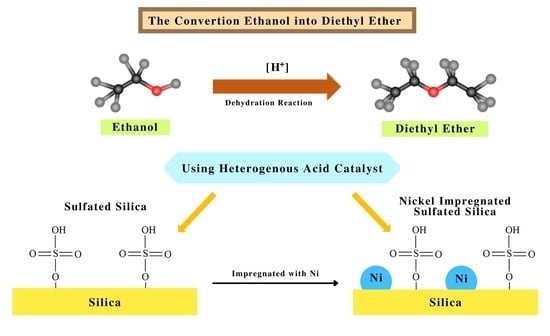
Synthesis, Characterizations and Catalysis of Sulfated Silica and Nickel Modified Silica Catalysts for Diethyl Ether (DEE) Production from Ethanol towards Renewable Energy Applications
Abstract
:1. Introduction
2. Results and Discussion
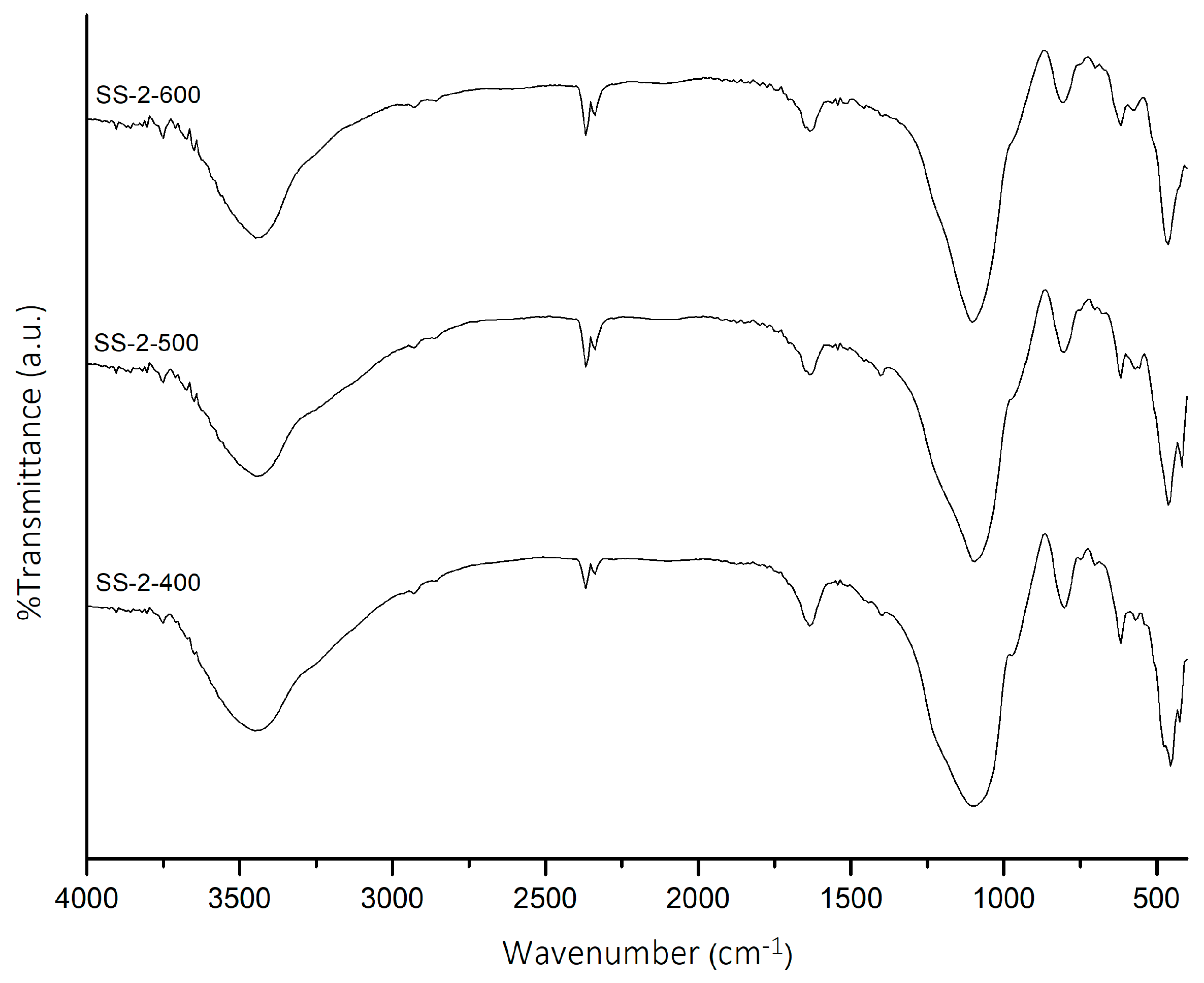
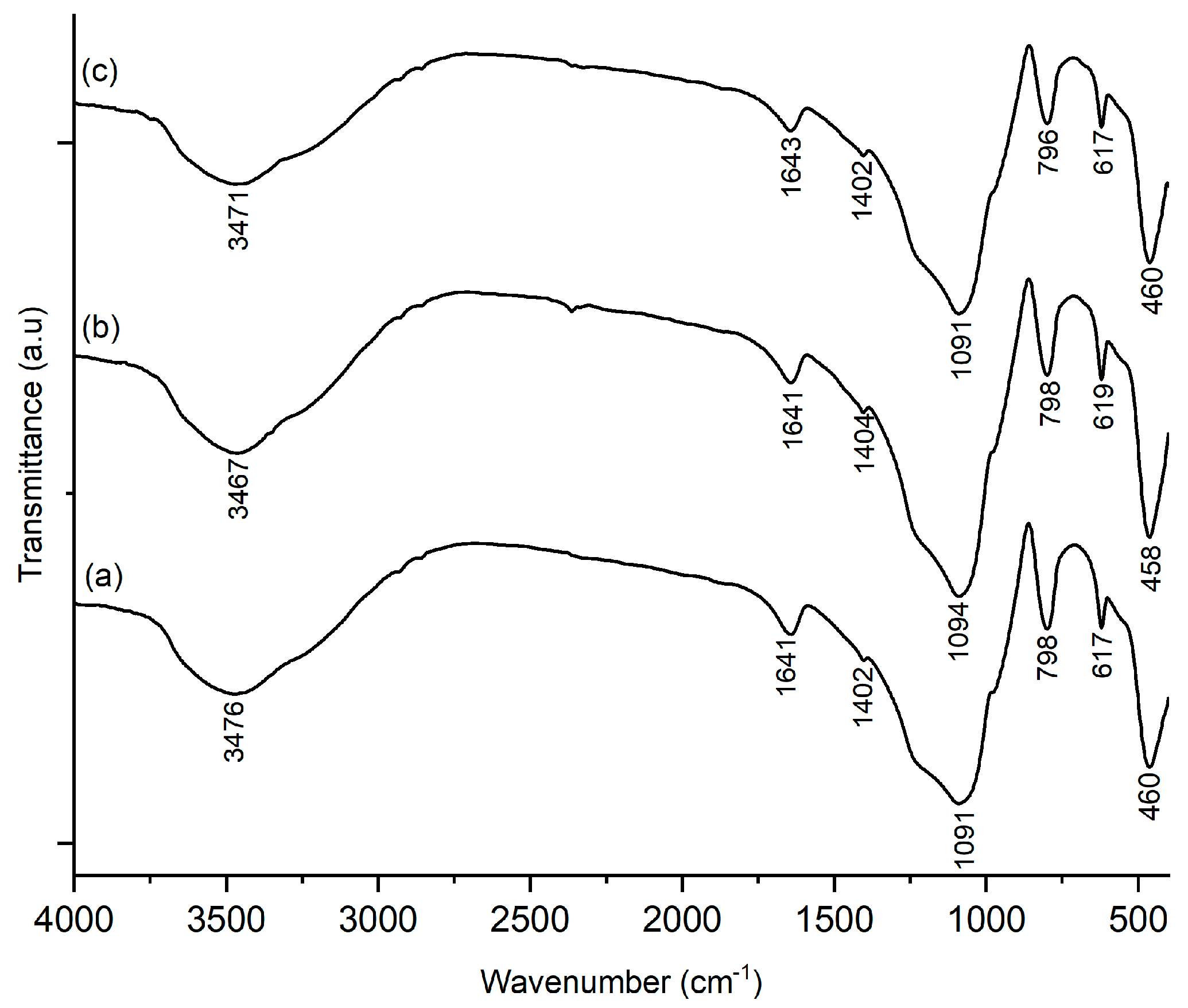
2.1. FTIR Analysis
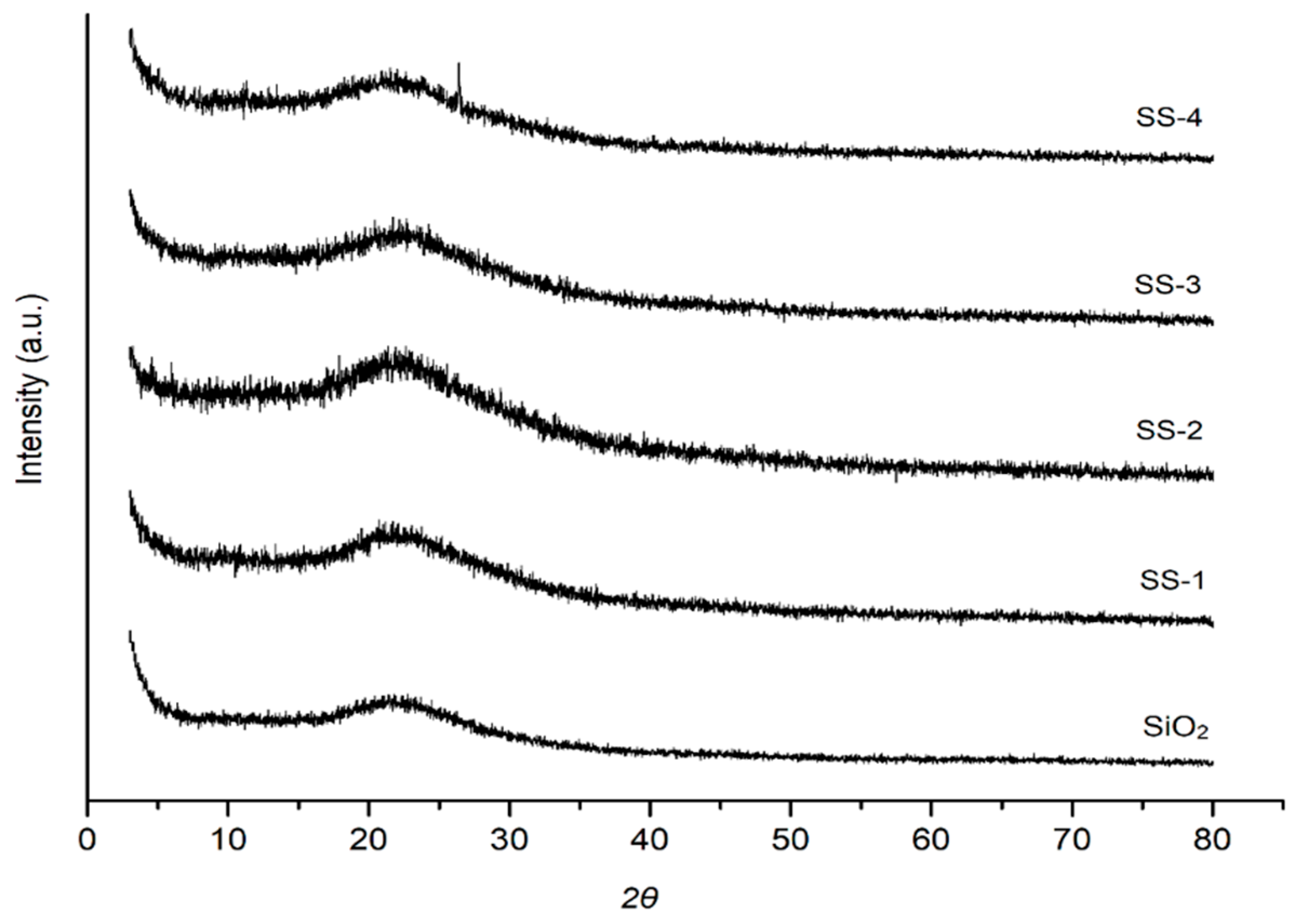
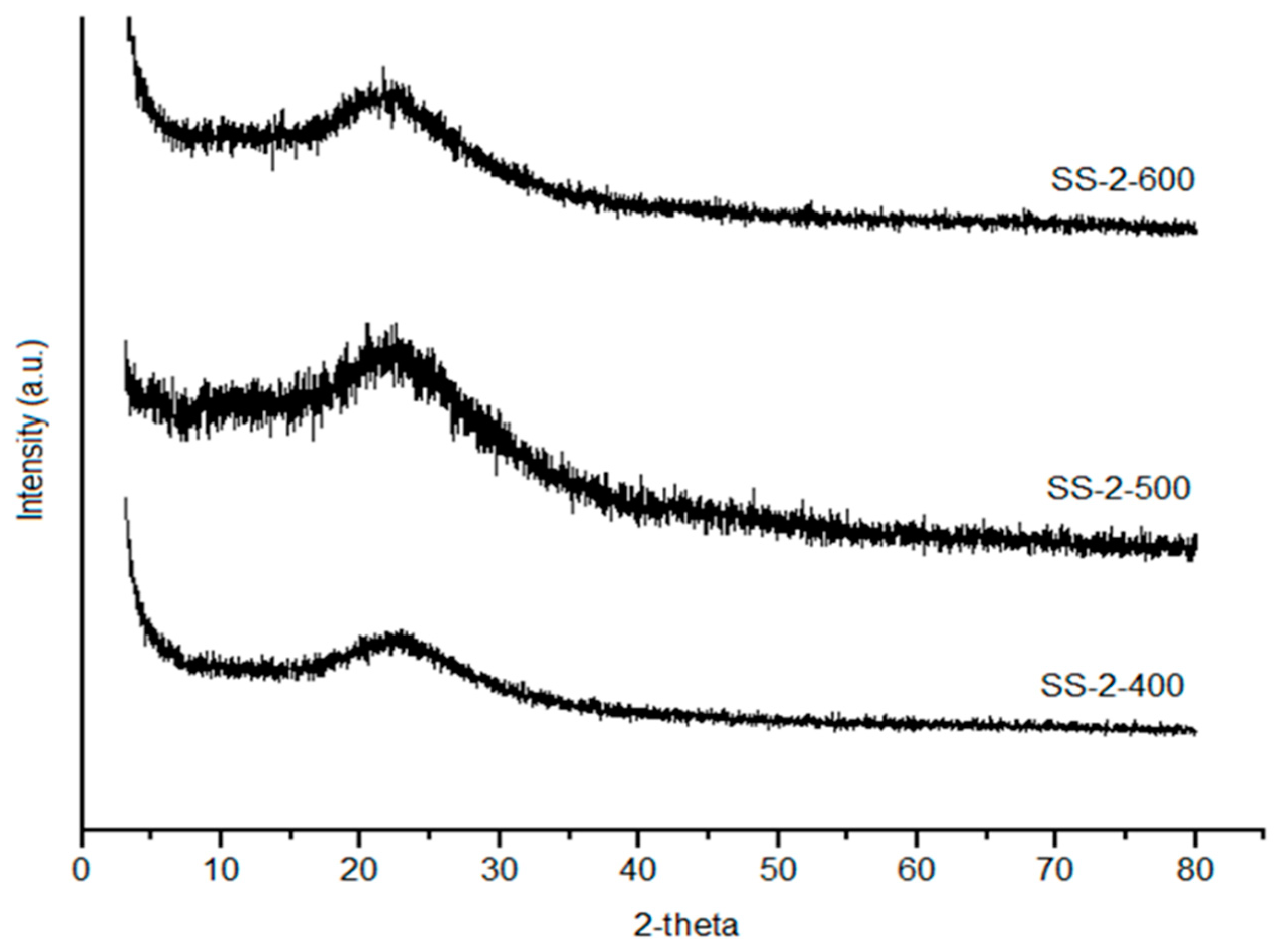
2.2. XRD Analysis
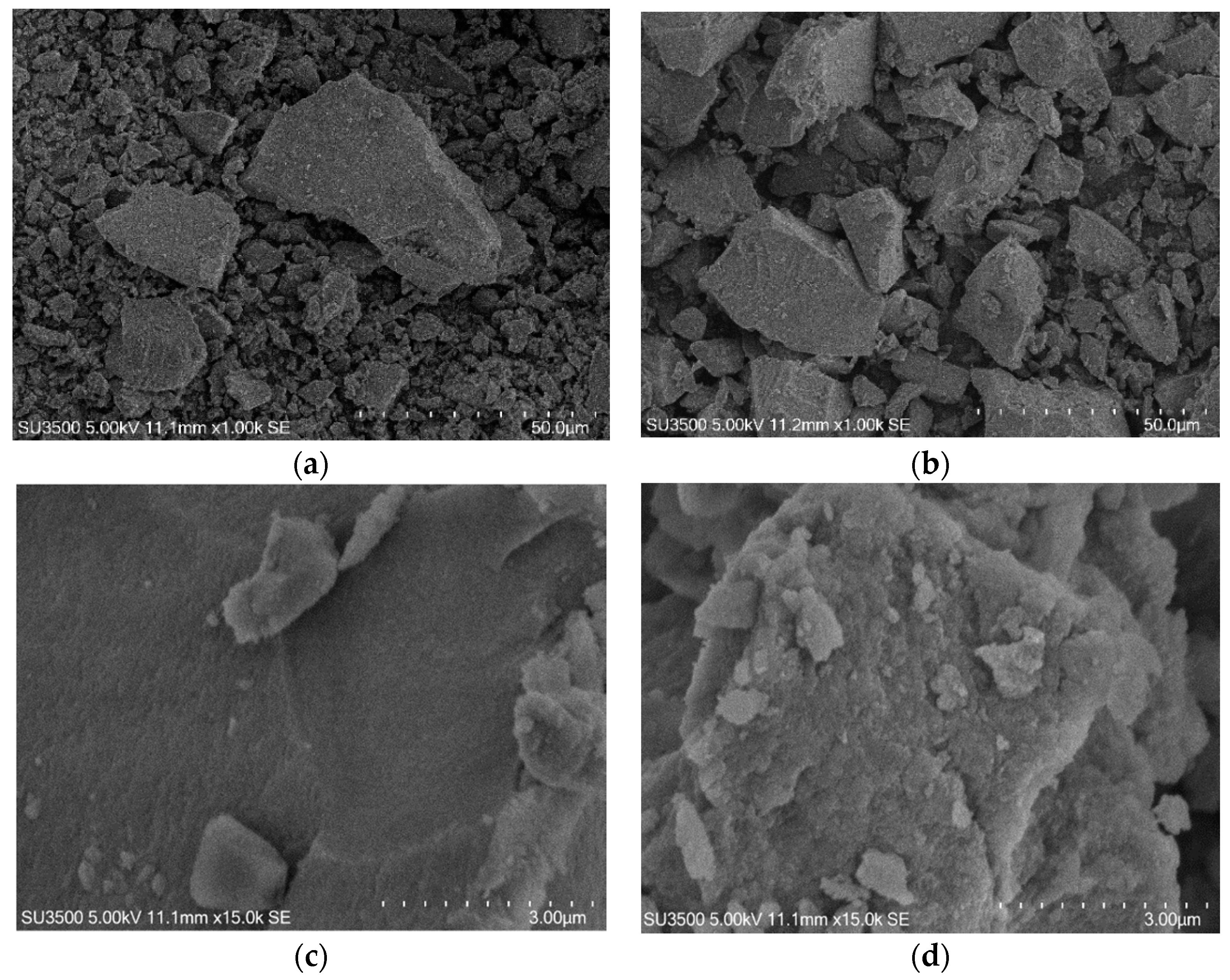
2.3. Surface Morphology
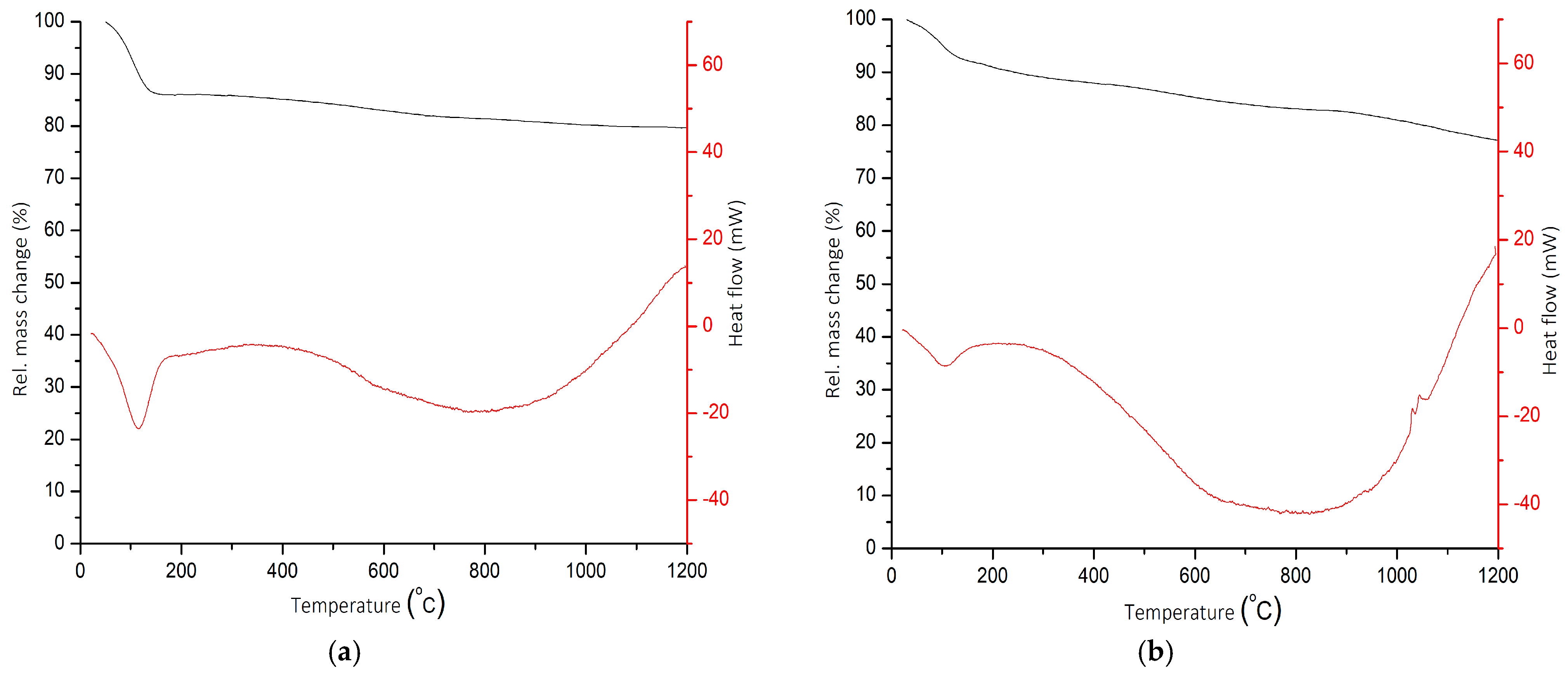
2.4. TGA/DSC Analysis
2.5. Surface Area and Porosity Analysis
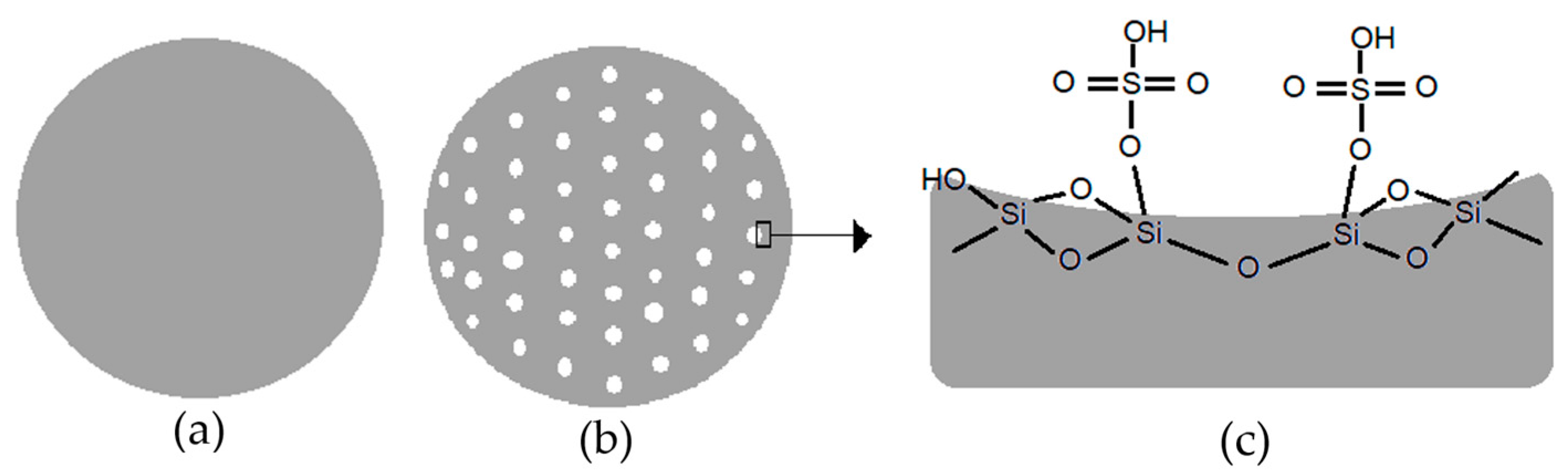
2.6. Characterization of Ni-SO4/SiO2 Catalyst
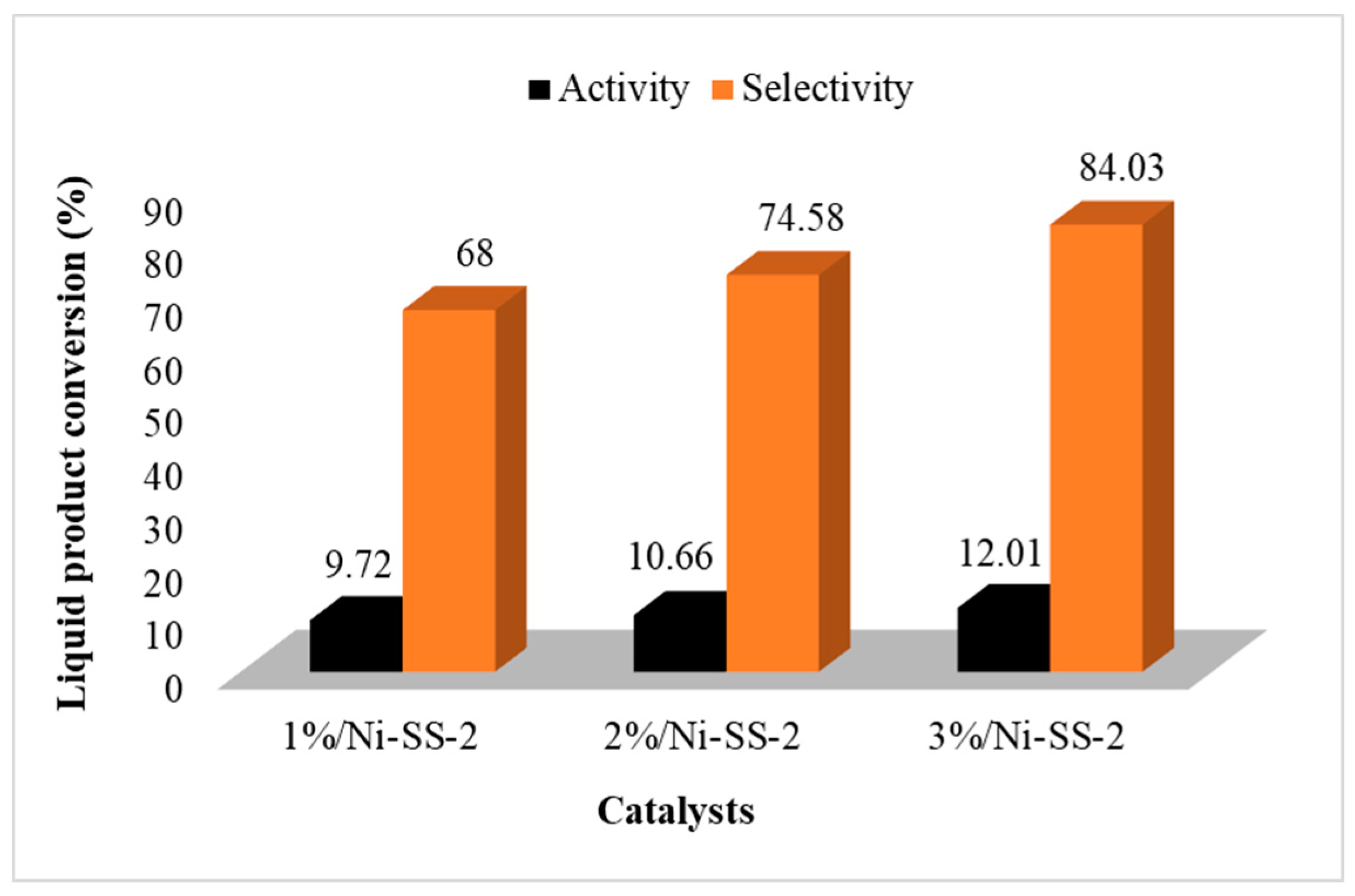
2.7. Study of Activity and Selectivity of SiO2, SO4/SiO2 and Ni-SO4/SiO2 Catalysts
3. Experimental
3.1. Materials
3.2. SO4/SiO2 Catalyst Preparation
3.3. Ni-SO4/SiO2 Catalyst Preparation
3.4. Ethanol Dehydration into Diethyl Ether
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abidin, S.Z.; Haigh, K.F.; Saha, B. Esterification of free fatty acids in used cooking oil using ion-exchange resins as catalysts: An efficient pretreatment method for biodiesel feedstock. Ind. Eng. Chem. Res. 2012, 51, 14653–14664. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An alternative to conventional fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef] [Green Version]
- Kapasi, Z.A.; Nair, A.R.; Somawane, S. Biofuel an alternative source of energy for presence and future. J. Adv. Sci. Technol. 2010, 13, 105–108. [Google Scholar]
- Alharbi, W.; Brown, E.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydration of ethanol over heteropoly acid catalysts in the gas phase. J. Catal. 2014, 319, 174–181. [Google Scholar] [CrossRef]
- Rahmanian, A.; Ghaziaskar, H.S. Continuous dehydration of ethanol to diethyl ether over aluminum phosphate-hydroxyapatite catalyst under sub and supercritical condition. J. Supercrit. Fluids 2013, 78, 34–41. [Google Scholar] [CrossRef]
- Varisli, D.; Dogu, T.D.G. Ethylene and diethyl-ether production by dehydration reaction of ethanol over different heteropolyacid catalysts. Chem. Eng. Sci. 2007, 62, 5349–5352. [Google Scholar] [CrossRef]
- Takahara, I.; Saito, M.; Inaba, M.; Murata, K. Dehydration of ethanol into ethylene over solid acid catalysts. Catal. Lett. 2005, 105, 249–252. [Google Scholar] [CrossRef]
- Ibrahim, A. Investigating the Effect of using diethyl ether as a fuel additive on diesel engine performance and combustion. Appl. Therm. Eng. 2016, 107, 853–862. [Google Scholar] [CrossRef]
- Al-Faze, R.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Diethyl ether conversion to ethene and ethanol catalyzed by heteropoly acids. ACS Omega 2021, 6, 9310–9318. [Google Scholar] [CrossRef] [PubMed]
- Winata, W.F.; Wijaya, K.; Mara, A.; Kurniawati, W. Conversion of bioethanol to diethyl ether catalyzed by sulfuric acid and zeolite. J. Idn. Chem. Soc. 2020, 3, 151–157. [Google Scholar] [CrossRef]
- Bergna, H.E.; Roberts, W.O. Colloidal silica: Fundamentals and applications, in Surfactant Science Series. Mech. Eng. J. 2006, 22, 9–37. [Google Scholar]
- Fricke, J.; Emmerling, A. Aerogels, preparation, properties, applications. In Structure and Bonding 77: Chemistry, Spectroscopy and Applications of Sol-Gel Glasses; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Lion, M.; Maache, M.; Lavalley, J.C.; Ramis, G.; Busca, G.; Rossi, P.F.; Lorenzelli, V. FT-IR study of the brønsted acidity of phosphated and sulphated silica catalysts. J. Mol. Struct. 1990, 218, 417–422. [Google Scholar] [CrossRef]
- Zhuang, Q.; Miller, J.M. One-pot sol-gel synthesis of sulfated ZrO2-SiO2 catalysts for alcohol dehydration. Can. J. Chem. 2001, 79, 1220–1223. [Google Scholar] [CrossRef]
- Fu, B.; Gao, L.; Niu, L.; Wei, R.; Xiao, G. Biodiesel from waste cooking oil via heterogeneous superacid catalyst SO42−/ZrO2. Energy Fuels 2009, 23, 569–572. [Google Scholar] [CrossRef]
- Feng, R.; Gao, X.J.; Yang, Q.; Li, M.J.; Zhang, J.F.; Song, F.; Zhang, Q.D.; Han, Y.Z.; Tan, Y.S. Effects of calcination temperature on the catalytic performance of Ti(SO4)2/CS for DME direct oxidation to polyoxymethylene dimethyl ethers. J. Fuel Chem. Technol. 2021, 49, 72–79. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Zugic, D.L.; Perovic, I.M.; Saponjic, A.B.; Babic, B.M.; Pasti, I.A.; Kaninski, M.P.M. Investigation of tungsten carbide supported Pd or Pt as anode catalysts for PEM fuel cells. Int. J. Hydrogen Energy 2013, 38, 11340–11345. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q. Nickel phyllosilicate derived Ni/SiO2 catalysts for CO2 methanation: Identifying effect of silanol group concentration. J. CO2 Util. 2021, 50, 101587. [Google Scholar] [CrossRef]
- Susi, E.P.; Wijaya, K.; Pratika, R.A.; Hariani, P.L. Effect of nickel concentration in natural zeolite as catalyst in hydrocracking process of used cooking oil. Asian J. Chem. 2020, 32, 2773–2777. [Google Scholar] [CrossRef]
- Sarve, D.T.; Singh, S.K.; Ekhe, J.D. Kinetic and mechanistic study of ethanol dehydration to diethyl ether over Ni-ZSM-5 in a closed batch reactor. React. Kinet. Mech. Catal. 2020, 131, 261–281. [Google Scholar] [CrossRef]
- Wijaya, K.; Syoufian, A.; Ariantika, S. Hydrocracking of used cooking oil into biofuel catalyzed by nickel-bentonite. Asian J. Chem. 2014, 26, 6097–6100. [Google Scholar] [CrossRef]
- Radwan, N.R.E.; Hagar, M.; Afifi, T.H.; Al-wadaani, F.; Okasha, R.M. Catalytic activity of sulfated and phosphated catalysts towards the synthesis of substituted coumarin. Catalysts 2018, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.W.; Niu, L.Y.; Jia, X.J.; Kang, Q.X.; Ma, Z.H.; Lei, Z.Q. Preparation of silica-supported sulfate and its application as a stable and highly active solid acid catalyst. Catal. Commun. 2011, 12, 798–802. [Google Scholar] [CrossRef]
- Du, Y.; Sun, Y.; Di, Y.; Zhao, L.; Liu, S.; Xiao, S.F. Ordered mesoporous sulfated silica-zirconia materials with high zirconium contents in the structure. J. Porous. Mater. 2006, 13, 163–171. [Google Scholar] [CrossRef]
- Sunajadevi, K.R.; Sugunan, S. Synthesis, Characterization and benzylation activity of nanocrystalline chromia loaded sulfated titania prepared via sol-gel route. Catal. Commun. 2004, 5, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.I.; El-Hakam, S.A.; Samra, S.E.; L-Khouly, A.A.E.; Khder, A.S. Structural characterization of sulfated zirconia and their catalytic activity in dehydration of ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 62–70. [Google Scholar] [CrossRef]
- Pratika, R.A.; Wijaya, K.; Trisunaryanti, W. Hydrothermal treatment of SO4/TiO2 and TiO2/CaO as heterogeneous catalysts for the conversion of Jatropha oil into biodiesel. J. Environ. Chem. Eng. 2021, 9, 106547. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Effect of calcination temperature on the structure and catalytic performance of ZrO2 catalyst in phenol degradation. Mater. Today Proc. 2019, 19, 1533–1536. [Google Scholar] [CrossRef]
- Wijaya, K.; Saputri, W.D.; Aziz, I.T.A.; Heraldy, E.; Hakim, L.; Suseno, A.; Utami, M. Mesoporous silica preparation using sodium bicarbonate as template and application of the silica for hydrocracking of used cooking oil into biofuel. Silicon 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Aneu, A.; Wijaya, K.; Syoufian, A. Silica-based solid acid catalyst with different concentration of H2SO4 and calcination temperature: Preparation and characterization. Silicon 2020, 13, 2265–2270. [Google Scholar] [CrossRef]
- Patel, A.; Brahmkhatri, V.; Singh, N. Biodiesel production by esterification of free fattyacid over sulfated zirconia. Renew. Energy 2013, 51, 227–233. [Google Scholar] [CrossRef]
- Utami, M.; Trisunaryanti, W.; Shida, K.; Tsushida, M.; Kawakita, H.; Ohto, K.; Wijaya, K.; Tominaga, M. Hydrothermal preparation of a platinum-loaded sulphated nanozirconia catalyst for the effective conversion of waste low density polyethylene into gasoline-range hydrocarbons. RSC Adv. 2019, 9, 41392. [Google Scholar] [CrossRef] [Green Version]
- Aboul-Gheit, A.K.; Gad, F.K.; Abdel-Aleem, G.M.; El-Desouki, D.S.; Abdel-Hamid, S.M.; Ghoneim, S.A.; Ibrahim, A.H. Pt, Re and incorporation in sulfated zirconia as catalyst for n-pentane isomerization. Egypt. Pet. 2014, 23, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Charmas, B.; Kucio, K.; Sydorchuk, V.; Khalameida, S.; Zięzio, M.; Nowicka, A. Characterization of multimodal silicas using TG/DTG/DTA, Q-TG, and DSC methods. Colloids Interfaces 2018, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Guo, S. Pore characterization of marine-continental transitional shale in permian shanxi formation of the southern north china basin. Energy Explor. Exploit. 2020, 38, 2199–2216. [Google Scholar] [CrossRef]
- Nurmalasari, W.T.; Sutarno, I.I.F. Mesoporous silica impregnated by Ni and NiMo as catalysts for hydrocracking of waste lubricant. Int. J. ChemTech Res. 2016, 9, 607–614. [Google Scholar] [CrossRef]
- Niwa, M.; Nishinikawa, S.; Katada, N. IRMS–TPD of ammonia for characterization of acid site in b-zeolite. Microporous Mesoporous Mater. 2005, 82, 105–112. [Google Scholar] [CrossRef]
- Wijaya, K.; Kurniawan, M.A.; Saputri, W.D.; Trisunaryanti, W.; Mirzan, M.; Hariani, P.L.; Tikoalu, A.D. Synthesis of nickel catalyst supported on ZrO2/SO4 pillared bentonite and its application for conversion of coconut oil into gasoline via hydrocracking process. J. Environ. Chem. Eng. 2021, 9, 105399. [Google Scholar] [CrossRef]
- Amin, K.A.; Wijaya, K.; Trisunaryanti, W. The catalytic performance of ZrO2-SO4 and Ni/ZrO2-SO4 prepared from commercial ZrO2 in Hydrocracking of LDPE plastic waste into liquid fuels. Orient. J. Chem. 2018, 34, 3070. [Google Scholar] [CrossRef] [Green Version]



| Catalysts | Acidity (mmol/g) |
|---|---|
| SiO2 | 2.08 |
| SS-1 | 2.13 |
| SS-2 | 2.22 |
| SS-3 | 2.05 |
| SS-4 | 1.68 |
| SS-2-400 | 2.87 |
| SS-2-500 | 2.22 |
| SS-2-600 | 1.76 |
| Elements | Atom (%) | |
|---|---|---|
| SiO2 | SS-2-400 | |
| Si | 35.4 | 40.6 |
| O | 63.7 | 57.2 |
| S | - | 2.1 |
| Catalyst | Surface Area (m2/g) | Total Pore Volume (cc/g) | Pore Diameter (nm) |
|---|---|---|---|
| SiO2 | 772.153 | 0.732 | 3.672 |
| SS-2-400 | 236.093 | 0.407 | 6.624 |
| Catalyst | Micropore Volume (cc/g) | Average Micropore Diameter (nm) |
|---|---|---|
| SiO2 | 0.325 | 0.626 |
| SS-2-400 | 0.407 | 0.806 |
| Catalysts | Ni Metal Content (AAS/wt%) |
|---|---|
| 1%/Ni-SS-2 | 0.74 |
| 2%/Ni-SS-2 | 1.55 |
| 3%/Ni-SS-2 | 2.18 |
| Catalysts | Acidity (mmol/g) |
|---|---|
| SS-2-400 | 2.87 |
| 1%/Ni-SS-2 | 5.16 |
| 2%/Ni-SS-2 | 6.38 |
| 3%/Ni-SS-2 | 7.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, K.; Lammaduma Malau, M.L.; Utami, M.; Mulijani, S.; Patah, A.; Wibowo, A.C.; Chandrasekaran, M.; Rajabathar, J.R.; Al-Lohedan, H.A. Synthesis, Characterizations and Catalysis of Sulfated Silica and Nickel Modified Silica Catalysts for Diethyl Ether (DEE) Production from Ethanol towards Renewable Energy Applications. Catalysts 2021, 11, 1511. https://doi.org/10.3390/catal11121511
Wijaya K, Lammaduma Malau ML, Utami M, Mulijani S, Patah A, Wibowo AC, Chandrasekaran M, Rajabathar JR, Al-Lohedan HA. Synthesis, Characterizations and Catalysis of Sulfated Silica and Nickel Modified Silica Catalysts for Diethyl Ether (DEE) Production from Ethanol towards Renewable Energy Applications. Catalysts. 2021; 11(12):1511. https://doi.org/10.3390/catal11121511
Chicago/Turabian StyleWijaya, Karna, Melynatri Laura Lammaduma Malau, Maisari Utami, Sri Mulijani, Aep Patah, Arief Cahyo Wibowo, Murugesan Chandrasekaran, Jothi Ramalingam Rajabathar, and Hamad A. Al-Lohedan. 2021. "Synthesis, Characterizations and Catalysis of Sulfated Silica and Nickel Modified Silica Catalysts for Diethyl Ether (DEE) Production from Ethanol towards Renewable Energy Applications" Catalysts 11, no. 12: 1511. https://doi.org/10.3390/catal11121511
APA StyleWijaya, K., Lammaduma Malau, M. L., Utami, M., Mulijani, S., Patah, A., Wibowo, A. C., Chandrasekaran, M., Rajabathar, J. R., & Al-Lohedan, H. A. (2021). Synthesis, Characterizations and Catalysis of Sulfated Silica and Nickel Modified Silica Catalysts for Diethyl Ether (DEE) Production from Ethanol towards Renewable Energy Applications. Catalysts, 11(12), 1511. https://doi.org/10.3390/catal11121511