA-15 | Epoxidized canola oil, acetic anhydride | 130 °C/5 h | 100 | [207] |
| H2SO4 | Epoxidized soybean oil, Guerbet alcohols | 110 °C/20 h/0.47 mol alcohol | - | [208] |
| CaO | Epoxidized FA UCO ME, methanol, isooctadecanol | 90–140 °C | - | [209] |
| Pyridine | 9,10-hydroxyacyloxy-stearic acid ME, CCl4, acylchlorides | 50 °C/5 h | 66–88 | [210] |
| p-toluensulphonic acid | Monoepoxide linoleic acid, oleic acid | 70–80 °C oleic acid added over 1.5 h then heated to 90–110 °C over 3–6 h | - | [211] |
| Amberlyst-15 | Epoxidized canola oil, n-butanol | 100 °C/15 h | - | [212] |
| p-toluensulphonic acid | Epoxidized linseed oil, oleic acid, xylene | 150 °C/4–5 h | - | [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts 2021, 11, 1118. https://doi.org/10.3390/catal11091118
Hájek M, Vávra A, de Paz Carmona H, Kocík J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts. 2021; 11(9):1118. https://doi.org/10.3390/catal11091118
Chicago/Turabian StyleHájek, Martin, Aleš Vávra, Héctor de Paz Carmona, and Jaroslav Kocík. 2021. "The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review" Catalysts 11, no. 9: 1118. https://doi.org/10.3390/catal11091118
APA StyleHájek, M., Vávra, A., de Paz Carmona, H., & Kocík, J. (2021). The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts, 11(9), 1118. https://doi.org/10.3390/catal11091118







