1. Introduction
Biomass is an important source of renewable energy. The possibilities of its conversion using non-combustion technologies into further utilizable, energy-rich products (bio-oils, solid carbonaceous residues and energy gases) are far from being explored. The reasons are that the conversion process must be highly efficient while not being energy-intensive, and the usability of the products obtained must be clearly determined on the basis of their energy content, chemical composition and the possibilities for the further processing and commercialization of the final products.
One promising conversion method is low-temperature pyrolysis [
1,
2], providing bio-oil, biochar and a gaseous mixture containing mostly CO
2, CO, methane and hydrogen. Bio-oil can be utilized not only as a clean fuel but also as a source of commercially useful chemicals, whereas biochar can be used as a smokeless fuel, and alternatively as a fertilizer or sorbent. The employment of the gas produced is a question. Depending on the method of thermal treatment and the type of biomass, the gas may contain predominantly CO
2 and CO and can thus be a source of these gases in the production of synthetic methane by the Power-to-Gas technology [
3], designed on the basis of the Sabatier reaction [
4,
5]. If the methane content in the resulting gas is higher, e.g., 20–30 vol.%, it is possible to consider its subsequent conversion to hythane, in our case in the presence of a catalyst.
Hythane is a gaseous mixture consisting of 10–30 vol.% of hydrogen and 70–90 vol.% of methane with a possible application mainly for automobiles. Hydrogen helps improve the performance of the combustion engine and reduce CO
2 and NO
x emissions in the atmosphere. However, another, very important use is the additivation of natural gas by direct injection of hythane into natural-gas networks or natural-gas storage tanks. It makes it possible to store hythane and with it the chemical energy that it contains. This energy can be used to generate electricity and contribute to the stabilization of the power grid. This is particularly important at the moment because the operation of solar and wind power plants is highly weather-dependent, as a result of which also the power supply fluctuates considerably and needs to be stabilized. In contrast, the operation of the gas grid is entirely stable and power plants using natural gas to generate electricity can compensate for fluctuations in the power grid. Nevertheless, depending on the weather, there can be fluctuations in the power grid in terms of excess or lack of electricity. The use of hythane in the case of electricity excess is addressed, for example, by the authors of [
6]: Hydrogen produced by electrolysis is mixed with biogas to form a hythane-like product that can be used in automotive transport. The concentration of CO
2 in the biogas has been reduced from the original 40% to less than 15% and the heating value of the gaseous product has increased from 534 kJ/mol to more than 669 kJ/mol. A preliminary techno-economic analysis has proven favorable in the case of the excess electricity from renewable sources.
The hythane-formation process offers a new method for the storage and upgrade of renewable energy. In the case of the lack of electricity, which is currently very topical, the use of hythane has not been addressed despite the fact that hythane can be useful in such a case as well. It is thus expedient to investigate the possibilities of converting waste into hythane, in our case the use of biomass to obtain hythane or a hythane-like gaseous product by low-temperature pyrolysis using catalysts.
Biomass pyrolysis takes place in three stages, including dehydration, primary decomposition and a secondary reaction of volatile products. The main (and competing) primary-decomposition processes are dehydrogenation, depolymerization, the subsequent fragmentation of molecules produced by depolymerization, and the formation of low-molecular-weight compounds and gases [
7]. These stages need to be taken into account in the design of the reactor and pyrolysis apparatus. Secondary reactions are the least described. If they are to be characterized responsibly, they need to be investigated further. Since they are essential for the formation of hythane, they will be dealt with in greater detail in the
Section 3. When addressing the conversion of biomass into hythane, it is necessary to take into account also the different chemical compositions of different types of biomass. Therefore, the pyrolysis of two contrasting biomass types, namely shavings from the European white birch (
Betula pendula) and apricot (
Prunus armeniaca, also
Armeniaca vulgaris) stone waste, have been investigated. The contrast between the two types results from the content of the key elements C, O and N in the organic part of the waste. (The waste can be characterized by the content of water, the inorganic part–ash matter (inherent ash), and the organic part, expressed by the content of the organically bound elements C, H, N, S
org and O.) While the shavings analyzed contained 44.8 wt.% of C, 33.7 wt.% of O and 0.65 wt.% of N, in the case of the apricot stones it was 56.1 wt.% of C, 22.17 of wt.% O and 6.6 wt.% of N (a received basis). These data already show the difference between the two types of biomass used (see the
Section 2 for more detail). However, they are the secondary reactions providing low-molecular-weight compounds and gases that are the most important for the task concerned. Although gases are also provided by the primary decomposition, the final character of the resulting gaseous mixture is largely determined by the secondary reactions. The key parameters affecting the secondary reactions are temperature, pressure and residence time, which must also be taken into account in the design of the reactor. In addition, it is necessary to consider the role of catalysts. A suitably selected catalyst or catalyst system can facilitate some secondary reactions, selectively promote the desired conversion of CO
2 to methane and show some resistance to catalyst poisoning by catalytic poisons (H
2S). All of these functions are essential for the conversion under consideration.
For these reasons, the reactor for the pyrolysis of both types of biomass was designed as a sealed pressure reactor, equipped with a catalyst with the expected effect of promoting secondary reactions (Raney nickel) and with a catalyst for the selective conversion of CO
2 to methane by hydrogen using the Sabatier reaction (Ni/Al
2O
3) or with a catalyst less selective but significantly accelerating this reaction (NiCo/Al
2O
3) or with a catalyst resistant to sulfur compounds (NiMo/Al
2O
3). The reactor was operated in a low-temperature regime, i.e., the temperature in the sample and in the reactor did not exceed 400 °C. These conditions were determined on the basis of our experience with low temperature regime [
8] and a study of the methanation [
9].
An important item in the conversion is pressure hydrogen, which creates a reducing environment and, above all, enables the methanation of CO2 and CO. It is an advantage that the methanation conditions have been brought to an industrial scale, so that information from this area can be used for the more complex problem of the methanation of the volatile products of biomass decomposition. The formation of methane, CO2 and CO is an essential step in the conversion of biomass to hythane.
The purpose of the work. The purpose of this work is to determine the process conditions for the catalyzed conversion of biomass represented by white-birch shavings and apricot stones to hythane or a hythane-like gaseous product under low-temperature conditions. The results may contribute to the knowledge of the synthesis of hythane, which can be as useful as the synthetic methane produced by the power-to-gas technology. The aim of this work is to compare the efficiency of the catalysts mentioned above in the process of the low-temperature conversion of two contrasting types of biomass. The hypothesis tested is that the key to the thermal treatment of biomass to hythane are the catalytically promoted secondary reactions of the volatile products of primary decomposition, providing mainly CO2 and CO; these carbon oxides are then converted by the Sabatier reaction to methane, which together with hydrogen provides the final hythane product. By using a suitable combination of Raney nickel and the above catalysts Ni, NiCo and NiMo on alumina, it is possible to reduce substantially the concentration of sulfur compounds in the resulting gas.
3. Results and Discussion
Two contrasting types of biomass have been selected for the experiments: birch-wood shavings and ground apricot stones. The reason is that the efficiency of catalysts should first be tested on biomass samples with a completely different composition and only after identifying the influence of the catalysts and their compositions, it is possible to proceed to systematic experiments with a wider spectrum of biomass. While the birch shavings contained holocellulose (62 wt.%), lignin (20.5 wt.%) and resins (2.5 wt.%), the apricot stones were formed by saccharides (68 wt.%), proteins (21.5 wt.%) and lipids (3 wt.%). (For proximate and ultimate analyses, see the
Section 2). These materials were subjected to low-temperature pressure pyrolysis in a hydrogen atmosphere, and the resulting carbon oxides were catalytically methanized using catalysts, the properties of which are summarized in
Table 2.
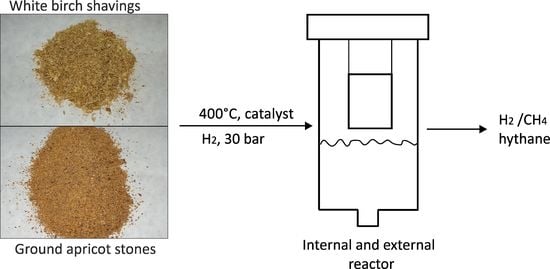
The synthesis of hythane from biomass must handle two opposing steps: endothermic decomposition of biomass with the highest possible yield of CO
2 and CO and exothermic catalyzed methanation of CO
2 and CO with the highest possible yield of CH
4. This was reflected in the design of the reactor vessel. The inner part (internal reactor,
Figure 1) contained the sample to be decomposed and a layer of powdered Raney nickel; the outer part (outer reactor,
Figure 1) comprised a catalyst (Ni/γ-Al
2O
3 or NiCo/γ-Al
2O
3 or NiMo/γ-Al
2O
3) enabling the methanation of CO
2 and CO. In the internal reactor, the sample thus underwent thermal degradation, but the secondary reactions of volatile products were promoted by Raney nickel (R-Ni). The presence of R-Ni facilitated the secondary reactions as well as increased the gas yield, as illustrated below. In the absence of R-Ni, the gas yield was only 15–20 wt.%, on contrary, if R-Ni was used, the gas yield was 26–36 wt.% (
Table 3 and
Table 4).
The effect of secondary reactions on the volatile products of biomass pyrolysis has been studied by the authors of [
13]. They have concluded that the key parameter for their formation and composition is the residence time, whereas the crucial parameter for the yield of liquid hydrocarbons and solid carbonaceous residue (char) is pressure. However, if the product of interest is a gas, the secondary reactions need to be promoted not only by an appropriate residence time but also by a suitable additive further enhancing the cleavage of the volatile products. In the case concerned, the residence time was 5 min at a temperature of 400 °C and a pressure of 30 bar. Nevertheless, this was not sufficient to obtain a satisfactory gas yield and it was necessary further to promote the cleavage of the volatiles in the internal reactor with Raney nickel. As above, a yield higher than 25 wt.% was considered satisfactory (
Table 3 and
Table 4).
Another issue was the composition of the resulting gas. For this, it was necessary to convert the released carbon oxides with hydrogen to methane by Sabatier reactions (1) and (2):
Both reactions are reversible and exothermic and their initiation requires initial activation energy. Important parameters for the catalytic methanation of carbon dioxide by hydrogen include the efficiency of the catalyst and the molar ratio of H2:CO2 in the converted gas. The molar ratio of H2:CO2 significantly affects the composition of the resulting gas and its properties. At low ratios, there was a tendency to produce gaseous products with higher molecular weight, while higher ratios led to the desirable higher production of methane. The optimum ratio for the selectivity and higher production of methane is 4:1. In the case of biomass, this ratio is quite difficult to achieve, but it can be approximated by an appropriate choice of charge volume and treatment, hydrogen pressure and reactor design. In the given case, it was necessary to work with the content of carbon oxides in the amount of approximately 45 vol.% of CO2 and about 5 vol.% of CO in the gas released by pressure pyrolysis of the biomass types considered. The experimentally determined hydrogen pressure that would methanize this amount while ensuring an acceptable composition of the resulting gas (e.g., ~50–70 vol.% of CH4 and 10–30 vol.% of H2) was 30 bar.
The methanation of carbon dioxide and carbon monoxide occurs at temperatures in the range of 125–675 °C depending on the type of catalyst used. Generally, higher yields are obtained at temperatures above 145 °C, but in some cases the maximum reaction rates and methane selectivity are achieved at temperatures as high as 325–425 °C. In the case under study, a temperature of 400 °C was chosen to ensure the decomposition of the biomass and a satisfactory degree of the methanation of the pyrolysis gas without an undesirable water–gas shift reaction.
For methanation, nickel-based catalysts are used because of their reasonable cost and high selectivity to methane; other catalysts used are cobalt and molybdenum. The catalytic activity of Ni is quite high, with almost no higher hydrocarbons produced during methanation. Nickel-based catalysts are often applied to γ-Al2O3 or SiO2 supports. The main disadvantage of Ni-catalysts is their high susceptibility to deactivation, which can be caused by sulfur compounds, in this case H2S. Nickel is particularly sensitive to deactivation by this compound. Another catalyst used for methanation is cobalt. Cobalt-based catalysts are not as selective to methane as Ni-based catalysts, but they have higher catalytic activity than Ni, which is particularly important in the case of biomass pyrolysis. Cobalt, like nickel, is most commonly applied to γ-Al2O3 or SiO2. Molybdenum-based catalysts have high resistance to sulfur compounds including H2S, but they have lower catalytic activity than nickel- and cobalt-based catalysts. In general, another disadvantage is their higher selectivity to higher hydrocarbons, but in the case of biomass this is not a disadvantage as they increase the higher and lower heating values of the resulting gas. Molybdenum is also usually applied to γ-Al2O3 or SiO2. In the given case, all of these catalysts have been tested both for catalytic activity and for H2S content in the resulting gas. Biomass generally contains sulfur, in this case 0.38 wt.% in the case of birch and 0.17 wt.% in the case of apricot stones, and it was necessary to ensure minimum H2S production or to consider desulfurization measures.
The results obtained are summarized in
Table 5 and
Table 6. The best results were achieved using the NiCo/γ-Al
2O
3 catalyst, which produced a gas with almost 70 vol.% of CH
4 and 10 vol.% of H
2. (
Table 5). The H
2S content was 0.13 vol.%, indicating that the gas will have to be further purified.
However, the removal of H
2S is feasible and further research can focus on the choice of the desulfurization method. A slightly lower catalytic activity was exhibited by the Ni/γ-Al
2O
3 catalyst, which provided gas with 57 vol.% of CH
4 and 23 vol.% of H
2 (
Table 5). Nevertheless, the H
2S content was similar as in the previous case and the measures will thus be the same. The lowest activity was observed in the case of the mixed NiMo/γ-Al
2O
3 catalyst. In addition, the H
2S content in the obtained gas was higher than expected in this case (
Table 6). This phenomenon requires further research into their causes.
To explain the data obtained (
Table 5 and
Table 6), a reaction scheme was suggested and the dependence of the normalized CH
4 production on the standard reduction potential of the considered catalytic metals was constructed. The reaction schemes developed so far are summarized and evaluated in the work [
9]. Based on that and the work [
14], the probable reaction mechanism can be expressed as a two-stage process involving: (1) the activation of hydrogen on the surface of the catalyst to form a reactive (formerly nascent) hydrogen (H
react) and (2) the subsequent reactions of reactive hydrogen with CO
2 and the formed formic acid and the reaction of hydrogen with the oxygen intermediate in the gas phase to form water and methane. The reaction scheme can then be suggested via Equations (3)–(6), where Equation (3) represents the activation of hydrogen by the catalytic metal (Ni, Co, Mo) on the catalyst surface and Equations (4)–(6) constitutes the gas-phase reactions.
It follows from Equation (3) that a catalytic metal must have a sufficient reduction potential to provide electrons or, better, a certain electron density to activate the hydrogen molecules. The efficiency of the catalyst thus depends on the reduction potential of the catalytic metal; but it is necessary to underline that reactions in the gas phase are simplified, because they are influenced or complicated by the composition of the initial biomass. From this point of view, reactions (4)–(6) must be seen as a simplification of the real situation. Furthermore, other reaction mechanisms have been postulated and the description of reactions can be different [
15,
16,
17].
The dependence of the catalyst efficiency on the reduction potential of the catalytic metal is shown in
Figure 2, where the catalyst efficiency is expressed as the CH
4/CO
2 ratio in the resulting gas (
Table 5 and
Table 6) related to 1 g of the catalytic metal (Ni) or metals (Ni, Co or Ni, Mo); the reduction potential of the catalytic metal here is the standard reduction potential (-E) of the influencing metal, i.e., in the case of the Ni-catalyst it is Ni, in the case of NiCo it is Co, and for NiMo it is Mo. The standard reduction potentials have been taken from the handbook [
18].
It follows from
Figure 2 that the NiCo catalyst showed the highest efficiency and that the nickel catalyst had a lower but comparable efficiency. The NiMo catalyst showed the lowest efficiency. In the study [
19], the maximum catalytic activity was exhibited by the Ni-based catalyst, whereas the efficiency of the other catalysts decreased in the order Ni > Co > Mo > Fe. In our case, this order was found to be NiCo ≥ Ni > NiMo. It can be said that these independent findings do not contradict each other. Therefore, for further research into the efficiency of catalysts in biomass pyrolysis, it will be best to focus on nickel and cobalt, possibly on the mixed NiCo catalyst.
A similar conclusion can be reached when assessing the activity of catalysts according to methane production.
Figure 3 (based on data in
Table 5 and
Table 6) shows that for both types of the input biomass, the highest methane production was recorded in the case of the NiCo catalyst. The Ni catalyst showed a slightly lower activity and the NiMo catalyst was the least effective.