Ionic Liquid-Modified Porous Organic Polymers as Efficient Metallocene Catalyst Supports
Abstract
:1. Introduction
2. Experimental
2.1. Materials
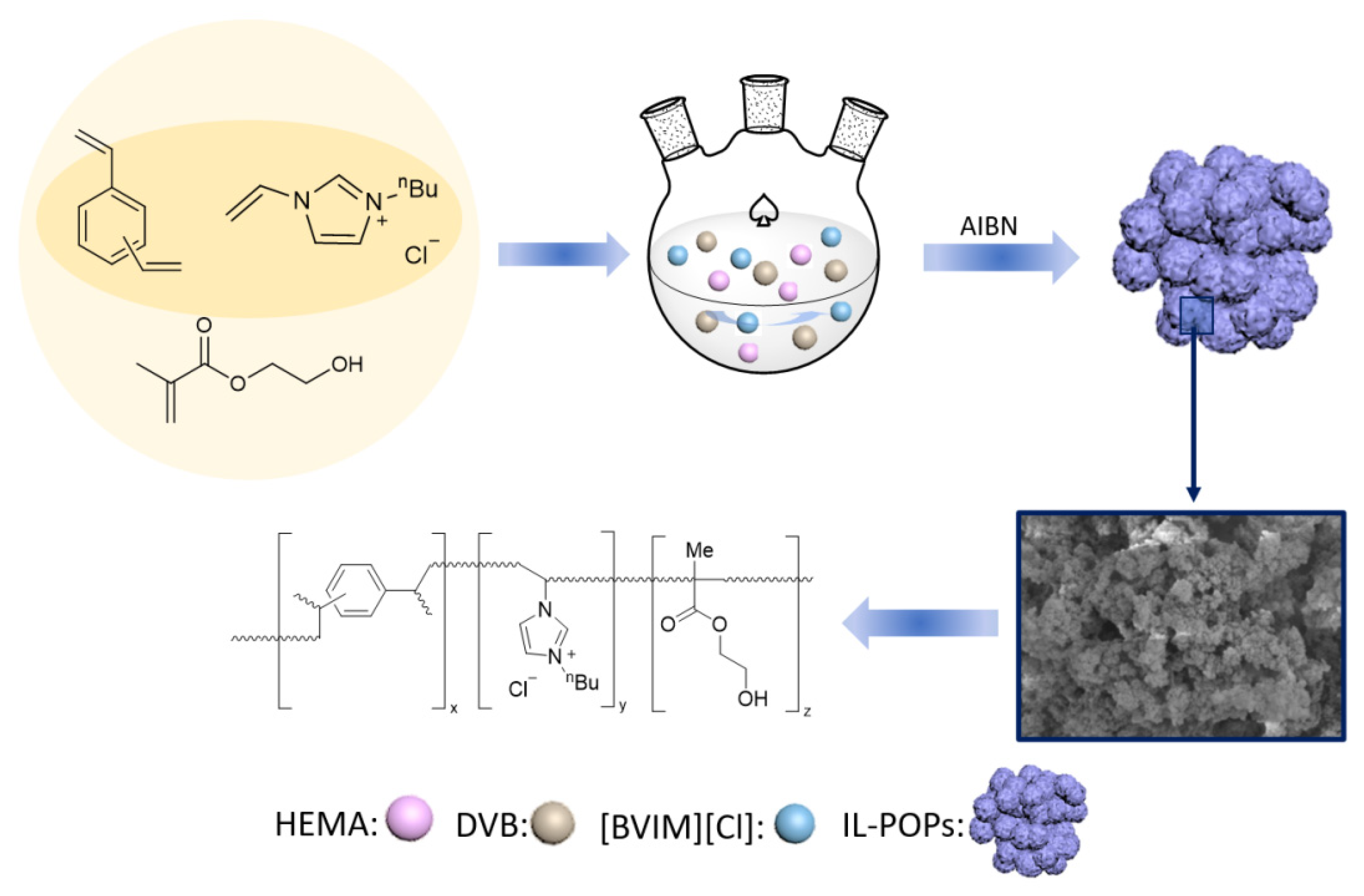
2.2. Preparation of Ionic Liquid-Modified Porous Organic Polymer Supports
2.3. Preparation of Ionic Liquid-Modified POPs Supported Catalysts
2.4. Ethylene Polymerization in Slurry Process
2.5. Characterization
3. Results and Discussion
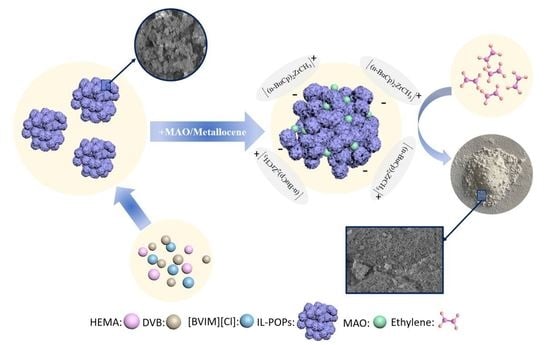
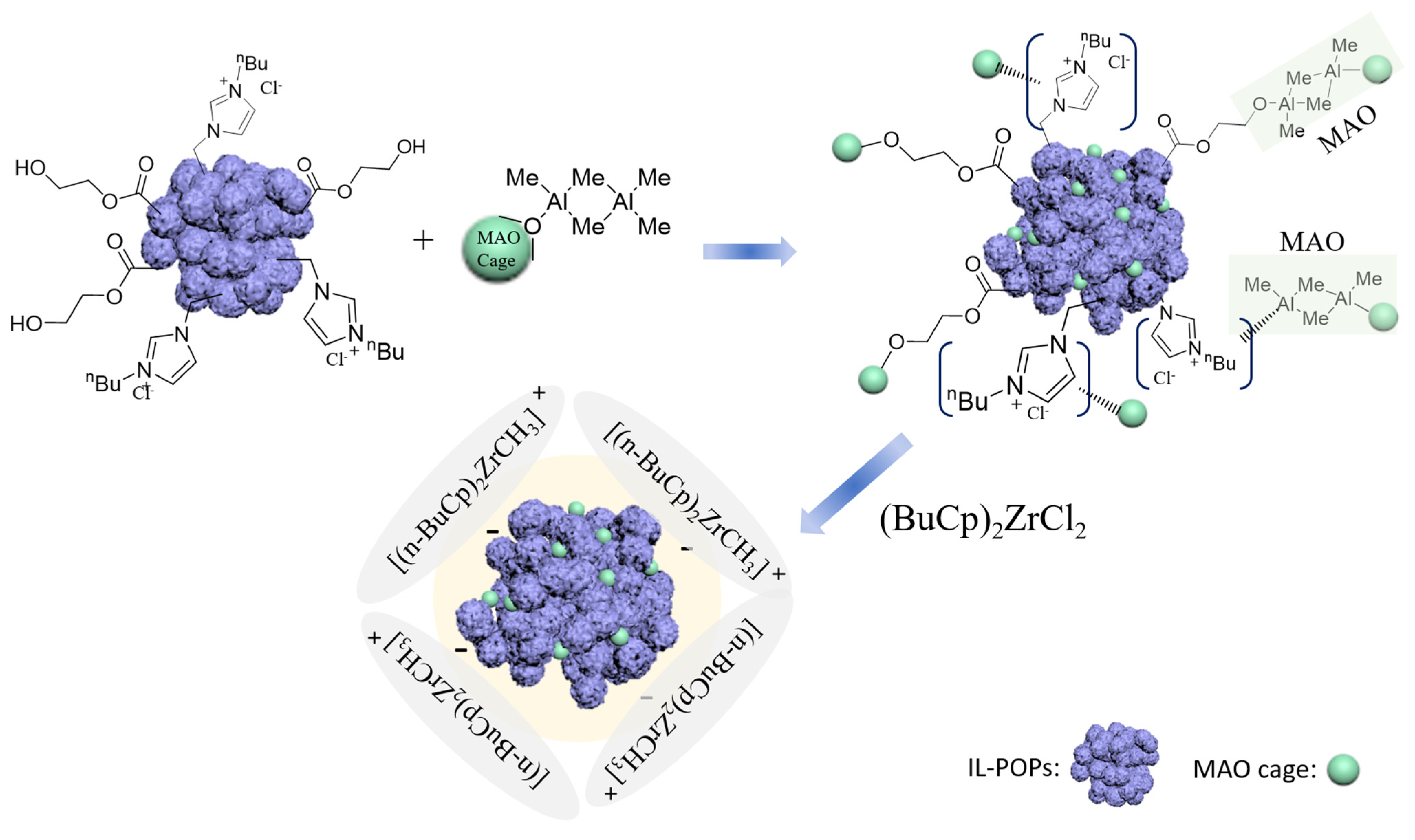
3.1. Preparation of Ionic Liquid-Modified Porous Organic Polymer
3.2. Pore Structure of Ionic Liquid-Modified Porous Organic Polymer
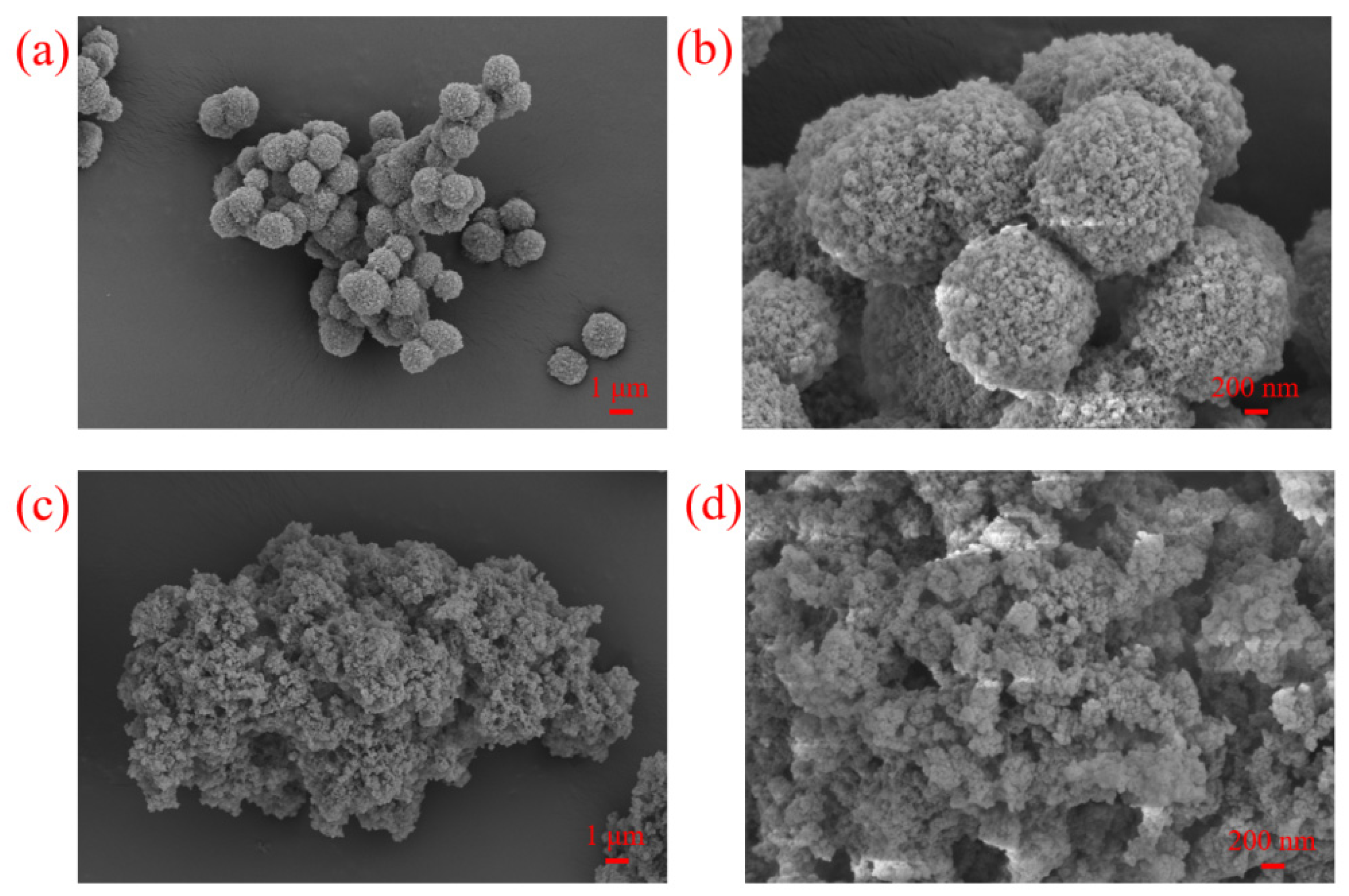
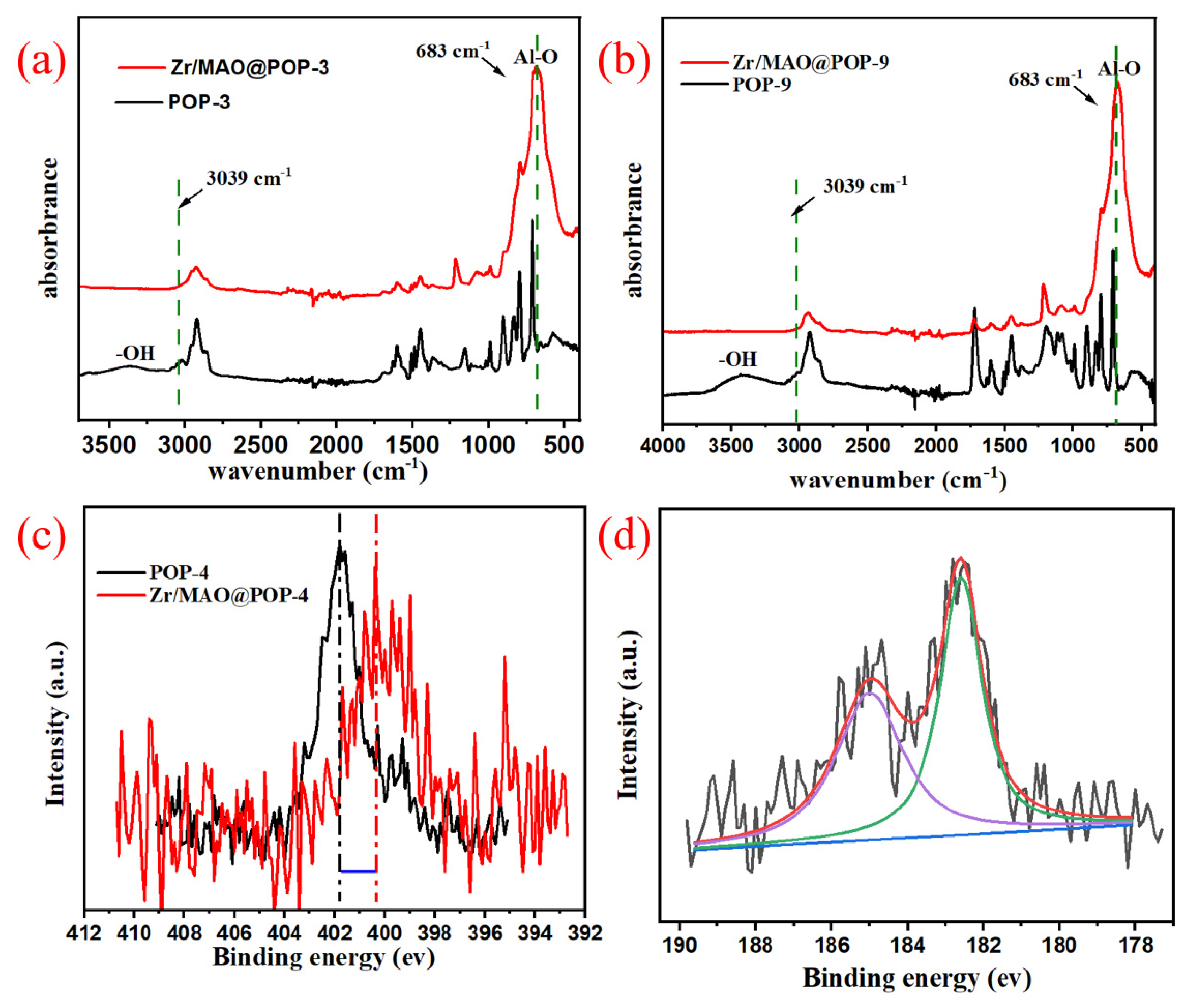
3.3. IR, TGA and SEM Analysis of IL-POPs
3.4. IL-POPs Supported Zr/MAO Catalysts
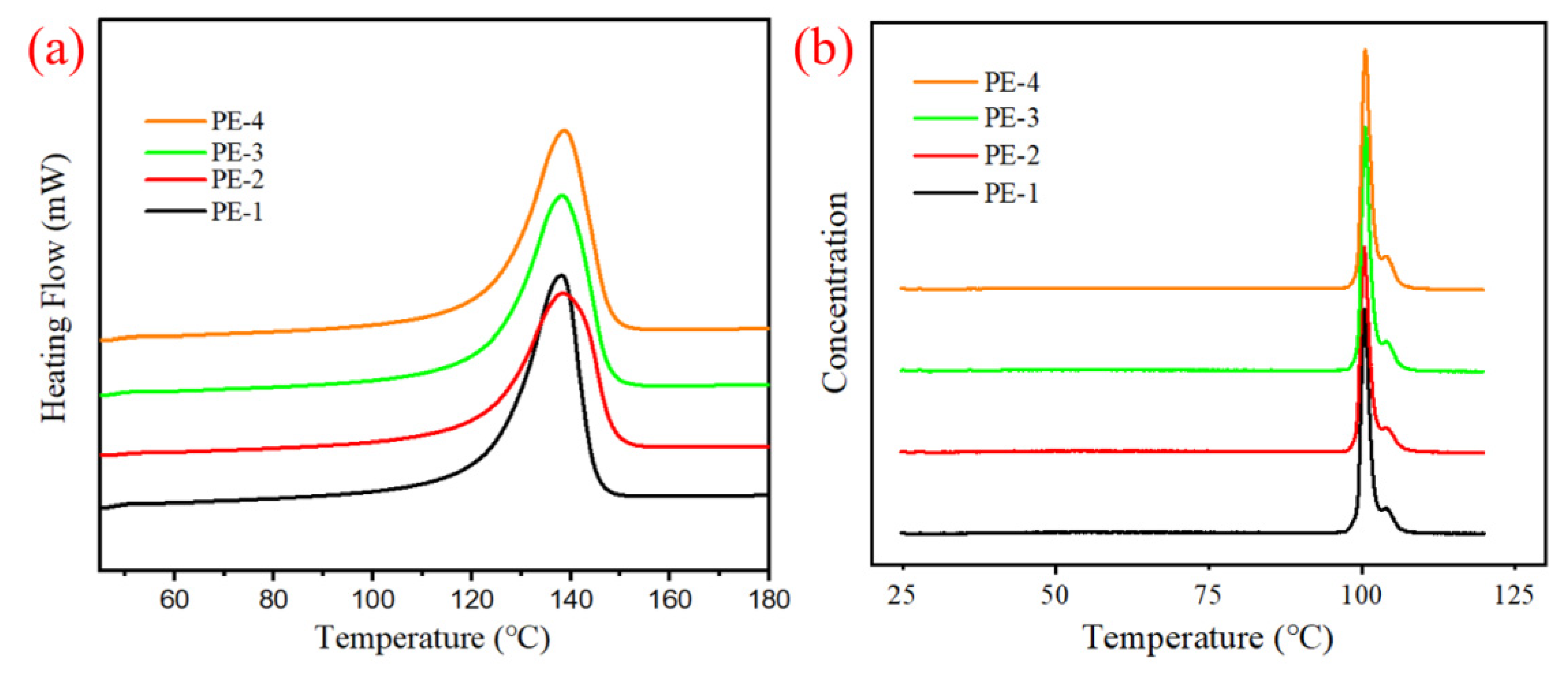
3.5. Characterization of Prepared Polyethylene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaminsky, W. The discovery of metallocene catalysts and their present state of the art. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Kaminsky, W. Polymerisation Catalysis. Springer Ser. Chem. Phys. 2004, 75, 403–440. [Google Scholar]
- Kaminsky, W. The discovery and evolution of metallocene-based olefin polymerization catalysts. Rend. Lincei 2017, 28, 87–95. [Google Scholar] [CrossRef]
- Kaminsky, W. Production of polyolefins by metallocene catalysts and their recycling by pyrolysis. Macromol. Symp. 2016, 360, 10–22. [Google Scholar] [CrossRef]
- Bae, S.M.; Sun, M.J.; Baek, J.W.; Lee, H.J.; Kim, H.; Yoon, Y.; Chung, S.; Lee, B.Y. Dinuclear Metallocene Complexes for High-Performance Supported Catalysts. Eur. Polym. J. 2020, 144, 110243. [Google Scholar] [CrossRef]
- Hejazi-Dehaghani, Z.A.; Arabi, H.; Thalheim, D.; Vidakovic, D.; Haghighi, M.N.; Veith, L.; Klapper, M. Organic Versus Inorganic Supports for Metallocenes: The Influence of Rigidity on the Homogeneity of the Polyolefin Microstructure and Properties. Macromolecules 2021, 54, 2667–2680. [Google Scholar] [CrossRef]
- Naundorf, C.; Ferrari, D.; Rojas, G.; Fink, G.; Klapper, M.; Müllen, K. Hard versus Soft Materials as Supports for Metallocene and Post-Metallocene Catalysts. Macromol. React. Eng. 2009, 3, 456–466. [Google Scholar] [CrossRef]
- Conte, A.; Marques, M.F.V. Magnesium chloride supported metallocene catalysts in olefin polymerizations. Eur. Polym. J. 2001, 37, 1887–1893. [Google Scholar] [CrossRef]
- Fátima, V.; Marques, M.; Alcantara, M. Alumina as support for metallocene catalyst in ethylene polymerization. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 9–21. [Google Scholar]
- Covarrubias, C.; Quijada, R.; Rojas, R. Ethylene polymerization using dealuminated ZSM-2 zeolite nanocrystals as an active metallocene catalyst support. Appl. Catal. A Gen. 2008, 347, 223–233. [Google Scholar] [CrossRef]
- Koch, M.; Stork, M.; Klapper, M.; Müllen, K.; Gregorius, H. Immobilization of metallocenes through noncovalent bonding via MAO to a reversibly cross-linked polystyrene. Macromolecules 2000, 33, 7713–7717. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, Y.; Xu, Q.; Yi, J. Preparation, characterization, and activity of α-Ti(HPO4)2 supported metallocene catalysts. Appl. Surf. Sci. 2016, 383, 126–132. [Google Scholar] [CrossRef]
- Maria, V.M.; Silva, O.F.C.; Coutinho, A.C.; de Araujo, A.S. Ethylene polymerization catalyzed by metallocene supported on mesoporous materials. Polym. Bull. 2008, 61, 415–423. [Google Scholar]
- Bashir, M.A.; Vancompernolle, T.; Gauvin, R.M.; Delevoye, L.; Merle, N.; Monteil, V.; Taoufik, M.; McKenna, T.F.L.; Boisson, C. Silica/MAO/(n-BuCp)2ZrCl2 catalyst: Effect of support dehydroxylation temperature on the grafting of MAO and ethylene polymerization. Catal. Sci. Technol. 2016, 6, 2962–2974. [Google Scholar] [CrossRef]
- Heurtefeu, B.; Bouilhac, C.; Cloutet, E.; Taton, D.; Deffieux, A.; Cramail, H. Polymer support of “single-site” catalysts for heterogeneous olefin polymerization. Prog. Polym. Sci. 2011, 36, 89–126. [Google Scholar] [CrossRef]
- Britcher, L.; Rahiala, H.; Hakala, K.; Mikkola, A.P.; Rosenholm, J.B. Preparation, characterization, and activity of silica supported metallocene catalysts. Chem. Mater. 2004, 16, 5713–5720. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Zhu, B.; Li, Y.; Han, X. Metal oxide as a template in the preparation of porous poly (2-hydroxyethylmethylacrylate-co-divinylbenzene) particles as a metallocene catalyst support. RSC Adv. 2016, 6, 52464–52474. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Zhu, B.; Li, Y.; Ma, Y. Synthesis and characterization of functional porous organic polymers as efficient metallocene catalyst supports. New J. Chem. 2016, 40, 8324–8333. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Han, X.; Han, Z.; Bai, Y. Highly tunable porous organic polymer (POP) supports for metallocene-based ethylene polymerization. Appl. Surf. Sci. 2017, 420, 496–503. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Liu, W.; Zhang, P. Feasibility study on the design and synthesis of functional porous organic polymers with tunable pore structure as metallocene catalyst supports. Polymers 2018, 10, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Han, X.; Ren, F.; Xu, R.; Bai, Y. Porous organic polymers-supported metallocene catalysts for ethylene/1-hexene copolymerization. Catalysts 2018, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kang, W.; Gao, L.; Li, G.; Chen, X.; Guo, Y. Highly Flowable Nano TiO2/Porous Organic Polymer (POP) Supports for Efficient Metallocene Catalysts. Nanomaterials 2021, 11, 60. [Google Scholar] [CrossRef]
- Wang, X.; Kang, W.; Li, G.; Zhang, P.; Jia, H.; Gao, D. Porous organic polymer/MMT hybrid supports for efficient metallocene catalysts. J. Mater. Sci. 2021, 56, 19253–19266. [Google Scholar] [CrossRef]
- Xiao, Y.; Dai, X.; Wang, K.; Zhou, G. High Melting Point of Linear, Spiral Polyethylene Nanofibers and Polyethylene Microspheres Obtained Through Confined Polymerization by a PPM-Supported Ziegler-Natta Catalyst. ChemistryOpen 2020, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Babucci, M.; Fang, C.Y.; Hoffman, A.S.; Bare, S.R.; Gates, B.C.; Uzun, A. Tuning the selectivity of single-site supported metal catalysts with ionic liquids. ACS Catal. 2017, 7, 6969–6972. [Google Scholar] [CrossRef]
- Sadjadi, S.; Tavakolian, M. Halloysite-Poly (ionic liquid) Nanocomposite as an Efficient Catalyst Support: Study of the Effects of Ionic Liquid Nature and Content on the Catalytic Activity. ChemistrySelect 2019, 4, 3369–3375. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Sharghi, H.; Shiri, P.; Aberi, M. An overview on recent advances in the synthesis of sulfonated organic materials, sulfonated silica materials, and sulfonated carbon materials and their catalytic applications in chemical processes. Beilstein J. Org. Chem. 2018, 14, 2745–2770. [Google Scholar] [CrossRef]
- Ochędzan-Siodłak, W.; Dziubek, K. Metallocenes and post-metallocenes immobilized on ionic liquid-modified silica as catalysts for polymerization of ethylene. Appl. Catal. A Gen. 2014, 484, 134–141. [Google Scholar] [CrossRef]
- Nuckowski, Ł.; Zalesińska, E.; Dzieszkowski, K.; Rafiński, Z.; Studzińska, S. Poly (ionic liquid) s as new adsorbents in dispersive micro-solid-phase extraction of unmodified and modified oligonucleotides. Talanta 2021, 221, 121662. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, B.; Qin, L.; Zhang, Y.; Gao, G.; He, M. Cross-linked poly (ionic liquid) as precursors for nitrogen-doped porous carbons. RSC Adv. 2019, 9, 8137–8145. [Google Scholar] [CrossRef] [Green Version]
- Eilertsen, J.L.; Rytter, E.; Ystenes, M. In situ FTIR spectroscopy during addition of trimethylaluminium (TMA) to methylaluminoxane (MAO) shows no formation of MAO–TMA compounds. Vib. Spectrosc. 2000, 24, 257–264. [Google Scholar] [CrossRef]
- Engelhard, M.H.; Droubay, T.C.; Du, Y. X-ray Photoelectron Spectroscopy Applications; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA; Environmental Molecular Sciences Laboratory (EMSL): Richland, WA, USA, 2017. [Google Scholar]
- Vayá, V.I.C.; Belelli, P.G.; Santos, J.H.Z.D.; Ferreira, M.L.; Damiani, D.E. Influence of acidic support in metallocene catalysts for ethylene polymerization. J. Catal. 2001, 204, 1–10. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Alves, M.C.M.; Cardoso, M.B.; Dos Santos, J.H.Z. Hybrid silica based catalysts prepared by the encapsulation of zirconocene compound via non-hydrolytic sol-gel method for ethylene polymerization. Appl. Catal. A Gen. 2018, 560, 225–235. [Google Scholar] [CrossRef]
- Galland, G.B.; Seferin, M.; Guimarães, R.; Rohrmann, J.A.; Stedile, F.C.; dos Santos, J.H. Evaluation of silica-supported zirconocenes in ethylene/1-hexene copolymerization. J. Mol. Catal. A Chem. 2002, 189, 233–240. [Google Scholar] [CrossRef]
- Kaminsky, W.; Sinn, H. Methylaluminoxane: Key Component for New Polymerization Catalysts. In Polyolefins: 50 Years after Ziegler and Natta II; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–28. [Google Scholar]
- Talsi, E.P.; Bryliakov, K.P.; Semikolenova, N.V.; Zakharov, V.A.; Ystenes, M.; Rytterc, E. 1H NMR characterization of intermediates formed by the activation of zirconocenes with methylaluminoxane at high Al/Zr ratios. Mendeleev Commun. 2003, 13, 46–48. [Google Scholar] [CrossRef]
- Rungswang, W.; Jarumaneeroj, C.; Parawan, T.; Jirasukho, P.; Juabrum, S.; Soontaranon, S.; Rugmai, S. Influences of molecular weight and thermal history on partial melting of polyethylene: Existence of non-lamellar crystallite. Polymer 2020, 211, 123096. [Google Scholar] [CrossRef]
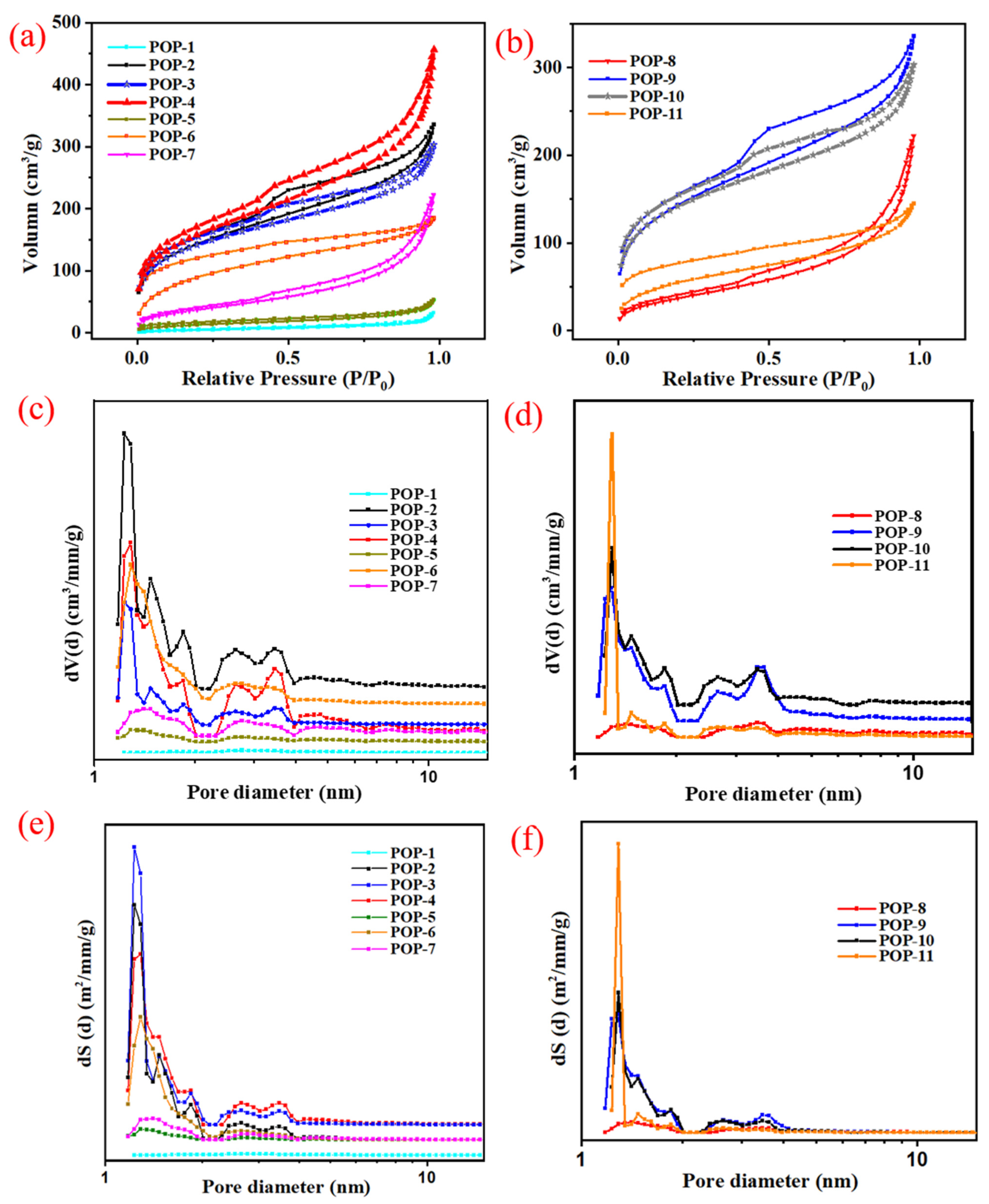


| POP No. | Solvent | FC | DVB:FC (Molar Ratio) | FCs Content (%) | SSA (m2/g) | TPV (cm3/g) | PD (nm) |
|---|---|---|---|---|---|---|---|
| POP-1 | EtOH | IL | 1:1 | 50 | 16.9 | 0.08 | 18.90 |
| POP-2 | EtOH | IL | 2:1 | 33 | 550.1 | 0.51 | 3.69 |
| POP-3 | EtOH | IL | 3:1 | 25 | 496.0 | 0.38 | 3.08 |
| POP-4 | EtOH | IL | 3:2 | 40 | 561.8 | 0.71 | 5.03 |
| POP-5 | EtOH | IL | 20:1 | 5 | 47.8 | 0.08 | 6.80 |
| POP-6 | EtOH | IL | 4:1 | 20 | 331.5 | 0.29 | 3.47 |
| POP-7 | EtOH:H2O/(9:1) | IL | 4:1 | 20 | 147.4 | 0.29 | 8.00 |
| POP-8 | EtOH | IL/HEMA (FC1/FC2) | DVB:FC1:FC2 = 4:1:1 | 43 | 139.7 | 0.34 | 9.83 |
| POP-9 | EtOH | IL/HEMA | 6:1:1 | 25 | 506.9 | 0.52 | 4.10 |
| POP-10 | EtOH | IL/HEMA | 4:1:1 | 33 | 492.7 | 0.47 | 3.80 |
| POP-11 | EtOH:H2O/(9:1) | IL/HEMA | 6:1:1 | 33 | 199.2 | 0.22 | 4.49 |
| Entry | Zr (μmol/g) | Al/Zr Molar Ratio | Cat (mg) | Yield (g) | Activity (kg PE/mol·Zr·bar·h) |
|---|---|---|---|---|---|
| Zr/MAO@POP-3 | 43.8 | 94.7 | 123 | 38.9 | 3606 |
| Zr/MAO@POP-5 | 22.9 | 55.3 | 160 | 3.67 | 499 |
| Zr/MAO@POP-9 | 38.4 | 87.3 | 144 | 35.6 | 3219 |
| Zr/MAO@POP-10 | 35.1 | 95.4 | 159 | 41.3 | 3700 |
| Entry | Samples | Tm (°C) | Mw × 104 (g/mol) | Mn × 104 (g/mol) | PDI | ||
|---|---|---|---|---|---|---|---|
| Zr/MAO@POP-3 | PE-1 | 138.1 | 61.0 | 178.7 | 22.2 | 9.4 | 2.4 |
| Zr/MAO@POP-5 | PE-2 | 138.4 | 54.0 | 158.3 | 28.7 | 10.1 | 2.8 |
| Zr/MAO@POP-9 | PE-3 | 138.7 | 61.7 | 180.9 | 18.3 | 8.5 | 2.2 |
| Zr/MAO@POP-10 | PE-4 | 138.3 | 60.2 | 176.4 | 23.7 | 10.6 | 2.2 |
| Entry | SF(%) | Peak 1 | Peak 2 | Peak 3 | Peak 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | C (%) | T(°C) | C (%) | T (°C) | C (%) | T (°C) | C (%) | ||
| PE-1 | 0.9 | 50.5 | 3.9 | 56.1 | 1.3 | 71.5 | 2.4 | 100.6 | 91.5 |
| PE-2 | 0.8 | 49.4 | 2.8 | 62.7 | 1.2 | 70.7 | 1.9 | 100.5 | 93.3 |
| PE-3 | 0.8 | 49.3 | 3.5 | 66.9 | 2.3 | 75.2 | 2.5 | 100.7 | 90.8 |
| PE-4 | 1.1 | 42.5 | 1.1 | 45.8 | 1.0 | 55.2 | 1.8 | 100.7 | 95.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Chen, S.; Wang, X.; Li, G.; Han, X.; Da, M. Ionic Liquid-Modified Porous Organic Polymers as Efficient Metallocene Catalyst Supports. Catalysts 2022, 12, 270. https://doi.org/10.3390/catal12030270
Kang W, Chen S, Wang X, Li G, Han X, Da M. Ionic Liquid-Modified Porous Organic Polymers as Efficient Metallocene Catalyst Supports. Catalysts. 2022; 12(3):270. https://doi.org/10.3390/catal12030270
Chicago/Turabian StyleKang, Wenqian, Sheng Chen, Xiong Wang, Guangquan Li, Xiaoyu Han, and Minfeng Da. 2022. "Ionic Liquid-Modified Porous Organic Polymers as Efficient Metallocene Catalyst Supports" Catalysts 12, no. 3: 270. https://doi.org/10.3390/catal12030270