Abstract
The influence of Ce and W promoters on the performance of alumina-supported nickel catalysts in the CO2 methanation reaction was investigated. The catalysts were obtained by the co-impregnation method. Nitrogen low-temperature adsorption, temperature-programmed reduction, hydrogen desorption, transmission electron microscopy, X-ray diffraction, and photoelectron spectroscopy studies were used for catalyst characterization. An introduction of Ce and W promoters (1–5 wt %) led to the decrease in mean Ni crystallite size. Gradual increase in the active surface area was observed only for Ce-promoted catalysts. The increase in CO2 conversion in methanation reaction at low-reaction temperatures carried out over Ce-promoted catalysts was attributed to the increase in the active surface area and changes in the redox properties. The introduction of small amounts of tungsten led to an increase in the activity of catalysts, although a decrease in the active surface area was observed. Quasi in situ XPS studies revealed changes in the oxidation state of tungsten under CO2 methanation reaction conditions, indicating the participation of redox promoter changes in the course of surface reactions, leading to an improvement in the activity of the catalyst.
1. Introduction
Mitigation of climate change affected by emission of greenhouse gases belongs to the most important challenges [1]. It requires multi-directional measures including, capture, storage, or utilization of carbon dioxide. The idea of converting captured CO2 into methane using hydrogen produced by renewable energy is currently receiving widespread attention [2,3]. This concept can be used in a variety of economic sectors, including energy and chemical production, as well as the agro-food industry. Although hydrogenation of carbon dioxide can be conducted towards valuable products such as methanol, dimethyl ether, or formic acid, its conversion to methane (reaction 1) is currently the most mature technology and is likely to find wide application [4,5,6].
CO2 + 4H2 → CH4 + 2H2O ∆H0298K = −165 kJ mol−1
Despite years of research studies, new low-cost catalysts with high activity and selectivity towards methane at low temperatures and high resistance to sintering and poisoning are still being sought [2,3,4,5,6]. The selection of a suitable catalyst is often a compromise between its catalytic properties and costs, which are influenced by the type of metal active phase, properties of supports, and the preparation methods. The most active catalysts in CO2 methanation reaction include those containing Ru and Rh nanoparticles dispersed over oxide supports. On the other hand, the lower activity of catalysts based on non-noble metals, such as Ni or Co, is often compensated by using a higher metal content, introduction of promoters, and application of suitable supports. A number of catalytic systems have been discussed in the literature [2,3,4,5,6]. Nickel catalysts with supports containing rare earth metal oxides are among the most active and selective at low temperatures. Their high activity was often ascribed to the enhanced activation of CO2 on redox or basic sites on the support. Such effects were often boosted by introduction of perturbations in the lattice ordering, the formation of vacancies or the use of specific preparation methods leading to an increase in the specific surface area, e.g., by the introduction of secondary metals, as in the Ce1-xZrxO2-type oxides. High activity of catalysts can also be achieved with less expensive carriers, such as alumina or silica. In this case, CO2 can be activated with the participation of acid–base centers. The proposed reaction mechanisms assume often stepwise transformation of intermediate carbon containing surface species towards methane with participation of hydrogen atoms adsorbed on metallic sites.
One of the simplest methods of improving catalysts’ activity is the introduction of promoters. They can be divided into several groups according to the most visible effect induced by their presence. However, it should be borne in mind that they can often act in complex ways, not fully explained yet. Promoters may increase the reducibility of nickel oxide phases (e.g., Ru and Pt) and influence intrinsic properties of crystallites through electronic interactions or the alternation of their morphology (e.g., formation of NiFe and NiRu alloys), changing the way of activation of CO2 through formation of additional acid-base or redox sites, e.g., caused by introduction of alkali or alkaline earth metals, such as K and Mg, and on the other hand addition of the small amounts of rare earth metals, such as Ce, La, Y.
The positive effects of Ce promoter were reported in several studies of CO2 metahantion catalysts [7,8,9,10,11,12]. Cerium oxide species in nickel catalysts have often been considered as structure-directing agents [13]. Their presence leads to the formation of new redox sites, causing enhancement of carbon dioxide activation [7,8,9,10,11,12]. However, their role has not been fully explained yet. In contrast, the effects of tungsten in catalysts used for CO2 methanation have not been widely discussed in the literature, although the beneficial role of tungsten has been revealed in other reactions, including the steam and dry reforming of hydrocarbons [14,15,16]. The impact of tungsten promoter was mainly associated with an improvement in the sintering and coking resistance of the catalysts [14,15,16]. Early work of Kelley indicated that pure tungsten can be active in CO methanation reaction at low temperatures [17]. Zhang et al. suggested that the presence of cobalt–tungsten interaction may increase the activity of catalysts in CO methanation reaction [18]. More recent studies of Ai et al. of W-doped ordered mesoporous Ni/Al2O3 catalysts in CO methanation indicated that tungsten may increase reducibility of catalysts and thus increase the number of active sites and activity of catalysts if special preparation methods are applied [19].
We have recently presented detailed studies of the influence of nickel content and support type on the structural and surface properties of catalysts and their catalytic performance in CO2 methanation reaction [20,21,22]. It was evidenced that an increase in the activity of alumina supported nickel catalysts at low-reaction temperature can be achieved by the increase in nickel content from 10 to 40 wt %. We have also revealed more advantageous properties of highly loaded alumina supported nickel catalysts than similar ceria supported catalysts (which showed very high activity at low temperatures), especially with regards of H2S poisoning resistance [12].
The aim of the present study was comparison of the catalytic properties of alumina-supported nickel catalysts promoted with cerium and tungsten in CO2 methanation reaction and elucidation of the role of promoters in the formation of active phase and the course of the CO2 methanation reaction.
2. Results and Discussion
2.1. Structural and Surface Properties of Catalysts after Calcination
The properties of alumina-supported nickel catalysts promoted with cerium and tungsten are presented in Table 1. The content of promoters varied from approximately 1 to 5 wt %. The effects of Ce and W introduction is compared for catalysts contained around 40 wt % Ni. Additionally, the influence of Ce is presented for catalysts contained smaller amounts of Ni (around 20 wt %).

Table 1.
Catalyst composition determined by the XRF method, specific surface area (SBET), mean pore diameter (DBJH), and total pore volume (Vp) of support and catalysts determined after calcination. Mean nickel crystallite size estimated form the XRD data ( ), an active surface area of catalysts (Sa) determined after reduction of catalysts at 600 °C for 2 h.
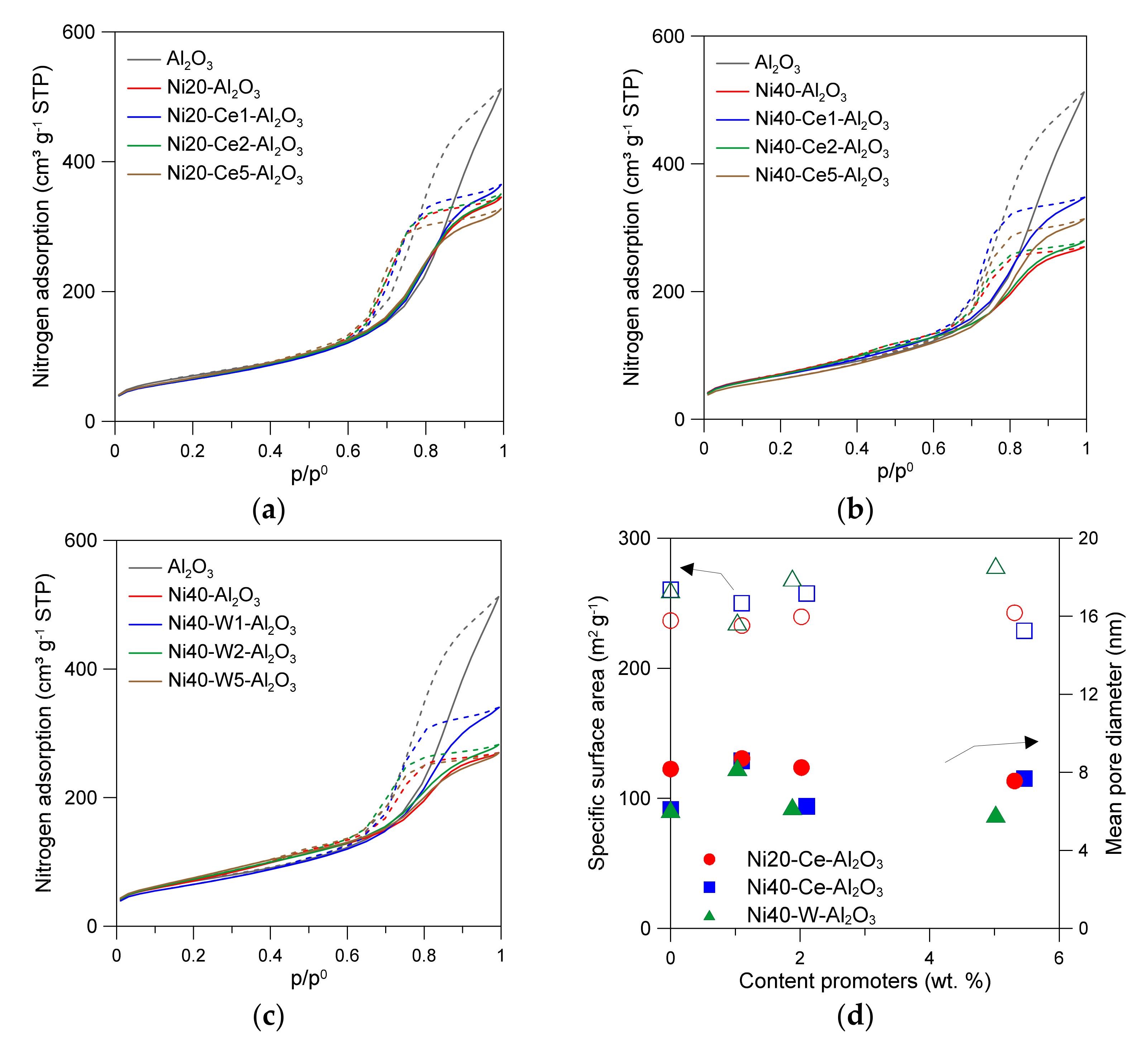
The γ-Al2O3 used as support showed high specific surface area (251.7 m2/g) (Table 1). An introduction of 20 wt % Ni led to the decrease in specific surface area to 236.7 m2/g. The course of nitrogen adsorption/desorption isotherms of the support and catalysts presented in Figure 1 was similar, and can be considered as IV-type, in agreement with the IUPAC recommendations [23]. The observed hysteresis loops, classified as H2(b), indicate the presence of mesopores. The decrease of mean pore diameter (DBJH) and total pore volume (Vp) can be assigned to partial blockage of small pores of alumina support by nickel oxide or nickel aluminate species.
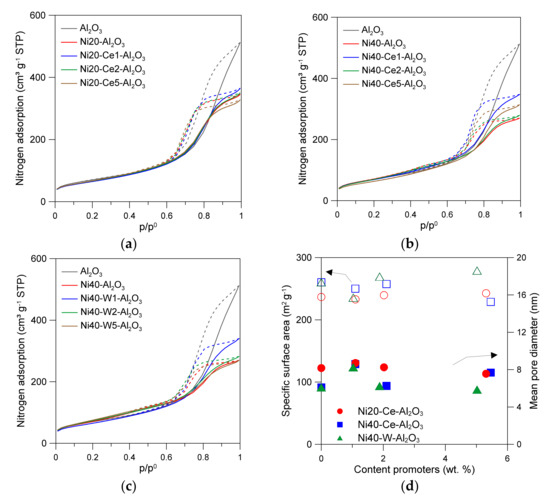
Figure 1.
Low-temperature nitrogen adsorption/desorption isotherms of catalysts (a–c); relations between content of promoters and the specific surface area, as well as and mean pore volume of catalysts (d).
An introduction of larger amount of nickel (36.9 wt % Ni) increased the specific surface area, as well as further reducing the mean pore diameter and total pore volume (Table 1). Such effects can be attributed to the formation of additional surface sites on the nickel oxide species, changes in the specific arrangement of small alumina particles around NiO nanoparticles and, at the same time, a decrease in the number of surface sites of the support due to the blockage of small pores by NiO nanoparticles. The introduction of promoters resulted in insignificant changes in the porosity of the catalysts or their specific surface area (Figure 1, Table 1). Small changes in the properties of the catalysts can be ascribed to variation in the size of nickel crystallites and/or formation of oxide phases of Ce and W.
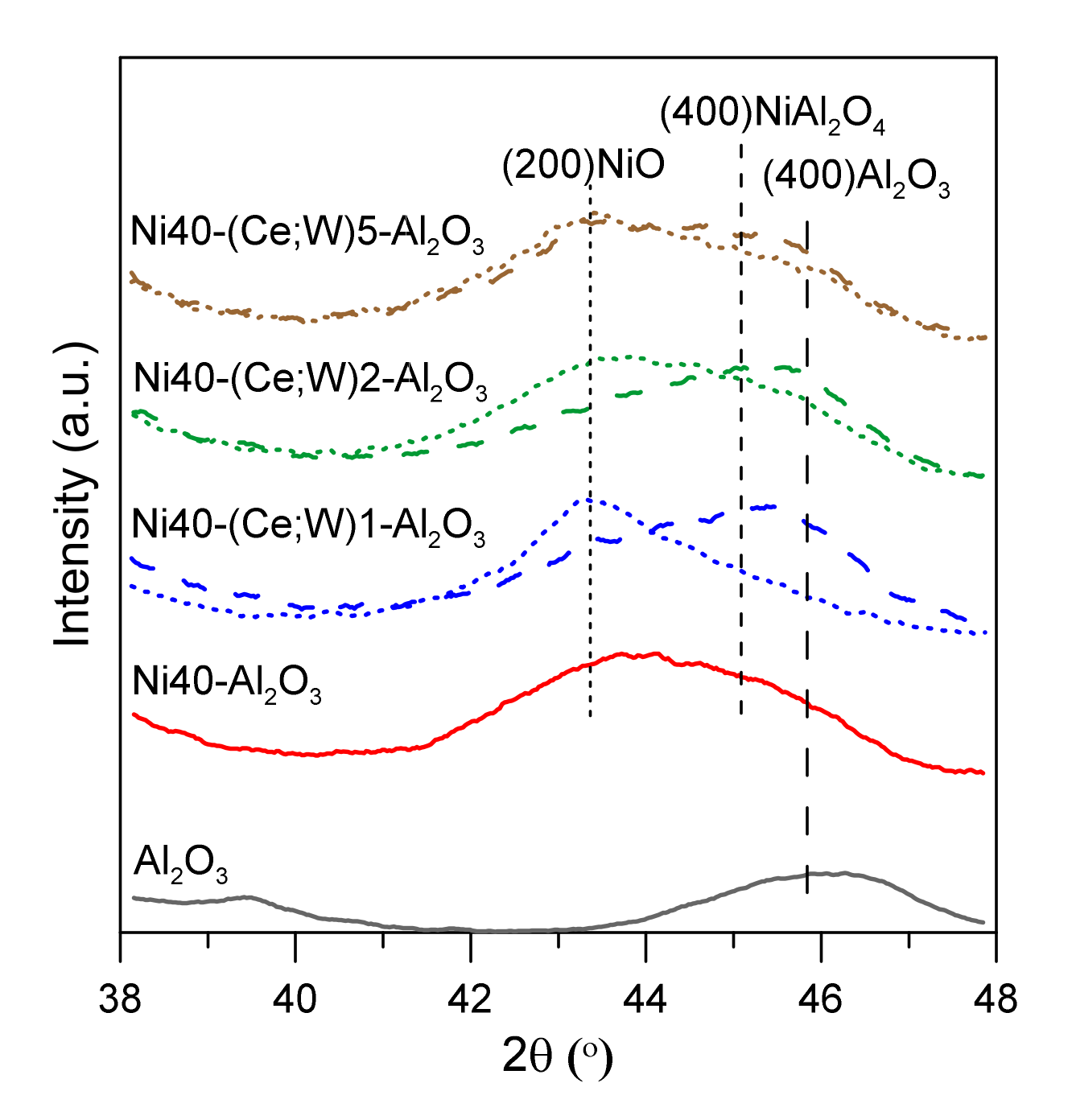
The structural and surface properties of γ-Al2O3 support, as well as the applied preparation method, especially the addition of citric acid to impregnating solution, facilitated formation of relatively small NiO crystallites. X-ray diffraction curves of alumina support and nickel catalysts after calcination are presented in Figure S1 in the Supplementary Materials. The presence of wide reflection peaks of γ-Al2O3 (PDF4 + 00-001-1303) point out small particle size of alumina support. The approximate size of Al2O3 domains was in the range of 4 nm. The reflection peaks of alumina partially overlapped NiO peaks (PDF4 + 04-016-6318), and hence it is difficult to determine the size of NiO crystallites with high accuracy. Their size was approximately in the range of 3–4 nm.
It is also difficult to unambiguously reject the possibility of the coexistence of dispersed nickel aluminate phases (NiAl2O4) due to similar position of reflection lines of such phases and width of X-ray diffraction peaks. Isolated XRD peaks of cerium and tungsten containing phases, such as CeO2, WO3, nickel aluminates, cerium aluminates, and aluminum or nickel tungstates, were not visible, indicating the presence of very small nanoparticles and their non-crystalline or amorphous nature. However, in comparing the narrow region of XRD curves for promoted Ni40-Al2O3 catalysts (Figure 2), we were able to distinguish weaker shift of overlapping NiO and Al2O3 peaks towards lower angles for W-promoted catalysts (dashed lines) in comparison to unpromoted and Ce-promoted nickel catalysts (solid and dotted lines, respectively). This effect can be attributed to the presence of smaller NiO particles in W-promoted catalysts or participation of Ni in the dispersed nickel tungstate phases.
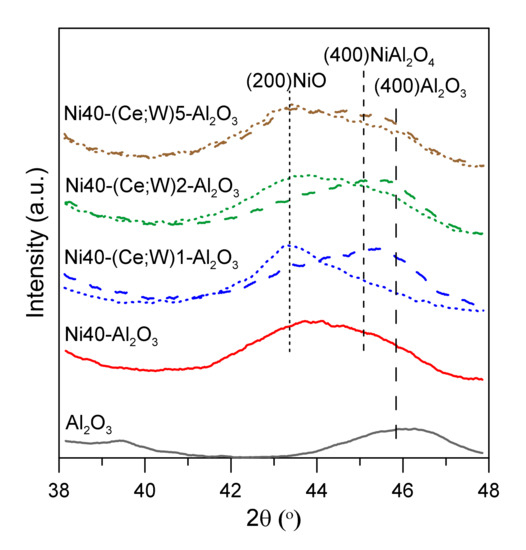
Figure 2.
X-ray diffraction patterns of catalysts after calcination in selected region of 2 theta angle; Ce-promoted nickel catalysts—dotted lines, W-promoted catalysts—dashed lines.
The crystalline structure of γ-Al2O3 is often presented as a cubic defect spinel. The oxygen atoms are in cubic close packing arrangement, and aluminum atoms occupy part of octahedral and tetrahedral sites [24]. Nickel or cerium ions during initial stages of impregnation and thermal treatment can be in part located in the vacant sites of alumina spinel. Similar suggestions were recently proposed for more complex systems, including Ni/xCeO2-Al2O3 [25]. Moreover, such effects may result in the corresponding changes of the oxidation state of Ce. Damyanova et al. indicated the possibility of decrease in the Ce3+/(Ce3+ + Ce4+) ratio as result of CeO2 agglomeration with an increase of CeO2 content [25]. Treatment of tungsten precursor during calcination may lead to the formation of different oxide forms WOx, 2 ≤ x ≤ 3. Bulk tungsten (VI) oxide WO3 contains WO6-octahedra connected by corners, while edge-shared octahedral are present in WO2. Other intermediate oxide phases may show more complex structure with linear defects. Ostromecki et al. suggested that tungsten oxide surface species are deposited on the surface alumina at low loadings as tetrahedrally coordinated monomers and can form a mixture of tetrahedrally and octahedrally coordinated surface polymers at high loadings [26]. They also indicated that the tungsten oxide species during impregnation may anchor to the alumina support through interactions with surface hydroxyls, whereas nickel oxides preferentially interact with the coordinatively unsaturated Al3+ Lewis acid surface sites. This mutual spatial dispersion of ionic species in the initial stages of synthesis of catalysts may retard the growth rate of clusters of nickel precursors [16]. Although the formation of nickel tungstates was reported in literature as a result of high temperature treatment (above 400 °C, i.e., the calcination temperature of catalysts used in this work), the presence of such surface or subsurface phases cannot be excluded, especially considering the high degree of dispersion and the presence of very small NiO crystallites formed under co-impregnation conditions [27].
The obtained catalysts may likewise attain high nickel dispersion due to the large specific surface area of γ-Al2O3 and the presence of some steric hindrances, resulting from the micro-mesoporous structure and presence of promoter oxide species, which hinder agglomeration of nickel surface species during thermal treatment. Relatively high nickel dispersion, ascribed to citric acid presence in the impregnating solution, was recently presented in several articles [20,28,29].
Citric acid has three carboxylic groups that can participate in the formation of bonds with nickel and cerium or tungsten. The equilibrium between citric acid and its deprotonated forms is related to the pH of the solution and nature of the ions [13,15,16]. In the solutions of low pH, undissociated citric acid and nickel aqua complexes [Ni(H2O)6]2+ may prevail. An increase in pH may facilitate the successive formation of additional nickel citrate complexes, such as [Ni(H2Cit)(H2O)5]+, [Ni(HCit)(H2O)4], [Ni(HCit)(Cit)(H2O)4]3−, and [Ni(Cit)2(H2O)4]4−, which can be adsorbed on alumina support [30]. The presence of citric acid may also influence viscosity during drying and calcination stages, hindering the migration of precursors, enhancing formation of “gel-like” rather than “crystalline-like” phases [31].
Moreover, the impregnation at low pH or in the presence of some complexing agents may result in partial dissolution of the support (via etching) and redistribution of metal ions. Hence, during calcination of binary (Ni-Al) and ternary (Ni-Ce-Al or Ni-W-Al) systems, the formation of complex oxides, phase segregation, and mutual diffusion of nickel, cerium, tungsten, and aluminum ions can occur. The individual ions my diffuse through the interfacial region of oxide phases. The diffusion rate in the mixed oxides systems depends on temperature, the nature of oxide lattice, content of metals, the size of the ions, and the extent of the interfacial border. Therefore, the increase in NiO dispersion simultaneously leads to increase in the interface border and thus to the enhanced formation of nickel aluminate phases, as well as nickel tungstate phases (NiWO4) in the W-doped catalysts.
Nickel aluminate and tungstate species show low reducibility; hence, to restrict formation of such phases, the calcination of catalysts was performed at relatively low temperature (400 °C) [20,21,32]. It seems, that the degree of the oxidation state of Ni2+ in NiO crystallites essentially does not change during thermal treatment; however, it is worth noting that Ni3+ sites can be formed under certain conditions [33]. In turn, Ce3+ ions introduced during impregnation of the alumina support can change the oxidation state to Ce4+ in the drying and calcination stages. Formation of solid solution between NiO and CeOx is relatively difficult, but potentially possible. The ionic radius of Ce ions is larger than Ni; VIIIr(Ce3+) = 1.14 Å, VIIIr(Ce4+) = 0.97 Å, and VIr(Ni2+) = 0.69 Å, respectively [34]. An incorporation of Ni2+ ions in the CeO2 lattice would cause large distortion of the crystal lattice. The solubility of nickel in the particles with a micrometric size of CeO2 is therefore relatively weak and falls below 1 atom % [35]. However, several recent studies have reported the possibility of increasing the solubility limits of Ni2+ in CeO2 nanoparticles [36,37,38]. In turn, formation other possible binary compounds, such as cerium aluminates (CeAlO3), cannot be completely ruled out [39,40].
2.2. Active Phase Formation
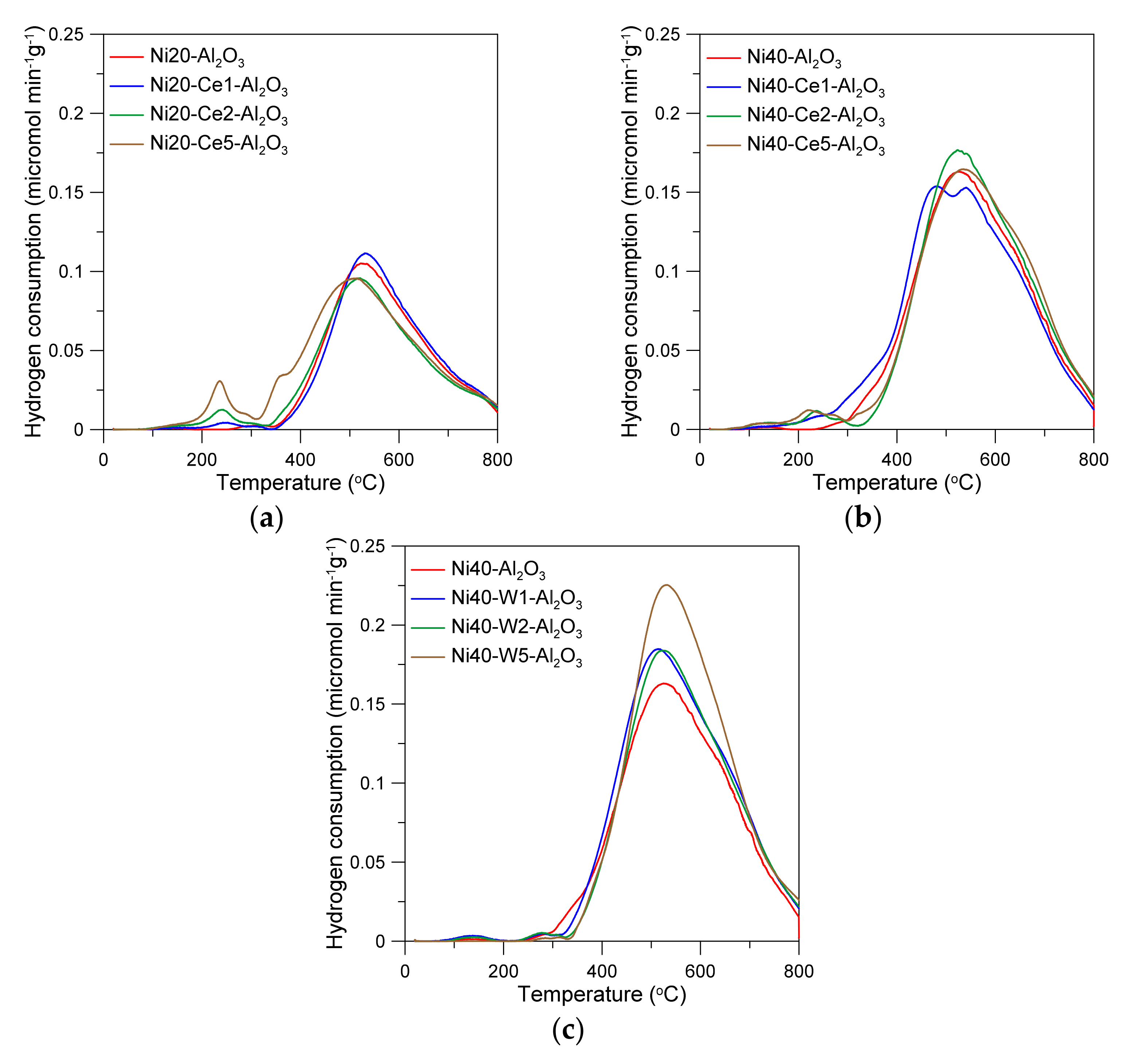
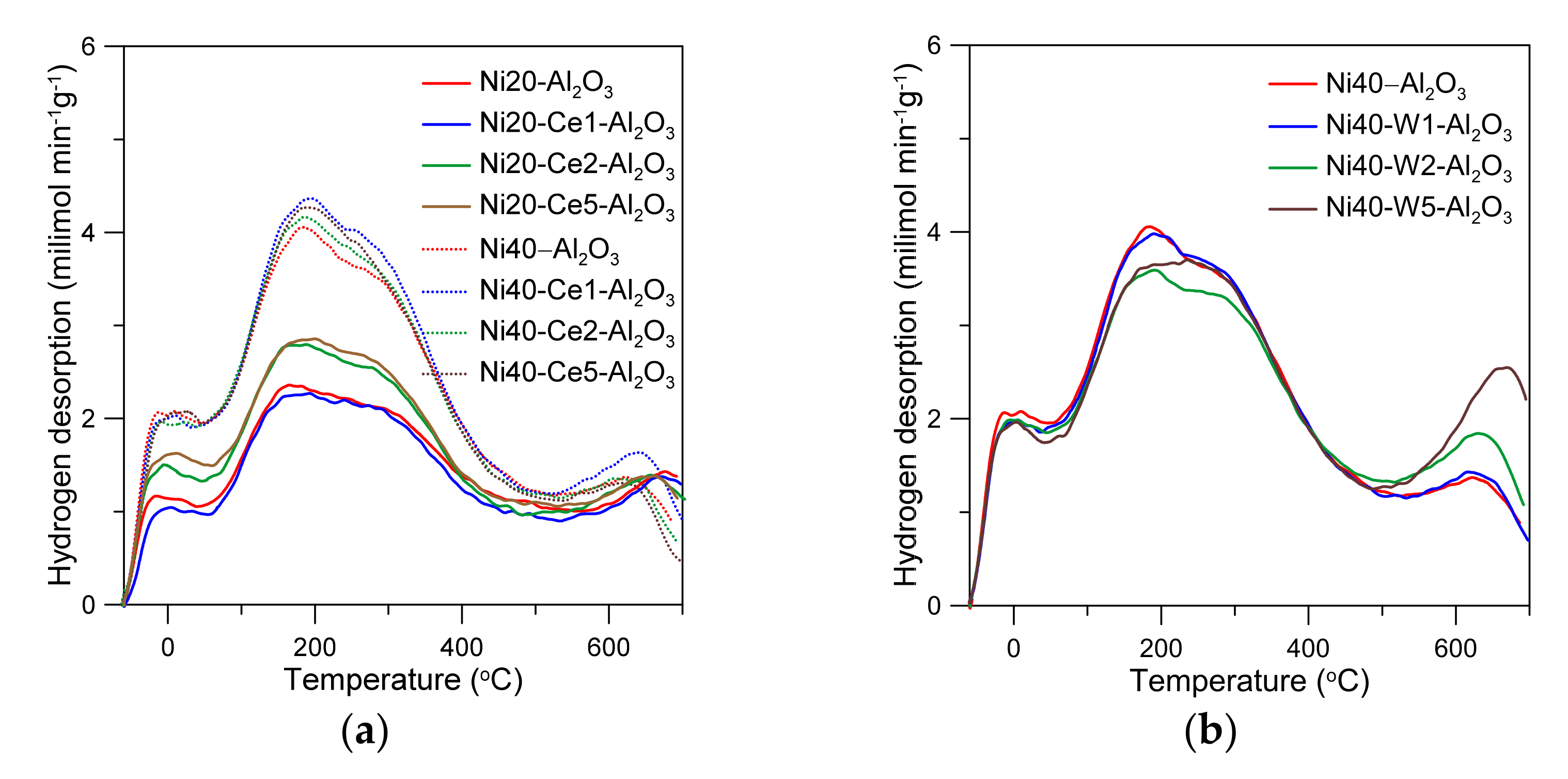
The temperature-programed reduction curves of alumina-supported nickel catalysts are presented in the Figure 3. The catalysts were reduced in a wide range of temperatures. One can observe small TPR peaks on the curve of Ni40-Al2O3 catalyst, located in the range from 250 to 350 °C. The predominant reduction peaks occurred between 350 and 800 °C, with large maximum at around 520 °C. The reduction of cerium-promoted catalysts depended on the content of Ni and Ce. The effects of the presence of cerium were more visible in the catalysts with low nickel loading and may have directly resulted from differences in the Ce/Ni ratio in the catalysts. The introduction of the small amounts of Ce to the catalysts with 20 wt % Ni led to the increase in reducibility. This effect was especially manifested in the increase in the intensity of low-temperature peaks and small shift of high-temperature reduction peaks to the lower temperatures. Similar effects were visible for the catalysts with high nickel loading, but the extent was much weaker. Low-temperature peaks were not visible in the W-promoted catalysts. The increase in the main peak intensity with an increase in W content was observed. In the literature, low-temperature reduction peaks on the TPR curves of nickel catalysts have been often attributed to the consumption of hydrogen in the reduction of “free NiO” crystallites, weakly interacting with alumina support, accordingly to the reaction NiO + H2 → Ni + H2O [41,42]. On the other hand, high-temperature peaks were attributed to the reduction of nickel oxide phases strongly interacting with alumina. High reduction temperature may also lead to the formation of oxygen vacancies and partial reduction of cerium (Ce4+ → Ce3+) in CeO2 species [43].
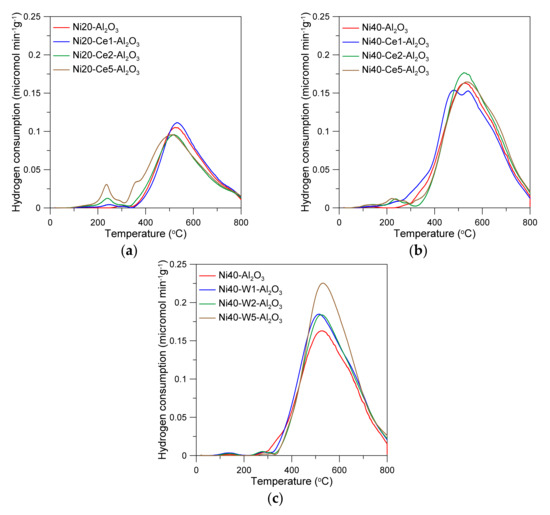
Figure 3.
Temperature-programmed reduction curves of catalysts (a–c).
Low reducibility of alumina-supported catalysts has often been reported as the effect of the presence of surface and bulk nickel aluminate spinels [44]. It is well established that the extent of interactions between NiO and Al2O3 may increase with an increase in NiO dispersion and increase in calcination temperature. The application of co-precipitation, sol–gel, or high-surface area support impregnation methods can lead to the formation of highly dispersed oxide systems. The presence of difficult-to-reduce phases allows formation of relatively small nickel crystallites, mainly due to retardation of diffusion processes during reduction stage.
It was discussed above that high calcination temperature may lead to the formation of spinel oxide species or solid solutions, which complete reduction can be achieved at very high temperatures, often above 800 °C [44]. Cerium and nickel precursors used in the synthesis of Ni-Ce/Al2O3 catalysts by the co-impregnation method are simultaneously deposited on the alumina support in the presence of citric acid, and hence the formation of mixed Ni and Ce oxide phases can be expected. Thus, gradual increase in reducibility of catalysts with an increase in Ce content (in the low-temperature region) may result from the increase of the contribution of the direct interactions between NiO and CeO2 phases [25], while the shift of high-temperature reduction peaks to lower temperatures can be attributed to the decrease in the interactions between nickel oxide and alumina support or aluminate species. Such effects are more pronounced and observed for catalysts with high nickel loading, where the Ce/Ni ratio is much smaller.
Although in the obtained catalysts the molar ratio of W/Ni is relatively small, nickel and tungsten precursors and then corresponding oxide phases may interact during impregnation, drying, and calcination stages, leading to the formation of mixed nickel and tungsten oxides (NiO, WO3) and nickel tungstate species (NiWO4), together with suitable compounds of Ni and W with alumina, including NiAl2O4 and Al2(WO4)3 [45,46]. Reduction of Ni-W-Al2O3 catalysts may occur through reduction of NiO species to metallic Ni0 and successive transformation of WO3 and NiWO4 to WO2, with tungsten on the lower oxidation state, accordingly to the reaction equation NiWO4 + 2H2 → Ni + WO2 + 2H2O, following WO2 + 2H2 → W + 2H2O, and finally formation of Ni–W alloys. According to literature data, transformation of NiWO4 and reduction of supported WO3 and Al2(WO4)3 species occurs at high temperatures, above 700 °C [45,46]. However, this does not exclude the possibility of partial reduction of tungsten phases at lower temperatures, facilitated by hydrogen atoms, activated on nickel crystallites, which can spillover to WOx species (see further discussion on XPS results). This may explain the increase in the size of the TPR peaks in the temperature range 500–700 °C with increasing tungsten content. As a result, the catalysts after activation for 2 h at 600 °C in hydrogen may contain nickel crystallites, as well as partially reduced tungsten oxide phases or probably even metallic W and Ni–W alloys. Such species may interact with CO2 or H2O during methanation reaction and participate in the successive elementary surface reactions via redox-type mechanism, making W-promoted nickel catalysts similar to cerium promoted or ceria supported catalysts. Although the presence of WOx or unreduced nickel aluminate species may prevent fast migration of nickel nuclei during reduction stage, leading to the formation of high-active catalysts, an increase in reduction temperature can simultaneously facilitate diffusion of surface atoms and increase the risk of catalysts sintering. An application of high reduction temperature (700–800 °C) may also bring some technical problems of the operation of methanation units.
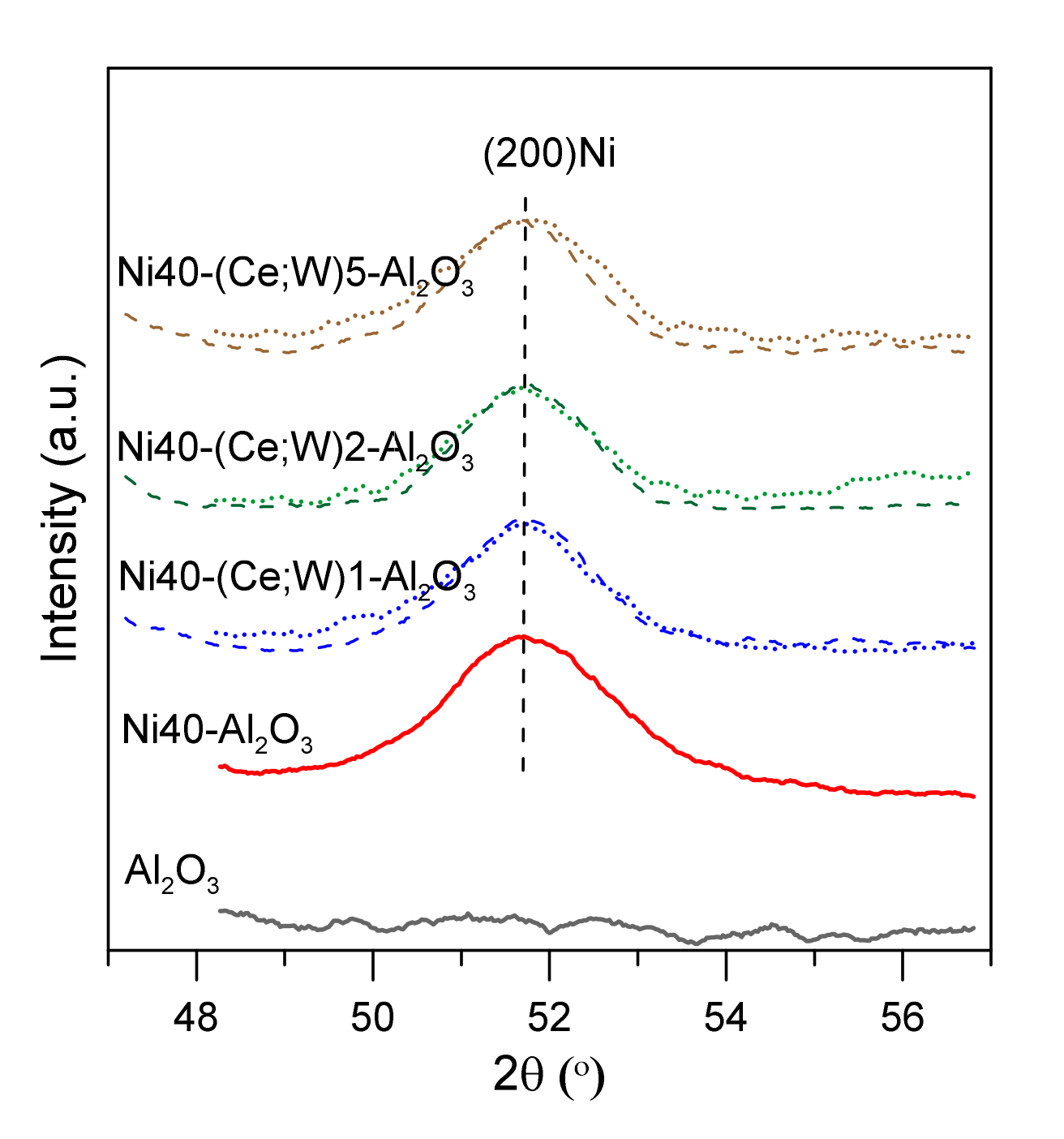
The XRD curves of the catalysts after reduction at 600 °C for 2 h are shown in the Figure S2. Wide reflection peaks of γ-Al2O3 and metallic nickel are partially overlapped, indicating the presence of the small Ni crystallites. Reflection lines of cerium and tungsten phases are not visible. Consequently, it is difficult to unambiguously estimate the size of nickel crystallites, despite advanced Rietveld method. Mean size of Ni crystallites, determined from the XRD results in the catalysts with ca. 20 wt % Ni, was equal to 4.2 nm (Table 1). An increase in Ni loading to ca. 40 wt % led to the increase in mean Ni crystallite size to 6.3 nm. XRD studies showed no strong changes in Ni crystallite size with increasing Ce or W content (Figure 4, Table 1).
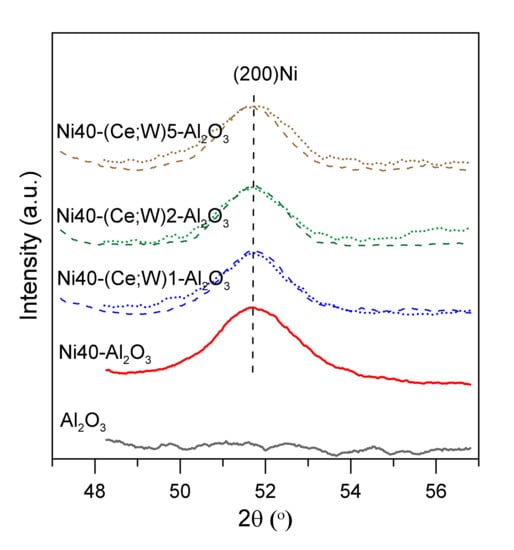
Figure 4.
X-ray diffraction patterns of catalysts after reduction in selected region of 2 theta angle; Ce-promoted nickel catalysts—dotted lines, W-promoted catalysts—dashed lines.
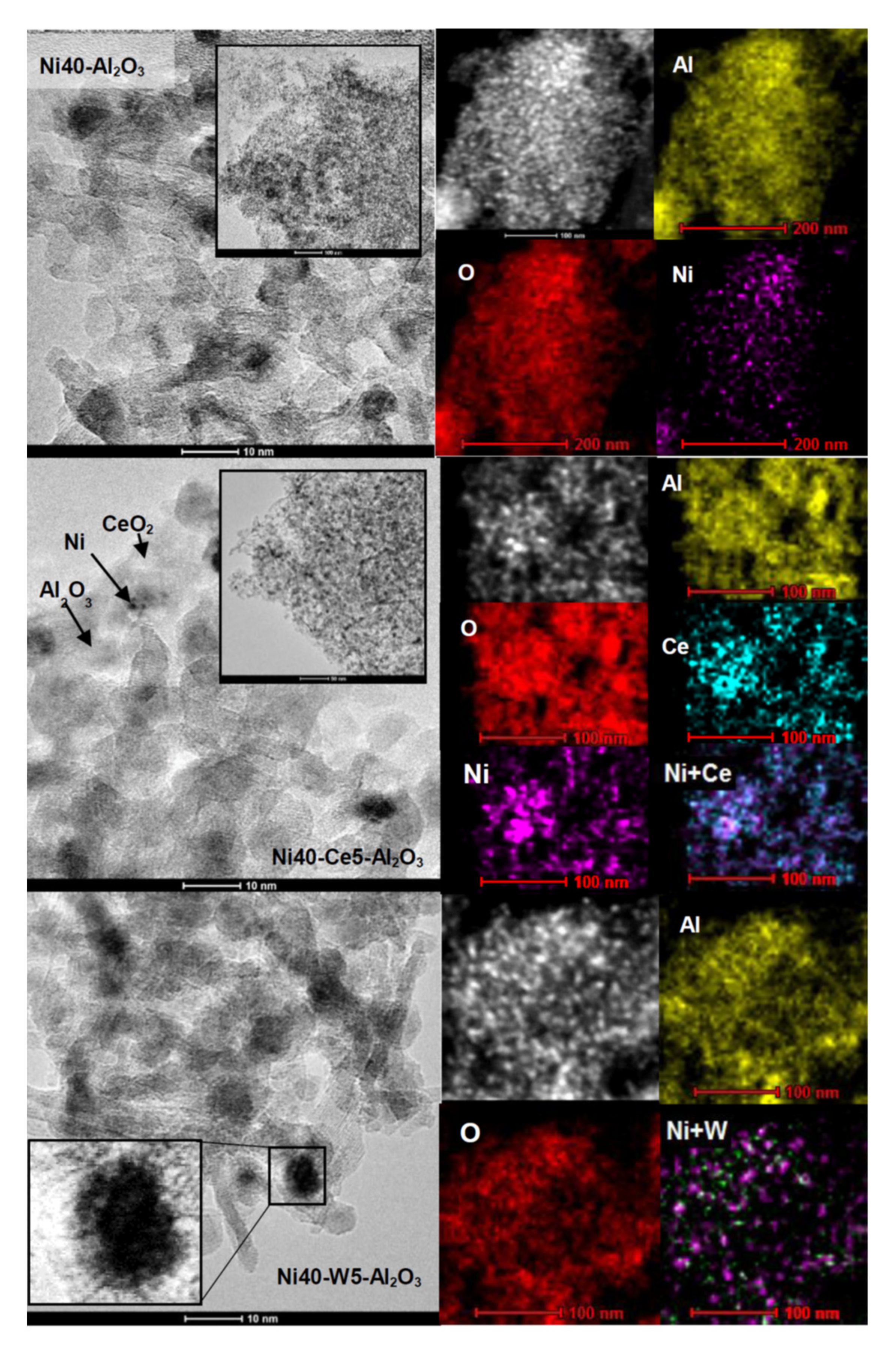
Transmission electron microscopy images of selected catalysts after reduction at 600 °C are presented in Figure 5. Ni40-Al2O3 catalyst contained round nickel crystallites deposited on highly dispersed alumina particles. More detailed inspection of catalysts’ morphology revealed the presence of some surface roughness and irregularities in the shape of nickel crystallites. Some crystallites appeared to be partially covered by the support. The catalyst promoted with cerium and tungsten (Ni40-Ce5-Al2O3 and Ni40-W5-Al2O3, respectively) showed similar morphology (Figure 5). The inset in Figure 5 reveals the presence of nickel particle in the Ni40-W5-Al2O3 catalyst partially covered by amorphous–like phases; however, it is difficult to state their chemical nature. STEM images confirmed strong mutual mixing of Ni, Al, Ce, and W elements in the catalysts.
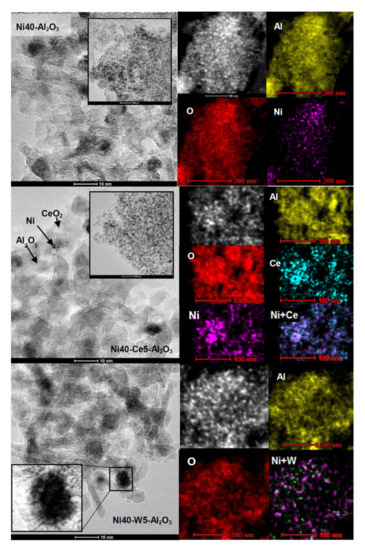
Figure 5.
TEM (left) and STEM (right) images of Ni40-Al2O3, Ni40-Ce5-Al2O3, and Ni40-W5-Al2O3 catalysts after reduction.
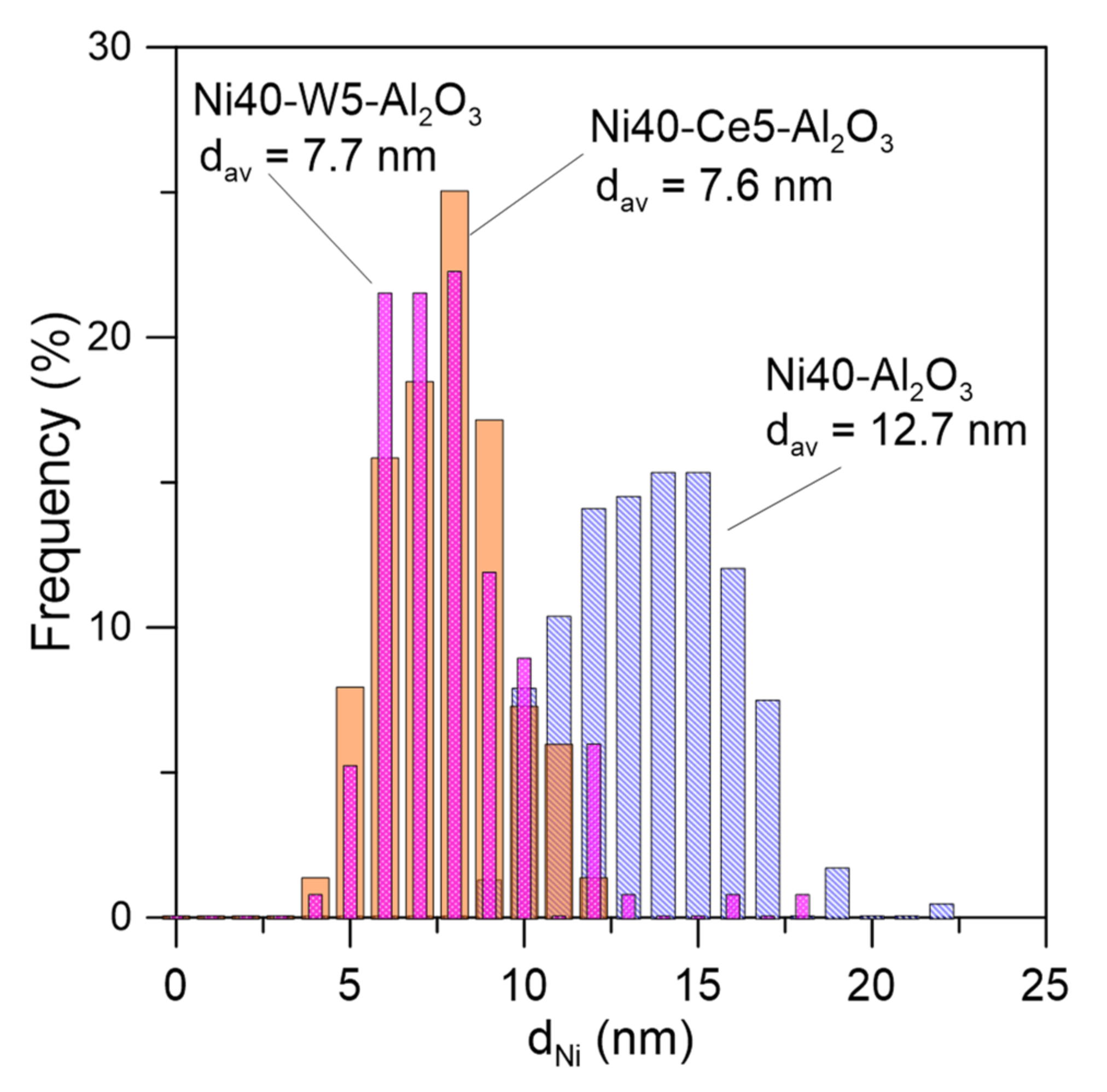
The Ce and W promoters were in part located close to Ni, suggesting direct interaction between nickel particles and promoters. TEM images of promoted catalysts disclosed the presence of some irregular nickel crystallites/agglomerates of the size around 6–8 nm and very small, nearly atomically dispersed Ni crystallites. Electron microscopic studies indicated the decrease of the mean size of nickel crystallites from around 12.7 nm in Ni40-Al2O3 catalyst to 7.7 and 7.6 nm in Ni40-Ce5-Al2O3 and Ni40-W5-Al2O3 catalysts, respectively (Figure 6).
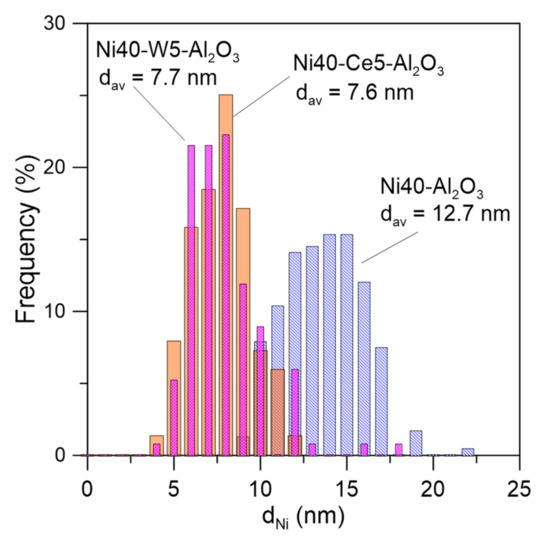
Figure 6.
Nickel crystallite size distribution in selected catalysts after reduction determined from TEM studies.
The active surface area of catalysts (Sa) determined from hydrogen chemisorption studies gradually increased from 12.5 m2/g for Ni20-Al2O3 catalyst with an increase in Ce content to 14.1 m2/g for Ni20-Ce5-Al2O3 (Table 1). Ni40-Al2O3 catalyst showed higher active surface area than Ni20-Al2O3 and similar increase in the active surface area of catalysts after introduction of cerium promoter was observed. The values of Sa varied from 18.1 m2/g for N40-Al2O3 to 19.6 m2/g for Ni40-Ce5-Al2O3 catalyst. The opposite direction was visible for tungsten-promoted catalysts. An increase in the content of tungsten from 1 to 5 wt % led to the decrease in the active surface area to 11.6 m2/g. In the further part of the article, we pointed out the complex role of the tungsten promoter in catalysts, the possibility of the occurrence of metallic tungsten, tungsten alloys, and tungsten oxides, limiting the hydrogen chemisorption.
The curves of hydrogen temperature-programmed desorption are presented in Figure 7. In the case of Ni20-Al2O3 catalyst, the overlapped peaks were observed between −50 and 700 °C. The peaks below 500 °C can be in general ascribed to the desorption of hydrogen from the sites of different strengths on the surface of nickel crystallites [47,48]. High-temperature peaks, above 500 °C, in the literature have been often ascribed to the re-oxidation of nickel by the contribution of hydroxyl groups from the support [49]. The intensity of low temperature peak (−50–100 °C) and wide multiple peaks located between 100 and 500 °C increased with an increase in Ce content for Ni20-Ce-Al2O3 catalysts. Such effects indicate an increase in the number of active nickel surface sites, resulting from the decrease in nickel particle size. The intensity of desorption peaks increased with an increase in Ni loading (Ni40-Ce-Al2O3).
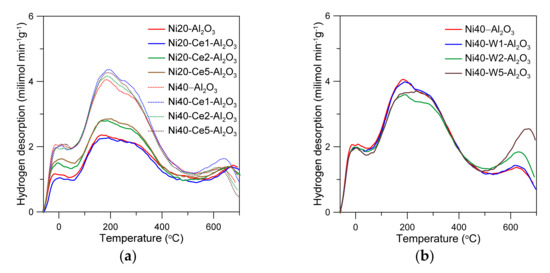
Figure 7.
Temperature-programmed desorption of hydrogen from the Ce- and W-promoted nickel catalysts (a,b).
The shape of desorption peaks and position of maxima for unpromoted and Ce-promoted catalysts containing different amounts of nickel were similar, which may indicate that cerium promoter does not strongly influence the strength of nickel–hydrogen bonds and distribution of individual types of Ni sites [50]. In turn, an introduction of the small amounts of tungsten led to the decrease in the intensity of the TPD peaks, indicating the decrease in the number of active surface sites, which is consistent with static hydrogen chemisorption studies presented above. Note that the intensity of high-temperature maxima increased with an increase with W content. This effect can be ascribed to the enhanced re-oxidation of nickel active phase or reduced tungsten species by the participation of surface hydroxyl groups or water desorbed at high temperatures from the support (Ni + H2O → NiO + H2, WOx + yH2O → WOx+y + yH2), suggesting changes in nickel dispersion, enhancement of interactions between alumina, and reduced tungsten oxide phases and nickel surface sites.
2.3. Catalytic Performance and the State of Catalysts
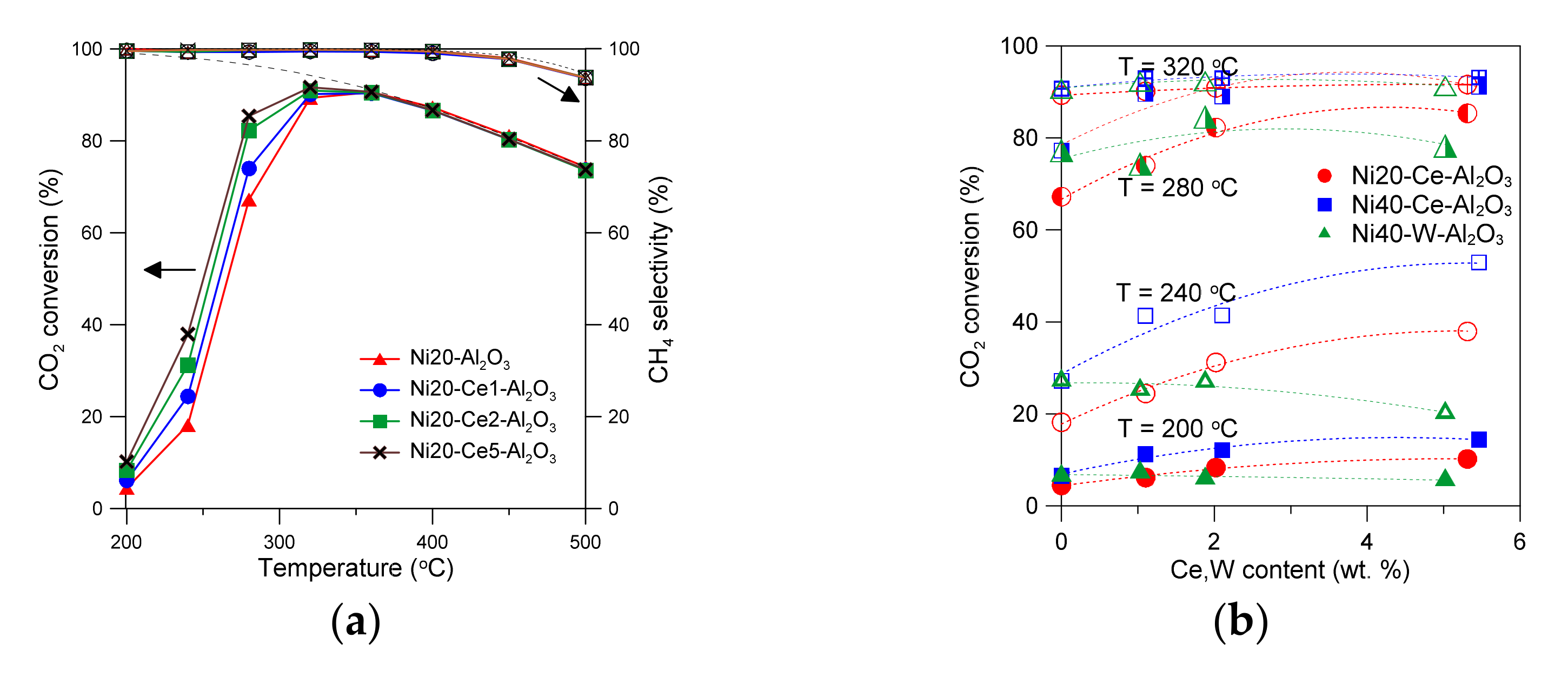
Conversion of carbon dioxide and selectivity towards methane determined at different temperatures in the CO2 methanation reaction in the presence of Ce-promoted Ni20-Al2O3 catalysts are presented in Figure 8a. Thermodynamic constrains are represented here by the dashed lines [21]. The catalysts show high activity at low reaction temperatures. CO2 conversion over Ni20-Al2O3 catalyst approached thermodynamic limit below 350 °C. The drop in CO2 conversion and selectivity to methane was observed above 350 °C. Such effects are consistent with thermodynamic limits [21]. Thermodynamic values of CO2 conversion decreased from around 100% at 200 °C to around 65% at 600 °C (under 2 bar pressure). The catalysts showed excellent selectivity to methane at low reaction temperatures (close to 100%) [21]. The selectivity to CH4 decreased at higher temperatures, in line with the thermodynamics of the CO2 methanation reaction. The only by-product of the reaction was carbon monoxide [21]. An introduction of the small amount of Ce led to the increase in CO2 conversion at low reaction temperatures. Recently, we have demonstrated high durability of unpromoted and Ce-promoted alumina-supported nickel catalysts in time-on-stream of CO2 methanation reaction [12,21]. Figure 8b shows the changes in CO2 conversion at selected temperatures (200, 240, 280, 320 °C) in the presence of catalysts containing different Ni and promoter amounts. An increase in Ce content up to 5 wt % leads to increase in CO2 conversion at low reaction temperatures. An almost linear increase in CO2 conversion with an increase in Ce content up to around 2 wt % was visible at low temperatures for Ni20-Ce-Al2O3 and Ni40-Ce-Al2O3 catalysts. An introduction of larger amounts of Ce led to the less pronounced changes, especially at higher temperatures, 280 and 320 °C, wherein the activity of Ni40-Ce-Al2O3 catalysts was very high. The effects of the presence of tungsten in the catalysts on CO2 conversion were more complex. Although selectivity changes towards methane with an increase in reaction temperature were the same as in the case of unpromoted catalyst, CO2 conversion reached maximum at certain tungsten loading (at low reaction temperatures) (Figure S3 in Supplementary Materials). A maximum of CO2 conversion at different reaction temperatures was attained at 2 wt % of W.
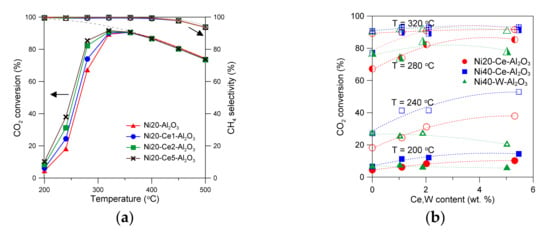
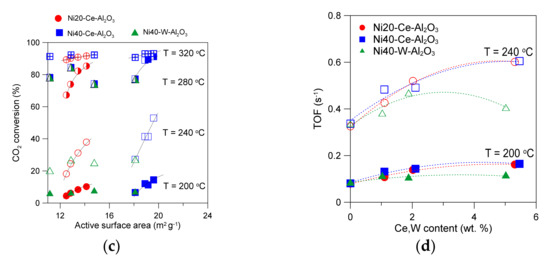
Figure 8.
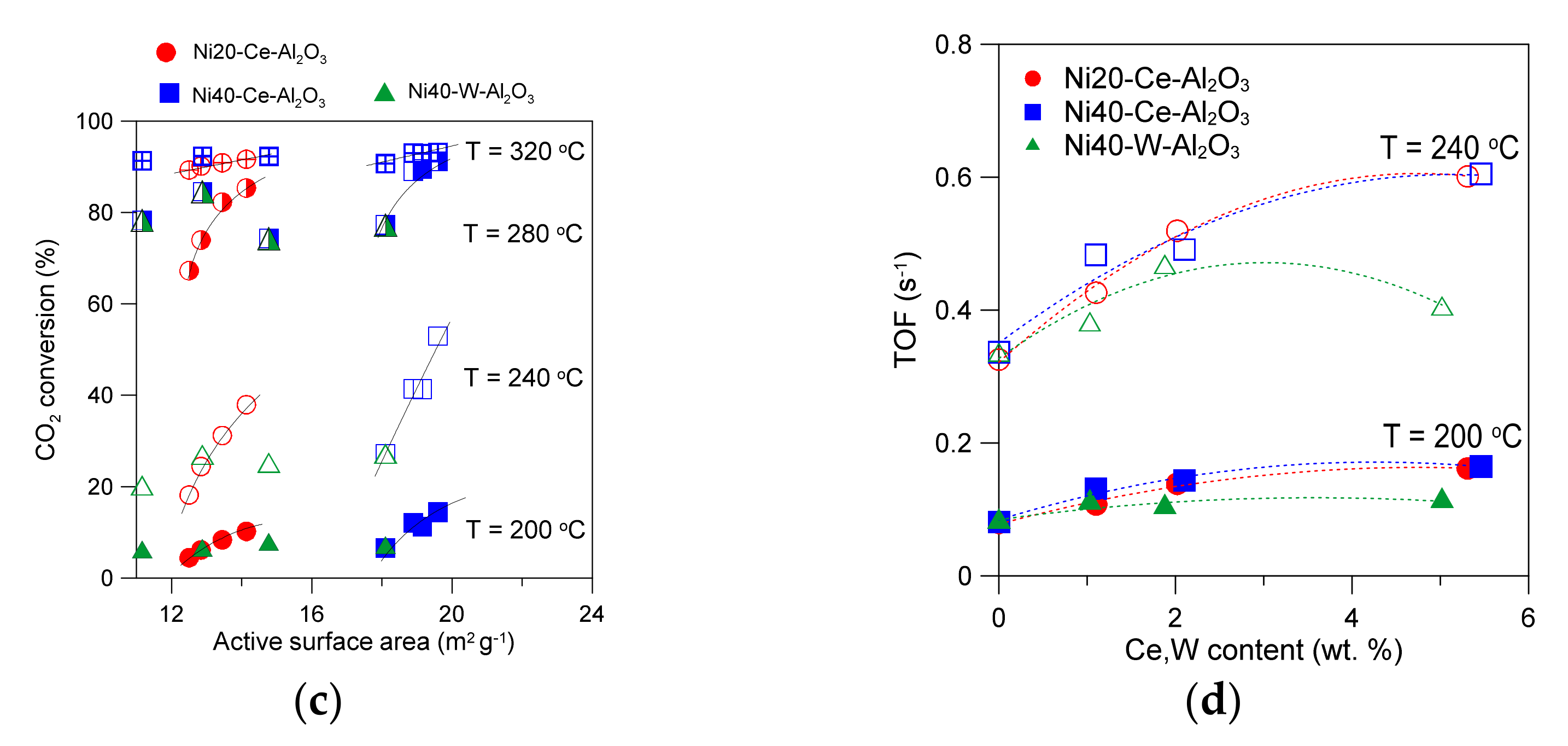
CO2 conversion and selectivity to methane for alumina-supported catalysts promoted with Ce, containing 20 wt % Ni (a). Influence of catalyst composition on CO2 conversion at selected temperatures (b). The effects of active surface area on CO2 conversion (c). The influence of Ce or W content in catalysts on TOF values at selected temperatures (d).
It is worth referring at this point to the methanation reaction mechanisms presented in the literature [51,52,53,54,55,56]. The overall process has been often regarded as the set of surface reactions, starting from hydrogen and carbon dioxide dissociative adsorption on the surface of metals; subsequent formation of hydrogen (Had), carbonyl (COad), and oxygen species (Oad); and then, depending on the assumed models, dissociation of carbonyl groups to oxygen (Oad) and carbon (Cad) hydrogenated next to CHx species (carbide mechanism) or hydrogenation of CO(ad) (hydrogen-assisted mechanism) to corresponding intermediates, such as HCO and H2CO species. Therefore, in accordance with this model, an increase in the activity of catalysts at low temperatures has been ascribed to the modification of the nature of metal surface sites or to an increase in their number. However, the role of peculiar surface sites and facets is still a matter of discussion [57,58]. Zhou et al. indicated that methanation reaction is sensitive to the presence of low-coordinated Ni atoms that enhances the C–O bond scission and correlated its rate on nickel planes in following order Ni(211) > Ni(100) > Ni(111) [57]. On the other hand, Zhen et al. underlined the positive role of Ni(111) planes in the increase in activity of catalyst during methanation reaction with regards of low CO2 dissociation barrier [58]. Numerous studies of CO2 methanation reaction were performed using supported metal catalysts, indicating the role of support in the activation and transformation of CO2 to methane [59,60]. The associative reaction mechanism assumes that suitable carbonate, hydroxycarbonate, or carbonyl groups are formed on the support in the first step, which are then hydrogenated to methane with participation of hydrogen atoms adsorbed on the surface of metallic crystallites [59,60]. According to this model, the improvement in catalysts activity can be achieved not only by modification of support properties but also by increase in the dispersion of metal crystallites, which result in the increase in the total number of periphery atoms. In our opinion, similar effects may appear in the case of cerium-promoted alumina supported nickel catalysts, given that cerium oxide species can be found in close proximity to nickel crystallites.
The obtained results of CO2 methanation reaction performed in the presence of alumina-supported catalysts promoted with cerium indicate that the activity of catalysts is related to the number of active nickel surface sites. Indeed, a good correlation between the increase in active surface area of catalysts and increase in CO2 conversion at selected reaction temperatures can be found for cerium-promoted catalysts (Figure 8c). An increase in the active surface area of alumina supported catalysts (Ni20-Ce-Al2O3) from 12.5 to 14.1 m2/g corresponds with an increase in CO2 conversion from 4.5 to 10.2% at 200 °C (for Ni20-Al2O3 and Ni20-Ce5-Al2O3 catalysts, respectively). The increase in conversion is more evident at 240 °C (an increase from 18.2 to 37.9% is observed). The relative changes in CO2 conversion become less evident with further increase in reaction temperature. Similar trends were observed for the second group of catalysts of higher Ni loading, promoted with cerium. An increase in the active surface area of Ni40-Al2O3 catalyst from 18.1 to 19.6 m2/g observed in Ni40-Ce5-Al2O3 corresponded with an increase in CO2 conversion from 6.6 to 14.4% at 200 °C, and from 27.2 to 53.0% at 240 °C, respectively. However, one can observe that some catalysts within Ni20-Ce-Al2O3 group, even with the lower active surface area, showed better catalytic performance than the catalysts containing 40 wt % Ni of high active surface area. For example, compare CO2 conversion for Ni20-Ce5-Al2O3 catalyst and Ni40-Al2O3 at 240 °C (Figure 8c).
The changes discussed above were not apparent for W-promoted catalysts. A decrease in the active surface area with increasing tungsten content was observed, but the CO2 conversion decreased only after exceeding a certain W content (Figure 8c). It was even possible to see an increase in CO2 conversion at low W content for selected catalysts.
The turnover frequency values (TOF) determined at low conversion of CO2 increased with increasing Ce content in Ni20-Ce(x)-Al2O3 catalysts (Figure 8d). A similar trend was observed for Ni40-Ce(x)-Al2O3 catalysts. Such effects suggest that improved properties of cerium-promoted catalysts are not only related to the increased number of nickel surface sites, although an increase in the active surface area may increase the ability of the adsorption and dissociation of H2 and CO2 molecules, which are transformed to COad or Cad species, hydrogenated to formates, and finally transformed to CHx species and desorbed from the surface of nickel crystallites [61]. It seems probable that formation of oxygen vacancy sites or decrease in the oxidation state of Ce4+ to Ce3+ in the species located in the close proximity of nickel crystallites or on their surface may occur under reductive conditions of the methanation reaction. Such changes were proposed for ceria-supported nickel catalysts [20,21,62,63]. Hence, one can expect that the presence of vacancies or Ce3+ sites in the dispersed cerium oxide species or their basic nature may facilitate activation of CO2 molecules, dissociative adsorption, and conversion to carbonyl and formate species.
An increase in TOF was also observed for catalysts with low content of tungsten (Figure 8d). In this case, the role of additional factors influencing activity of catalysts became even more evident. The catalysts (with low tungsten content) did not lose their activity, despite the decrease in active surface area. In our opinion a similar redox mechanism as in the Ce-promoted catalysts discussed above may occur in W-promoted catalysts. The oxidation state of tungsten may change under reaction conditions, leading to the facilitation of the activation and then conversion of CO2. To confirm the presence of implied changes in the catalysts under different operation conditions, we applied quasi in situ XPS studies.
Survey XPS scans of selected catalysts (Ni40-Al2O3, Ni40-Ce5-Al2O3, and Ni40-W5-Al2O3) after calcination, activation in hydrogen at 600 °C, and after CO2 methanation reaction performed at 350 °C are shown in Figure S4 in the Supplementary Materials. The catalysts after reduction and methanation prior to the spectra recording were not exposed to air. The results of deconvolutions of corresponding spectra are presented in Table S1.
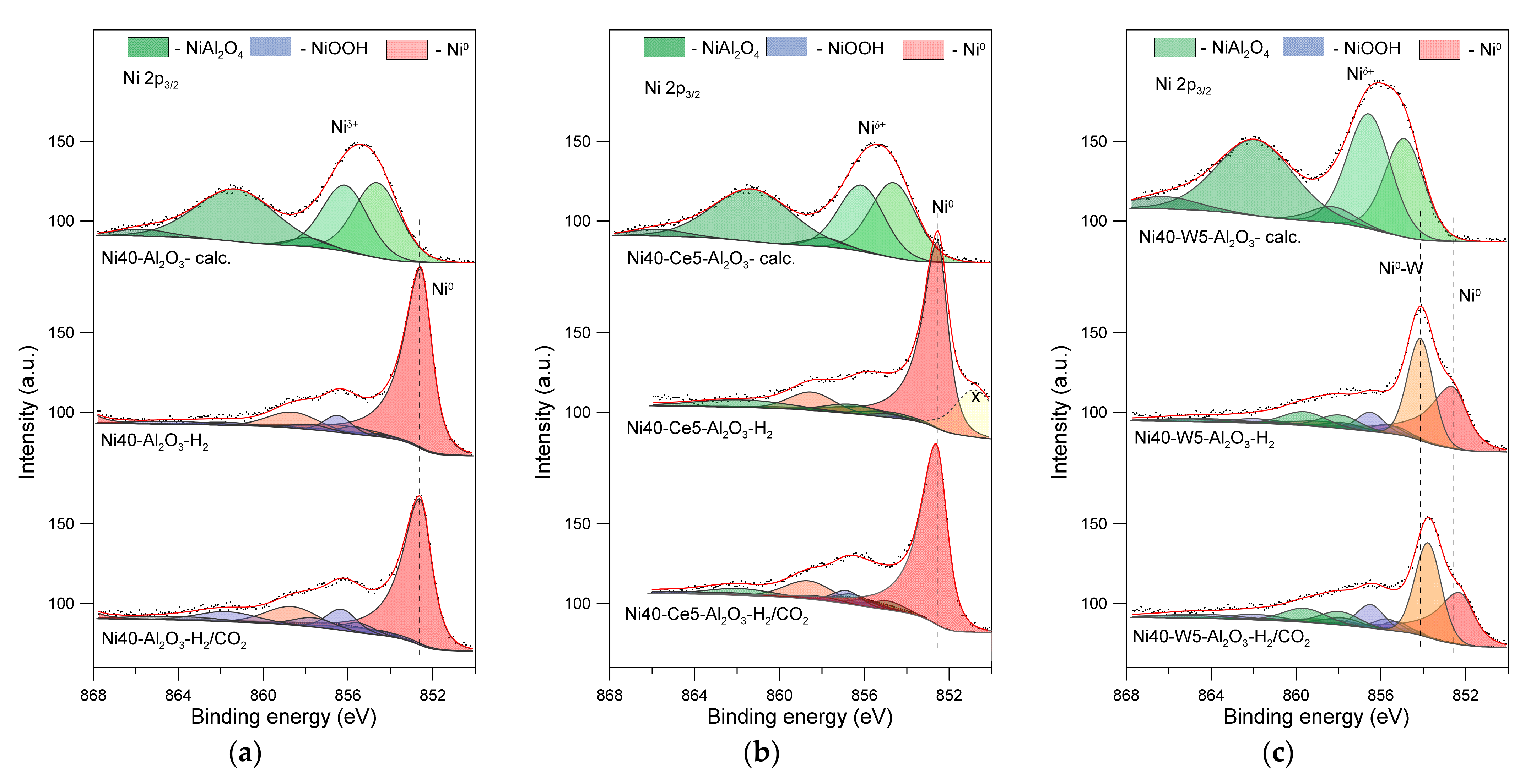
High resolution Ni 2p XPS spectra are presented in Figure 9. A good fit of the Ni 2p XPS peaks in the spectra of calcined Ni40-Al2O3 catalyst based on the peaks positions and energy separation was achieved, assuming the presence of surface NiAl2O4 spinel. Similar positions and shape of corresponding peaks can be found booth on the spectra of Ni40-Al2O3 and Ni40-Ce-5-Al2O3 calcined catalysts. The main component of Ni 2p3/2 observed at 854.6 eV (FWHM equal 2.54 eV), with strong accompanied satellite peaks at 856.1 and 861.3 eV, was located in a slightly higher binding energy than corresponding peak of bulk NiO reported by Biesinger [64,65]. However, it should be stressed that chemical state of Ni2+ in NiO phases on alumina support can be different from that in bulk NiO. As an example, Arranz et al. identified Ni 2p3/2 peaks for NiO grown on polycrystalline aluminum at 854.0 and 856.2 eV (with FWHM value of 2.2 eV) [66]. Simultaneously, they also observed the presence additional broader peak at around 856 eV (with FWHM value of 2.6 eV) attributed to Niint located at the aluminum oxide–nickel oxide interface. Hence, one can infer that nickel oxide particles in the obtained catalysts can be decorated by nickel aluminate species, which is in good agreement with the TPR results. The same fitting model was used for deconvolution of the spectra of calcined Ni40-W5-Al2O3 catalyst. In this case, the main and satellite lines were slightly shifted to higher energies (Table S1). Moreover, the intensity of Ni 2p satellite peaks was much higher than on the spectra of unpromoted catalyst. Similar effects were reported by Solsona et al. for Ni–W–O mixed metal oxide catalysts and attributed to a different local environment of the Ni atoms resulting from the presence of tungsten in the structure of catalysts [67]. However, in contrast to the mentioned work, we observed a very weak intensity of W 4f photoelectrons (Figure 10), which could be attributed to the presence of diverse oxide species with tungsten in different chemical environment or the specific locations of tungsten species in the catalyst [67,68]. Similarly, very low intensities were observed for cerium, which prevented correct interpretation of the spectra (Figure S4).
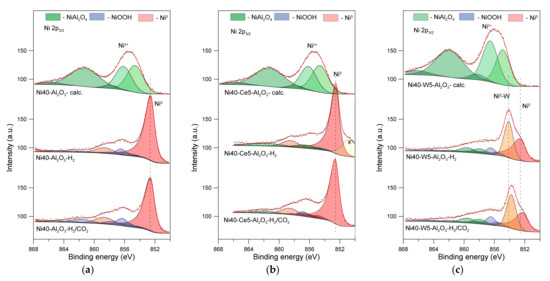
Figure 9.
Ni 2p3/2 XPS spectra of catalysts after calcination (-calc.) (a), activation in hydrogen (-H2) (b), and CO2 methanation reaction (-H2/CO2) (c).
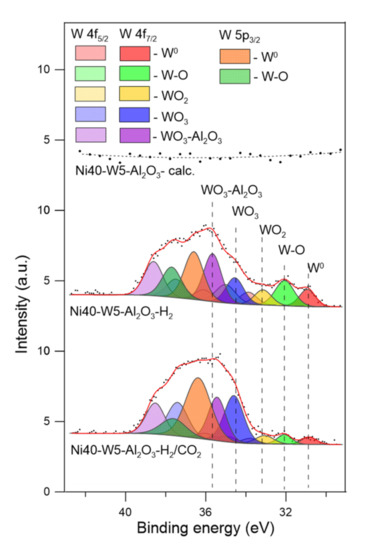
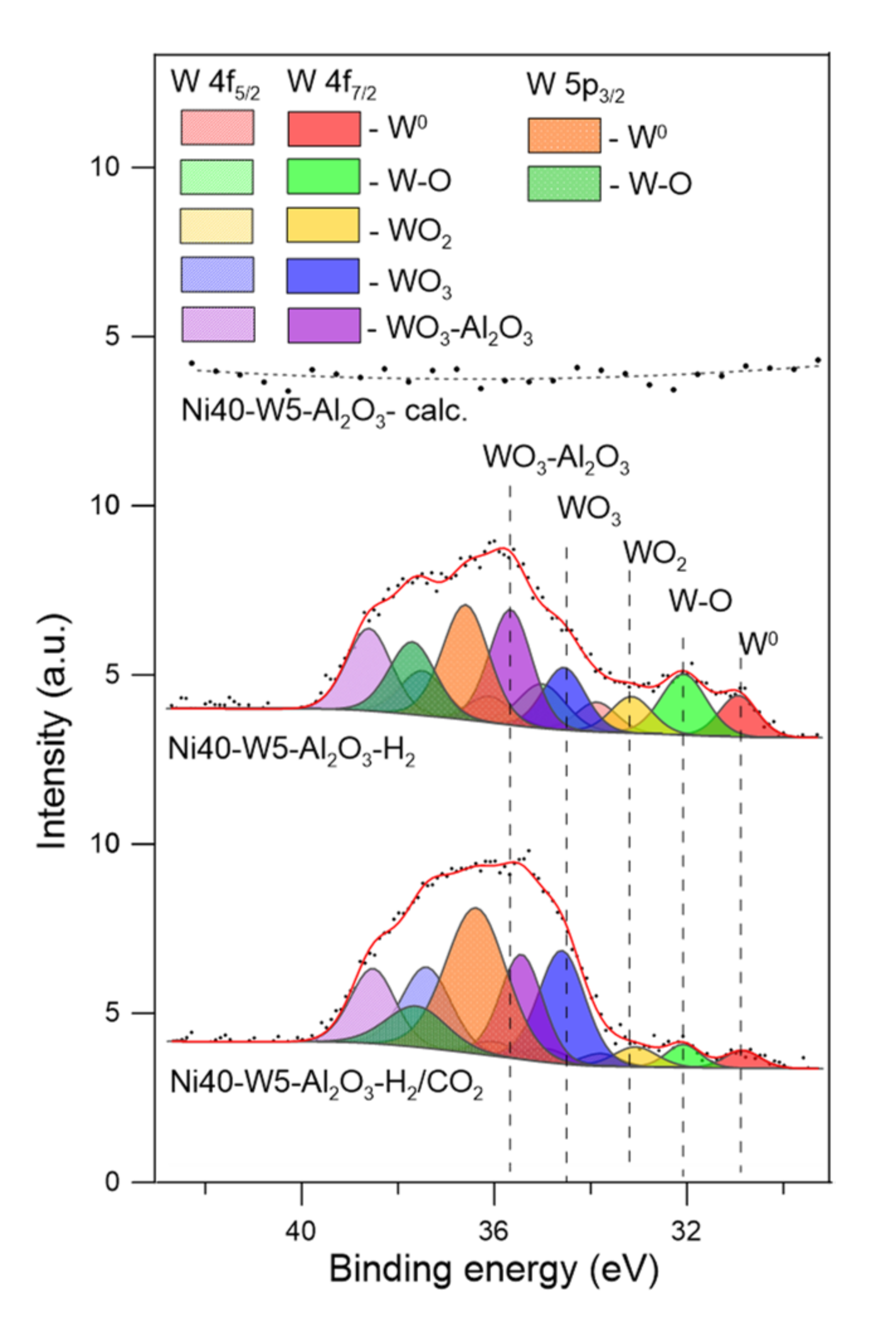
Figure 10.
W 4f and W 5p XPS spectra of catalysts after calcination (-calc.), activation in hydrogen (-H2), and CO2 methanation reaction (-H2/CO2).
The spectra of reduced alumina-supported catalyst (denoted here as Ni40-Al2O3–H2) showed distinct Ni 2p3/2 peak located at 852.5 eV with corresponding smaller satellite peaks at 856.2 and 858.6 eV, which can be attributed to the presence of metallic nickel (Ni0) [64,65]. In addition, it was possible to distinguish several peaks located at higher energies, ascribed to Niδ+, the nature of which is difficult to determine unambiguously (Figure 9). The best fit was obtained through assuming the presence of NiOOH-like phases. The formation of such species could result from the reoxidation of surface nickel atoms lying in the immediate vicinity of the alumina support (or alumina species decorated the surface of crystallites) by the participation of hydroxyl groups from Al2O3. Similar phases were identified by the analysis of the XPS spectrum of Ni40-Ce5-Al2O3-H2 catalyst sample. A good fit was achieved by additional assumption of the presence of small amounts of NiAl2O4 (Table S1). Slightly smaller contribution of NiOOH phases could be a consequence of the presence of dispersed CeO2 particles lying in the direct vicinity of surface nickel atoms (Table S1).
XPS studies evidenced that activation of Ni40-Al2O3 and Ni40-Ce5-Al2O3 catalysts prior to the reaction resulted in the formation of metallic nickel crystallites and small amounts of nickel oxide or nickel aluminate species. More complex changes were identified in tungsten promoted catalyst. The first Ni 2p3/2 peak on the XPS spectrum of Ni40-W5-Al2O3-H2 catalyst was broader and its maximum was shifted to higher energies in comparison to the discussed above spectra. The best fit was achieved through assuming the presence of two types of metallic nickel atoms, which can be attributed to metallic nickel Ni0 identified by the presence of the peak at 852.6 eV, together with accompanying satellite peaks at similar position as in the unpromoted nickel catalyst, as well as metallic nickel atoms of different chemical environments, probably in contact with tungsten, denoted here as Ni0-W (where W is necessarily not a metallic tungsten). Additionally, small amounts of nickel aluminate spinel and oxide species were identified.
The same samples after collecting the XPS spectra were transferred to the reaction chamber and exposed to the reaction mixture of H2 and CO2 at 350 °C for 1 h. The XPS spectra of catalysts, denoted here as Ni40-Al2O3-H2-CO2 and Ni40-Ce-5-Al2O3-H2-CO2, recorded after methanation reaction, revealed slight changes in the oxidation state of nickel, identified mainly by the increase in the contribution of NiOOH-type species (Table S1). Such effects can be explained by partial reoxidation of surface nickel sites with surface hydroxyl groups or water formed during methanation reaction.
More visible changes were observed in the case of tungsten-promoted catalyst. In addition to the reoxidation of nickel phases discussed above, a decrease in the contribution of the Ni0-W peak was observed. This peak was also shifted towards lower energies. Such effect can be associated with a decrease in the contribution of W being in direct contact with nickel, probably due to reoxidation of tungsten and/or Ni0-W phase segregation.
Such changes correspond with perturbation of the W 4f XPS spectra (Figure 10). The spectra of tungsten were fitted with the doublets of 4 f7/2 and 4 f5/2 peaks, together with corresponding W 5p3/2 peaks of tungsten at different chemical environments, attributed to metallic tungsten (W0), metallic tungsten interacting with surface oxygen atoms (W-O), tungsten dioxide (WO2), and tungsten trioxide (WO3) of different interactions with alumina support [67,68,69,70].
The XPS results indicate that reduction of tungsten-promoted catalysts may lead to the formation of oxides with the lower oxidation state of tungsten (WOx, x < 3) and also development of metallic tungsten. Such deep reduction can be ascribed to the interaction between dispersed tungsten oxide species and nickel crystallites, as well as enhanced breaking of W–O bonds by participation of hydrogen atoms activated on metallic nickel surface sites. Thus, metallic tungsten may interact with nickel (Ni0), forming corresponding alloys or composite oxide systems (Ni0-W). The presence of multiple forms of tungsten oxides may indicate that their contribution will depend on the presence of reducing and oxidizing agents in the reaction zone, including H2 and CH4, as well as CO, CO2, and H2O. The XPS spectra of catalysts recorded after the methanation reaction revealed the decrease in the contribution of metallic tungsten (W0) (which is line with discussed above Ni 2p spectra for Ni0-W forms), W–O-type species, and WO2, and simultaneously an increase in the contribution of WO3-like forms.
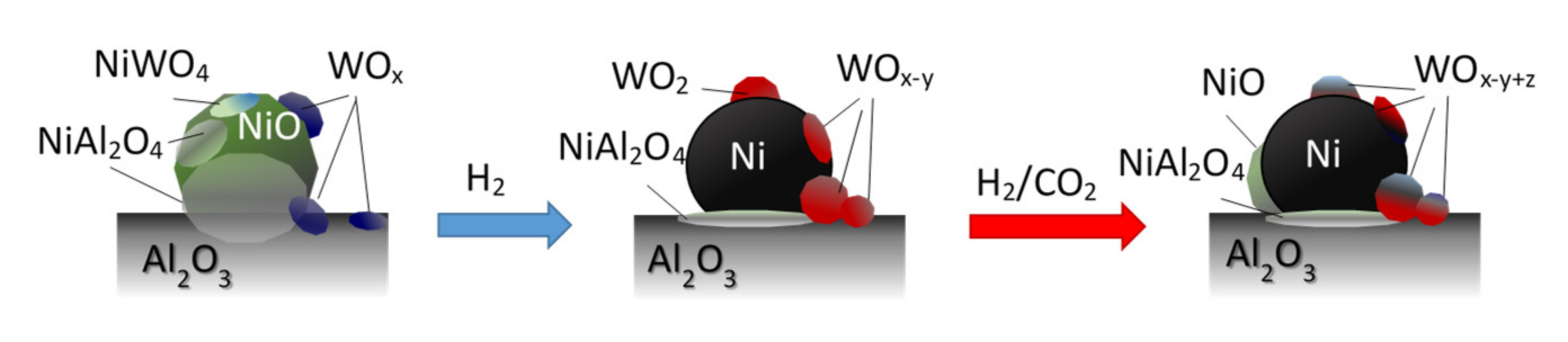
The increased activity of Ce-containing nickel catalysts has been often ascribed to the improved activation of CO2 on the specific sites on CeO2, regarded as redox and basic Lewis centers. In the case of W-promoted catalysts, the nature of oxides with different oxidation state of tungsten (and/or crystallographic structure of oxides) may vary, depending on the reaction conditions, including local and temporal changes in the concentration of the reducing and oxidizing agents over the catalysts surface (H2, CH4, H2O, and CO2). Such species may also participate in the elementary stages of the methanation reaction, affecting the rightward shift of the equilibrium state of surface reactions. It may be thought that under methanation reaction conditions, partially reduced forms of tungsten oxides (formed under activation steps of catalysts, WOx + yH2 → WOx−y + yH2O) or even metallic (W0) or intermetallic Ni0-W phases may undergo oxidation through binding surface oxygen atoms produced during dissociative adsorption of CO2 or via the reaction with hydroxyl groups or water formed in the intermediate surface reactions (CO2(ad) → CO(ad) + O(ad); WOx−y + zO(ad) → WOx−y+z; WOx−y + zH2O → WOx−y+z + zH2) (schematically presented in Figure 11) [46,67,68,69,70,71,72].
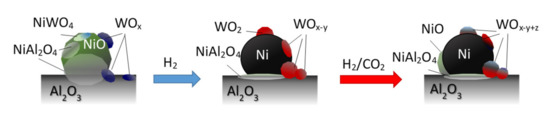
Figure 11.
Schematic illustration of oxide phase transformations in alumina-supported, tungsten-promoted nickel catalysts under reduction and CO2 methanation reaction conditions.
XPS studies indicated that intermediate oxide species can be also formed on the surface of nickel crystallites. They can be reduced again with the participation of hydrogen atoms activated on the nickel surface sites in subsequent steps of the redox cycle (NiO(surf) + 2H(ad) → Ni0 + H2O; WOx + yH2 → WOx−y + yH2O). However, additional experimental and/or theoretical studies are necessary to verify such hypothesis.
Studies pointed out that positive effect of tungsten can be observed if suitable balance between corresponding surface oxidation-reduction reactions is achieved. They can participate in the redox cycles, enhancing activation and transformation of surface intermediate products. On the other hand, an increase in tungsten loading may lead to the partial coverage of nickel crystallites; formation of less-active W0 or WOx sites; and decrease in the number of Ni0 surface sites, capable for activation of H2 and CO2, thus participating in successive oxidation-reduction cycles of tungsten oxide species. It is likely that an increase in the concentration of oxidizing agents in the gas phase interacting with the catalyst surface of catalysts or the presence of catalysts additives hindering reversible redox changes may act in a similar direction.
3. Materials and Methods
3.1. Catalysts Preparation
Two series of alumina-supported nickel catalysts promoted with cerium and tungsten were prepared by the modified impregnation method. The nominal nickel content in the catalysts was 40 wt %. In addition, the effect of Ni loading was investigated for Ce-promoted catalysts by the preparation of catalysts containing 20 wt % Ni. The alumina support (γ-Al2O3, p.a., Alfa Aesar, 1/8” pellets) was initially finely ground in an agate mortar to a grain size of approximately 0.1–0.3 mm and then dried at 110 °C for 12 h. Pure alumina-supported nickel catalysts were obtained through dissolving proper amounts of nickel nitrate hexahydrate (Ni(NO3)2·6H2O, p.a., Merck Sp. z o.o., Poland) and citric acid (p.a., Merck Sp. z o.o., Poland) in 300 mL of demineralized water, keeping the molar ratio of nickel nitrate to citric acid equal 1:1, as described earlier [20]. Then, 5 g of support was introduced into the resulting aqueous solutions. The pH of the initial solutions was approximately 1. Water was slowly evaporated at around 90 °C on a water bath heater over several hours. The samples were dried overnight at 120 °C. To avoid formation of hardly reducible oxide species, we calcined the catalysts at 400 °C for 2 h. Alumina-supported nickel catalysts promoted with cerium or tungsten were prepared in a similar way using an aqueous solutions containing nickel nitrate, citric acid, and cerium nitrate hexahydrate (Ce(NO3)3·6H2O, p.a., Merck Sp. z o.o., Poland) or ammonium metatungstate hydrate ((NH4)6H2W12O40·H2O, p.a., Merck Sp. z o.o., Poland), keeping the molar ratio of metal salts to citric acid equal to 1:1. Similar thermal treatment was used as in the case of unpromoted catalysts [12,20]. The cerium and tungsten content varied from 1 to 5 wt %.
3.2. Characterization of Catalysts
The elemental composition of catalysts was determined by the use of X-ray fluorescence method employing ED-XRF Canberra-Packard 1510 spectrometer (data presented in Table 1). Nitrogen adsorption/desorption isotherms were collected at −196 °C using ASAP 2405N analyzer (Micromeritics Instrument Corp., Norcross, GA, USA) [20]. The specific surface area (SBET) after outgassing of catalysts under the pressure of around 10−2 Pa at 200 °C was calculated from the adsorption data using the standard Brunauer–Emmett–Teller (BET) method. The total pore volume (Vp) of catalysts was calculated from the single point adsorption at p/p0 = 0.98. Pore size distribution and mean pore diameter (DBJH) was determined by the application of the Barret–Joyner–Halenda (BJH) method from the data of the desorption branch of isotherms.
X-ray diffraction studies (XRD) of catalysts after their calcination and reduction were carried out using Empyrean, PANalytical diffractometer with Cu Kα radiation (λ = 1.5418 Å). Samples were reduced in the flow of hydrogen at 600 °C for 2 h and then passivated at room temperature. The Rietveld method with HighScore Plus software was used for determination of mean nickel crystallite size (). The catalysts after reduction at 600 °C were studied by the transmission electron microscopy (TEM) using a Tecnai G2 20 X-TWIN microscope (FEI Company, Hillsboro, OR, USA), which was equipped with an LaB6 source, HAADF detector, and EDS spectrometer with an accelerating voltage of the electron beam 200 kV [20]. The spatial distribution of elements was determined using the STEM method. On the basis of EDS spectra collected from each image point, we constructed element distribution maps consisting of a matrix of pixels of appropriate color and intensity related to the concentration of elements.
Temperature-programmed reduction (TPR) and hydrogen desorption studies (TPD) were performed by the application of the Autochem II 2920 (Micromeritics Instrument Corp., Norcross, GA, USA), equipped with the TCD detector [20]. Reduction of the catalysts (sample weight m = 0.05 g) was carried in the flow of 5% vol. H2 in Ar mixture (30 mL min−1) with 10 °C/min ramp rate. In the TPD studies, samples (m = 0.1 g) were initially reduced at 600 °C for 2 h. To overcome hydrogen adsorption activation barrier, we cooled down the catalysts in the flow of hydrogen from 600 to −60 °C. Desorption was carried out in the flow of Ar (30 mL/min) with the linear ramp rate 10 °C/min. Water evolved during reduction and desorption steps was removed in a cold trap maintained in the liquid nitrogen (LN2)–isopropyl alcohol mixture at around −90 °C, which was installed between the reactor and the detector.
The active surface area of catalysts (Sa) was estimated by the static volumetric chemisorption method using ASAP 2020C apparatus (Micromeritics Instr. Corp.). The catalysts were reduced at 600 °C for 2 h. The stoichiometry of H/Ni chemisorption was assumed to be 1/1, and the area occupied by one hydrogen atom was 0.0649 nm2 [20,21].
Quasi in situ X-ray photoelectron spectroscopy (XPS) studies were carried out by the application of the multi-chamber UHV system (PREVAC), which was equipped with the load lock, flow reactor, distribution, and analytical chambers [22]. The spectra were collected by the application of a Scienta SAX-100 X-ray source (Al Kα, 1486.6 eV, 0.8 eV band) with the XM 650 X-ray Monochromator (0.2 eV band) and a hemispherical Scienta R4000 electron analyzer. Survey spectra (with 500 meV step) were collected using the pass energy of the analyzer equal to 200 eV. High resolution spectra were recorded for selected regions, including Ni 2p, W 4f, and C 1s, using 50 eV pass energy. The base pressure in the analytical chamber was equal 2 × 10−9 mbar. The samples, prior to the XPS studies, were pre-reduced at 600 °C using argon of high purity and then passivated at room temperature in the external system (Autochem II 2920, Micromeritics). Next, the samples were pressed into thin wafers (10 mm diameter), mounted on the sample holders, and then inserted into the load lock. The catalysts were degassed for 16 h, then were transferred through the distribution chamber into the flow reactor chamber, which was coupled to the UHV system. Initially, the samples were reduced in the flow of a mixture consisting of H2 (40 mL min−1) and Ar (60 mL min−1) over 1 h at 350 °C under 1 bar pressure. Next, the temperature was decreased, and the reaction gases were pumped out. The entire holders were transferred to the analytical XPS chamber under vacuum conditions (without exposure to air). The samples after collection of the XPS spectra were transferred again to the reactor chamber and heated up in the flow of the reaction mixture consisting of H2 (40 mL min−1), CO2 (10 mL min−1), and Ar (50 mL min−1) (H2/CO2 = 4/1) to 350 °C. The samples after a treatment at 350 °C for 1 h were cooled down to room temperature again, and then the reaction mixture was replaced by Ar. Subsequent XPS spectra were collected after pumping out the gas and transferring the samples to the analytical chamber. Data processing was performed using CasaXPS software (v2.3.23 PR1.0). The spectra were charge-corrected for a C 1s peak binding energy equal 284.7 eV. The deconvolution and fitting procedures of Ni 2p spectra were similar to that proposed in [22].
Activity and selectivity studies of catalysts in the CO2 methanation reaction were carried out by the application of PID Eng&Tech automated reactor system, which was equipped with fixed-bed continuous-flow quartz reactor, worked under the pressure of 1.9 × 105 Pa [12,20,21]. The catalysts samples (0.200 g) were mixed with quartz crumbs and introduced to the reactor. The samples were reduced in the flow of hydrogen stream at 600 °C for 2 h. The temperature after activation was decreased to 200 °C; then, hydrogen was replaced by the reaction mixture. The total flow rate was equal 100 mL min−1. The stream was composed from hydrogen (61.6 vol %), carbon dioxide (15.4 vol %), and Ar (23 vol %) with H2/CO2 ratio equal to 4/1. The space velocity referenced to the total flow rate of reaction mixture divided by the catalyst weight was equal 30,000 mL h−1g−1. The temperature of the catalyst bed was steeply increased from 200 to 600 °C. The customized Bruker’s Rapid Refinery Gas Analyzer was utilized for determination of the composition of the outlet stream. Water vapor was removed from the stream in a cold trap separator prior to the GC analysis. CO2 conversion and selectivity towards CH4 and CO were calculated from the following equations:
where and are concentrations of CO2 in the feed stream and in the post-reaction mixture, and and are concentrations of CH4 and CO in the post-reaction mixture, suitably corrected, taking into account changes in the volume of the reaction mixture, using Ar as an internal standard [21]. Thermodynamic limits in the CO2 methanation reaction were identified by the application of Gibbs free energy minimization method, using Gibbs reactor model and Soave–Redlich–Kwong equation of state [21]. The estimated turnover frequency (TOF) values were calculated at low CO2 conversion (determined at 200 °C and 240 °C) as number of CO2 molecules consumed per number of nickel surface sites in the unit time (s−1). The number of nickel surface sites was taken from the static volumetric hydrogen chemisorption studies.
4. Conclusions
Alumina-supported nickel catalysts containing 20 and 40 wt % Ni were prepared by the impregnation of γ-Al2O3 support using an aqueous solution of nickel nitrate and citric acid. The catalysts were promoted with cerium and tungsten (1–5 wt %) by applying the co-impregnation method. The catalysts after calcination showed high nickel oxide dispersion. The presence of Ce and W oxide species with small crystallite size was inferred from XRD, TEM, and XPS studies. Temperature-programmed reduction studies indicated an increase in reducibility of catalysts with an increase in Ce content. An opposite direction was observed for W-promoted catalysts. Quasi in situ XPS studies evidenced partial reduction of tungsten oxide species and formation of metallic tungsten during activation in hydrogen. Chemisorption and temperature-programmed hydrogen desorption studies indicated that an active surface area of catalysts was increased with an increase in Ce content and decreased with an increase in W content. In turn, electron microscopy studies pointed out an increase in nickel dispersion induced both by the introduction of cerium and tungsten. An increase in CO2 conversion in the methanation reaction at low temperatures with an increase in Ce content was evidenced. This effect was correlated with increase in the active surface area of catalysts. More detailed analysis of the methanation reaction based on TOF changes pointed out that an increase in activity was additionally influenced by the presence of dispersed cerium oxide species, which enhance activation and hydrogenation of CO2 molecules. In turn, a slight increase in CO2 conversion in the presence of W promoter, despite the decrease in active surface area of catalysts, was observed. Such an effect was ascribed to the participation of tungsten oxide species in the transformation of carbon dioxide with hydrogen to methane through the successive oxidation–reduction surface reaction. Quasi in situ XPS studies evidenced changes in the oxidation state of tungsten under methanation reaction conditions. In contrast to Ce promoter, the positive effect was observed in the presence of small amounts of tungsten, at around 2 wt % W in the catalysts containing 30–40 wt % Ni.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal12010013/s1, Figure S1: X-ray diffraction patterns of catalysts after calcination (a–c). Figure S2: X-ray diffraction patterns of catalysts after reduction (a–c). Figure S3: CO2 conversion (a,c,e) and selectivity to CH4 (b,d,f) of cerium and tungsten-promoted, alumina-supported nickel catalysts in CO2 methanation reaction. Figure S4a: Survey XPS scans of Ni40-Al2O3 catalyst after calcination (-calc.), activation in hydrogen (-H2), and CO2 methanation reaction (-H2/CO2). Figure S4b: Survey XPS scans of Ni40-Ce5-Al2O3 catalyst after calcination (-calc.), activation in hydrogen (-H2), and CO2 methanation reaction (-H2/CO2). Figure S4c: Survey XPS scans of Ni40-W5-Al2O3 catalyst after calcination (-calc.), activation in hydrogen (-H2), and CO2 methanation reaction (-H2/CO2). Table S1: The analysis of XPS peaks from the XPS studies of catalysts after calcination (-calc.), activation in hydrogen (-H2), and CO2 methanation reaction (-H2/CO2).
Author Contributions
Conceptualization, methodology, investigation, writing: W.G.; investigation: W.Z., M.G., M.R., G.S. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the National Centre for Research and Development (NCBR) under ERA-NET Bioenergy project “Development of an Innovative Concept for Carbon Dioxide Utilization as Side Stream of Integrated Bio-refinery Concepts” (ICOCAD), contract no. BIOENERGY/ICOCAD/04/2016.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The research was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Innovative Economy Operational Programme (project no. POIG.02.01.00-06-024/09 Centre for Functional Nanomaterials).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Special Report on Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 30 November 2021).
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Jalama, K. Carbon dioxide hydrogenation over nickel-, ruthenium-, and copper-based catalysts: Review of kinetics and mechanism. Catal. Rev. 2017, 59, 95–164. [Google Scholar] [CrossRef]
- Sabatier, P.; Senderens, J.-B. Hydrogénation directe des oxydes du carbone eu présence de divers métaux divisés. Compt. Rend. Acad. Sci. 1902, 134, 689–691. [Google Scholar]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent advances in CO2 capture and utilization. ChemSusChem 2008, 11, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of promoted nickel catalysts supported on mesoporous nanocrystalline gamma alumina for carbon dioxide methanation reaction. J. Ind. Eng. Chem. 2014, 20, 4176–4182. [Google Scholar] [CrossRef]
- Ahmad, W.; Younis, M.N.; Shawabkeh, R.; Ahmed, S. Synthesis of lanthanide series (La, Ce, Pr, Eu & Gd) promoted Ni/γ-Al2O3 catalysts for methanation of CO2 at low temperature under atmospheric pressure. Catal. Commun. 2017, 100, 121–126. [Google Scholar] [CrossRef]
- Ding, M.; Tu, J.; Zhang, Q.; Wang, M.; Tsubaki, N.; Wang, T.; Ma, L. Enhancement of methanation of bio-syngas over CeO2-modified Ni/Al2O3 catalysts. Biomass Bioenergy 2016, 85, 12–17. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 addition on Ni/Al2O3 catalysts for methanation of carbon dioxide with hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- Nie, W.; Zou, X.; Shang, X.; Wang, X.; Ding, W.; Lu, X. CeO2-assisted Ni nanocatalysts supported on mesoporous γ-Al2O3 for the production of synthetic natural gas. Fuel 2017, 202, 135–143. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Słowik, G.; Greluk, M. CO2 methanation in the presence of Ce-promoted alumina supported nickel catalysts: H2S deactivation studies. Top. Cat. 2019, 62, 524–534. [Google Scholar] [CrossRef] [Green Version]
- Borowiecki, T.; Ryczkowski, J. Promoters of the catalysts for methane conversion into synthesis gases. In Focus on Catalysis Research; Bevy, L.P., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2006; pp. 101–146. ISBN 1-59454-810-2. [Google Scholar]
- Borowiecki, T.; Gołębiowski, A. Influence of molybdenum and tungsten additives on the properties of nickel steam reforming catalyst. Cat. Lett. 1994, 25, 309–313. [Google Scholar] [CrossRef]
- Panczyk, M.; Giecko, G.; Gac, W.; Pasieczna, S.; Stasińska, B.; Borowiecki, T. Nickel-promoted catalysts in the reforming of n-butane with CO2 or H2O. Adsorp. Sci. Technol. 2001, 19, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Vroulias, D.; Gkoulemani, N.; Papadopoulou, C.; Matralis, H. W–modified Ni/Al2O3 catalysts for the dry reforming of methane: Effect of W loading. Catal. Today 2020, 355, 704–715. [Google Scholar] [CrossRef]
- Kelley, R.D.; Madey, T.E.; Yates, J.T., Jr. Activity of tungsten as a methanation catalyst. J. Catal. 1997, 50, 301–305. [Google Scholar] [CrossRef]
- Zhang, R.; Schwarz, A.A.; Datye, A.; Baltrus, J.P. The effect of second-phase oxides on the catalytic properties of dispersed metals: Cobalt supported on 12% WO3/Al2O3. J. Catal. 1992, 135, 200–222. [Google Scholar] [CrossRef]
- Ai, H.; Liu, Q.; Yang, H. W-doped ordered mesoporous Ni/Al2O3 catalyst for methanation of carbon monoxide. Int. J. Hydrog. Ener. 2019, 44, 23975–23982. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of support composition on the performance of nickel catalysts in CO2 methanation reaction. Catal. Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Pennemann, H.; Neuberg, S.; Zapf, R.; Kolb, G. Direct conversion of carbon dioxide to methane over ceria- and alumina-supported nickel catalysts for biogas valorization. ChemPlusChem 2021, 86, 889–903. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Słowik, G.; Kuśmierz, M.; Dzwigaj, S. The state of BEA zeolite supported nickel catalysts in CO2 methanation reaction. Appl. Surf. Sci. 2021, 564, 150421. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2014, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.K.; Garcia-Garcia, F.J.; Häussermann, U. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Capel Sanchez, M.C.; Tyulieva, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Ostromecki, M.M.; Burcham, L.J.; Wachs, I.E. The influence of metal oxide additives on the molecular structures of surface tungsten oxide species on alumina. II. In situ conditions. J. Mol. Catal. A 1998, 132, 59–71. [Google Scholar] [CrossRef]
- Quintana-Melgoza, J.M.; Cruz-Reyes, J.; Avalos-Borja, M. Synthesis and characterization of NiWO4 crystals. Mater. Lett. 2001, 47, 314–318. [Google Scholar] [CrossRef]
- Bentaleb, F.; Che, M.; Dubreuil, A.C.; Thomazeau, C.; Marceau, E. Influence of organic additives on the properties of impregnation solutions and on nickel oxide particle size for Ni/Al2O3catalysts. Catal. Today 2014, 235, 250–255. [Google Scholar] [CrossRef]
- Shanmugam, V.; Neuberg, S.; Zapf, R.; Pennemann, H.; Kolb, G. Effect of support and chelating ligand on the synthesis of Ni catalysts with high activity and stability for CO2 methanation. Catalysts 2020, 10, 493. [Google Scholar] [CrossRef]
- Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; de los Reyes, J.A.; Vázquez-Zavala, A.; Vrinat, M.; Geantet, C. Influence of the solution pH in impregnation with citric acid and activity of Ni/W/Al2O3 catalysts. J. Mol. Catal. A 2015, 404–405, 36–46. [Google Scholar] [CrossRef]
- Jos van Dillen, A.; Terörde, R.J.A.M.; Lensveld, D.J.; Geus, J.W.; de Jong, K.P. Synthesis of supported catalysts by impregnation and drying using aqueous chelated metal complexes. J. Catal. 2003, 216, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Vargas, P.E.; Salinas-Gutiérrez, J.M.; Meléndez-Zaragoza, M.J.; Pantoja-Espinoza, M.J.; López-Ortiz, A.; Collins-Martinez, V. Reduction and oxidation kinetics of NiWO4 as an oxygen carrier for hydrogen storage by a chemical looping process. RSC Adv. 2021, 11, 29453–29465. [Google Scholar] [CrossRef]
- Jang, W.L.; Lu, Y.M.; Hwang, W.S.; Hsiung, T.L.; Wang, H.P. Point defects in sputtered NiO films. Appl. Phys. Lett. 2009, 94, 062103. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Thurber, A.; Reddy, K.M.; Shutthanandan, V.; Engelhard, M.H.; Wang, C.; Hays, J.; Punnoose, A. Ferromagnetism in chemically synthesized CeO2 nanoparticles by Ni doping. Phys. Rev. B 2007, 76, 165206. [Google Scholar] [CrossRef]
- Barrio, L.; Kubacka, A.; Zhou, G.; Estrella, M.; MartínezArias, A.; Hanson, J.C.; Fernández-Garcíıa, M.; Rodriguez, J.A. Unusual physical and chemical properties of Ni in Ce1-xNixO2-y oxides: Structural characterization and catalytic activity for the water gas shift reaction. J. Phys. Chem. C 2010, 114, 12689–12697. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Acuna, L.M.; Albornoz, C.A.; Leyva, A.G.; Sousa, N.; Figueiredo, F.M. Structural, physical and chemical properties of nanostructured nickel-substituted ceria oxides under reducing and oxidizing conditions. RSC Adv. 2016, 6, 64861–64870. [Google Scholar] [CrossRef] [Green Version]
- Kaufherr, N.; Mendelovici, L.; Steinberg, M. The preparation of cerium(III) aluminate at lower temperatures: IR, X-ray and electron spin resonance study. J. Less Common Met. 1958, 107, 281–289. [Google Scholar] [CrossRef]
- Venâncio, S.A.; de Miranda, P.V. Synthesis of CeAlO3/CeO2-Al2O3 for use as a solid oxide fuel cell functional anode material. Ceram. Int. 2011, 37, 3139–3152. [Google Scholar] [CrossRef]
- Hurst, N.W.; Gentry, S.J.; Jones, A.; McNicol, B.D. Temperature programmed reduction. Catal. Rev. Sci. Eng. 1982, 24, 233–309. [Google Scholar] [CrossRef]
- Zieliński, J. Morphology of nickel/alumina catalysts. J. Catal. 1982, 76, 157–163. [Google Scholar] [CrossRef]
- Strunk, J.; Vining, W.C.; Bell, A.T. Synthesis of different CeO2 structures on mesoporous silica and characterization of their reduction properties. J. Phys. Chem. C 2011, 115, 4114–4126. [Google Scholar] [CrossRef]
- Borowiecki, T.; Gac, W.; Denis, A. Effects of small MoO3 additions on the properties of nickel catalysts for the steam reforming of hydrocarbons: III. Reduction of Ni-Mo/Al2O3 catalysts. Appl. Catal. A 2004, 270, 27–36. [Google Scholar] [CrossRef]
- Sridhar, S.; Sichen, D.; Seetharaman, S. Investigation of the kinetics of reduction of nickel tungstate by hydrogen. Metal. Mater. Trans. B 1994, 25, 391–396. [Google Scholar] [CrossRef]
- Southmayd, D.W.; Contescut, C.; Schwarz, J.A. Temperature-programmed reduction and oxidation of nickel supported on WO3-Al2O3 Composite Oxides. J. Chem. Soc. Faraday Trans. 1993, 89, 2075–2083. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Hydrogen adsorption on supported cobalt, iron, and nickel. Catal. Lett. 1990, 7, 27–52. [Google Scholar] [CrossRef]
- Ewald, S.; Standl, S.; Hinrichsen, O. Characterization of nickel catalysts with transient methods. Appl. Catal. A 2018, 549, 93–101. [Google Scholar] [CrossRef]
- Znak, L.; Zieliński, J. Interaction of hydrogen with unsupported and supported nickel. Langmuir 2006, 22, 8758–8763. [Google Scholar] [CrossRef]
- Somorjai, G.A. The surface science of heterogeneous catalysis. Surf. Sci. 1994, 299–300, 849–866. [Google Scholar] [CrossRef] [Green Version]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.; Yang, J.; Qin, Z.; Lin, J.; Li, Z. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.P.; Abild-Pedersen, F.; Remediakis, I.N.; Bligaard, T.; Jones, G.; Engbæk, J.; Lytken, O.; Horch, S.; Nielsen, J.H.; Sehested, J.; et al. Structure sensitivity of the methanation reaction: H2-induced CO dissociation on nickel surfaces. J. Catal. 2008, 255, 6–19. [Google Scholar] [CrossRef]
- Shetty, S.; Jansen, A.P.J.; van Santen, R.A. Direct versus hydrogen-assisted CO dissociation. J. Am. Chem. Soc. 2009, 131, 12874–12875. [Google Scholar] [CrossRef] [PubMed]
- Wind, T.L.; Falsig, H.; Sehested, J.; Moses, P.G.; Nguyen, T.T.M. Comparison of mechanistic understanding and experiments for CO methanation over nickel. J. Catal. 2016, 342, 105–116. [Google Scholar] [CrossRef]
- Zhou, M.; Le, T.N.-M.; Huynh, L.K.; Liu, B. Effects of structure and size of Ni nanocatalysts on hydrogen selectivity via water-gas-shift reaction—A first-principles based kinetic study. Catal. Today 2017, 280, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Zhen, W.; Gao, F.; Tian, B.; Ding, P.; Deng, Y.; Li, Z.; Gao, H.; Lu, G. Enhancing activity for carbon dioxide methanation by encapsulating (111) facet Ni particle in metalorganic frameworks at low temperature. J. Catal. 2017, 348, 200–211. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Wambach, J.; Lling, I.; Freund, H.-J. CO2 activation and reaction with hydrogen on Ni (110): Formate formation. Chem. Phys. Lett. 1991, 184, 239–244. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Liu, Z.; Duchoň, T.; Wang, H.; Grinter, D.C.; Waluyo, I.; Zhou, J.; Liu, Q.; Jeong, B.; Crumlin, E.J.; Matolín, V.; et al. Ambient pressure XPS and IRRAS investigation of ethanol steam reforming on Ni-CeO2(111) catalysts: An in situ study of C–C and O–H bond scission. Phys. Chem. Chem. Phys. 2016, 18, 16621–16628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Arranz, A.; Palacio, C. Interaction of Ni/Al interfaces with oxygen. Langmuir 2002, 18, 1695–1701. [Google Scholar] [CrossRef]
- Solsona, B.; López Nieto, J.M.; Concepción, P.; Dejoz, A.; Ivars, F.; Vázquez, M.I. Oxidative dehydrogenation of ethane over Ni–W–O mixed metal oxide catalysts. J. Catal. 2011, 280, 28–39. [Google Scholar] [CrossRef]
- Reinhoudt, H.R.; Crezee, E.; van Langeveld, A.D.; Kooyman, P.J.; van Veen, J.A.R.; Moulijn, J.A. Characterization of the active phase in NiW/γ-Al2O3 catalysts in various stages of sulfidation with FTIR(NO) and XPS. J. Cat. 2000, 196, 315–329. [Google Scholar] [CrossRef]
- Salvatl, L.; Makovsky, L.E.; Stencel, J.M.; Brown, F.R.; Hercules, D.M. Surface Spectroscopic Study of Tungsten-Alumina Catalysts Using X-ray Photoelectron, Ion Scattering, and Raman Spectroscopies. J. Phys. Chem. 1981, 85, 3700–3707. [Google Scholar] [CrossRef]
- Xie, F.Y.; Gong, L.; Liu, X.; Tao, F.Y.; Zhang, W.H.; Chen, S.H.; Meng, H.; Chen, J. XPS studies on surface reduction of tungsten oxide nanowire film by Ar+ bombardment. J. Electron Spectros. Relat. Phenom. 2012, 185, 112–118. [Google Scholar] [CrossRef]
- Hossain, E.; Rothgeb, D.W.; Jarrold, C.C. CO2 reduction by group 6 transition metal suboxide cluster anions. J. Chem. Phys. 2010, 133, 024305. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the Versatility of Tungsten Oxide Coatings. Phys. Status Solidi A 2019, 216, 1900047. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).