Synthesis of Brominated Alkanes via Heterogeneous Catalytic Distillation over Al2O3/SO42−/ZrO2
Abstract
:1. Introduction
2. Results
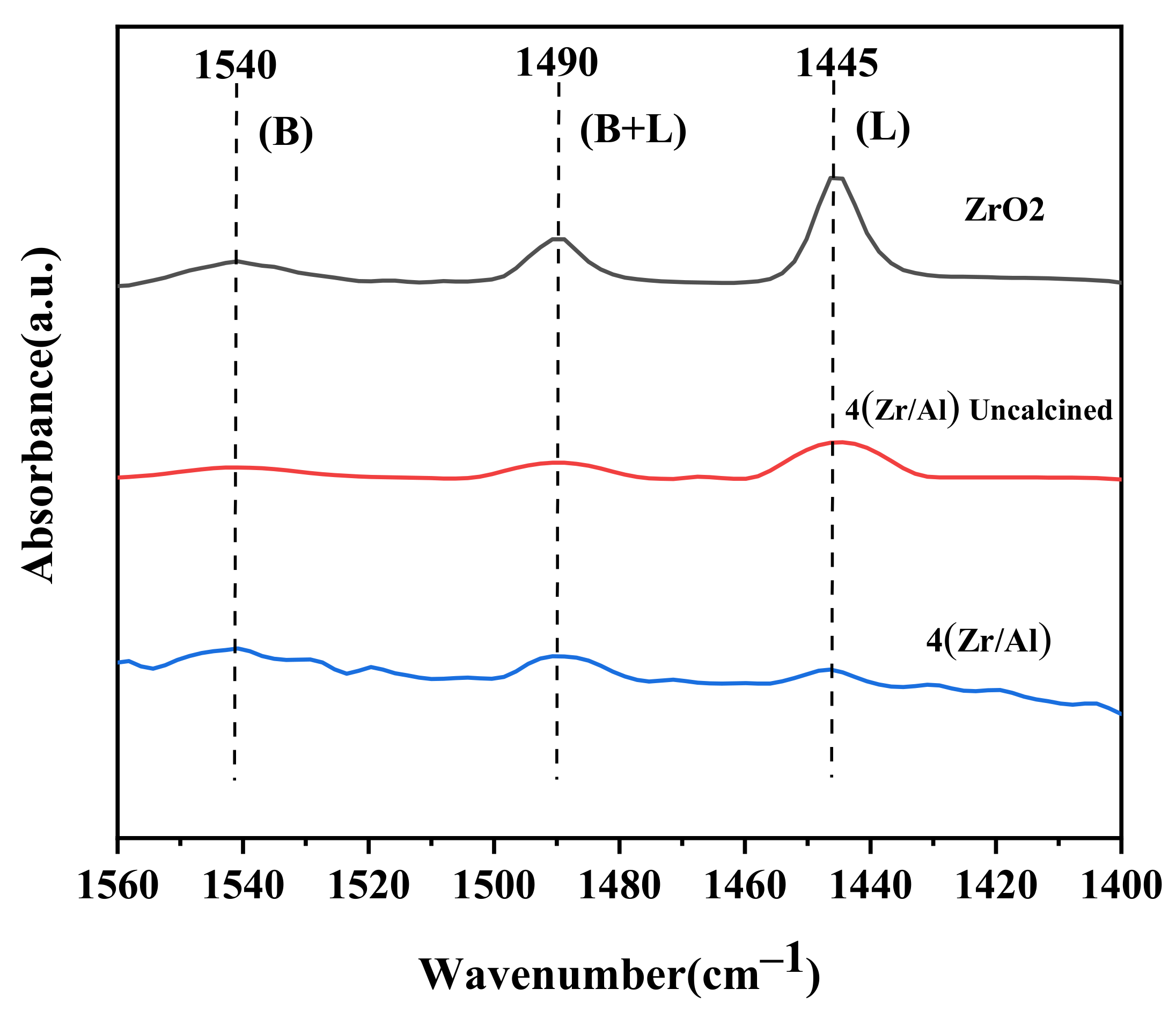
2.1. Characterization of the Catalysts
2.2. Catalytic Activity of Al2O3/SO42−/ZrO2
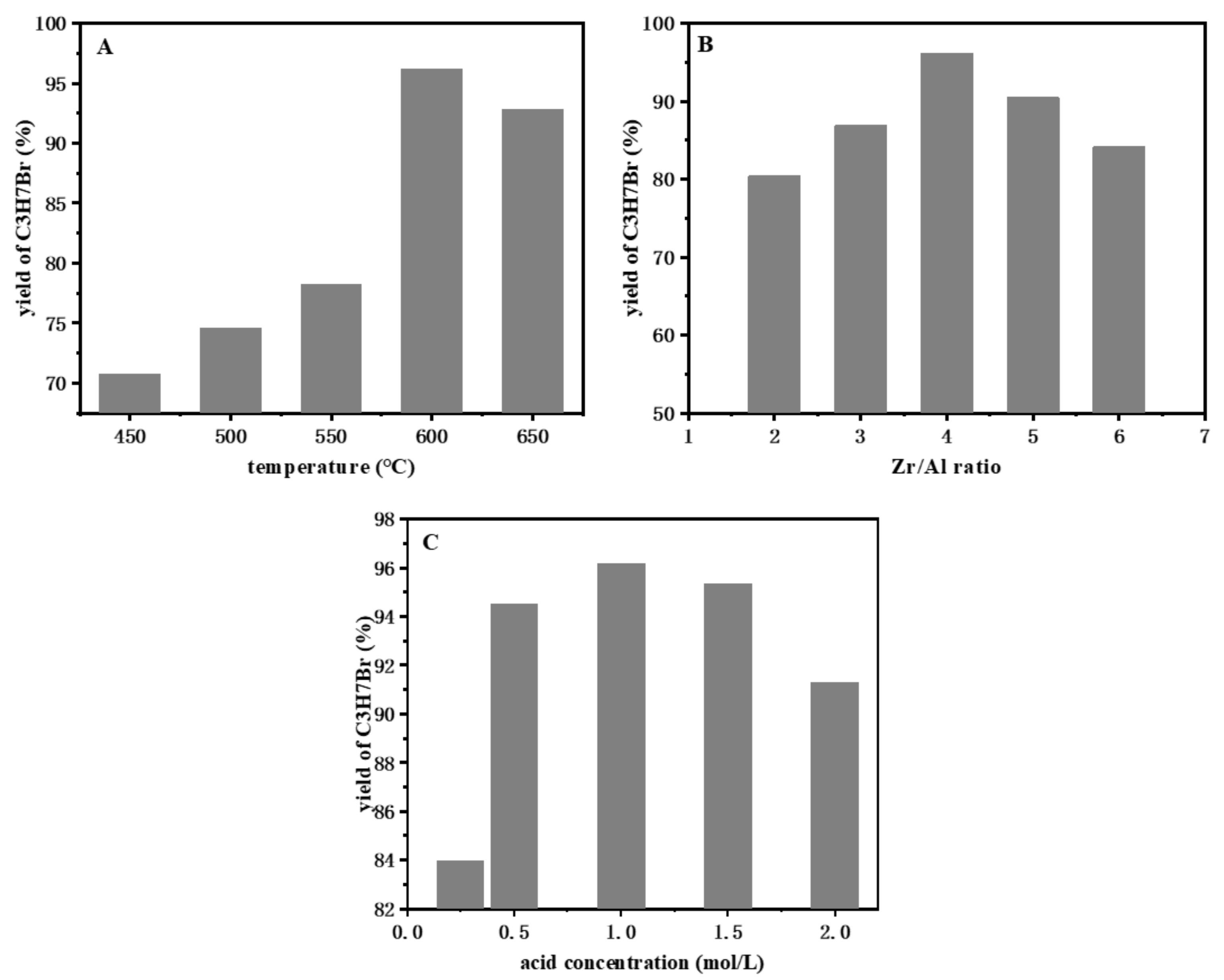
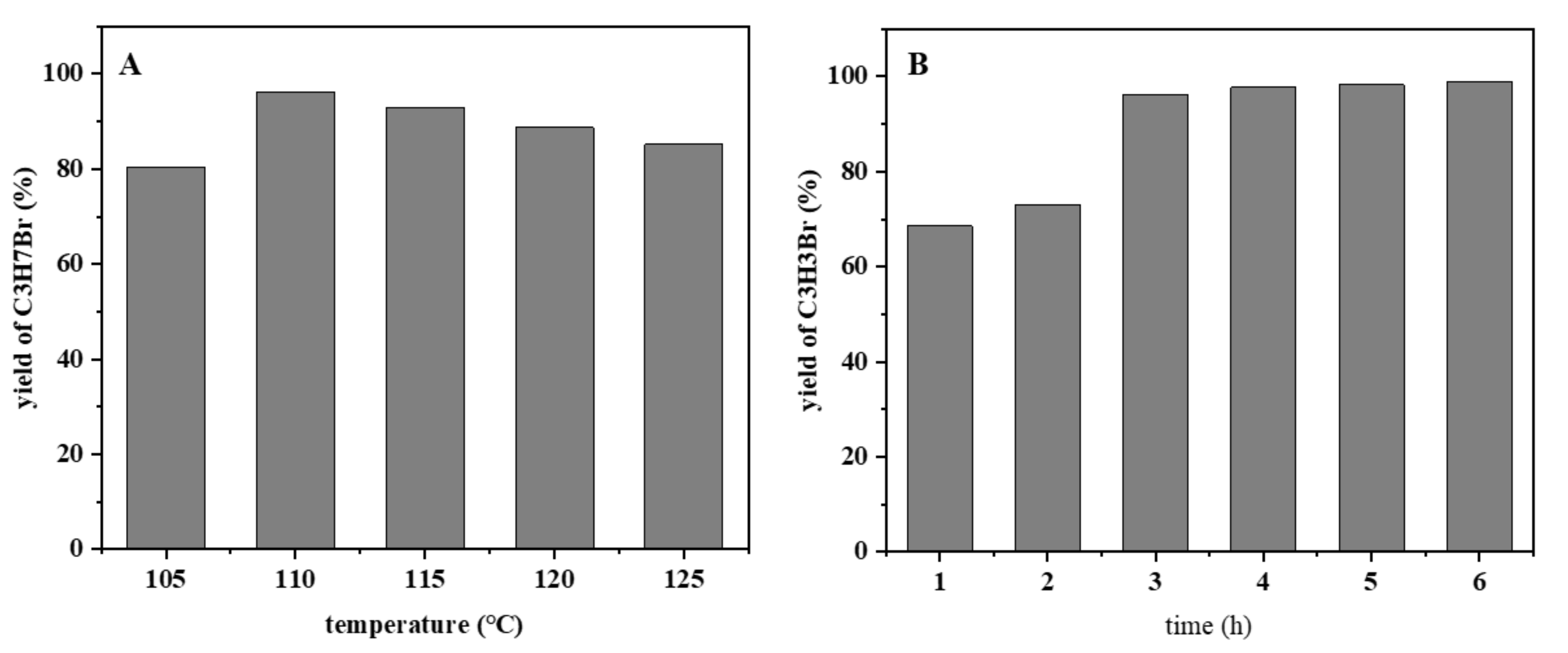
2.3. Optimization of Reaction Parameters
2.3.1. The Effect of Catalyst Preparation Conditions on the Bromopropane Yield
2.3.2. The Effect of Reaction Temperature and Reaction Time on Bromopropane Yield
2.3.3. The Effect of Substrate Concentration and Catalyst Dosage on Bromopropane Yield
2.4. Catalytic Stability of Al2O3/SO42−/ZrO2
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Performance Research
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid acid catalysts for biodiesel production—Towards sustainable energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Reddy, B.M.; Patil, M.K. Organic syntheses and transformations catalyzed by sulfated zirconia. Chem. Rev. 2009, 109, 2185–2208. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.X.; Zhou, X.L.; Tang, C.; Li, C.L.; Wang, J.A.; Novaro, O. A comparative study of the synthesis approaches and catalytic behaviors of Pt/SO42−/ZrO2-Al2O3 catalysts for n-hexane hydro isomerization. Catal. Commun. 2008, 9, 1770–1774. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Hino, M.; Arata, K. Synthesis of the solid superacid catalyst of tin oxide treated with sulfate ion. Appl. Catal. 1990, 59, 205–212. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. Synthesis of highly active superacids of SO4/ZrO2 with Ir, Pt, Rh, Ru, Os, and Pd substances for reaction of butane. Catal. Lett. 1994, 30, 25–30. [Google Scholar] [CrossRef]
- Song, H.; Wang, N.; Song, H.L.; Li, F. La–Ni modified S2O82−/ZrO2-Al2O3 catalyst in n-pentane hydroisomerization. Catal. Commun. 2015, 59, 61–64. [Google Scholar] [CrossRef]
- Canton, P.; Olindo, R.; Pinna, F.; Strukul, G.; Riello, P.; Meneghetti, M.; Cerrato, G.; Morterra, C.; Benedetti, A. Alumina-promoted sulfated zirconia system: Structure and microstructure characterization. Chem. Mater. 2001, 13, 1634–1641. [Google Scholar] [CrossRef]
- Song, H.; Wang, N.; Song, H.L.; Li, F.; Jin, Z.S. Effect of Al content on the isomerization performance of solid superacid Pd-S2O82-/ZrO2-Al2O3. Chin. J. Chem. Eng. 2014, 22, 1226–1231. [Google Scholar] [CrossRef]
- Kamoun, N.; Younes, M.K.; Ghorbel, A.; Mamede, A.S.; Rives, A. Comparative study of aerogels nanostructured catalysts: Ni/ZrO2-SO42− and Ni/ZrO2-Al2O3-SO42−. Ionics 2015, 21, 221–229. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lohitharn, N.; Goodwin, J.G., Jr.; Olindo, R.; Pinna, F.; Canton, P. The effect of Al2O3-promotion of sulfated zirconia on n-butane isomerization: An isotopic transient kinetic analysis. Catal. Commun. 2006, 7, 209–213. [Google Scholar] [CrossRef]
- Cao, C.J.; Han, S.; Chen, C.L.; Xu, N.P.; Mou, C.Y. Hydroisomerization of n-hexane over gallium-promoted sulfated zirconia. Catal. Commun. 2003, 4, 511–515. [Google Scholar] [CrossRef]
- Cao, C.J.; Yu, X.Z.; Chen, C.L.; Xu, N.P.; Wang, Y.R.; Mou, C.Y. Comparison of the promoting effects of gallium and aluminum on the n-butane isomerization activity of sulfated zirconia. React. Kinet. Catal. Lett. 2004, 83, 85–92. [Google Scholar] [CrossRef]
- Atlas, C. Search for anomalous production of prompt same-sign lepton pairs and pair-produced doubly charged Higgs bosons with √s = 8 TeV pp collisions using the ATLAS detector. J. High Energy Phys. 2015, 3, 41. [Google Scholar]
- Kanemura, S.; Yagyu, K.; Yokoya, H. First constraint on the mass of doubly charged Higgs bosons in the same-sign diboson decay at the LHC. Phys. Lett. 2013, 726, 316–319. [Google Scholar] [CrossRef] [Green Version]
- Li, X.B.; Nagaoka, K.; Lercher, J.A. Labile sulfates as key components in active sulfated zirconia for n-butane isomerization at low temperatures. J. Catal. 2004, 227, 130–137. [Google Scholar] [CrossRef]
- Ma, Z.M.; Meng, X.; Liu, N.W.; Yang, C.; Shi, L. Preparation, characterization, and isomerization catalytic performance of palladium loaded zirconium hydroxide/sulfated zirconia. Ind. Eng. Chem. Res. 2018, 57, 14377–14385. [Google Scholar] [CrossRef]
- Zalewski, D.J.; Alerasool, S.; Doolin, P.K. Characterization of catalytically active sulfated zirconia. Catal. Today 1999, 53, 419–432. [Google Scholar] [CrossRef]
- Mercera, P.D.L.; Ommen, J.G.V.; Doesburg, E.B.M.; Burggraaf, A.J.; Boss, J.R.H. Zirconia as a support for catalysts: Evolution of the texture and structure on calcination in air. Appl. Catal. 1990, 57, 127–148. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yang, S.W.; Kondo, J.N.; Domen, K.; Yamada, T.; Hattori, H. Spectroscopic study of H2 and CO adsorption on platinum-promoted sulfated zirconia catalysts. J. Phys. Chem. B 2003, 107, 11951–11959. [Google Scholar] [CrossRef]
- Chen, W.H.; Ko, H.H.; Sakthivel, A.; Huang, S.J.; Liu, S.H.; Lo, A.Y.; Tsai, T.C.; Liu, S.B. A solid-state NMR, FT-IR and TPD study on acid properties of sulfated and metal-promoted zirconia: Influence of promoter and sulfation treatment. Catal. Today 2006, 116, 111–120. [Google Scholar] [CrossRef]
- Wang, Z.C.; Shui, H.F.; Zhang, D.X.; Gao, J.S. A comparison of FeS, FeS + S and solid superacid catalytic properties for coal hydro-liquefaction. Fuel 2007, 86, 835–842. [Google Scholar] [CrossRef]
- Cavani, F.; Guidetti, S.; Trevisanut, C.; Ghedini, E.; Signoretto, M. Unexpected events in sulfated zirconia catalyst during glycerol-to-acrolein conversion. Appl. Catal. A Gen. 2011, 409, 267–278. [Google Scholar] [CrossRef]
- Song, H.; Wang, N.; Song, H.L.; Li, F. Effect of Pd content on the isomerization performance over Pd-S2O82−/ZrO2-Al2O3 catalyst. Res. Chem. Intermed. 2016, 42, 951–962. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Balakrishna, G.; Rajesh, B.; Jayasri, V.; Sikhwivhilu, L.M.; Coville, N.J. Alkylation of catechol with methanol to give guaiacol over sulphate-modified zirconia solid acid catalysts: The influence of structural modification of zirconia on catalytic performance. Catal. Commun. 2008, 9, 2422–2427. [Google Scholar] [CrossRef]
- Appay, M.D.; Manoli, J.M.; Potvin, C.; Muhler, M.; Wild, U.; Pozdnyakova, O.; Paal, Z. High-resolution electron microscopic, spectroscopic, and catalytic studies of intentionally sulfided Pt/ZrO2–SO4 catalysts. J. Catal. 2005, 222, 419–428. [Google Scholar] [CrossRef]
- Breitkopf, C.; Matysik, S.; Papp, H. Selective poisoning of active centers of sulfated zirconia monitored by TAP, XPS, and DRIFTS. Appl. Catal. A Gen. 2006, 301, 1–8. [Google Scholar] [CrossRef]
- Comelli, R.A.; Vera, C.R.; Parera, J.M. Influence of ZrO2 crystalline structure and sulfate Ion concentration on the catalytic activity of SO42−-ZrO2. J. Catal. 1995, 151, 96–101. [Google Scholar] [CrossRef]
- Fottinger, K.; Zorn, K.; Vinek, H. Influence of the sulfate content on the activity of Pt containing sulfated zirconia. Appl. Catal. A Gen. 2005, 284, 69–75. [Google Scholar] [CrossRef]
- Barrera, A.; Montoya, J.A.; Viniegra, M.; Navarrete, J.; Espinosa, G.; Vargas, A.; Angel, P.D.; Perez, G. Isomerization of n-hexane over mono-and bimetallic Pd–Pt catalysts supported on ZrO2–Al2O3–WOx prepared by sol–gel. Appl. Catal. A Gen. 2005, 290, 97–109. [Google Scholar] [CrossRef]
- Zhukov, Y.M.; Efimov, A.Y.; Shelyapina, M.G.; Petranovskii, V.; Zhizhin, E.V.; Burovikhina, A.; Zvereva, I.A. Effect of preparation method on the valence state and encirclement of copper exchange ions in mordenites. Microporous Mesoporous Mater. 2016, 224, 415–419. [Google Scholar] [CrossRef]
- Hua, W.M.; Xia, Y.D.; Yue, Y.H.; Gao, Z. Promoting effect of Al on SO42−/MxOy (M = Zr, Ti, Fe) catalysts. J. Catal. 2000, 196, 104–114. [Google Scholar] [CrossRef]
- Wang, J.H.; Mou, C.Y. Alumina-promoted mesoporous sulfated zirconia: A catalyst for n-butane isomerization. Appl. Catal. A Gen. 2005, 286, 128–136. [Google Scholar] [CrossRef]
- Yang, Y.C.; Weng, H.S. Al-promoted Pt/SO42−/ZrO2 with low sulfate content for n-heptane isomerization. Appl. Catal. A. Gen. 2010, 384, 94–100. [Google Scholar] [CrossRef]
- Taouli, A.; Klemt, A.; Breede, M.; Reschtilowski, W. Acidity investigations and determination of integrated molar extinction coefficients for infrared absorption bands of ammonia adsorbed on acids sites of MCM-41. Stud. Surf. Sci. Catal. 1999, 125, 307–314. [Google Scholar]
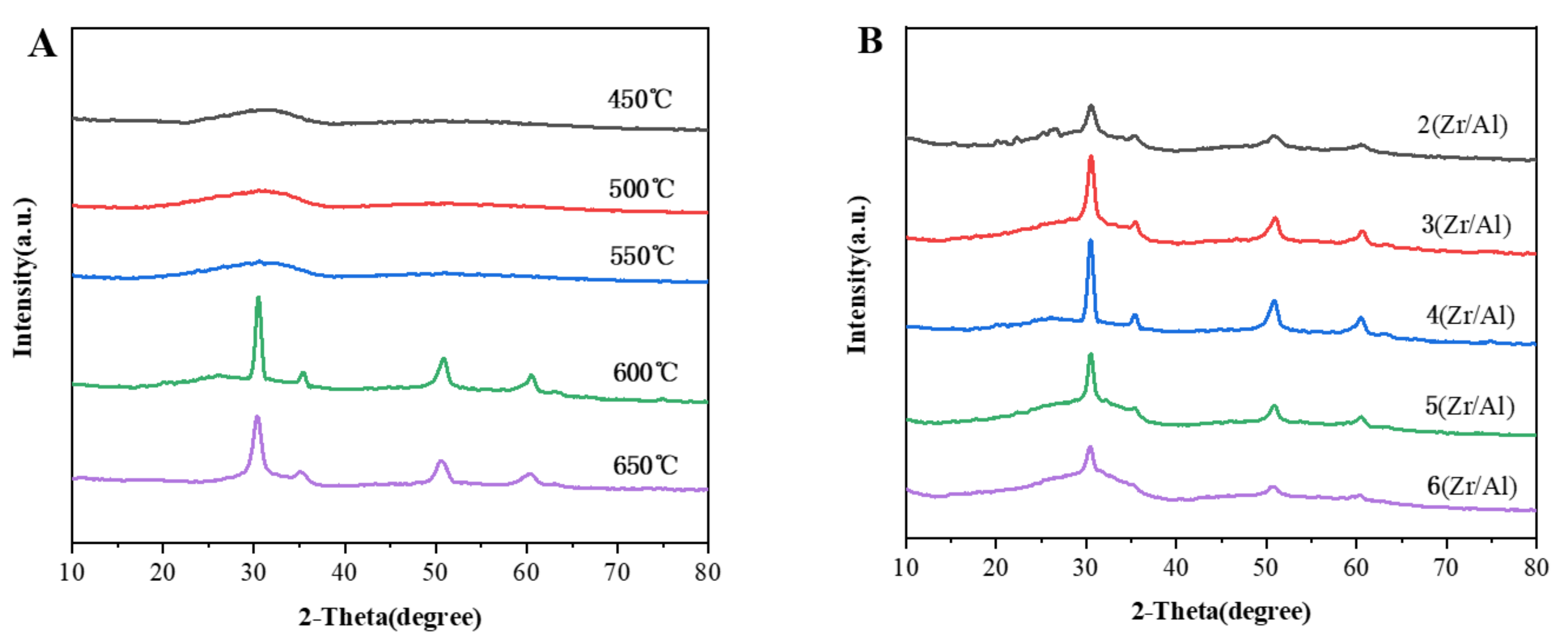
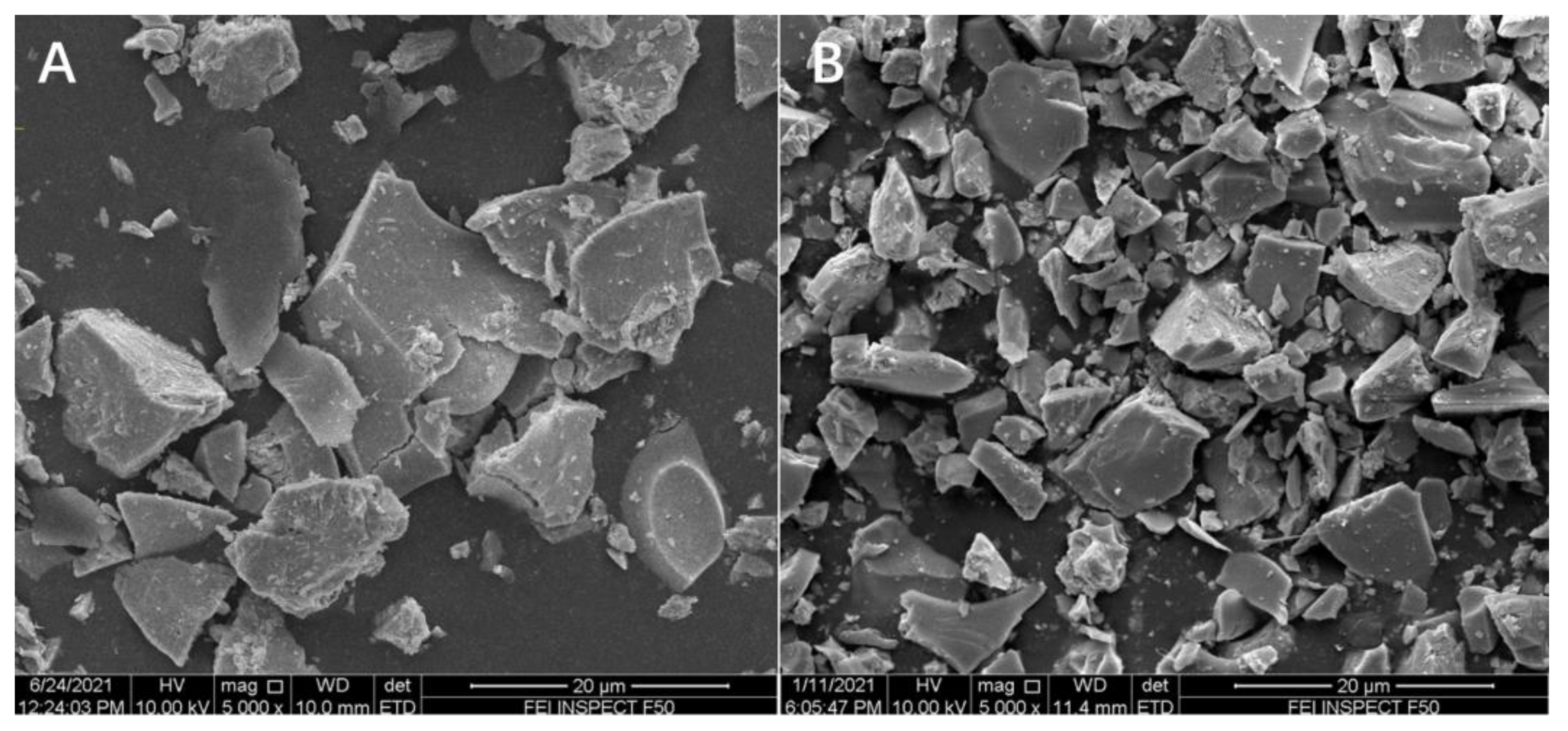
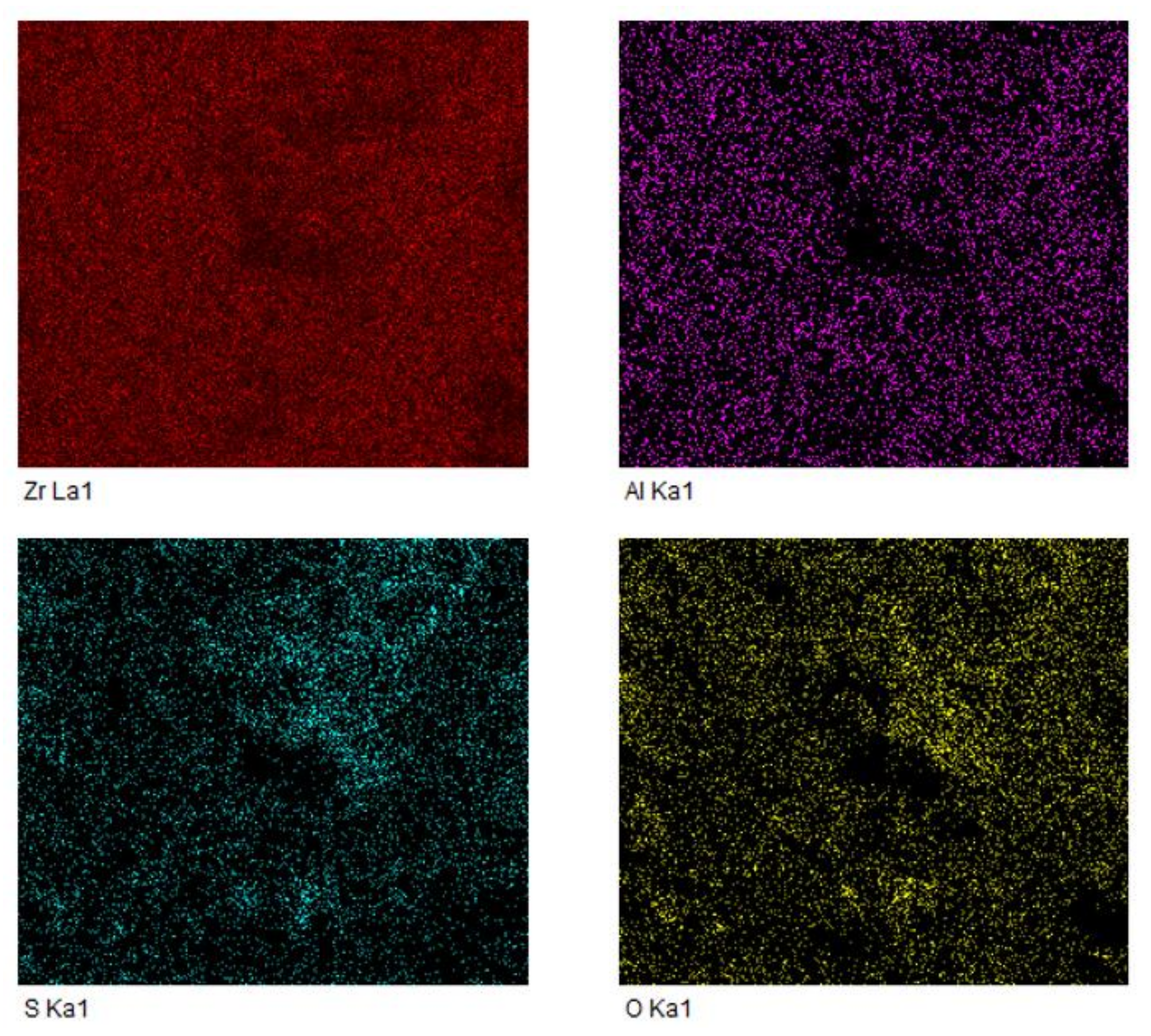
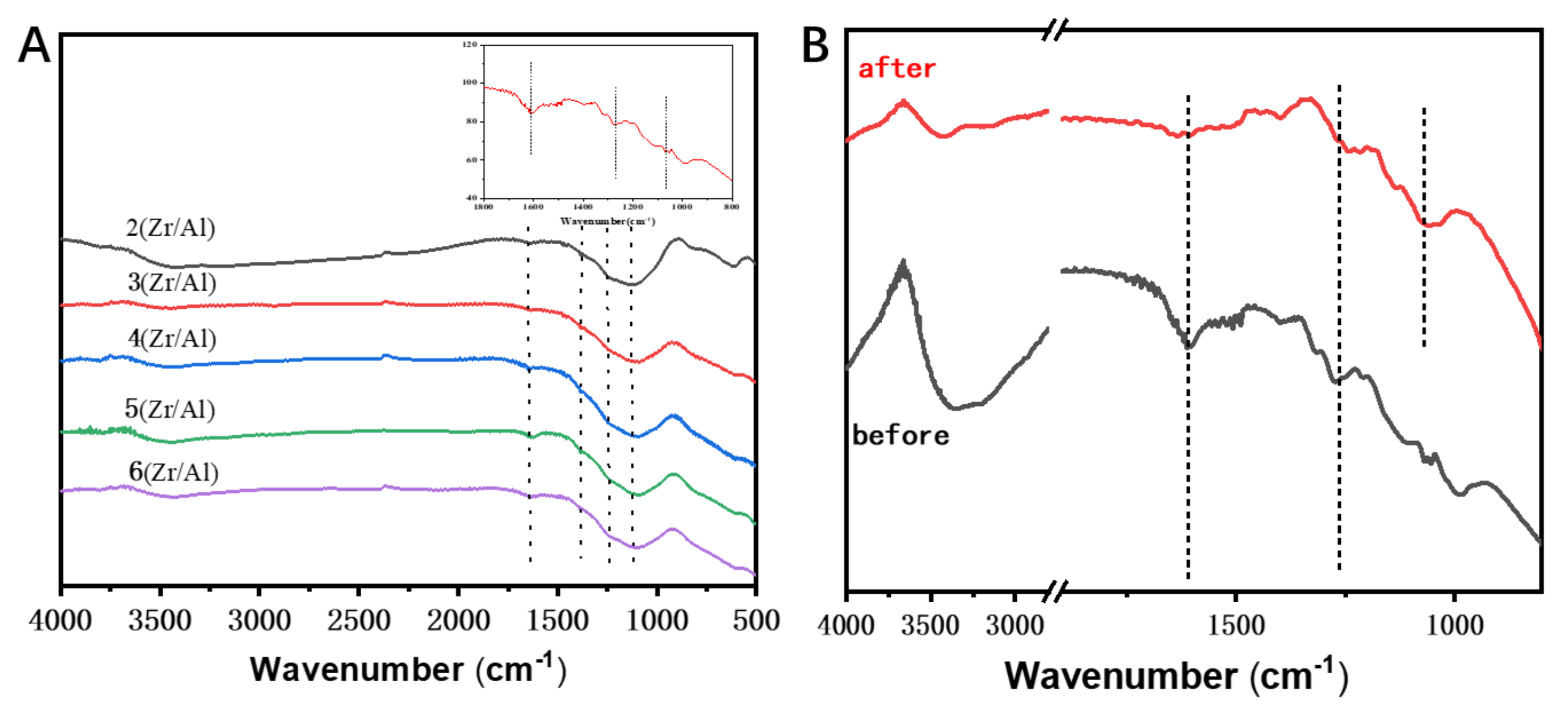



| Catalysts | SBET a (m2/g) | Dmean b (nm) |
|---|---|---|
| ZrO2 | 43.5 | 50.27 |
| 2(Zr/Al) | 69.6 | 33.65 |
| 3(Zr/Al) | 57.8 | 37.60 |
| 4(Zr/Al) | 105.9 | 26.80 |
| 5(Zr/Al) | 98.1 | 22.23 |
| 6(Zr/Al) | 87.9 | 8.98 |
| Catalysis | Brønsted Acid Sites (µmol/g) | Lewis Acid Sites (µmol/g) | Total Acids (µmol/g) | Ratio of Brønsted to Lewis |
|---|---|---|---|---|
| ZrO2 | 21.77 | 39.90 | 61.67 | 0.55 |
| (4)Zr/Al Uncalcined | 12.69 | 22.27 | 24.96 | 0.57 |
| (4)Zr/Al | 8.69 | 5.62 | 14.31 | 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Guo, X.; Pan, X.; Gu, L.; Liu, X.; Gao, L.; Xiao, G. Synthesis of Brominated Alkanes via Heterogeneous Catalytic Distillation over Al2O3/SO42−/ZrO2. Catalysts 2021, 11, 1464. https://doi.org/10.3390/catal11121464
Yang S, Guo X, Pan X, Gu L, Liu X, Gao L, Xiao G. Synthesis of Brominated Alkanes via Heterogeneous Catalytic Distillation over Al2O3/SO42−/ZrO2. Catalysts. 2021; 11(12):1464. https://doi.org/10.3390/catal11121464
Chicago/Turabian StyleYang, Su, Xiaoxuan Guo, Xiaomei Pan, Liuyu Gu, Xueping Liu, Lijing Gao, and Guomin Xiao. 2021. "Synthesis of Brominated Alkanes via Heterogeneous Catalytic Distillation over Al2O3/SO42−/ZrO2" Catalysts 11, no. 12: 1464. https://doi.org/10.3390/catal11121464
APA StyleYang, S., Guo, X., Pan, X., Gu, L., Liu, X., Gao, L., & Xiao, G. (2021). Synthesis of Brominated Alkanes via Heterogeneous Catalytic Distillation over Al2O3/SO42−/ZrO2. Catalysts, 11(12), 1464. https://doi.org/10.3390/catal11121464







