TiCl4/MgCl2/MCM-41 Bi-Supported Ziegler–Natta Catalyst: Effects of Catalyst Composition on Ethylene/1-Hexene Copolymerization
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization of Supports and Catalysts
2.2. Polymerization Activity
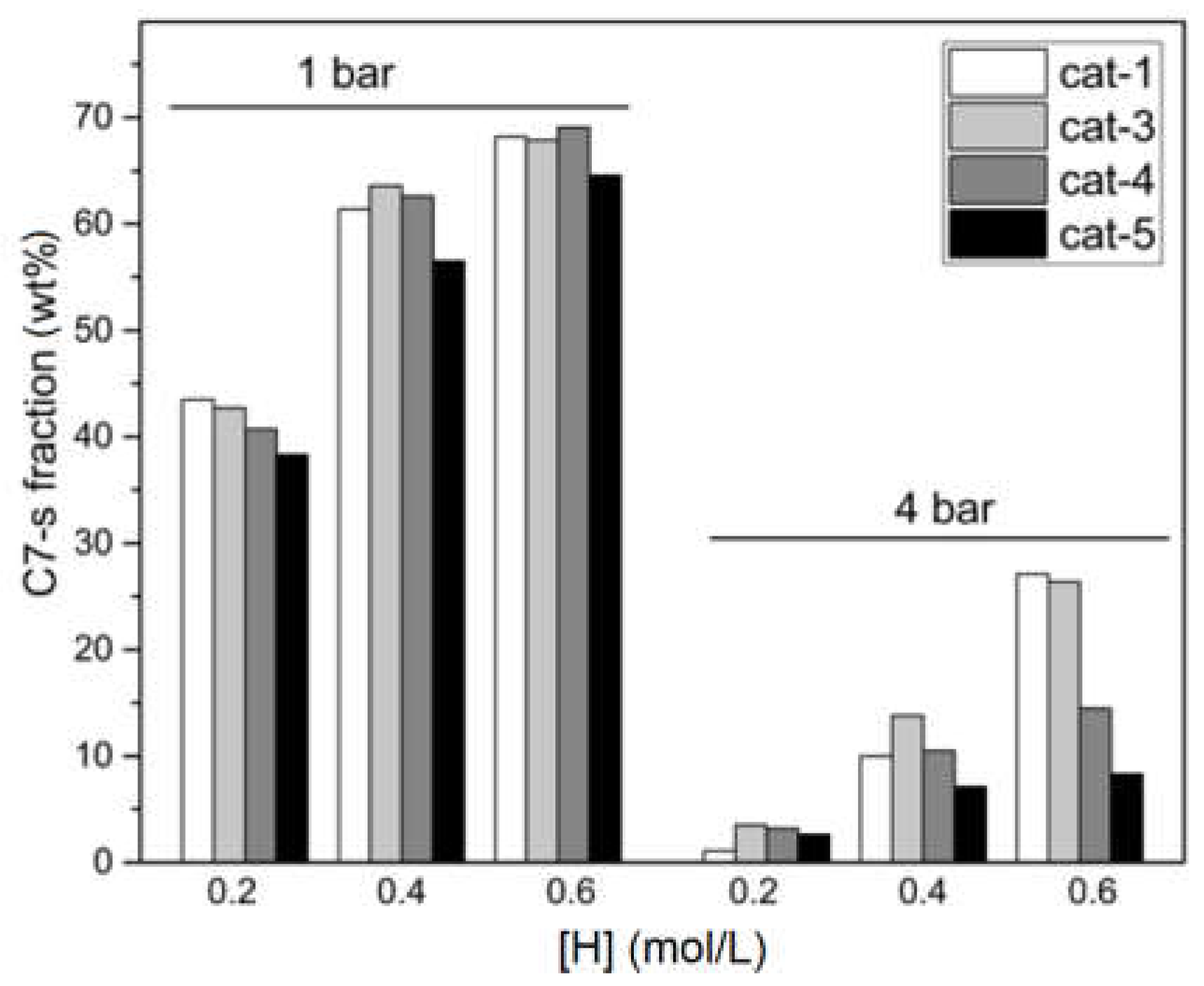
2.3. Polymer Structure
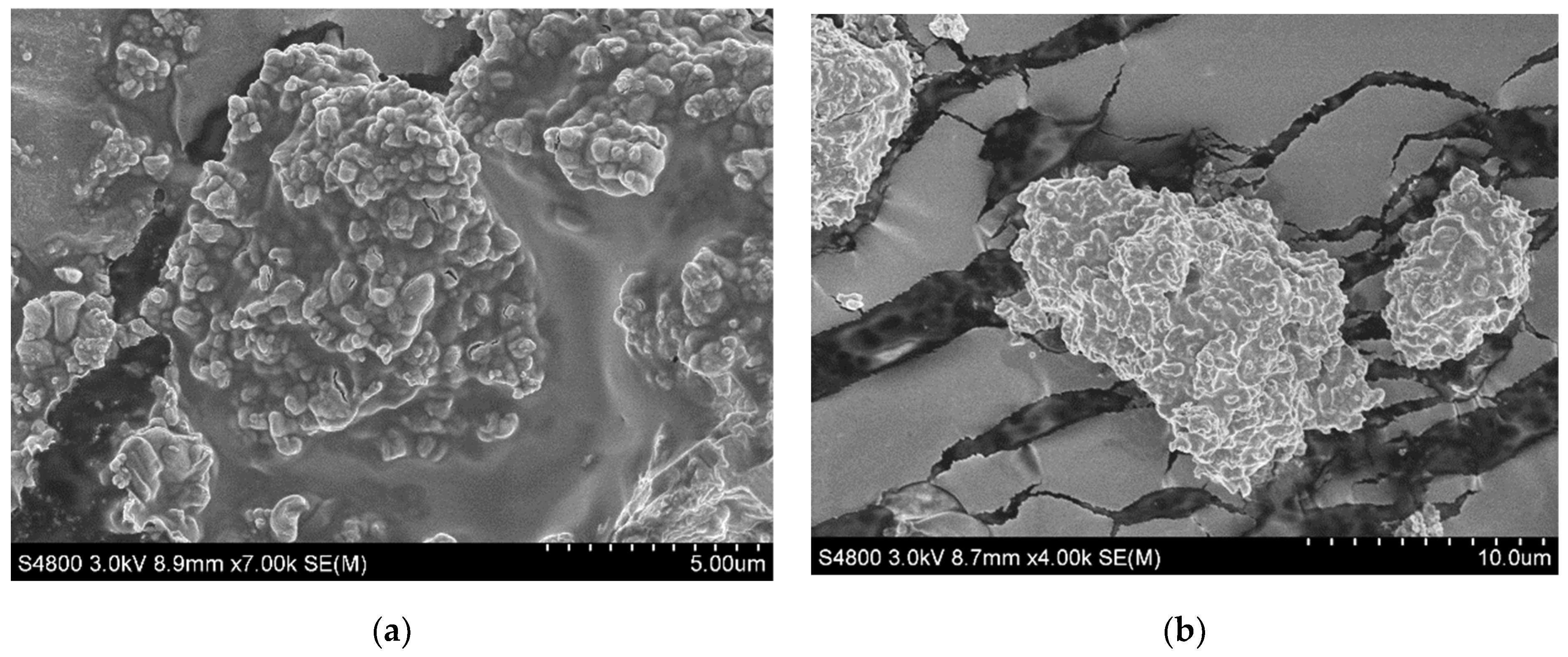
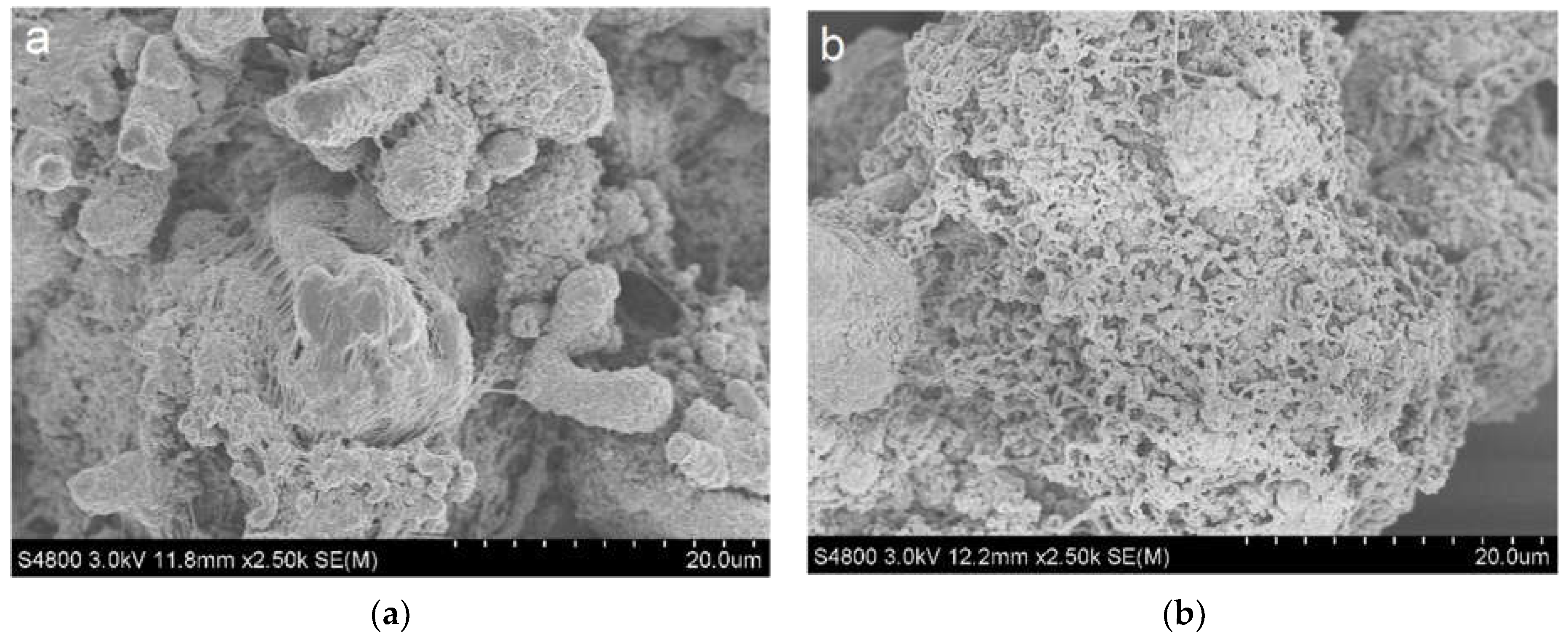
2.4. Morphology of Nascent Polymer Particles
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of MgCl2 and MgCl2/MCM-41 Support
3.3. Preparation of Ziegler-Natta Catalysts
3.4. Ethylene Polymerization and Ethylene/1-Hexene Copolymerization
3.5. Polymer Fractionation
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mülhaupt, R. Catalytic Polymerization and Post Polymerization Catalysis Fifty Years After the Discovery of Ziegler’s Catalysts. Macromol. Chem. Phys. 2003, 204, 289–327. [Google Scholar] [CrossRef]
- Claverie, J.P.; Schaper, F. Ziegler-Natta Catalysis: 50 Years After the Nobel Prize. MRS Bull. 2013, 38, 213–218. [Google Scholar] [CrossRef]
- Kakugo, M.; Miyatake, T.; Mizunuma, K. Chemical Composition Distribution of Ethylene-1-Hexene Copolymer Prepared with Titanium Trichloride-diethylaluminum Chloride Catalyst. Macromolecules 1991, 24, 1469–1472. [Google Scholar] [CrossRef]
- Chen, Y.P.; Fan, Z.Q. Ethylene/1-hexene copolymerization with TiCl4/MgCl2/AlCl3 catalyst in the presence of hydrogen. Eur. Polym. J. 2006, 42, 2441–2449. [Google Scholar] [CrossRef]
- Zhang, L.T.; Fan, Z.Q.; Fu, Z.S. Dependence of the Distribution of Active Centers on Monomer in Supported Ziegler-Natta Catalysts. Chin. J. Polym. Sci. 2008, 26, 605–610. [Google Scholar] [CrossRef]
- Tso, C.C.; DesLauriers, P.J. Comparison of Methods for Characterizing Comonomer Composition in Ethylene 1-Olefin Copolymers: 3D-TREF vs. SEC-FTIR. Polymer 2004, 45, 2657–2663. [Google Scholar] [CrossRef]
- Wharry, S.M. Randomness in Ziegler–Natta Olefin Copolymerizations as Determined by 13C-NMR Spectroscopy—The Influence of Chain Heterogeneity. Polymer 2004, 45, 2985–2989. [Google Scholar] [CrossRef]
- Ko, Y.S.; Han, T.K.; Sadatoshi, H.; Woo, S.I. Analysis of Microstructure of Ethylene–1-Hexene Copolymer Prepared over Thermally Pretreated MgCl2/THF/TiCl4 Bimetallic Catalyst. Polym. Sci. A Polym. Chem. 1998, 36, 291–300. [Google Scholar] [CrossRef]
- Chu, K.J.; Soares, J.B.P.; Penlidis, A.; Ihm, S.K. Effect of Prepolymerization and Hydrogen Pressure on the Microstructure of Ethylene/1-Hexene Copolymers Made with MgCl2-supported TiCl3 Catalysts. Eur. Polym. J. 2000, 36, 3–11. [Google Scholar] [CrossRef]
- Mulhaupt, R.; Ovenall, D.W.; Ittel, S.D. Control of Composition in Ethylene Copolymerizations Using Magnesium Chloride Supported Ziegler–Natta Catalysts. J. Polym. Sci. A Polym. Chem. 1988, 26, 2487–2500. [Google Scholar] [CrossRef]
- Dupuy, J.; Spitz, R. Modification of Ziegler-Natta Catalysts by Cyclopentiadienyl-type Ligands: Activation of Titanium-based Catalysts. Appl. Polym. Sci. 1997, 165, 2281–2288. [Google Scholar] [CrossRef]
- Kang, K.K.; Oh, J.K.; Jeong, Y.T.; Shiono, T.; Ikeda, T. Highly Active MgCl2-supported CpMCl3 (M = Ti, Zr) Catalysts for Ethylene Polymerization. Macromol. Rapid Commun. 1999, 20, 308–311. [Google Scholar] [CrossRef]
- Yuan, K.; Yi, J.J.; Dou, X.L.; Liu, W.J.; Huang, Q.G.; Gao, K.J.; Yang, W.T. With Different Structure Ligands Heterogeneous Ziegler–Natta Catalysts for the Preparation of Copolymer of Ethylene and 1-Octene with High Comonomer Incorporation. Polymer 2010, 51, 3859–3866. [Google Scholar]
- Lou, J.Q.; Liu, X.Y.; Fu, Z.S.; Wang, Q.; Xu, J.T.; Fan, Z.Q. Ethylene/1-hexene copolymerization with a diisopropylphenol modified supported Ziegler–Natta catalyst. Acta Polym. Sin. 2009, 8, 748–755. [Google Scholar] [CrossRef]
- Xia, S.J.; Fu, Z.S.; Huang, B.; Xu, J.T.; Fan, Z.Q. Ethylene/1-hexene copolymerization with MgCl2-supported Ziegler–Natta catalysts containing aryloxy ligands. Part I: Catalysts prepared by immobilizing TiCl3(OAr) onto MgCl2 in batch reaction. J. Mol. Catal. A Chem. 2012, 355, 161–167. [Google Scholar] [CrossRef]
- Yang, H.R.; Huang, B.; Fu, Z.S.; Fan, Z.Q. Ethylene/1-Hexene Copolymerization with Supported Ziegler–Natta Catalysts Prepared by Immobilizing TiCl3(OAr) onto MgCl2. J. Appl. Polym. Sci. 2015, 132, 41329. [Google Scholar] [CrossRef]
- Xia, S.J.; Fu, Z.S.; Liu, X.Y.; Fan, Z.Q. Copolymerization of Ethylene and 1-Hexene with TiCl4/MgCl2 Catalysts Modified by 2,6-Diisopropylphenol. Chin. J. Polym. Sci. 2013, 31, 110–121. [Google Scholar] [CrossRef]
- Kageyama, K.; Tamazawa, J.-I.; Aida, T. Extrusion Polymerization: Catalyzed Synthesis of Crystalline Linear Polyethylene Nanofibers Within a Mesoporous Silica. Science 1999, 285, 2113–2115. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Rao, R.R.; Pelgrims, J.; Schoonheydt, R.A.; Bodart, P.; Debras, G.; Collart, O.; Voort, P.V.D.; Vansant, E.F. Synthesis, Spectroscopy and Catalysis of [Cr(Acac)3] Complexes Grafted onto MCM-41 Materials: Formation of Polyethylene Nanofibres within Mesoporous Crystalline Aluminosilicates. Chem. A Eur. J. 2000, 6, 2960–2970. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Zhu, S.; Wang, W.-J.; Alsyouri, H.; Lin, Y.S. Morphological and Mechanical Properties of Nascent Polyethylene Fibers Produced via Ethylene Extrusion Polymerization with a Metallocene Catalyst Supported on MCM-41 Particles. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2433–2443. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Jiang, G.; Zhao, Z.; Sun, T.; Yu, H.; Wang, W. MCM-41 and SBA-15 Supported Cp2ZrCl2 Catalysts for the Preparation of Nano-Polyethylene Fibres via in Situ Ethylene Extrusion Polymerization. J. Mol. Catal. A Chem. 2005, 240, 239–244. [Google Scholar]
- Chen, S.; Guo, C.; Liu, L.; Xu, H.; Dong, J.; Hu, Y. Immobilization of a Zirconium Complex Bearing Bis(Phenoxyketimine) Ligand on MCM-41 for Ethylene Polymerization. Polymer 2005, 46, 11093–11098. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Wang, W.; Yu, H.; Wang, J.; Chen, T.; Zhao, Z. Preparation of Nano-Polyethylene Fibers and Floccules Using MCM-41-Supported Metallocene Catalytic System under Atmospheric Pressure. Eur. Polym. J. 2005, 41, 797–803. [Google Scholar] [CrossRef]
- Ghosh, S. Influence of Supported Vanadium Catalyst on Ethylene Polymerization Reactions: Influence of Vanadium Catalyst on Ethylene Polymerization. Polym. Int. 2008, 57, 262–267. [Google Scholar] [CrossRef]
- Wang, N.; Shi, Z.-X.; Zhang, J.; Wang, L. The Influence of Modification of Mesoporous Silica with Polyethylene Via In Situ Ziegler—Natta Polymerization on PE/MCM-41 Nanocomposite. J. Compos. Mater. 2008, 42, 1151–1157. [Google Scholar] [CrossRef]
- Xu, H.; Guo, C.-Y. Polymerization in the Confinement of Molecular Sieves: Facile Preparation of High Performance Polyethylene. Eur. Polym. J. 2015, 65, 15–32. [Google Scholar] [CrossRef]
- Kumkaew, P.; Wu, L.; Praserthdam, P.; Wanke, S.E. Rates and product properties of polyethylene produced by copolymerization of 1-hexene and ethylene in the gas phase with (n-BuCp)2ZrCl2 on supports with different pore sizes. Polymer 2003, 44, 4791–4803. [Google Scholar] [CrossRef]
- Ko, Y.S.; Woo, S.I. Shape and diffusion of the monomer-controlled copolymerization of ethylene and alpha-olefins over Cp2ZrCl2 confined in the nanospace of the supercage of NaY. J. Polym. Sci. A Polym. Chem. 2003, 41, 2171–2179. [Google Scholar] [CrossRef]
- Kumkaew, P.; Wanke, S.E.; Praserthdam, P.; Danumah, C.; Kaliaguine, S. Gas-phase ethylene polymerization using zirconocene supported on mesoporous molecular sieves. J. Appl. Polym. Sci. 2003, 87, 1161–1177. [Google Scholar] [CrossRef]
- Ko, Y.S.; Lee, J.S.; Yim, J.-H.; Jeon, J.-K.; Jung, K.Y. Influence of Nanopores of MCM-41 and SBA-15 Confining (n-BuCp)2ZrCl2 on Copolymerization of Ethylene-α-Olefin. J. Nanosci. Nanotechnol. 2010, 10, 180–185. [Google Scholar] [CrossRef]
- Paredes, B.; van Grieken, R.; Carrero, A.; Suarez, I.; Soares, J.B.P. Ethylene/1-Hexene Copolymers Produced with MAO/(nBuCp)2ZrCl2 Supported on SBA-15 Materials with Different Pore Sizes. Macromol. Chem. Phys. 2011, 212, 1590–1599. [Google Scholar] [CrossRef]
- Lee, J.S.; Yim, J.-H.; Jeon, J.-K.; Ko, Y.S. Polymerization of Olefins with Single-Site Catalyst Anchored on Amine-Functionalized Surface of SBA-15. Catal. Today 2012, 185, 175–182. [Google Scholar] [CrossRef]
- Lee, J.S.; Ko, Y.S. Control of the molecular structure of ethylene-1-hexene copolymer by surface functionalization of SBA-15 with different compositions of amine groups. J. Mol. Catal. A Chem. 2014, 386, 120–125. [Google Scholar] [CrossRef]
- Ahmadjo, S.; Arabi, H.; Zohuri, G.; Nekoomanesh, M.; Nejabat, G.; Mortazavi, S.M.M. Preparation of Ethylene/α-Olefins Copolymers Using (2-RInd)2ZrCl2/MCM-41 (R:Ph,H) Catalyst, Microstructural Study. J. Therm. Anal. Calorim. 2014, 116, 417–426. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.Y.; Ren, F.; Xu, R.W.; Bai, Y.X. Porous Organic Polymers-Supported Metallocene Catalysts for Ethylene/1-Hexene Copolymerization. Catalysts 2018, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Semsarzadeh, M.A.; Aghili, A. Novel Preparation of Polyethylene from Nano-extrusion Polymerization Inside the Nano-channels of MCM-41/MgCl2/TiCl4 Catalysts. J. Macromol. Sci. Part A 2008, 45, 680–686. [Google Scholar] [CrossRef]
- Jafariyeh-Yazdi, E.; Tavakoli, A.; Abbasi, F.; Parnian, M.J.; Heidari, A. Bi-supported Ziegler–Natta TiCl4/MCM-41/MgCl2 (Ethoxide Type) Catalyst Preparation and Comprehensive Investigations of Produced Polyethylene Characteristics. J. Appl. Polym. Sci. 2020, 137, 48553. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Zhang, R. Ethylene Polymerization Catalyzed by TiCl4/MgCl2/MCM-41 Catalytic System. Des. Monomers Polym. 2007, 10, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Lei, J.; Wang, H.; Jiang, M.; Zhou, G. Nanofibrous Ultrahigh Molecular Weight Polyethylene Synthesized Using TiCl4 as Catalyst Supported on MCM-41 and SBA-15. Polym. Bull. 2012, 68, 1565–1575. [Google Scholar] [CrossRef]
- Nejabat, G.-R.; Nekoomanesh, M.; Arabi, H.; Emami, M.; Aghaei-Nieat, M. Preparation of Polyethylene Nano-Fibres Using Rod-like MCM-41/TiCl4/MgCl2/THF Bi-Supported Ziegler-Natta Catalytic System. Iran. Polym. J. 2010, 19, 79–87. [Google Scholar]
- Yang, F.; Zhang, X.; Zhao, H.; Chen, B.; Huang, B.; Feng, Z. Preparation and Properties of Polyethylene/Montmorillonite Nanocomposites By in Situ Polymerization. J. Appl. Polym. Sci. 2003, 89, 3680–3684. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Yang, F.; Chen, B.; Jin, Y.; Li, G.; Feng, Z.; Huang, B. Synthesis and Characterization of Polpropylene/Montmorillonite Nanocomposites via an in-Situ Polymerization Approach. Chin. J. Polym. Sci. 2003, 21, 413–418. [Google Scholar]
- Di Noto, V.; Bresadola, S. New Synthesis of a Highly Active δ-MgCl2 for MgCl2/TiCl4/AlEt3 Catalytic Systems. Macromol. Chem. Phys. 1996, 197, 3827–3835. [Google Scholar] [CrossRef]
- Soga, K. Ziegler-Natta Catalysts for Olefin Polymerizations. Prog. Polym. Sci. 1997, 22, 1503–1546. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Wu, J.C.; Kuo, C.I. Magnesium chloride supported high-mileage catalysts for olefin polymerization. V. BET, porosimetry, and X-ray diffraction studies. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 737–750. [Google Scholar] [CrossRef]
- Nakayama, Y.; Bando, H.; Sonobe, Y.; Fujita, T. Development of single-site new olefin polymerization catalyst system using MgCl2-based activators: MAO-free MgCl2-supported FI catalyst systems. Bull. Chem. Soc. Jpn. 2004, 77, 617–625. [Google Scholar] [CrossRef]
- Busico, V.; Causà, M.; Cipullo, R.; Credendino, R.; Cutillo, F.; Friederichs, N.; Lamanna, R.; Segre, A.; Castelli, V.V.A. Periodic DFT and high-resolution magic-angle-spinning (HR-MAS) 1H NMR investigation of the active surfaces of MgCl2-supported Ziegler-Natta catalysts, The MgCl2 matrix. J. Phys. Chem. C 2008, 112, 1081–1089. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, S.; Makwana, U.; Patankar, R.B.; Gupta, V.K. Influence of internal donors on the performance and structure of MgCl2 supported titanium catalysts for propylene polymerization. Macromol. Chem. Phys. 2009, 210, 69–76. [Google Scholar] [CrossRef]
- Redzic, E.; Garoff, T.; Mardare, C.C.; List, M.; Hesser, G.; Mayrhofer, L.; Hassel, A.W.; Paulik, C. Heterogeneous Ziegler–Natta catalysts with various sizes of MgCl2 crystallites: Synthesis and characterization. Iran. Polym. J. 2016, 25, 321–337. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.A.; Liu, X.Y.; Jiang, B.Y.; Zhang, B.; Ali, A.; Fu, Z.S.; Fan, Z.Q. Effect of alkylaluminum cocatalyst on ethylene/1-hexene copolymerization and active center distribution of MgCl2-supported Ziegler-Natta catalyst. J. Macromol. Sci. Part A 2021, 58, 539–549. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Weng, Y.H.; Zhang, S.J.; Zhang, Z.; Fu, Z.S.; Fan, Z.Q. Kinetics and mechanism of ethylene polymerization with TiCl4/MgCl2 model catalysts: Effects of titanium content. J. Catal. 2018, 360, 57–65. [Google Scholar] [CrossRef]
- Xu, T.; Yang, H.R.; Fu, Z.S.; Fan, Z.Q. Effects of comonomer on active center distribution of MgCl2/TiCl4-AlEt3 catalyst in ethylene/1-hexene copolymerization. J. Organomet. Chem. 2015, 798, 328–334. [Google Scholar] [CrossRef]




| Support | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Radius (nm) |
|---|---|---|---|
| MgCl2 | 159.6 | 0.66 | 3.8 |
| MCM-41 | 1021.7 | 1.02 | 2.7 |
| MM4 | 606.1 | 0.88 | 2.7 |
| MM5 | 697.7 | 0.88 | 3.1 |
| Catalyst | Cat-1 | Cat-2 | Cat-3 | Cat-4 | Cat-5 |
|---|---|---|---|---|---|
| Ti, wt% | 5.20 | 6.00 | 3.63 | 5.85 | 4.84 |
| Mg, wt% | 13.20 | 0 | 7.49 | 6.98 | 4.95 |
| n(Mg)/n(Ti) b | 5.0 | 0 | 4.1 | 2.4 | 2.0 |
| m(MgCl2)/m(MCM-41) c | 1/0 | 0/1 | 1/1 | 1/2 | 1/3 |
| Entry | Catalyst | Pressure (Bar) | [H] (mol/L) | Activity (kgPE/(gTi·h)) | Mwc (kg/mol) | Ðc | Tmd (°C) | ΔHm d (J/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | cat-1 | 1 | 0 | 1.47 | 495 | 8.7 | 133 | 168.4 |
| 2 | cat-1 | 1 | 0.2 | 3.37 | 255 | 23.8 | 124 | 94.5 |
| 3 | cat-1 | 1 | 0.4 | 3.86 | 208 | 21.0 | 123 | 58.4 |
| 4 | cat-1 | 1 | 0.6 | 4.04 | 156 | 17.7 | 123 | 42.5 |
| 5 | cat-2 | 1 | 0 | 0.15 | − | − | 133 | 112.5 |
| 6 | cat-2 | 1 | 0.2 | 0.12 | − | − | 130 | 84.5 |
| 7 | cat-2 | 1 | 0.4 | 0.10 | − | − | 129 | 70.5 |
| 8 | cat-2 | 1 | 0.6 | 0.09 | − | − | 129 | 70.1 |
| 9 | cat-3 | 1 | 0 | 2.39 | 393 | 9.8 | 134 | 172.7 |
| 10 | cat-3 | 1 | 0.2 | 3.99 | 167 | 15.2 | 125 | 97.1 |
| 11 | cat-3 | 1 | 0.4 | 3.99 | 166 | 24.1 | 124 | 52.1 |
| 12 | cat-3 | 1 | 0.6 | 5.07 | 138 | 18.7 | 123 | 41.4 |
| 13 | cat-4 | 1 | 0 | 1.26 | 455 | 10.5 | 134 | 173.4 |
| 14 | cat-4 | 1 | 0.2 | 2.79 | 231 | 23.1 | 124 | 99.7 |
| 15 | cat-4 | 1 | 0.4 | 3.06 | 184 | 18.6 | 124 | 55.6 |
| 16 | cat-4 | 1 | 0.6 | 3.70 | 130 | 17.1 | 123 | 39.1 |
| 17 | cat-5 | 1 | 0 | 1.17 | 569 | 9.3 | 133 | 167.5 |
| 18 | cat-5 | 1 | 0.2 | 2.55 | 291 | 23.5 | 124 | 100.5 |
| 19 | cat-5 | 1 | 0.4 | 2.77 | 173 | 22.5 | 123 | 62.8 |
| 20 | cat-5 | 1 | 0.6 | 2.85 | 195 | 19.9 | 123 | 43.0 |
| 21 b | cat-1 | 4 | 0.2 | 13.58 | 414 | 5.5 | 127 | 131.4 |
| 22 | cat-1 | 4 | 0.4 | 14.47 | 287 | 8.9 | 124 | 114.6 |
| 23 | cat-1 | 4 | 0.6 | 14.01 | 239 | 10.0 | 123 | 97.2 |
| 24 | cat-3 | 4 | 0.2 | 21.18 | 362 | 8.2 | 127 | 132.5 |
| 25 | cat-3 | 4 | 0.4 | 20.33 | 295 | 10.7 | 124 | 117.0 |
| 26 | cat-3 | 4 | 0.6 | 20.07 | 274 | 9.1 | 123 | 98.0 |
| 27 | cat-4 | 4 | 0.2 | 30.34 | 405 | 7.4 | 129 | 129.8 |
| 28 | cat-4 | 4 | 0.4 | 32.60 | 372 | 11.1 | 126 | 116.0 |
| 29 | cat-4 | 4 | 0.6 | 48.43 | 310 | 8.9 | 124 | 113.4 |
| 30 | cat-5 | 4 | 0.2 | 30.27 | 389 | 6.5 | 128 | 130.8 |
| 31 | cat-5 | 4 | 0.4 | 34.07 | 417 | 7.9 | 127 | 120.9 |
| 32 | cat-5 | 4 | 0.6 | 34.47 | 399 | 7.3 | 125 | 111.4 |
| Entry | Catalyst | [H] (mol/L) | C6a (mol%) | Conversion (%) b | C7-s | C7-in | ||
|---|---|---|---|---|---|---|---|---|
| Fraction (wt%) | C6a (mol%) | Fraction (wt%) | C6a (mol%) | |||||
| 21 | cat-1 | 0.2 | 2.4 | 27.6 | 1.1 | - | 98.9 | 2.4 |
| 22 | cat-1 | 0.4 | 2.1 | 13.3 | 10.0 | 6.9 | 90.0 | 1.6 |
| 23 | cat-1 | 0.6 | 3.6 | 14.7 | 27.1 | 8.2 | 72.9 | 2.1 |
| 24 | cat-3 | 0.2 | 0.9 | 16.6 | 3.5 | - | 96.5 | 0.9 |
| 25 | cat-3 | 0.4 | 2.7 | 23.7 | 13.8 | 8.1 | 86.2 | 1.9 |
| 26 | cat-3 | 0.6 | 3.3 | 19.4 | 26.4 | 7.5 | 73.6 | 1.9 |
| 27 | cat-4 | 0.2 | 1.1 | 28.9 | 3.2 | - | 96.8 | 1.1 |
| 28 | cat-4 | 0.4 | 2.4 | 34.0 | 10.5 | 10.0 | 89.5 | 1.6 |
| 29 | cat-4 | 0.6 | 3.2 | 45.4 | 14.5 | 9.5 | 85.5 | 2.3 |
| 30 | cat-5 | 0.2 | 1.1 | 28.9 | 2.6 | - | 97.4 | 1.1 |
| 31 | cat-5 | 0.4 | 2.0 | 29.8 | 7.1 | 8.8 | 92.9 | 1.5 |
| 32 | cat-5 | 0.6 | 2.4 | 24.6 | 8.3 | 9.0 | 91.7 | 1.8 |
| Entry | Catalyst | [H] (mol/L) | C7-s | C7-in | ||
|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tm (°C) | ΔHm (J/g) | |||
| 21 | cat-1 | 0.2 | 114 | 104.3 | 127 | 132 |
| 22 | cat-1 | 0.4 | 119 | 60.5 | 124 | 123 |
| 23 | cat-1 | 0.6 | 116 | 53.0 | 123 | 115 |
| 24 | cat-3 | 0.2 | 117 | 73.8 | 127 | 137 |
| 25 | cat-3 | 0.4 | 118 | 59.3 | 124 | 125 |
| 26 | cat-3 | 0.6 | 118 | 50.1 | 123 | 112 |
| 27 | cat-4 | 0.2 | 117 | 54.5 | 129 | 134 |
| 28 | cat-4 | 0.4 | 116 | 41.3 | 125 | 125 |
| 29 | cat-4 | 0.6 | 117 | 41.3 | 124 | 120 |
| 30 | cat-5 | 0.2 | 116 | 41.7 | 128 | 124 |
| 31 | cat-5 | 0.4 | 117 | 42.3 | 124 | 126 |
| 32 | cat-5 | 0.6 | 117 | 46.1 | 125 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Guo, W.; Wang, X.; Guo, Y.; Zhang, B.; Fu, Z.; Wang, Q.; Fan, Z. TiCl4/MgCl2/MCM-41 Bi-Supported Ziegler–Natta Catalyst: Effects of Catalyst Composition on Ethylene/1-Hexene Copolymerization. Catalysts 2021, 11, 1535. https://doi.org/10.3390/catal11121535
Liu X, Guo W, Wang X, Guo Y, Zhang B, Fu Z, Wang Q, Fan Z. TiCl4/MgCl2/MCM-41 Bi-Supported Ziegler–Natta Catalyst: Effects of Catalyst Composition on Ethylene/1-Hexene Copolymerization. Catalysts. 2021; 11(12):1535. https://doi.org/10.3390/catal11121535
Chicago/Turabian StyleLiu, Xiaoyu, Wenqi Guo, Xueer Wang, Yintian Guo, Biao Zhang, Zhisheng Fu, Qi Wang, and Zhiqiang Fan. 2021. "TiCl4/MgCl2/MCM-41 Bi-Supported Ziegler–Natta Catalyst: Effects of Catalyst Composition on Ethylene/1-Hexene Copolymerization" Catalysts 11, no. 12: 1535. https://doi.org/10.3390/catal11121535
APA StyleLiu, X., Guo, W., Wang, X., Guo, Y., Zhang, B., Fu, Z., Wang, Q., & Fan, Z. (2021). TiCl4/MgCl2/MCM-41 Bi-Supported Ziegler–Natta Catalyst: Effects of Catalyst Composition on Ethylene/1-Hexene Copolymerization. Catalysts, 11(12), 1535. https://doi.org/10.3390/catal11121535







