Green Synthesis of Ag Nanoparticles for Plasmon-Assisted Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Results and Discussion
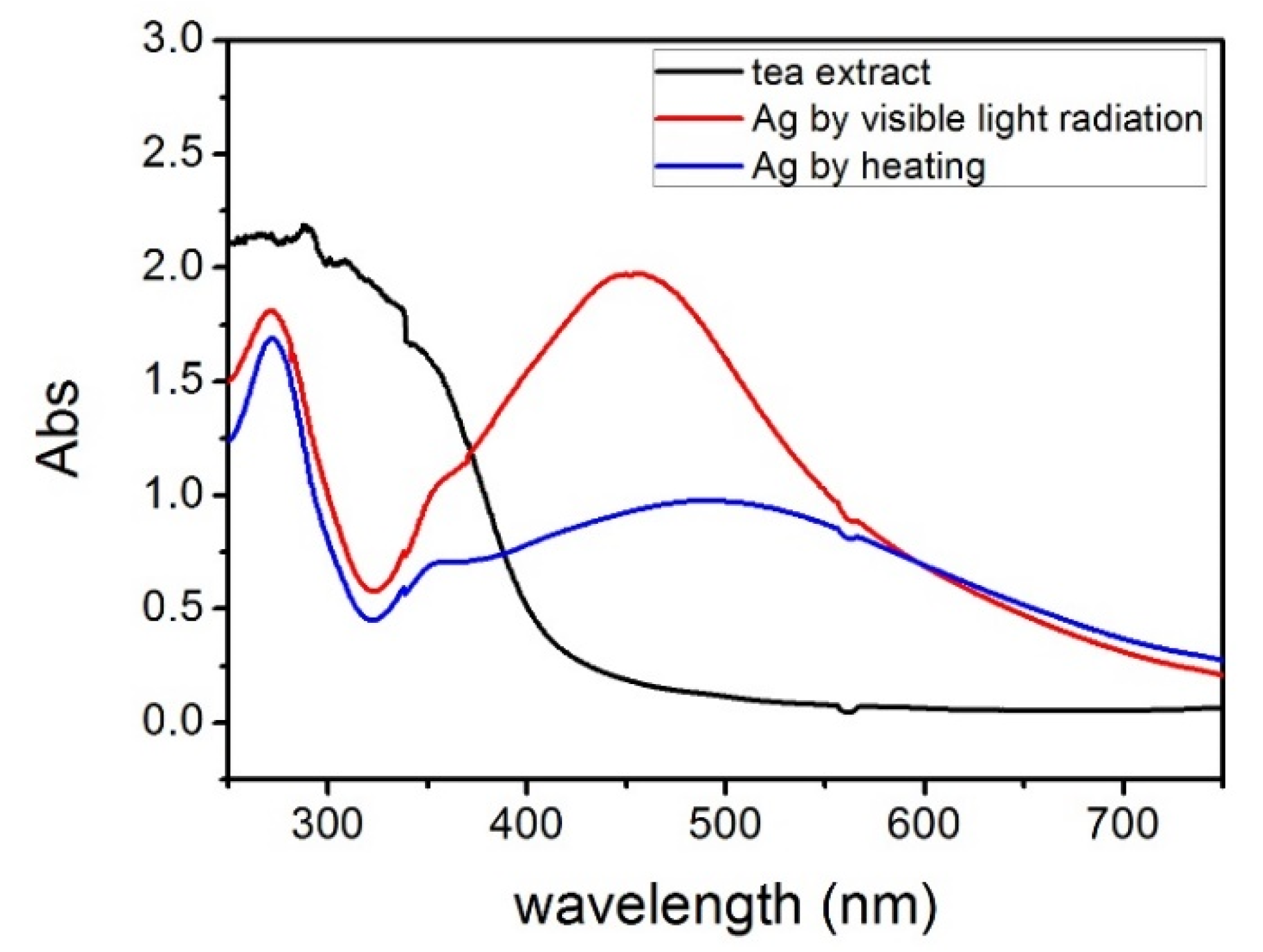
2.1. UV-Vis Spectroscopy and X-ray Diffraction
2.2. SEM Studies
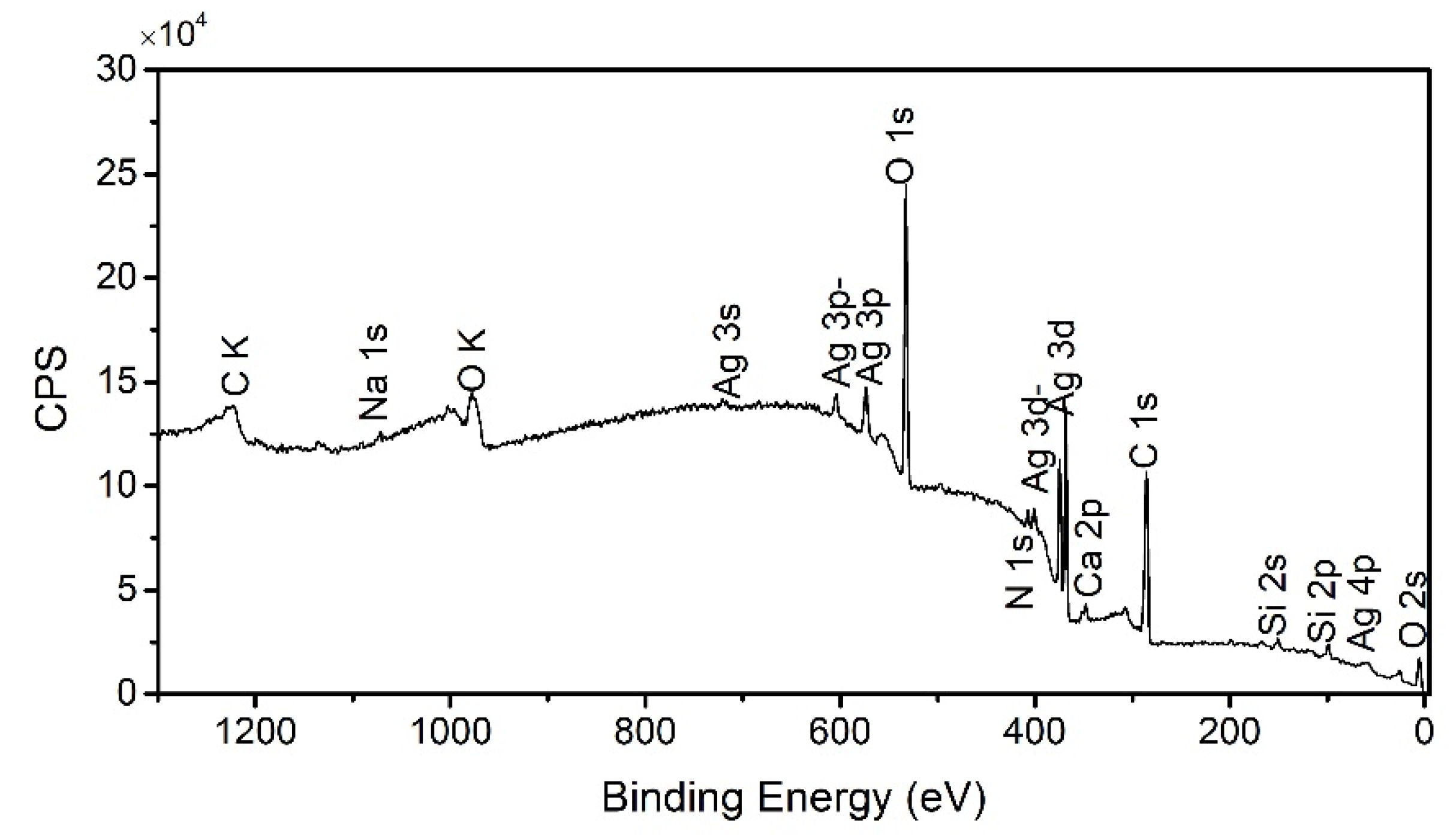
2.3. IR Spectra and XPS Analysis of Organic Shells
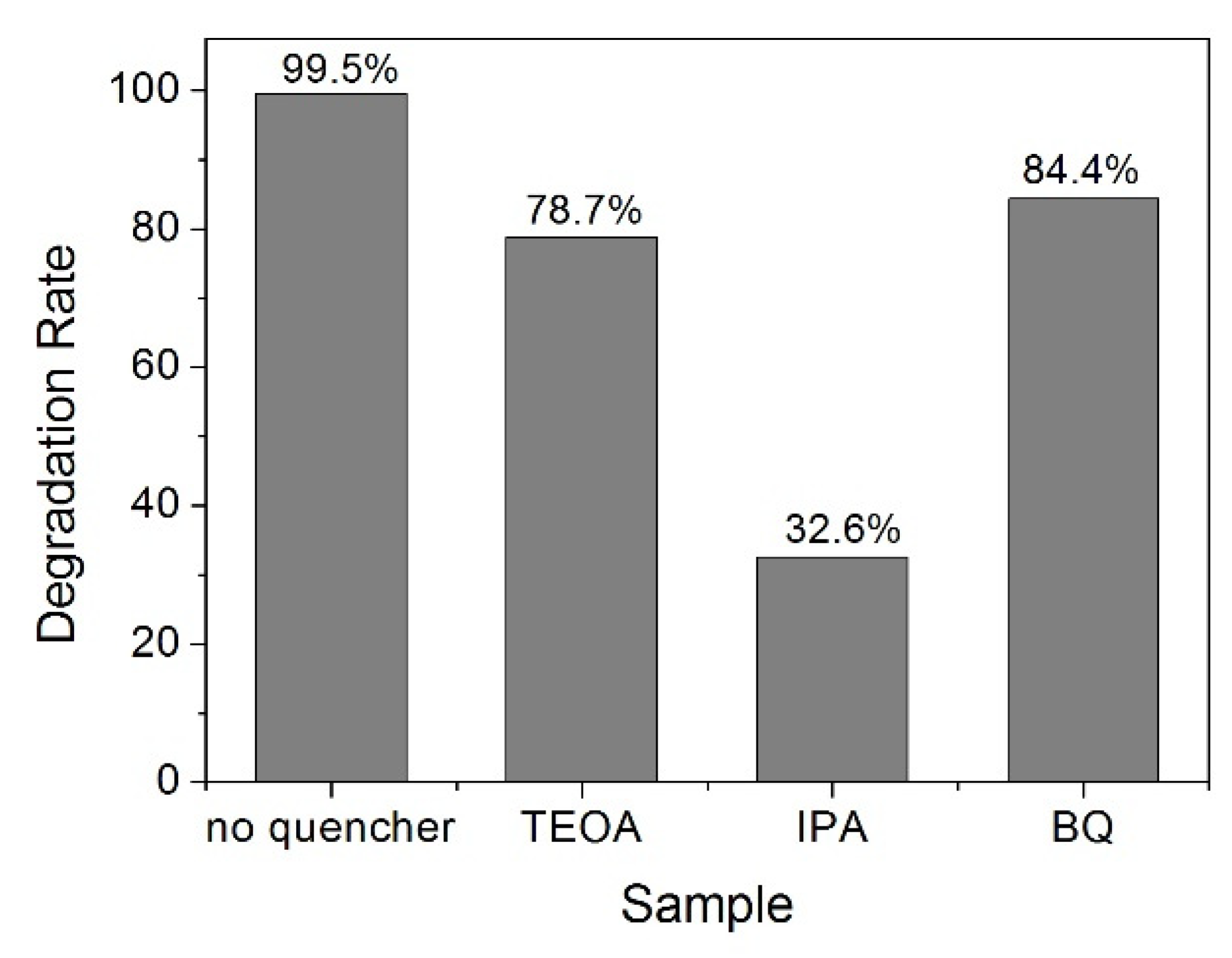
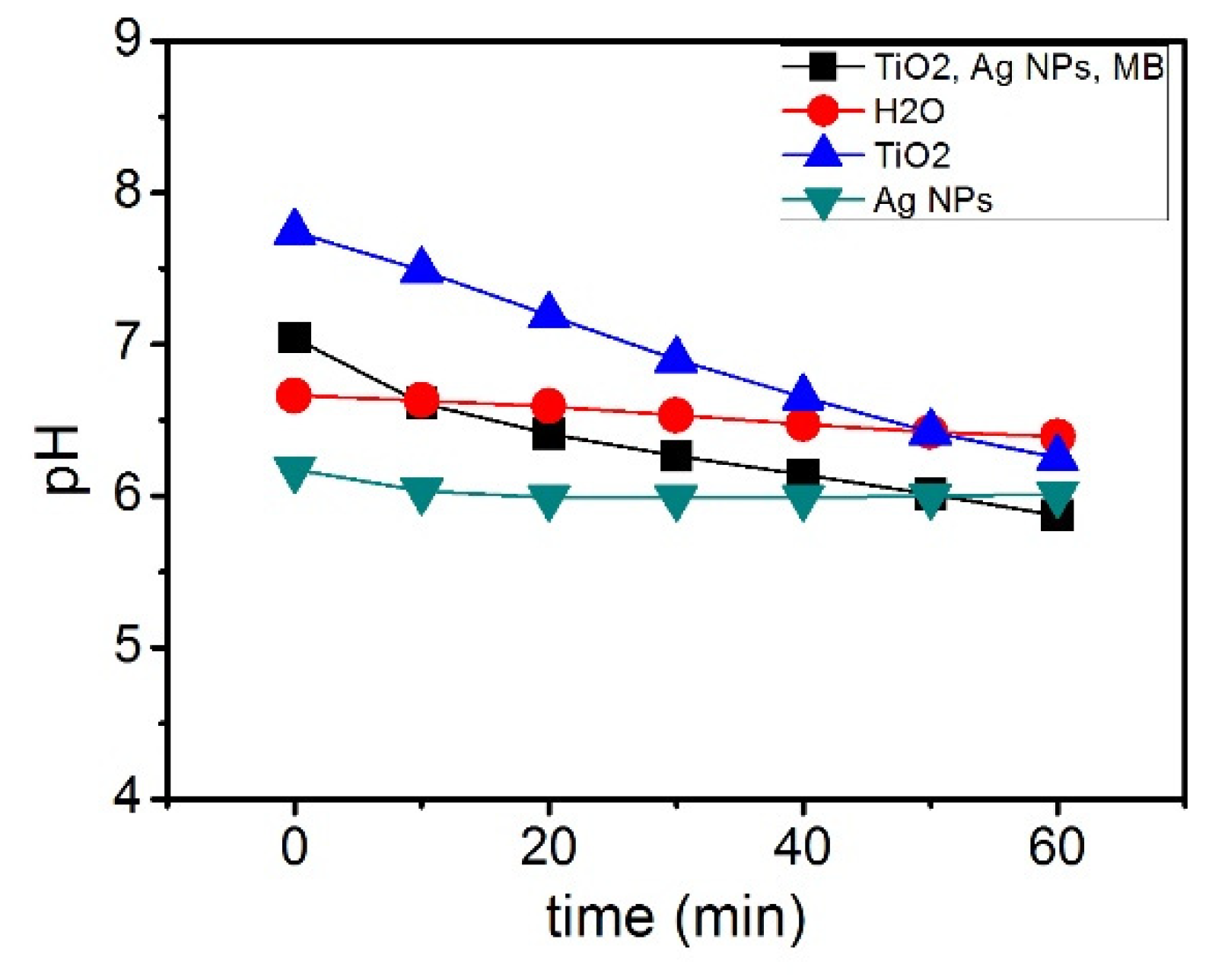
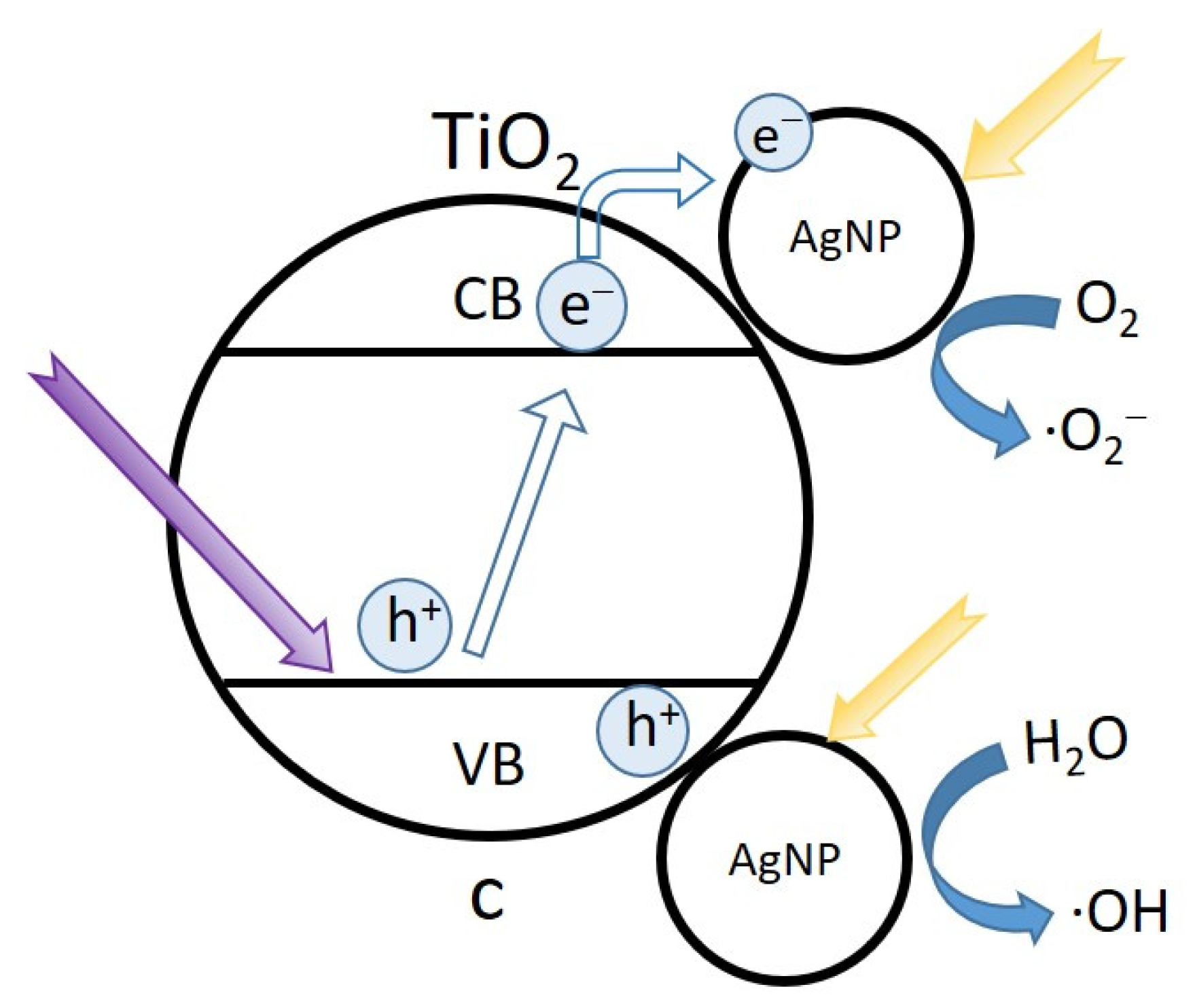
2.4. Photocatalytic Degradation of MB
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ag NPs
3.3. Characterization
3.4. Photocatalytic Degradation of Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, L.L.; Wei, M.; Shang, L.; Yang, Y.W. Cucurbiturils-mediated noble metal nanoparticles for applications in sensing, sers, theranostics, and catalysis. Adv. Funct. Mater. 2021, 31, 2007277. [Google Scholar] [CrossRef]
- Hu, A.; Li, R.; Bai, S.; Yu, Y.; Zhou, W.; Bridges, D.; Zhang, L. Introduction to Laser Micro-to-Nano Manufacturing. In Laser Micro-Nano-Manufacturing and 3D Microprinting; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–74. [Google Scholar]
- Siegel, J. Progressive approach for metal nanoparticle synthesis. Mater. Lett. 2012, 89, 47–50. [Google Scholar] [CrossRef]
- Hu, A.; Guo, J.Y.; Alarifi, H. Low temperature sintering of Ag nanoparticles for flexible electronics packaging. Appl. Phys. Lett. 2010, 97, 153117. [Google Scholar] [CrossRef]
- Yan, J.F.; Zou, G.S.; Hu, A. Preparation of PVP coated Cu NPs and the application for low-temperature bonding. J. Mater. Chem. 2011, 21, 15981–15986. [Google Scholar]
- Klaus, T.; Joerger, R.; Olsson, E. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, A. Diversity of bacterial synthesis of silver nanoparticles. BioNanoScience 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Khan, A.U. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Echavarría, J. Synthesis of silver nanoparticles using white-rot fungus Anamorphous Bjerkandera sp. R1: Influence of silver nitrate concentration and fungus growth time. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Du, L.; Xu, Q.; Huang, M. Synthesis of small silver nanoparticles under light radiation by fungus Penicillium oxalicum and its application for the catalytic reduction of methylene blue. Mater. Chem. Phys. 2015, 160, 40–47. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Mochochoko, T.; Leo, A.J.; Mohan, S.; Jumbam, D.N.; Songca, S.P. Microwave irradiation synthesis of silver nanoparticles using cellulose from Eichhornia crassipes plant shoot. Mater. Lett. 2016, 185, 576–579. [Google Scholar] [CrossRef]
- Jayaprakash, N. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 169, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato (Solanum tuberosum) infusion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 146, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, M.; Rajmohan, R. Green synthesis of silver nanoparticles using Ixora coccinea leaves extract. Mater. Lett. 2013, 97, 141–143. [Google Scholar] [CrossRef]
- Mo, Y.-Y. Green synthesis of silver nanoparticles using eucalyptus leaf extract. Mater. Lett. 2015, 144, 165–167. [Google Scholar] [CrossRef]
- Ahmadi, S. Anti-bacterial/fungal and anti-cancer performance of green synthesized Ag nanoparticles using summer savory extract. J. Exp. Nanosci. 2020, 15, 363–380. [Google Scholar] [CrossRef]
- Hu, A.; Liang, R.; Zhang, X. Enhanced photocatalytic degradation of dyes by TiO2 nanobelts with hierarchical structures. J. Photochem. Photobiol. A Chem. 2013, 256, 7–15. [Google Scholar] [CrossRef]
- Liang, R.; Hu, A.; Hatat-Fraile, M. Development of TiO2 Nanowires for Membrane Filtration Applications. In Nanotechnology for Water Treatment and Purification; Hu, A., Allen, A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 47–77. [Google Scholar]
- Asahi, R. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Jafari, S.; Zhao, F.; Zhao, D. A comparative study for the removal of methylene blue dye by N and S modified TiO2 adsorbents. J. Mol. Liq. 2015, 207, 90–98. [Google Scholar] [CrossRef]
- Yu, S. Carbon-doped TiO2 nanoparticles wrapped with nanographene as a high performance photocatalyst for phenol degradation under visible light irradiation. Appl. Catal. B Environ. 2014, 144, 893–899. [Google Scholar] [CrossRef]
- Bayan, E.M. Fluorine-doped titanium dioxide: Synthesis, structure, morphology, size and photocatalytic activity. In Advanced Materials; Springer International Publishing: Cham, Switzerland, 2017; pp. 17–24. [Google Scholar]
- Kondo, K. Development of highly efficient sulfur-doped TiO2 photocatalysts hybridized with graphitic carbon nitride. Appl. Catal. B Environ. 2013, 142, 362–367. [Google Scholar] [CrossRef]
- Li, W.; Liang, R.; Hu, A. Generation of oxygen vacancies in visible light activated one-dimensional iodine TiO2 photocatalysts. RSC Adv. 2014, 4, 36959–36966. [Google Scholar] [CrossRef]
- Jiang, B. A facile and green synthesis route towards two-dimensional TiO2@Ag heterojunction structure with enhanced visible light photocatalytic activity. Crystengcomm 2013, 15, 648–657. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Sohrabnezhad, S.; Seifi, A. The green synthesis of Ag/ZnO in montmorillonite with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 386, 33–40. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Hu, A.; Peng, P.; Alarifi, H. Femtosecond laser welded nanostructures and plasmonic devices. J. Laser Appl. 2012, 24, 042001. [Google Scholar] [CrossRef] [Green Version]
- Shi, X. Plasmon-enhanced photocurrent generation and water oxidation with a gold nanoisland-loaded titanium dioxide photoelectrode. J. Phys. Chem. C 2013, 117, 2494–2499. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 with metal cocatalysts exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation under irradiation of visible light. ACS Catal. 2013, 3, 79–85. [Google Scholar] [CrossRef]
- Nanaji, K.; Janardhana, R.K.S.K.; Rao, T.N.; Anandan, S. Energy level matching for efficient charge transfer in Ag doped-Ag modified TiO2 for enhanced visible light photocatalytic activity. J. Alloy. Compd. 2019, 794, 662–671. [Google Scholar] [CrossRef]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. Seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Bai, S.; Zhou, W.; Lin, Y. Ultraviolet pulsed laser interference lithography and application of periodic structured Ag-nanoparticle films for surface-enhanced Raman spectroscopy. J. Nanoparticle Res. 2014, 16, 1–14. [Google Scholar] [CrossRef]
- Mie, G. Contribution to the optical properties of turbid media, in particular of colloidal suspensions of metals. Ann. Phys. 1908, 25, 377–452. [Google Scholar] [CrossRef]
- Shankar, S.; Rai, A.; Ahmad, A. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Vanaja, M.; Paulkumar, K.; Baburaja, M. Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 742356–742364. [Google Scholar] [CrossRef] [PubMed]
- Horžić, D.; Komes, D.; Belščak, A. The composition of polyphenols and methylxanthines in teas and herbal infusions. Food Chem. 2009, 115, 441–448. [Google Scholar] [CrossRef]
- Konieczynski, P.; Wesolowski, M. Phytate, inorganic and total phosphorus and their relations to selected trace and major elements in herbal teas. Acta Pol. Pharm. 2014, 71, 85–93. [Google Scholar] [PubMed]
- Aoshima, H.; Hirata, S.; Ayabe, S. Antioxidative and anti-hydrogen peroxide activities of various herbal teas. Food Chem. 2007, 103, 617–622. [Google Scholar] [CrossRef]
- Romand, M.; Roubin, M.; Deloume, J.P. ESCA studies of some copper and silver selenides. J. Electron Spectrosc. Relat. Phenom. 1978, 13, 229–242. [Google Scholar] [CrossRef]
- Lee, S.H.; Jo, J.S.; Park, J.H.; Lee, S.W.; Jang, J.W. A hot-electron-triggered catalytic oxidation reaction of plasmonic silver nanoparticles evidenced by surface potential mapping. J. Mater. Chem. A 2018, 6, 20939–20946. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg 2+ biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Hu, A.; Li, W. Enhanced degradation of persistent pharmaceuticals found in wastewater treatment effluents using TiO2 nanobelt photocatalysts. J. Nanoparticle Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Hsiao, H.-M. Preparation of iodine doped titanium dioxide to photodegrade aqueous bisphenol A under visible light. Process. Saf. Environ. Prot. 2015, 95, 265–270. [Google Scholar] [CrossRef]
- Madhavi, V.; Kondaiah, P. Influence of silver nanoparticles on titanium oxide and nitrogen doped titanium oxide thin films for sun light photocatalysis. Appl. Surf. Sci. 2018, 436, 708–719. [Google Scholar]
- Salomatina, E.V. Preparation and photocatalytic properties of titanium dioxide modified with gold or silver nanoparticles. J. Environ. Chem. Eng. 2021, 9.5, 106078. [Google Scholar] [CrossRef]
- Lee, J.E.; Bera, S.; Choi, Y.S.; Lee, W.I. Size-dependent plasmonic effects of M and M@ SiO2 (M= Au or Ag) deposited on TiO2 in photocatalytic oxidation reactions. Appl. Catal. B Environ. 2017, 214, 15–22. [Google Scholar] [CrossRef]
- Kowalska, E. Silver-modified titania with enhanced photocatalytic and antimicrobial properties under UV and visible light irradiation. Catal. Today 2015, 252, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Xi, J.; Li, M.-D.; Chan, H.T. The degradation mechanism of methyl orange under photo-catalysis of TiO2. Phys. Chem. Chem. Phys. 2012, 14, 3589–3595. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Poncheri, A.; Badr, Y. Photocatalytic degradation of methyl red dye. S. Afr. J. Sci. 2009, 105, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Zhang, X.; Oakes, K.D. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef]




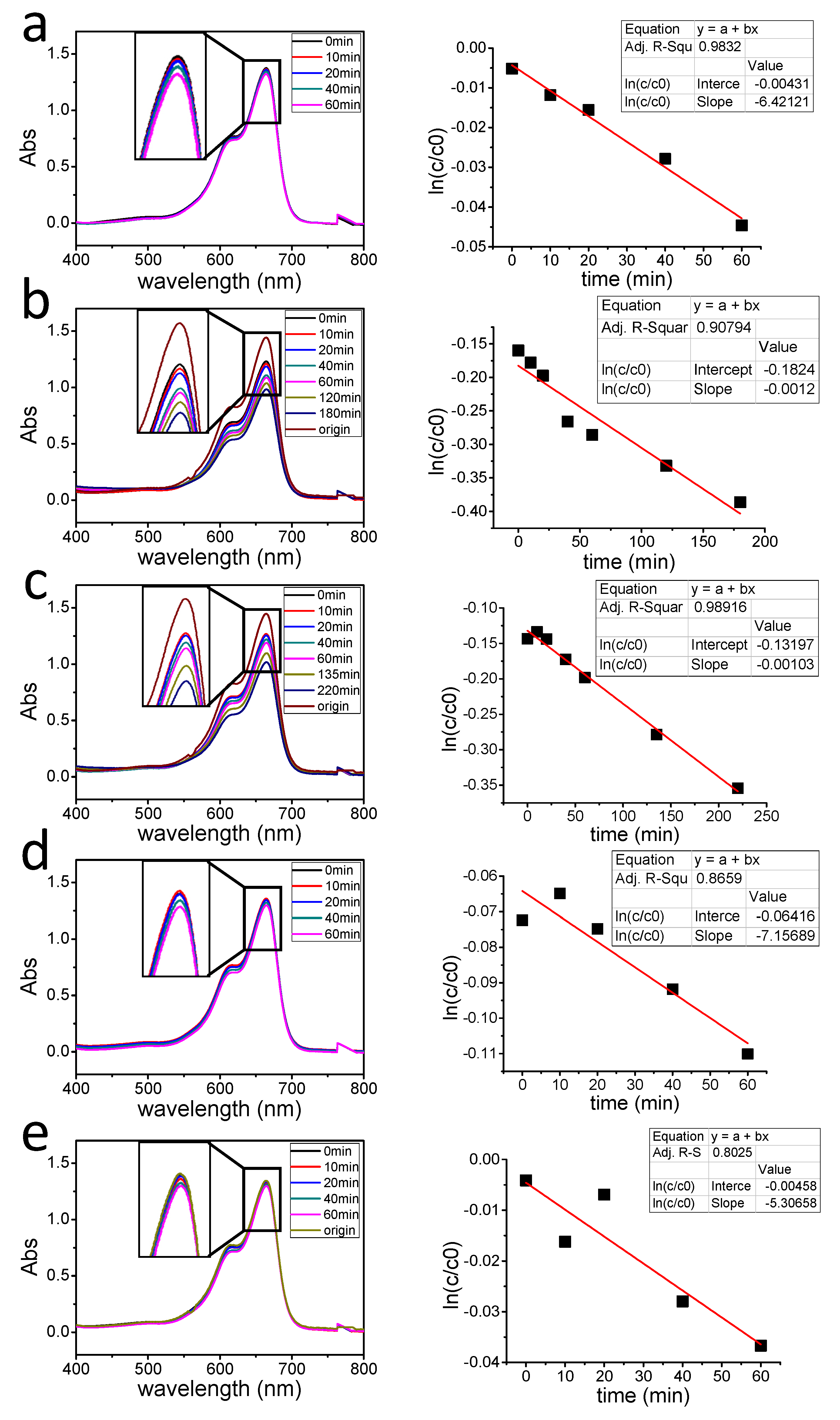
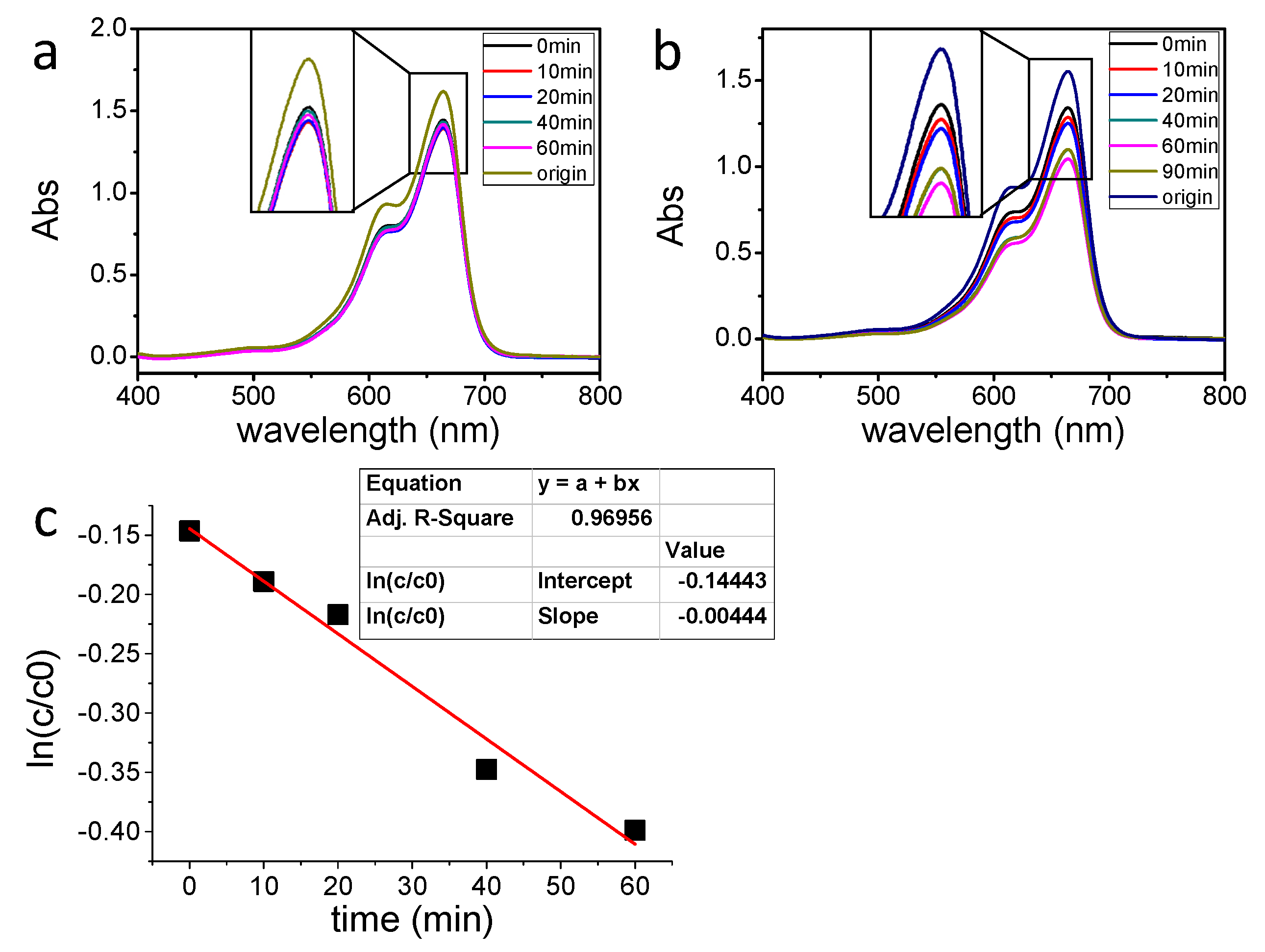
| Sample | Synthesis Method | Degradation Rate K (min−1) | Average Particle Size (nm) |
|---|---|---|---|
| 1 | By heating | 0.00064 | 71.43 |
| 2 | Irradiating for 0.5 h | 0.0012 | 25.34 |
| 3 | Irradiating for 1 h | 0.00103 | 32.88 |
| 4 | Irradiating for 1.5 h | 0.000715 | 33.24 |
| 5 | Irradiating for 2 h | 0.00053 | 40.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Tao, L.; Bai, S.; Hu, A. Green Synthesis of Ag Nanoparticles for Plasmon-Assisted Photocatalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1499. https://doi.org/10.3390/catal11121499
Ma Y, Tao L, Bai S, Hu A. Green Synthesis of Ag Nanoparticles for Plasmon-Assisted Photocatalytic Degradation of Methylene Blue. Catalysts. 2021; 11(12):1499. https://doi.org/10.3390/catal11121499
Chicago/Turabian StyleMa, Ying, Liyiming Tao, Shi Bai, and Anming Hu. 2021. "Green Synthesis of Ag Nanoparticles for Plasmon-Assisted Photocatalytic Degradation of Methylene Blue" Catalysts 11, no. 12: 1499. https://doi.org/10.3390/catal11121499





