Single-Atom Catalysts: A Review of Synthesis Strategies and Their Potential for Biofuel Production
Abstract
1. Introduction
2. SAC and SAA Systems and Configuration
3. Stabilized SAC and SAA Strategies
3.1. Stabilization of SACs
3.1.1. Mutual Metal–Support Interaction
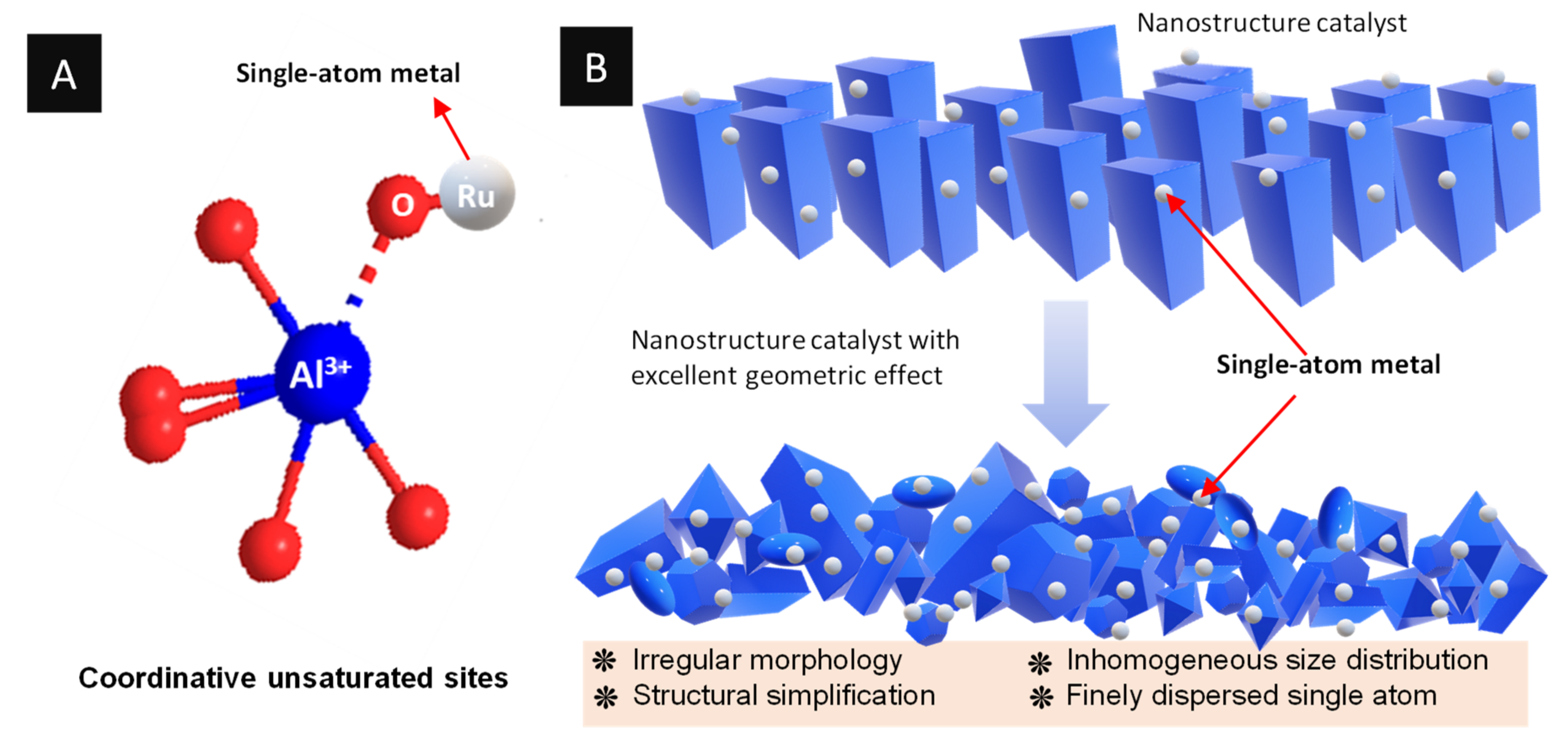
3.1.2. Coordination Geometric Effects
3.2. Stabilisation of SAAs
4. SAC and SAA Support
5. Synthesis of SACs and SAAs
5.1. Bottom-Up Synthesis Strategies of SACs and SAAs
5.2. Top-Down Synthesis Strategies of SACs and SAAs
6. The Challenge and Opportunities of SACs and SAAs in Biofuel Production
- Large-scale and controllable SACs and SAAs preparation
- 2.
- Stability of SACs and SAAs
- 3.
- Additional SACs and SAAs applications in the environmental sector
- 4.
- Complex SACs and SAAs structures for specific applications
- 5.
- Further studies on the active sites and reaction mechanisms of SACs and SAAs
7. Conclusions and Remark
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Thanh, L.T.; Okitsu, K.; Van Boi, L.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and application of biochar-based catalysts for biofuel production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.M.J.-M. From first- to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Mohr, A.; Raman, S. Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Effic. Sustain. Biofuel Prod. Environ. Land-Use Res. 2015, 63, 281–310. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.S.; Min, B.; Jeon, B.H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Dutta, K.; Daverey, A.; Lin, J.G. Evolution retrospective for alternative fuels: First to fourth generation. Renew. Energy 2014, 69, 114–122. [Google Scholar] [CrossRef]
- Havlík, P.; Schneider, U.A.; Schmid, E.; Böttcher, H.; Fritz, S.; Skalský, R.; Aoki, K.; De Cara, S.; Kindermann, G.; Kraxner, F.; et al. Global land-use implications of first and second generation biofuel targets. Energy Policy 2011, 39, 5690–5702. [Google Scholar] [CrossRef]
- Serrano, D.P.; Melero, J.A.; Morales, G.; Iglesias, J.; Pizarro, P. Progress in the design of zeolite catalysts for biomass conversion into biofuels and bio-based chemicals. Catal. Rev. Sci. Eng. 2018, 60, 1–70. [Google Scholar] [CrossRef]
- Picazo-Espinosa, R.; Gonzalez-Lopez, J.; Manzaner, M. Bioresources for Third-Generation Biofuels. Biofuel’s Eng. Process Technol. 2011. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Luo, X.; Jin, Y.; Li, J.; Zhang, H.; Zhang, A.; Xie, J. Heterogeneous sulfur-free hydrodeoxygenation catalysts for selectively upgrading the renewable bio-oils to second generation biofuels. Renew. Sustain. Energy Rev. 2018, 82, 3762–3797. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous catalysis: A central science for a sustainable future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef]
- Pálinkó, I. Heterogeneous Catalysis: A Fundamental Pillar of Sustainable Synthesis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128095492. [Google Scholar]
- Lam, M.K.; Lee, K.T. Scale-Up and Commercialization of Algal Cultivation and Biofuel Production. In Biofuels from Algae; Elsevier B.V.: Amsterdam, The Netherlands, 2013; pp. 261–286. ISBN 9780444595584. [Google Scholar]
- Thakuria, H.; Borah, B.M.; Das, G. Macroporous metal oxides as an efficient heterogeneous catalyst for various organic transformations-A comparative study. J. Mol. Catal. A Chem. 2007, 274, 1–10. [Google Scholar] [CrossRef]
- Asikin Mijan, N.; Lee, H.V.; Abdulkareem Alsultan, G.; Afandi, A.; Taufiq-Yap, Y.H. Production of green diesel via cleaner catalytic deoxygenation of Jatropha curcas oil. J. Clean. Prod. 2017, 167, 1048–1059. [Google Scholar] [CrossRef]
- Gates, B.C.; Flytzani-Stephanopoulos, M.; DIxon, D.A.; Katz, A. Atomically dispersed supported metal catalysts: Perspectives and suggestions for future research. Catal. Sci. Technol. 2017, 7, 4259–4275. [Google Scholar] [CrossRef]
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloy Catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Liu, W.; Wang, A.; Zhang, T. Single-atom catalyst: A rising star for green synthesis of fine chemicals. Natl. Sci. Rev. 2018, 5, 653–672. [Google Scholar] [CrossRef]
- Mitchell, S.; Pérez-Ramírez, J. Single atom catalysis: A decade of stunning progress and the promise for a bright future. Nat. Commun. 2020, 11, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Walsh, A.G.; Yu, J.; Zhang, P. Single-atom alloy catalysts: Structural analysis, electronic properties and catalytic activities. Chem. Soc. Rev. 2021, 50, 569–588. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, G.; Feng, H.; Chen, L.; Wang, H.; Wang, B.; Zhang, X.; Zheng, L.; Hong, S.; Wei, M. Platinum–copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun. 2019, 10, 5812. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Mirjalili, A.; Wheeler, J.; Xie, W.; Jang, B.W.L. Investigation of the preparation methodologies of Pd-Cu single atom alloy catalysts for selective hydrogenation of acetylene. Front. Chem. Sci. Eng. 2015, 9, 442–449. [Google Scholar] [CrossRef]
- Xing, F.; Jeon, J.; Toyao, T.; Shimizu, K.I.; Furukawa, S. A Cu-Pd single-atom alloy catalyst for highly efficient NO reduction. Chem. Sci. 2019, 10, 8292–8298. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Liu, S.; Norouzi Banis, M.; Song, Z.; Li, J.; Yang, L.; Markiewicz, M.; Zhao, Y.; Li, R.; et al. Pt/Pd Single-Atom Alloys as Highly Active Electrochemical Catalysts and the Origin of Enhanced Activity. ACS Catal. 2019, 9, 9350–9358. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Cheng, Y.; Sun, Q.; Gan, T.; He, Q.; He, X.; Ji, H. Quasi-continuous synthesis of cobalt single atom catalysts for transfer hydrogenation of quinoline. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Chen, D.; Hu, T.; Duan, X.; Shakir, I.; Huang, Y.; Duan, X. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Liu, P.; Fu, G.; Zheng, N. Strategies for Stabilizing Atomically Dispersed Metal Catalysts. Small Methods 2018, 2, 1700286. [Google Scholar] [CrossRef]
- Janampelli, S.; Darbha, S. Effect of support on the catalytic activity of WOxpromoted Pt in green diesel production. Mol. Catal. 2018, 451, 125–134. [Google Scholar] [CrossRef]
- Gamal, M.S.; Asikin-Mijan, N.; Arumugam, M.; Rashid, U.; Taufiq-yaq, Y.H.; Safa Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Rashid, U.; Taufiq-Yap, Y.H. Solvent-free catalytic deoxygenation of palm fatty acid distillate over cobalt and manganese supported on activated carbon originating from waste coconut shell. J. Anal. Appl. Pyrolysis 2019, 144, 104690. [Google Scholar] [CrossRef]
- Khalit, W.N.A.W.; Asikin-Mijan, N.; Marliza, T.S.; Gamal, M.S.; Shamsuddin, M.R.; Saiman, M.I.; Taufiq-Yap, Y.H. Catalytic deoxygenation of waste cooking oil utilizing nickel oxide catalysts over various supports to produce renewable diesel fuel. Biomass Bioenergy 2021, 154, 106248. [Google Scholar] [CrossRef]
- Lai, W.H.; Miao, Z.; Wang, Y.X.; Wang, J.Z.; Chou, S.L.; Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; et al. Atomic-Local Environments of Single-Atom Catalysts: Synthesis, Electronic Structure, and Activity. Adv. Energy Mater. 2019, 9, 1900722. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Meng, G.; Zhang, J.; Li, X.; Wang, D.; Li, Y. Electronic structure regulations of single-atom site catalysts and their effects on the electrocatalytic performances. Appl. Phys. Rev. 2021, 8, 021321. [Google Scholar] [CrossRef]
- Ren, Y.; Tang, Y.; Zhang, L.; Liu, X.; Li, L.; Miao, S.; Sheng Su, D.; Wang, A.; Li, J.; Zhang, T. Unraveling the coordination structure-performance relationship in Pt1/Fe2O3 single-atom catalyst. Nat. Commun. 2019, 10, 4500. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.T.; Jones, T.E.; Beeg, S.; Zwiener, L.; Scherzer, M.; Girgsdies, F.; Piccinin, S.; Armbrüster, M.; Knop-Gericke, A.; Schlögl, R. Free-atom-like d states in single-atom alloy catalysts. Nat. Chem. 2018, 10, 1008–1015. [Google Scholar] [CrossRef]
- Thirumalai, H.; Kitchin, J.R. Investigating the Reactivity of Single Atom Alloys Using Density Functional Theory. Top. Catal. 2018, 61, 462–474. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Ma, Z.; Chen, J.; Tang, X. Fabrication, characterization, and stability of supported single-atom catalysts. Catal. Sci. Technol. 2017, 7, 4250–4258. [Google Scholar] [CrossRef]
- Kim, Y.T.; Ohshima, K.; Higashimine, K.; Uruga, T.; Takata, M.; Suematsu, H.; Mitani, T. Fine size control of platinum on carbon nanotubes: From single atoms to clusters. Angew. Chem. Int. Ed. 2006, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong Metal-Support Interactions. Group 8 Noble Metals Supported on TiO2. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong interactions in supported-metal catalysts. Science (80-) 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Alayoglu, S.; Musselwhite, N.; Plamthottam, S.; Melaet, G.; Lindeman, A.E.; Somorjai, G.A. Enhanced CO oxidation rates at the interface of mesoporous oxides and Pt nanoparticles. J. Am. Chem. Soc. 2013, 135, 16689–16696. [Google Scholar] [CrossRef]
- An, N.; Li, S.; Duchesne, P.N.; Wu, P.; Zhang, W.; Lee, J.F.; Cheng, S.; Zhang, P.; Jia, M.; Zhang, W. Size effects of platinum colloid particles on the structure and CO oxidation properties of supported Pt/Fe2O3 catalysts. J. Phys. Chem. C 2013, 117, 21254–21262. [Google Scholar] [CrossRef]
- Najafishirtari, S.; Guardia, P.; Scarpellini, A.; Prato, M.; Marras, S.; Manna, L.; Colombo, M. The effect of Au domain size on the CO oxidation catalytic activity of colloidal Au-FeOx dumbbell-like heterodimers. J. Catal. 2016, 338, 115–123. [Google Scholar] [CrossRef]
- Deng, D.; Chen, X.; Yu, L.; Wu, X.; Liu, Q.; Liu, Y.; Yang, H.; Tian, H.; Hu, Y.; Du, P.; et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462. [Google Scholar] [CrossRef]
- Vilé, G.; Albani, D.; Nachtegaal, M.; Chen, Z.; Dontsova, D.; Antonietti, M.; López, N.; Pérez-Ramírez, J. A Stable Single-Site Palladium Catalyst for Hydrogenations. Angew. Chem. Int. Ed. 2015, 54, 11265–11269. [Google Scholar] [CrossRef]
- Chen, Z.; Pronkin, S.; Fellinger, T.P.; Kailasam, K.; Vilé, G.; Albani, D.; Krumeich, F.; Leary, R.; Barnard, J.; Thomas, J.M.; et al. Merging Single-Atom-Dispersed Silver and Carbon Nitride to a Joint Electronic System via Copolymerization with Silver Tricyanomethanide. ACS Nano 2016, 10, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Daelman, N.; Capdevila-Cortada, M.; López, N. Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts. Nat. Mater. 2019, 18, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, T.Y.; Kwon, G.; Yi, J.; Lee, H. Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion. J. Am. Chem. Soc. 2017, 139, 17694–17699. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, Z.; Yang, W.; Liu, S.; Zhang, X.; Yu, Y.; Cheong, W.C.; Zheng, L.; Ren, F.; Ying, G.; et al. MXene (Ti3C2) Vacancy-Confined Single-Atom Catalyst for Efficient Functionalization of CO2. J. Am. Chem. Soc. 2019, 141, 4086–4093. [Google Scholar] [CrossRef]
- Figueroba, A.; Kovács, G.; Bruix, A.; Neyman, K.M. Towards stable single-atom catalysts: Strong binding of atomically dispersed transition metals on the surface of nanostructured ceria. Catal. Sci. Technol. 2016, 6, 6806–6813. [Google Scholar] [CrossRef]
- O’Connor, N.J.; Jonayat, A.S.M.; Janik, M.J.; Senftle, T.P. Interaction trends between single metal atoms and oxide supports identified with density functional theory and statistical learning. Nat. Catal. 2018, 1, 531–539. [Google Scholar] [CrossRef]
- Hou, C.C.; Wang, H.F.; Li, C.; Xu, Q.; Xu, Q.; Xu, Q. From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications. Energy Environ. Sci. 2020, 13, 1658–1693. [Google Scholar] [CrossRef]
- Liu, D.; Ni, K.; Ye, J.; Xie, J.; Zhu, Y.; Song, L. Tailoring the Structure of Carbon Nanomaterials toward High-End Energy Applications. Adv. Mater. 2018, 30, 1–13. [Google Scholar] [CrossRef]
- Liu, D.; Ding, S.; Wu, C.; Gan, W.; Wang, C.; Cao, D.; Rehman, Z.U.; Sang, Y.; Chen, S.; Zheng, X.; et al. Synergistic effect of an atomically dual-metal doped catalyst for highly efficient oxygen evolution. J. Mater. Chem. A 2018, 6, 6840–6846. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.X.; Yan, Q.Q.; Xu, S.L.; Chu, S.Q.; Chen, P.; Lin, Y.; Liang, H.W. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv. 2019, 5, eaax6322. [Google Scholar] [CrossRef]
- Long, X.; Li, Z.; Gao, G.; Sun, P.; Wang, J.; Zhang, B.; Zhong, J.; Jiang, Z.; Li, F. Graphitic phosphorus coordinated single Fe atoms for hydrogenative transformations. Nat. Commun. 2020, 11, 4074. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dong, H.; Liu, C.; Li, Y. Two-dimensional π-conjugated metal-organic nanosheets as single-atom catalysts for the hydrogen evolution reaction. Nanoscale 2019, 11, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Bulushev, D.A.; Nishchakova, A.D.; Trubina, S.V.; Stonkus, O.A.; Asanov, I.P.; Okotrub, A.V.; Bulusheva, L.G. Ni-N4 sites in a single-atom Ni catalyst on N-doped carbon for hydrogen production from formic acid. J. Catal. 2021, 402, 264–274. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, L.; Zou, L.; Zou, Z.; Chen, C.; Hu, Z.; Yang, H. Single cobalt atom and N codoped carbon nanofibers as highly durable electrocatalyst for oxygen reduction reaction. ACS Catal. 2017, 7, 6864–6871. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, S.; Singh, A. Cobalt Single Atom Heterogeneous Catalyst: Method of Preparation, Characterization, Catalysis, and Mechanism. Cobalt Compd. Appl. 2019. [Google Scholar] [CrossRef]
- Hart, A.S.; Kc, C.B.; Gobeze, H.B.; Sequeira, L.R.; D’Souza, F. Porphyrin-sensitized solar cells: Effect of carboxyl anchor group orientation on the cell performance. ACS Appl. Mater. Interfaces 2013, 5, 5314–5323. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Controlled Functionalization of Graphene by Oxo-addends. Phys. Sci. Rev. 2019, 2, 1–24. [Google Scholar] [CrossRef]
- Liu, J.; Bunes, B.R.; Zang, L.; Wang, C. Supported single-atom catalysts: Synthesis, characterization, properties, and applications. Environ. Chem. Lett. 2018, 16, 477–505. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Tao, L.; Lu, Y.; Wang, S. Defect-Based Single-Atom Electrocatalysts. Small Methods 2019, 3, 1–17. [Google Scholar] [CrossRef]
- Tang, N.; Cong, Y.; Shang, Q.; Wu, C.; Xu, G.; Wang, X. Coordinatively Unsaturated Al3+ Sites Anchored Subnanometric Ruthenium Catalyst for Hydrogenation of Aromatics. ACS Catal. 2017, 7, 5987–5991. [Google Scholar] [CrossRef]
- Tian, H.; Song, A.; Tian, H.; Liu, J.; Shao, G.; Liu, H.; Wang, G. Single-atom catalysts for high-energy rechargeable batteries. Chem. Sci. 2021, 12, 7656–7676. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-Atom Catalysts: Emerging Multifunctional Materials in Heterogeneous Catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.F.; Wang, A.Q.; Li, L.; Liu, X.Y.; Zhang, T.; Mou, C.Y.; Li, J. Bimetallic Au-Pd alloy catalysts for N 2O decomposition: Effects of surface structures on catalytic activity. J. Phys. Chem. C 2012, 116, 6222–6232. [Google Scholar] [CrossRef]
- Song, W.; Jansen, A.P.J.; Hensen, E.J.M. A computational study of the influence of the ceria surface termination on the mechanism of CO oxidation of isolated Rh atoms. Faraday Discuss. 2013, 162, 281. [Google Scholar] [CrossRef]
- Darby, M.T.; Sykes, E.C.H.; Michaelides, A.; Stamatakis, M. Carbon Monoxide Poisoning Resistance and Structural Stability of Single Atom Alloys. Top. Catal. 2018, 61, 428–438. [Google Scholar] [CrossRef]
- Skriver, H.L.; Rosengaard, N.M. Surface energy and work function of elemental metals. Phys. Rev. B 1992, 46, 7157–7168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, F.; Jin, Y.; Wang, J.; Jin, T.; Kou, B. Alloying effect in silver-based dilute nanoalloy catalysts for oxygen reduction reactions. J. Catal. 2020, 384, 37–48. [Google Scholar] [CrossRef]
- Fu, Q.; Luo, Y. Catalytic activity of single transition-metal atom doped in Cu(111) surface for heterogeneous hydrogenation. J. Phys. Chem. C 2013, 117, 14618–14624. [Google Scholar] [CrossRef]
- Yang, K.; Yang, B.; Papanikolaou, K.G.; Darby, M.T.; Stamatakis, M. CO-Induced Aggregation and Segregation of Highly Dilute Alloys: A Density Functional Theory Study. Phys. Chem. Chem. Phys. 2019, 123, 9128–9138. [Google Scholar] [CrossRef]
- Yang, K.; Yang, B. Surface restructuring of Cu-based single-atom alloy catalysts under reaction conditions: The essential role of adsorbates. Phys. Chem. Chem. Phys. 2017, 19, 18010–18017. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, X.; Ren, G.; Yang, T.; Ren, Y.; Lee, A.F.; Su, Y.; Pan, X.; Zhang, J.; Chen, Z.; et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat. Commun. 2020, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-Atom Catalysts Based on the Metal-Oxide Interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Bagheri, A.; Dai, H.; Amal, R. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: A review. J. Mater. Chem. A 2017, 5, 8825–8846. [Google Scholar] [CrossRef]
- Védrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Liu, J.; Do-Thanh, C.L.; Chen, H.; Xu, S.; Lin, Q.; Jiao, Y.; Wang, J.; Wang, Y.; et al. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 2013, 135, 15314–15317. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Dong, J.; Si, R.; Sun, K.; Zhang, J.; Li, M.; Yu, N.; Zhang, B.; Humphrey, M.G.; Fu, Q.; et al. Synergistic effects for enhanced catalysis in a dual single-atom catalyst. ACS Catal. 2021, 11, 1952–1961. [Google Scholar] [CrossRef]
- Han, B.; Li, T.; Zhang, J.; Zeng, C.; Matsumoto, H.; Su, Y.; Qiao, B.; Zhang, T. A highly active Rh1/CeO2 single-atom catalyst for low-temperature CO oxidation. Chem. Commun. 2020, 56, 4870–4873. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, W.; Xie, Z.; You, Z.; Jiang, X.; Han, B.; Lang, R.; Wu, C. High-Loading Pt Single-Atom Catalyst on CeO2-Modified Diatomite Support. Chemistry 2021, 16, 2622–2625. [Google Scholar] [CrossRef]
- Luo, R.; Luo, M.; Wang, Z.; Liu, P.; Song, S.; Wang, X.; Chen, M. The atomic origin of nickel-doping-induced catalytic enhancement in MoS2 for electrochemical hydrogen production. Nanoscale 2019, 11, 7123–7128. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B.; et al. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Liu, J.; Shan, J.; Lucci, F.R.; Cao, S.; Sykes, E.C.H.; Flytzani-Stephanopoulos, M. Palladium-gold single atom alloy catalysts for liquid phase selective hydrogenation of 1-hexyne. Catal. Sci. Technol. 2017, 7, 4276–4284. [Google Scholar] [CrossRef]
- Yang, H.; Shang, L.; Zhang, Q.; Shi, R.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat. Commun. 2019, 10, 4585. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Han, Y.; Liu, S.; Yuan, B.; Liu, Y.; Ma, H. Porous Materials Confining Single Atoms for Catalysis. Front. Chem. 2021, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Dong, J.; Arellano-Jiménez, M.J.; Ye, G.; Dong Kim, N.; Samuel, E.L.G.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Frišcic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Cui, X.; Xiao, J.; Wu, Y.; Du, P.; Si, R.; Yang, H.; Tian, H.; Li, J.; Zhang, W.H.; Deng, D.; et al. A Graphene Composite Material with Single Cobalt Active Sites: A Highly Efficient Counter Electrode for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 6708–6712. [Google Scholar] [CrossRef]
- Jiang, X.H.; Zhang, L.S.; Liu, H.Y.; Wu, D.S.; Wu, F.Y.; Tian, L.; Liu, L.L.; Zou, J.P.; Luo, S.L.; Chen, B.B. Silver Single Atom in Carbon Nitride Catalyst for Highly Efficient Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 23112–23116. [Google Scholar] [CrossRef]
- Li, J.C.; Xiao, F.; Zhong, H.; Li, T.; Xu, M.; Ma, L.; Cheng, M.; Liu, D.; Feng, S.; Shi, Q.; et al. Secondary-Atom-Assisted Synthesis of Single Iron Atoms Anchored on N-Doped Carbon Nanowires for Oxygen Reduction Reaction. ACS Catal. 2019, 9, 5929–5934. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, Y.; Wang, L.; Mu, Z.; Zhu, H.; Zhu, X.; Xing, H.; Xia, H.; Huang, B.; et al. A General Method for Transition Metal Single Atoms Anchored on Honeycomb-Like Nitrogen-Doped Carbon Nanosheets. Adv. Mater. 2020, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, K.; Zong, L.; Zhang, Y.; Feng, D.; Hou, M.; Zhang, Q.; Zheng, D.; Chen, Y.; Wang, L. Fe, N-decorated three dimension porous carbonaceous matrix for highly efficient oxygen reduction reaction. Appl. Surf. Sci. 2020, 505, 144635. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Huang, L.B.; Liu, X.Z.; Zhang, Q.H.; He, C.; Wu, Z.Y.; Zhang, L.J.; Wu, J.; Yang, W.; et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Yi, X.; Zheng, A.; Deng, F.; Chen, C.; Ji, Y.; Liu, F.; Meng, X.; Xiao, F.S.; et al. Mesoporous ZSM-5 zeolite-supported ru nanoparticles as highly efficient catalysts for upgrading phenolic biomolecules. ACS Catal. 2015, 5, 2727–2734. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Yu, Q.; Chen, Y.; Chai, Z.; Zhao, G.; Liu, S.; Cheong, W.C.; Pan, Y.; Zhang, Q.; et al. A General Strategy for Fabricating Isolated Single Metal Atomic Site Catalysts in y Zeolite. J. Am. Chem. Soc. 2019, 141, 9305–9311. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Zhang, T.; Bai, R.; Mayoral, A.; Zhang, P.; Zhang, Q.; Terasaki, O.; Yu, J. Zeolite-Encaged Single-Atom Rhodium Catalysts: Highly-Efficient Hydrogen Generation and Shape-Selective Tandem Hydrogenation of Nitroarenes. Angew. Chem. Int. Ed. 2019, 58, 18570–18576. [Google Scholar] [CrossRef]
- Cui, Q.; Qin, G.; Wang, W.; Geethalakshmi, K.R.; Du, A.; Sun, Q. Novel two-dimensional MOF as a promising single-atom electrocatalyst for CO2 reduction: A theoretical study. Appl. Surf. Sci. 2020, 500, 143993. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Liu, P.; Song, X.; Mei, D.; Tang, Y.; Wang, X.; Zhong, C. Metal-organic framework encapsulated single-atom Pt catalysts for efficient photocatalytic hydrogen evolution. J. Catal. 2019, 375, 351–360. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, Y.; Wang, C.; Jiang, H.; Jiang, H.; Cheng, G.J.; Cheng, G.J. A Single-Atomic Noble Metal Enclosed Defective MOF via Cryogenic UV Photoreduction for CO Oxidation with Ultrahigh Efficiency and Stability. ACS Appl. Mater. Interfaces 2020, 12, 26068–26075. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Chen, W.; Tong, Y.; Zhao, C.; Lin, Y.; Jiang, Z.; Zhang, Q.; Xue, Z.; Cheong, W.C.; et al. Two-step carbothermal welding to access atomically dispersed pd1 on three-dimensional zirconia nanonet for direct indole synthesis. J. Am. Chem. Soc. 2019, 141, 10590–10594. [Google Scholar] [CrossRef] [PubMed]
- Nongbe, M.C.; Ekou, T.; Ekou, L.; Yao, K.B.; Le Grognec, E.; Felpin, F.X. Biodiesel production from palm oil using sulfonated graphene catalyst. Renew. Energy 2017, 106, 135–141. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Qiao, B.; Liu, J.; Wang, Y.G.; Lin, Q.; Liu, X.; Wang, A.; Li, J.; Zhang, T.; Liu, J. Highly Efficient Catalysis of Preferential Oxidation of CO in H2-Rich Stream by Gold Single-Atom Catalysts. ACS Catal. 2015, 5, 6249–6254. [Google Scholar] [CrossRef]
- Gu, X.K.; Qiao, B.; Huang, C.Q.; Ding, W.C.; Sun, K.; Zhan, E.; Zhang, T.; Liu, J.; Li, W.X. Supported single Pt1/Au1 atoms for methanol steam reforming. ACS Catal. 2014, 4, 3886–3890. [Google Scholar] [CrossRef]
- Li, P.; Wang, M.; Duan, X.; Zheng, L.; Cheng, X.; Zhang, Y.; Kuang, Y.; Li, Y.; Ma, Q.; Feng, Z. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th anniversary article: Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Boucher, M.B.; Zugic, B.; Cladaras, G.; Kammert, J.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.H.; Flytzani-Stephanopoulos, M. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys. Chem. Chem. Phys. 2013, 15, 12187–12196. [Google Scholar] [CrossRef]
- Shan, J.; Liu, J.; Li, M.; Lustig, S.; Lee, S.; Flytzani-Stephanopoulos, M. NiCu single atom alloys catalyze the C–H bond activation in the selective non-oxidative ethanol dehydrogenation reaction. Appl. Catal. B Environ. 2018, 226, 534–543. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.; Wimble, J.M.; Lucci, F.R.; Lee, S.; Michaelides, A.; Flytzani-stephanopoulos, M.; Stamatakis, M.; Sykes, E.C.H. Catalysts for efficient C–H activation. Nat. Chem. 2018, 10, 325–332. [Google Scholar] [CrossRef]
- Lucci, F.R.; Liu, J.; Marcinkowski, M.D.; Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective hydrogenation of 1,3-butadiene on platinum–copper alloys at the single-atom limit. Nat. Commun. 2015, 6, 8550. [Google Scholar] [CrossRef]
- Mao, J.; Yin, J.; Pei, J.; Wang, D.; Li, Y. Single atom alloy: An emerging atomic site material for catalytic applications. Nano Today 2020, 34, 100917. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, Z.J.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S.C.; et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454. [Google Scholar] [CrossRef]
- Aich, P.; Wei, H.; Basan, B.; Kropf, A.J.; Schweitzer, N.M.; Marshall, C.L.; Miller, J.T.; Meyer, R. Single-atom alloy Pd–Ag catalyst for selective hydrogenation of acrolein. J. Phys. Chem. C 2015, 119, 18140–18148. [Google Scholar] [CrossRef]
- Kim, J.; Roh, C.W.; Sahoo, S.K.; Yang, S.; Bae, J.; Han, J.W.; Lee, H. Highly Durable Platinum Single-Atom Alloy Catalyst for Electrochemical Reactions. Adv. Energy Mater. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement andmarket trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science (80-) 2012, 335, 1209–1212. [Google Scholar] [CrossRef]
- Lucci, F.R.; Darby, M.T.; Mattera, M.F.G.; Ivimey, C.J.; Therrien, A.J.; Michaelides, A.; Stamatakis, M.; Sykes, E.C.H. Controlling hydrogen activation, spillover, and desorption with Pd–Au single-atom alloys. J. Phys. Chem. Lett. 2016, 7, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Hannagan, R.T.; Giannakakis, G.; Réocreux, R.; Schumann, J.; Finzel, J.; Wang, Y.; Michaelides, A.; Deshlahra, P.; Christopher, P.; Flytzani-Stephanopoulos, M. First-principles design of a single-atom–alloy propane dehydrogenation catalyst. Science (80-) 2021, 372, 1444–1447. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Q.; Liu, W.; Lin, Y.; Guan, Q.; Zheng, X.; Pan, H.; Zhu, J.; Sun, Z.; Wei, S.; et al. Quasi Pd1Ni single-atom surface alloy catalyst enables hydrogenation of nitriles to secondary amines. Nat. Commun. 2019, 10, 4998. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Li, L.; Huang, Y.; Zhang, T.; Lee, J.W.; Jang, B.W.L.; Mou, C.-Y. Promotional effect of Pd single atoms on Au nanoparticles supported on silica for the selective hydrogenation of acetylene in excess ethylene. New J. Chem. 2014, 38, 2043–2051. [Google Scholar] [CrossRef]
- Trimpalis, A.; Giannakakis, G.; Cao, S.; Flytzani-Stephanopoulos, M. NiAu single atom alloys for the selective oxidation of methacrolein with methanol to methyl methacrylate. Catalysis Today. 2020, 355, 804–814. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, S.; Chen, W.; Huang, Z.-Q.; Wei, W.; Yao, T.; Liu, R.; Zang, K.; Wang, X.; Wu, G. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2019, 2, 304–313. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Wu, Y.; Li, Y. Fabrication of single-atom catalysts with precise structure and high metal loading. Adv. Mater. 2018, 30, 1801649. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Sophisticated construction of Au islands on Pt–Ni: An ideal trimetallic nanoframe catalyst. In Controlled Synthesis of Pt-Ni Bimetallic Catalysts and Study of Their Catalytic Properties; Springer: Berlin/Heidelberg, Germany, 2016; pp. 93–111. [Google Scholar]
- Balajii, M.; Niju, S. Biochar-derived heterogeneous catalysts for biodiesel production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.R.; Kim, H.J.; Yang, S.-Y.Y.; Song, H.-S.S.; Park, J.Y.; Park, Y.-L.L.; Han, Y.-H.H.; Choi, Y.-K.K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.M.; Grunwaldt, J.-D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Ventura, M.; Fonseca, I.M. Carbon-based materials for the development of highly dispersed metal catalysts: Towards highly performant catalysts for fine chemical synthesis. Catalysts 2020, 10, 1407. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Yang, N.; Deng, M.; Ibraheem, S.; Deng, J.; Li, J.; Li, L.; Wei, Z. Ultrahigh-loading zinc single-atom catalyst for highly efficient oxygen reduction in both acidic and alkaline media. Angew. Chem. Int. Ed. 2019, 58, 7035–7039. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. 2016, 128, 10958–10963. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic Exchange of Metal-Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Gan, T.; Liu, Y.; He, Q.; Zhang, H.; He, X.; Ji, H. Facile synthesis of kilogram-scale Co-alloyed Pt single-atom catalysts via ball milling for hydrodeoxygenation of 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2020, 8, 8692–8699. [Google Scholar] [CrossRef]
- Gan, T.; He, Q.; Zhang, H.; Xiao, H.; Liu, Y.; Zhang, Y.; He, X.; Ji, H. Unveiling the kilogram-scale gold single-atom catalysts via ball milling for preferential oxidation of CO in excess hydrogen. Chem. Eng. J. 2020, 389, 124490. [Google Scholar] [CrossRef]
- Liu, G.; Robertson, A.W.; Li, M.M.J.; Kuo, W.C.H.; Darby, M.T.; Muhieddine, M.H.; Lin, Y.C.; Suenaga, K.; Stamatakis, M.; Warner, J.H.; et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 2017, 9, 810–816. [Google Scholar] [CrossRef]
- Cao, W.; Luo, W.; Ge, H.; Su, Y.; Wang, A.; Zhang, T. UiO-66 derived Ru/ZrO2@C as a highly stable catalyst for hydrogenation of levulinic acid to γ-valerolactone. Green Chem. 2017, 19, 2201–2211. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lende, A.B.; Anbalagan, A.K.; Lee, C.-H.; Tan, C.-S. Development and Characterization of a One-Pot Synthesized Fe–Au–Pd Surface Alloy Catalyst for Highly Selective Conversion of Castor Oil to Octadecane via Hydrodeoxygenation. Energy Fuels 2021, 35, 16637–16652. [Google Scholar] [CrossRef]
- Akri, M.; El Kasmi, A.; Batiot-Dupeyrat, C.; Qiao, B. Highly active and carbon-resistant nickel single-atom catalysts for methane dry reforming. Catalysts 2020, 10, 630. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Xin, T.; Li, Y.; Wu, X.; Zhang, G. Single-atom V-N charge-transfer bridge on ultrathin carbon nitride for efficient photocatalytic H2 production and formaldehyde oxidation under visible light. Chem. Eng. J. 2022, 429, 132229. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.; Zhang, L.; Si, R.; Shen, M.; Wang, M.; Tian, J.; Shi, J. Electron Configuration Modulation of Nickel Single Atoms for Elevated Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 6827–6831. [Google Scholar] [CrossRef]
- Zhang, L.; Long, R.; Zhang, Y.; Duan, D.; Xiong, Y.; Zhang, Y.; Bi, Y. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. Int. Ed. 2020, 59, 6224–6229. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, F.; Li, N.; Zhang, Y.; Mu, Z.; Tang, Y.; Lai, J.; Chao, Y.; Luo, M.; Lin, F.; et al. Strengthening reactive metal-support interaction to stabilize high-density Pt single atoms on electron-deficient g-C3N4 for boosting photocatalytic H2 production. Nano Energy 2019, 56, 127–137. [Google Scholar] [CrossRef]
- Trofimovaite, R.; Parlett, C.M.A.; Kumar, S.; Frattini, L.; Isaacs, M.A.; Wilson, K.; Olivi, L.; Coulson, B.; Debgupta, J.; Douthwaite, R.E.; et al. Single atom Cu(I) promoted mesoporous titanias for photocatalytic Methyl Orange depollution and H2 production. Appl. Catal. B Environ. 2018, 232, 501–511. [Google Scholar] [CrossRef]
- Liang, J.X.; Lin, J.; Liu, J.; Wang, X.; Zhang, T.; Li, J. Dual Metal Active Sites in an Ir1/FeOx Single-Atom Catalyst: A Redox Mechanism for the Water-Gas Shift Reaction. Angew. Chem. Int. Ed. 2020, 59, 12868–12875. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Z.; Sa, R.; Adidharma, H.; Gasem, K.A.M.; Russell, A.G.; Fan, M.; Wu, K. Computation-predicted, stable, and inexpensive single-atom nanocatalyst Pt@Mo2C-an important advanced material for H2 production. J. Mater. Chem. A 2017, 5, 14658–14672. [Google Scholar] [CrossRef]
- He, Q.; Liu, D.; Lee, J.H.; Liu, Y.; Xie, Z.; Hwang, S.; Kattel, S.; Song, L.; Chen, J.G. Electrochemical Conversion of CO2 to Syngas with Controllable CO/H2 Ratios over Co and Ni Single-Atom Catalysts. Angew. Chem. Int. Ed. 2020, 59, 3033–3037. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Yang, X.; Zhang, L.; Wang, A.; Li, L.; Wang, H.; Wang, X.; Zhang, T. Performance of Cu-Alloyed Pd Single-Atom Catalyst for Semihydrogenation of Acetylene under Simulated Front-End Conditions. ACS Catal. 2017, 7, 1491–1500. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Wang, H.; Wang, Y. Toward efficient single-atom catalysts for renewable fuels and chemicals production from biomass and CO2. Appl. Catal. B Environ. 2021, 292, 120162. [Google Scholar] [CrossRef]
- Zhao, S.; Wen, Y.; Liu, X.; Pen, X.; Lü, F.; Gao, F.; Xie, X.; Du, C.; Yi, H.; Kang, D.; et al. Formation of active oxygen species on single-atom Pt catalyst and promoted catalytic oxidation of toluene. Nano Res. 2020, 13, 1544–1551. [Google Scholar] [CrossRef]
- Gu, H.; Liu, X.; Liu, X.; Ling, C.; Wei, K.; Zhan, G.; Guo, Y.; Zhang, L. Adjacent single-atom irons boosting molecular oxygen activation on MnO2. Nat. Commun. 2021, 12, 5422. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Mei, B.; Tong, Y.; Li, Y.; Ruan, M.; Song, P.; Sun, G.; Jiang, L.; Wang, Y.; et al. Carbon-Supported Divacancy-Anchored Platinum Single-Atom Electrocatalysts with Superhigh Pt Utilization for the Oxygen Reduction Reaction. Angew. Chem. 2019, 131, 1175–1179. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, J.; Wu, D.; Zhao, X.; Jing, Y.; Zhou, Z. A Ti-anchored Ti2CO2 monolayer (MXene) as a single-atom catalyst for CO oxidation. J. Mater. Chem. A 2016, 4, 4871–4876. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.; Lv, Y.; Wang, J.; Li, Q.; Zhang, Z. From nanoparticles to single atoms for Pt/CeO2: Synthetic strategies, characterizations and applications. J. Rare Earths 2020, 38, 850–862. [Google Scholar] [CrossRef]
- Rong, X.; Wang, H.J.; Lu, X.L.; Si, R.; Lu, T.B. Controlled Synthesis of a Vacancy-Defect Single-Atom Catalyst for Boosting CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, D.; Zhou, S.; Delariva, A.; Peterson, E.J.; Xiong, H.; Pereira-Hernández, X.I.; Purdy, S.C.; Ter Veen, R.; Brongersma, H.H.; Miller, J.T.; et al. Stabilizing High Metal Loadings of Thermally Stable Platinum Single Atoms on an Industrial Catalyst Support. ACS Catal. 2019, 9, 3978–3990. [Google Scholar] [CrossRef]
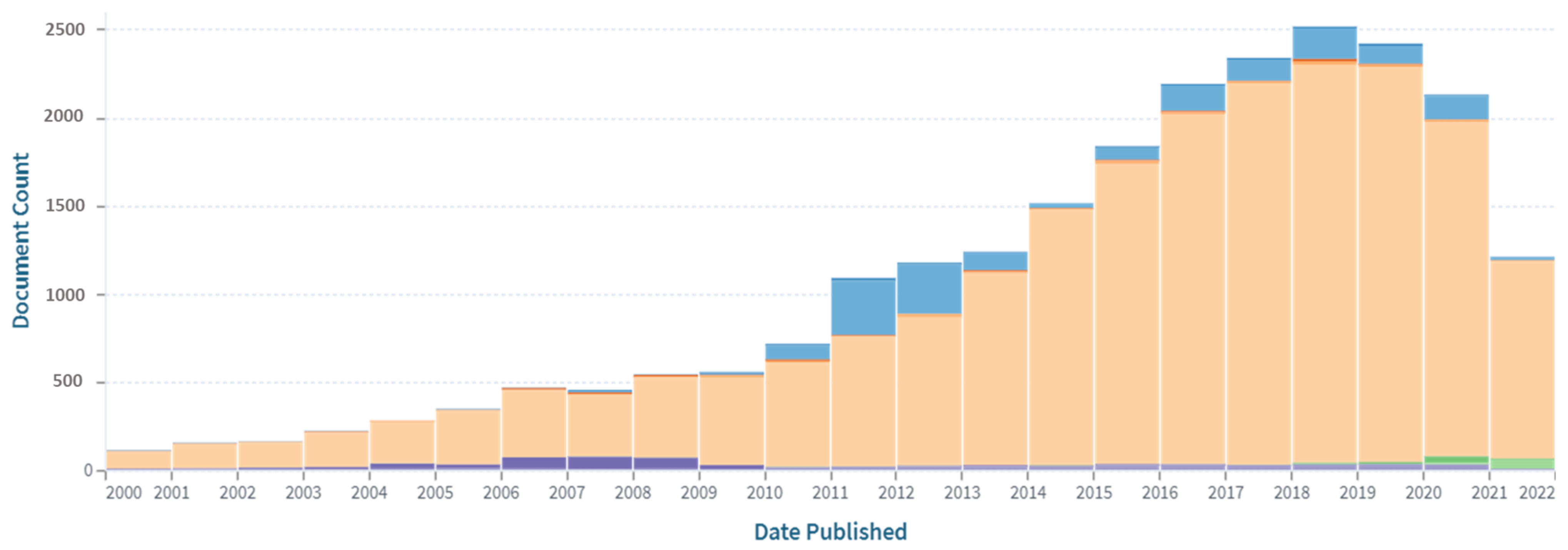
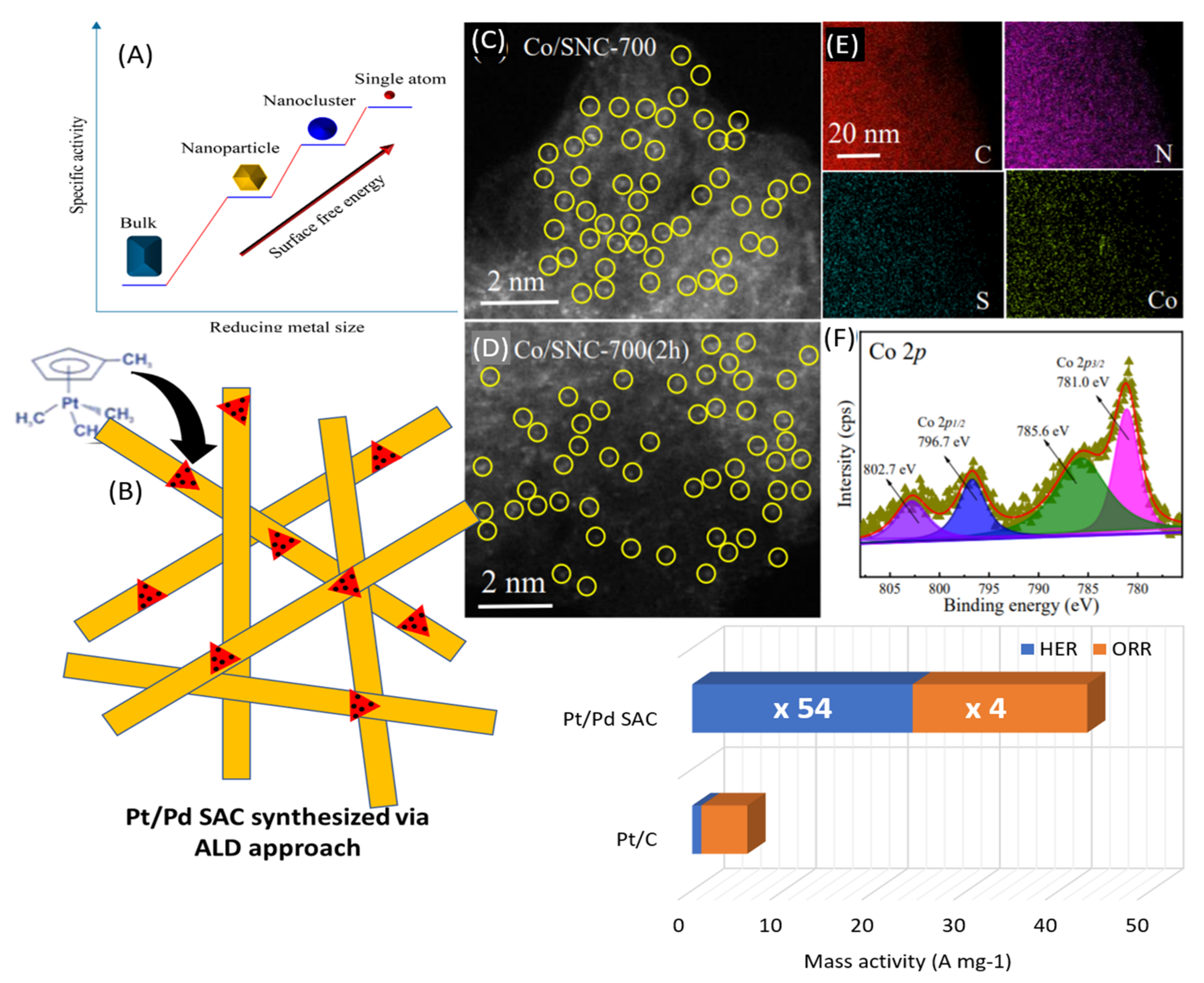
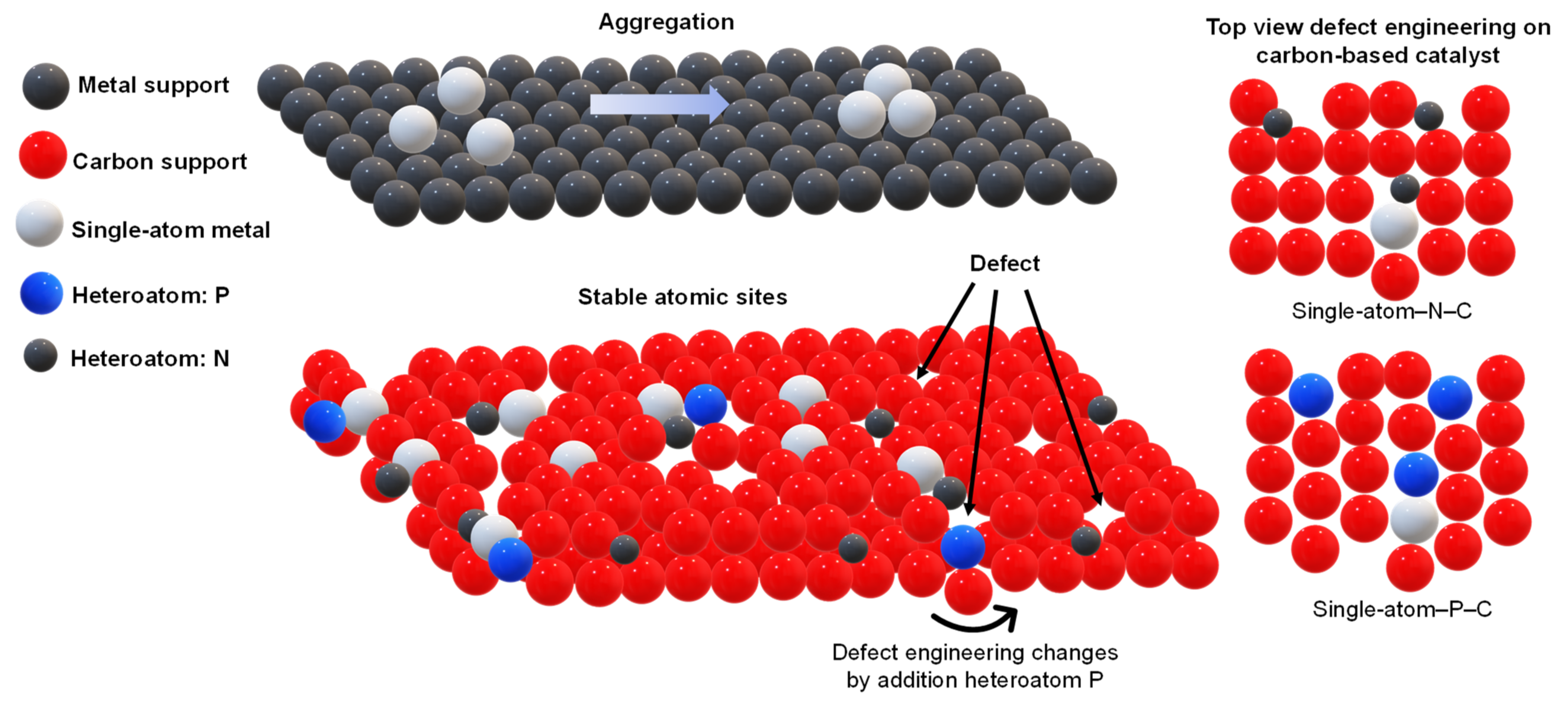
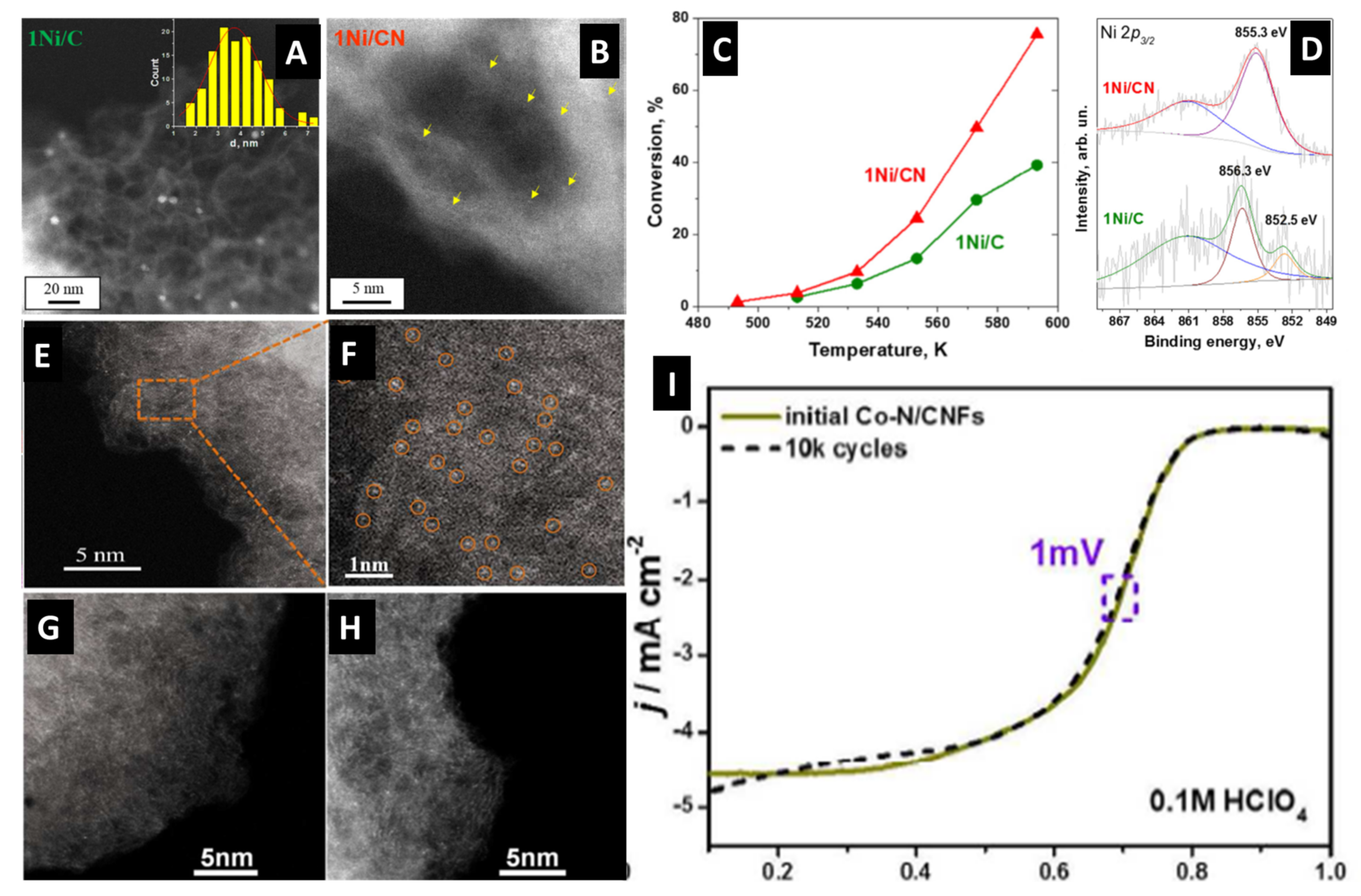

| Catalyst | Preparation | Performance | Ref. |
|---|---|---|---|
| Rh/zirconia SAC | i. Preparation of zirconia support by sol-gel method ii. Deposition of Rh on zirconia by a wet impregnation method | Activation of methane in mild conditions by single atomic Rh catalysts on zirconia support. | [52] |
| Pd/fluorite oxide (CeZrHfTiLa)Ox (HEFO)SAC | Combination of mechanical milling with calcination at 900 °C | Incorporation of single Pd atom into HEFO by forming stable Pd–O–M bonds (M=Ce/Zr/La). The reducibility of lattice oxygen has been improved with higher CO oxidation activity at low-temperature and superior resistance to thermal and hydrothermal degradation. | [85] |
| Ir/FeOx (Ir1/FeOx) SAC | Co-precipitation method Aqueous mixture of H2IrCl6 and Fe(NO3)3 with appropriate ratio was added dropwise to NaOH solution (pH of the final solution was maintained at around 8) | The activity is 1 order of magnitude higher than its Ir cluster or nanoparticle counterparts and even higher than those of the most active Au- or Pt-based catalysts. | [86] |
| Ir1Mo1/TiO2 SACs | i. TiO2 support was treated at 600 °C for 2 h under argon ii. Ir2Mo2(CO)10(η5-C5H5)2 was dissolved in dry n-pentane, forming an orange colour solution iii. TiO2 support was added to the previous orange solution, followed by vigorous stirring overnight until the orange colour was not observed iv. The solvent was removed by evacuation using Schlenk techniques, then was heated at 450 °C under argon | Dual single-atom catalyst supported on TiO2, Ir1Mo1/TiO2 SACs demonstrate a superior catalytic performance for selective hydrogenation of 4-nitrostyrene (4-NS) to 4-vinylaniline (4-VA) as compared to single-atom catalyst Ir1/TiO2. | [87] |
| Pt/CeO2 modified diatomite SAC | Dispersion of diatomite in the solution of 1,3,5-benzenetricarboxylic acid and Ce(NO3)3 mixture, followed by calcination. CeO2@diatomite was dispersed in solution of H2PtCl6 and further calcined in air at 300 °C | Highly active for hydrogenation of phenylacetylene to styrene | [89] |
| Rh1/CeO2 SAC | Co-precipitation method | Highly active and good stability for CO oxidation (TOF of 0.41 s−1 at 100 °C). | [88] |
| Ru/MoS2 SAC | Simple one-step impregnation methodRuCl3 was added into a MoS2 nanosheet dispersed in a mixed solution of ethanol and deionised water | Significantly improved hydrogen evolution reaction (HER) of 2D MoS2 (low overpotential of 76 mV at 10 mA cm−2 in alkaline media). | [91] |
| Ni/MoS2 SAC | MoS2 on carbon cloth was immersed into NiCl2 ethanol solution before being dried at 80 °C, followed by calcination at 300 °C for 1 h under 10% H2/Ar atm | Enhanced HER activity (98 mV and 110 mV in 1M KOH and 0.5M H2SO4, respectively. | [92] |
| PdAu SAA PdAu/silica SAA | Sequential reduction method (Pd/Au ratio of 1/250) Fumed silica was introduced into PdAu SAA solution in water and stirred overnight | Excellent selectivity and stable activity of PdAu SAA for the hydrogenation of 1-hexyne to 1-hexene. Silica support is not required to catalyse the reaction. | [93] |
| Method | Merits | Demerits | Catalysts | Application | Performance/Condition | Ref. |
|---|---|---|---|---|---|---|
| Bottom-up strategy | ||||||
| Atomic layer deposition (ALD) | Controllable size and dispersion of particles | High equipment cost Low yield | Pt/graphene SAC | Methanol oxidation | Pt/graphene SAC found to be effective for methanol oxidation at 0.59, 0.60, 0.62, and 0.7 V with order of ALD cycles 50–150. | [114] |
| Pd1Ni SAA | Hydrogenation | Successful hydrogenolysis of nitriles by Pd/Ni to secondary amines with yield > 94%, exhibiting excellent recyclability. | [131] | |||
| Physical vapour deposition (PVD) | Simple preparation | High equipment cost Low yield | Pd-Au SAAs | Hydrogenation | Pd−Au SAAs highly dissociate H2 at 85 K, and reaction better than that of Au (111). | [129] |
| RhCu/SiO2 SAA | Dehydrogenation | RhCu/SiO2 SAA catalyst showed propene selectivity 100% versus ~80% on Pt/Al2O3 and sustained transformation of propane to propene and hydrogen for more than 50 h on stream at 623 K. | [130] | |||
| Co-precipitation | Simple and quick preparation Low cost | Easy agglomeration Low metal loading | Au1/CeO2 SAC and Au1/FeOx SAC | Preferential oxidation of CO | Au1/CeO2 SAC and Au1/FeOx SAC effectively catalysed CO conversion at 60− 90 °C with excellent stability. | [115] |
| Pt1/ZnO SAC and Au1/ZnO SAC | Methanol steam reforming (MSR) | Pt1/ZnO SAC catalyst showed 43% conversion at the steady state, higher than that of Au1/ZnO SAC (28%). Au1/ZnO SAC showed higher CO2 selectivity (100%) than Pt1/ZnO (88%). | [116] | |||
| Ir1/FeOx SAC | Water–gas shift (WGS) | Under atmospheric pressure with different Ir loadings from 0.01, 0.22, 0.32, and 2.40, the Ir1/FeOx SAC showed high WGS activity. | [86] | |||
| Incipient wetness co-impregnation | Low cost Simplest method Varies metal choices | Low yield | Pd-Ag/SiO2 _SeqIWI SAA and Pd-Ag/SiO2_CoIWI SAA | Hydrogenation | Pd atom enhances acrolein adsorption through the C=C bond (1.36 eV) more dramatically than the C=O bond while acrolein adsorbs weakly to the monometallic pure Ag/SiO2 catalyst. | [125] |
| Pt1/ATO (antimony doped tin–oxide) SAA | Electrochemical reactions—formic acid oxidation reaction (FAOR) | Owing to the existence of single atomic Pt sites, which initiate the O–H bond of formic acid and have good resistance, the Pt1/ATO SAA catalyst exhibited superior FAOR activity. | [126] | |||
| Sequential reduction | Available complex structure | Complicated steps Uncontrollable structure | Ni0.005Au/SiO2 SAA | Selective oxidation | Ni0.005Au/SiO2 SAA showed superior activity with 100% conversion to methyl methacrylate (MMA) than NiAu/SiO2 (2%) for selective oxidation of methacrolein with methanol to MMA. | [133] |
| Ru1–PtCu3/AC SAA, Ru1–PtCu/AC SAA, and Ru1–Pt3Cu/AC SAA | Water oxidation Electrocatalysis | The PtCu alloy’s OER activity was low, with an oxidation potential of 410, implying that the atomically scattered Ru1 incorporated into the Pt–Cu alloys serve as, or at least participate in, the OER active sites. | [134] | |||
| Galvanic replacement | Special compositions and structures | Need difference reduction potential between two metals Limited choices | Pd1Cu SAA, Pt1Cu SAA, and NiCu SAA. | Ethanol dehydrogenation | NiCu SAA alloy exhibited higher activity than Pd1Cu SAA and Pt1Cu SAA due to the presence of Ni, which lowers the barrier for C–H activation and thus increases the activity. | [120] |
| Pt/Cu SAAs | C–H activation | Catalytic studies showed that Pt/Cu SAAs catalysed the exchange reaction at 250 °C, compared to ∼550 °C on Cu NPs, and are stable during the reaction. | [121] | |||
| Pt/Cu SAAs’ | Hydrogenation | Near 2D volcano plot, Ru1–Pt3Cu (111) showed the highest OER activity (η = 0.42 V) while Ru1–Cu (111), Ru1–PtCu3 (111), Ru1–PtCu(111), and Ru1–Pt (111) exhibited 0.82, 0.71 and 0.66, and 0.92 V, respectively, which are less dynamic. | [122] | |||
| Top-down strategy | ||||||
| High-temperature pyrolysis | Suitable large-scale production Easy to anchor heteroatoms | Dangerous etching (HF) High temperature needed | Co SAs/N-C, Co NPs-N/C, and Pt/C | Electroreduction of CO2 | CoSAs/N–C (900) had the strongest ORR activity, with an Eonset number that was almost comparable to Pt/C (0.982 V vs. RHE) and E1/2 (0.881 V) more favorable than that of Pt/C (0.811 V) and CoSAs/N–C (800) (0.863 V). | [142] |
| Ni SAs/N-C and Ni NPs/N-C | Electroreduction of CO2 | Ni SAs/N–C catalyst demonstrated higher amount of onset potential for reversible hydrogen electrode (RHE) than Ni NPs/N–C catalyst. | [143] | |||
| Ball milling | Easy scalable Environmentally friendly | High maintenance Time consuming | FeN4/GN SAAs | Catalytic oxidation benzene to phenol | FeN4/GN showed phenol yield (18.7%) and conversion (23.4%) for direct catalytic oxidation of benzene to phenol. The FeN4/GN also exhibited high stability by yielding 8.3% of phenol after 24 h of reaction time. | [48] |
| Pt1/Co SAAs | Hydrodeoxygenation | Pt1/Co SAAs showed high HMF conversion (100%) and were highly selective towards DMF (93%) for the catalyst. | [144] | |||
| Au1/CeO2 SAAs | Preferential oxidation of CO | Au1/CeO2 SAAs had 100% CO conversion after 160 h in the mainstream, showing that the catalyst has excellent stability and superior activity. | [145] | |||
| Catalyst/ Biofuel and Renewable Chemical | Reaction | Product | Remarks | Refs. |
|---|---|---|---|---|
| Liquid renewable chemicals/biofuels | ||||
| Co–MoS2 SAC | Hydrodeoxygenation | Toluene Hydrocarbon fuels | -High metal dispersion and stability associated to a strong interaction between Ru and nano tetragonal ZrO2. -The catalyst exhibited clear deactivation in the recyclability study. The deactivation of the catalyst was associated to the leaching. | [146] |
| Ru/ZrO2@C SAC | Hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) | γ-valerolactone (GVL) | -A strong covalent bond between Co atoms to monolayer MoS2 enhances the number of Co–S–Mo interfacial. -The catalyst is clearly effective and highly stable for 4-methylphenol to toluene transformation. | [147] |
| Fe–Au–Pd SAAs | Hydrodeoxygenation | Octadecane | Octadecane selectivity of 76% was obtained. | [148] |
| Gas biofuel | ||||
| Ni SACs | Dry methane reforming (DRM) | Carbon monoxide and hydrogen | -Ni SACs is coking-resistant agent. -PVP method during catalyst synthesis stabilised the catalyst. | [149] |
| SAVCN SAC | Photocatalytic hydrogen production and formaldehyde oxidation | Hydrogen | -SAVCN rendered excellent photo-catalytic activity at room temperature. | [150] |
| CN–0.2Ni–HO (CN: nitride) | Photocatalytic water splitting | Hydrogen | -Photocatalytic H2 production rate was the highest (354.9 μmol h−1 g−1), higher than that of reported CN photocatalysts SACs. | [151] |
| Pt–C3N4 SAC | Photocatalytic water splitting | Hydrogen | -High H2 production activity (14.7 mmol·h−1·g−1) was achieved over Pt/C3N4 SAC catalyst. The reaction activity was 20 times higher than that of metallic Pt–C3N4 catalyst. | [152] |
| PtNP-loaded g-C3N4 SAC | In situ photocatalytic reduction method at a sub-zero temperature | Hydrogen | -Excellent H2 evolution is due to RMSI. -RMSI contributes to the supra-high-density PtSAs on the electron- deficient g-C3N4. | [153] |
| Cu/meso-TiO2-500C SAC | Photocatalytic dye degradation | Hydrogen | -A synergy between Cu(I) and CuO increased photocatalytic H2 production four-fold relative to the mesoporous anatase scaffold. -Tailoring textural and photophysical properties positively affect chemical mass transport, energetics, and lifetime of charge carriers. | [154] |
| CoNi-NC SAC | The electrochemical CO2 reduction reaction (CO2RR) | Hydrogen, carbon monoxide | -The CoNi-NC catalysts maintained the high syngas yield. -Co and Ni with varies ratios co-existed in an SAC configuration exhibited to have controllable CO/H2 ratios. | [157] |
| Ir1/FeOx SAC | Water–gas shift | Hydrogen | -Fe and Ir1 single atoms play a synergetic effect to catalyse the WGS reaction. | [155] |
| Pt@Mo2C SAC | Water–gas shift | Hydrogen | -Pt@Mo2C SAC promoted WGS, demonstrating the advantages of lower cost and higher thermal stability. -The synergetic effect of bimetallic (Mo–Pt) is beneficial to H2 production. | [156] |
| Cu alloyed Pd SAC | Semi-hydrogenation of acetylene | Ethylene | -Ethylene selectivity of ~85% was achieved. -The isolation of Pd atoms by the IB metal played a key role in enhancing the ethylene selectivity, while the electronic effect had a relatively minor effect on their catalytic performances. | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asikin-Mijan, N.; Mohd Sidek, H.; AlSultan, A.G.; Azman, N.A.; Adzahar, N.A.; Ong, H.C. Single-Atom Catalysts: A Review of Synthesis Strategies and Their Potential for Biofuel Production. Catalysts 2021, 11, 1470. https://doi.org/10.3390/catal11121470
Asikin-Mijan N, Mohd Sidek H, AlSultan AG, Azman NA, Adzahar NA, Ong HC. Single-Atom Catalysts: A Review of Synthesis Strategies and Their Potential for Biofuel Production. Catalysts. 2021; 11(12):1470. https://doi.org/10.3390/catal11121470
Chicago/Turabian StyleAsikin-Mijan, Nurul, Haslinda Mohd Sidek, Abdulkareem G. AlSultan, Nurul Ahtirah Azman, Nur Athirah Adzahar, and Hwai Chyuan Ong. 2021. "Single-Atom Catalysts: A Review of Synthesis Strategies and Their Potential for Biofuel Production" Catalysts 11, no. 12: 1470. https://doi.org/10.3390/catal11121470
APA StyleAsikin-Mijan, N., Mohd Sidek, H., AlSultan, A. G., Azman, N. A., Adzahar, N. A., & Ong, H. C. (2021). Single-Atom Catalysts: A Review of Synthesis Strategies and Their Potential for Biofuel Production. Catalysts, 11(12), 1470. https://doi.org/10.3390/catal11121470









